Abstract
Background:
Surgical treatment of pituitary pathologies has evolved along the years, adding safety and decreasing morbidity related to the procedure. Advances in the field of radiology, coupled with stereotactic technology and computer modeling, have culminated in the contemporary and widespread use of image guidance systems, as we know them today. Image guidance navigation has become a frequently used technology that provides continuous three-dimensional information for the accurate performance of neurosurgical procedures. We present a discussion about the application of image guidance in pituitary surgeries.
Methods:
Major indications for image guidance neuronavigation application in pituitary surgery are presented and demonstrated with illustrative cases. Limitations of this technology are also presented.
Results:
Patients presenting a history of previous transsphenoidal surgeries, anatomical variances of the sphenoid sinus, tumors with a close relation to the internal carotid arteries, and extrasellar tumors are the most important indications for image guidance in pituitary surgeries. The high cost of the equipment, increased time of surgery due to setup time, and registration and the need of specific training for the operating room personnel could be pointed as limitations of this technology.
Conclusion:
Intraoperative image guidance systems provide real-time images, increasing surgical accuracy and enabling safe, minimally invasive interventions. However, the use of intraoperative navigation is not a replacement for surgical experience and a systematic knowledge of regional anatomy. It must be recognized as a tool by which the neurosurgeon can reduce the risk associated with surgical approach and treatment of pituitary pathologies.
Keywords: Image guidance, neuronavigation, pituitary, transsphenoidal
INTRODUCTION
The surgical management of pituitary lesions, as in many other fields of medicine, has evolved over time, with significant advances credited to the persistence and innovation of pioneer surgeons. Harvey Cushing advanced the field of pituitary surgery in the early 20th century with the transsphenoidal approach. However, he ended up abandoning the approach for unclear reasons most likely related to lack of technology.[12] More recently, substantial progress in the fields of radiology, endocrinology, neurosurgery, otolaryngology, and pathology has culminated in the comprehensive, multidisciplinary approach to pituitary disorders offered at contemporary medical centers. Synergistically, these advances played an essential role in decreasing morbidity and mortality of pituitary surgery.[1,9]
Radiological improvements have added accuracy and safety to all neurosurgical procedures. The first reports of preoperative imaging for sellar diseases date from 1899, when Hermann Oppenheim demonstrated that the sella turcica was enlarged in a patient with acromegaly.[1] The introduction of radiography to the management of sellar diseases validated the surgeon's pre-surgical diagnosis by allowing him or her to visualize the lesion responsible for the patient's clinical picture.
Continued advances occurred in the field of radiology, leading to better imaging, and consequently, better understanding of the pituitary gland and sellar pathologies. Information regarding the working distance between the carotid arteries, location of the optic chiasm and nerves, and exact characteristics of the disease could be well documented with these diagnostic imaging modalities; however, this did not necessarily translate to improved morbidity related to transsphenoidal surgery.[1]
Incessant efforts to reduce the surgical risks through the improvement in surgical techniques led to the innovative use of existing imaging techniques to guide the surgeon intraoperatively.[3,16] Image guidance in pituitary surgery began with the use of intraoperative air encephalography, whereby surgeons performed a lumbar puncture and injected air into the subarachnoid space in order to create contrast around pituitary tumors on intraoperative X-rays. This was particularly useful for tumors with suprasellar extension. The air not only helped to define the presence of residual tumor, but also increased the pressure in the cistern assisting any suprasellar residual tumor to be “pushed” down into the sella and surgical field allowing a better resection. Gerard Guiot added fluoroscopy to the armamentarium, which was a great improvement in transsphenoidal surgery navigation.[12] More recently, intraoperative fluoroscopy guidance added greatly to transsphenoidal surgery, allowing for frameless stereotactic navigation not only on the sagittal view, but also on a computer reconstructed coronal view.[7] For the first time in transsphenoidal image guidance, the surgeon received intraoperatively real-time information related to the laterality of the approach without the need of an AP X-ray. Such information is crucial to avoiding unplanned deviation during the approach with subsequent unexpected “sellar openings” on the face of the cavernous sinus and carotid artery.[7] Currently, the field continues to expand with the implementation of newer technologies such as computer-based stereotactic image guidance.[1,10]
Image-guided neurosurgery may be broadly defined as any neurosurgical procedure assisted by imaging, regardless of whether the images are acquired before or during the surgery.[8] Considering this, all neurosurgery could be considered “image guided.” In this paper, we will discuss the use of computer-based neuronavigation systems in the surgical management of pituitary lesions.
IMAGE GUIDANCE NAVIGATION SYSTEM
Three-dimensional image-guided navigation systems (IGNSs) have been increasingly utilized in many neurosurgical procedures.[17] Reported benefits include accuracy improvement, reduction of intervention time, improvement of quality of life, reduction of morbidity, decrease in intensive care stays and hospital costs.[2,4]
Initial intraoperative image guidance systems utilized a stereotactic frame, which required fixation of the patient's head with cranial screws during surgery. These systems evolved to frameless and wandless systems based on one of the two main tracking technologies: electromagnetic or optical. Electromagnetic tracking systems use a radiofrequency transmitter attached to a patient headset and an electromagnetic sensor integrated to a handpiece. Optical-based systems utilize an infrared camera array that localizes the instrument and head position through the detection of light-emitting diodes (LEDs) that are fixed to the surgical instrument and to a headset worn by the patient.[11,14,17]
Regardless of the tracking technology, most systems include a monitor, workstation, referential system (or receiver), and a localizer [Figure 1]. These systems provide real-time surgical navigation using preoperative computed tomographic and magnetic resonance images.[8,14]
Figure 1.
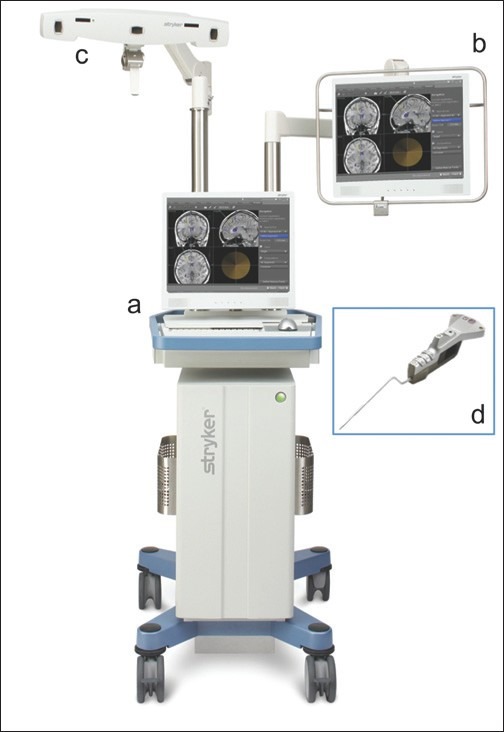
Example of image guidance neuronavigation system: (a) workstation; (b) monitor; (c) navigation camera (referential system); (d) straight pointer (localizer) [published with permission from Stryker Navigation (Kalamazoo, MI, USA)]
PITUITARY LESIONS AND INTRAOPERATIVE IMAGING NAVIGATION
Because of the close relationship to important orbital and intracranial structures, transsphenoidal approaches to the sella demand a high degree of surgical precision and an accurate understanding of the pertinent anatomical relationships. This task can be challenging in patients with poor anatomical landmarks, especially in those who have had previous surgery or who have disease invading or engulfing the surrounding structures.[9,11]
Both osseous and neurovascular structures may serve as important landmarks during transsphenoidal approaches to the sella. Hence, both computed tomography (CT) and magnetic resonance imaging (MRI) have their advantages and disadvantages – CT scans are generally optimal for identifying bone integrity and bony landmarks, while MRI is more suited for soft tissue differentiation. Accordingly, CT or MRI may be used independently or fused depending on the specific needs for a given case.[11,15] For example, surgery for microadenomas that are not apparent on routine preoperative MRI is unlikely to benefit from the use of intraoperative MRI guidance, as it will not aid in tumor identification. Therefore, CT is more prudent, as it is less costly and time consuming and offers sufficient information for safe transsphenoidal surgery. Conversely, large pituitary adenomas with suprasellar extension generally benefit from MRI navigation to better define the interface of tumor and normal anatomy. Patients with more invasive pituitary adenomas, particularly those invading the cavernous sinus, generally benefit from fusion of MRI and computed tomography angiography (CTA). Fusion of the two modalities allows for discrimination of the tumor not only with surrounding soft tissue, but also with the internal carotid artery (ICA).
Registration is the process by which digitized imaging data in the computer workstation are correlated with the patient anatomy. This is performed in the operating suite following induction of anesthesia and optimal patient positioning. Although the specific process varies depending on the manufacturer of the IGNS, registration is performed using surface landmarks. These landmarks are matched to corresponding anatomical landmarks on the MRI and / or CT. This process necessitates the use of a soft tissue window for registration to a CT scan. Following registration, the scan can be adjusted to a bony window. After completing registration and confirming accuracy, the IGNS can be properly used.[17] In general, the authors prefer to use the lateral orbital rim and the labial surface of the central maxillary incisors for confirmation of coronal plane accuracy; the maxillary incisors (mesial surface / midline), nasal tip, and nasal dorsum for sagittal plane accuracy; and the maxillary teeth (incisal surface), nasal tip, rhinion, and nasion for axial plane accuracy.
In order for the system to remain accurate, the anatomical landmarks used for registration must remain stable in reference to the localizer / tracker. Although pin-less systems offer convenience, this may come at the cost of accuracy if shifting of the patient / system occurs during the procedure. The use of a head pin holder not only allows the surgeon to minimize head movement during the case, but also helps to stabilize the relationship to the localizer / tracker for those systems that connect to the head pin device rather than the patient's head.
INDICATIONS
In our department, we currently use intraoperative navigation for all endoscopic endonasal procedures, including the transsphenoidal approaches. Although transsphenoidal surgery can be performed easily and without significant risk of complications in most patients, resection of even limited tumors may be challenging when adjacent anatomical landmarks are absent or obscured.[3]
IGNS is especially useful during revision surgery, where normal anatomical structures may be distorted.[1,5] In our experience, the presence of sphenoid sinus anomalies, lack of sphenoid pneumatization, lateral pneumatization, or multiple sphenoid sinus septae increases the chances that IGNS will be necessary [Figure 2]. Similarly, expansive and invasive macroadenomas may obliterate the normal anatomy and even displace the surrounding neurovascular structures. These tumors may expand superiorly to the suprasellar space, laterally to the cavernous sinus, or inferiorly to the clivus. Information regarding key anatomical landmarks can be provided by the intraoperative image guidance system and are valuable in order to achieve a safe tumor removal[17] [Figure 3].
Figure 2.
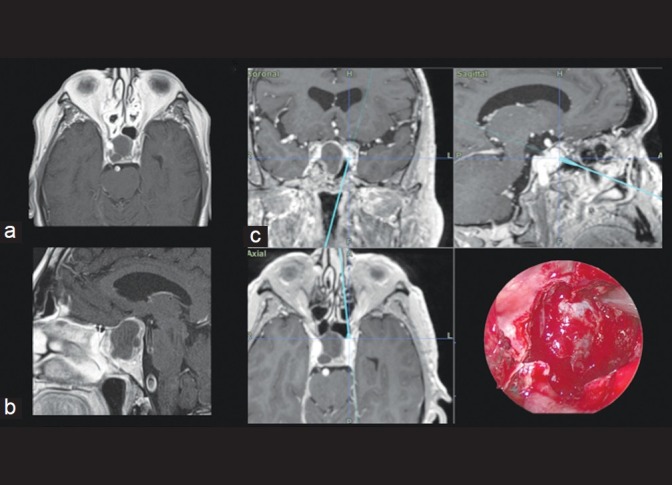
Axial (a) and coronal (b) T1-weighted MR images of a patient with a non-functioning macroadenoma and poorly pneumatized sphenoid sinus. Intraoperative neuronavigation image (c) shows sphenoid sinus presenting a left lateral pneumatization, which may make midline orientation difficult. The pneumatization leads directly to the left ICA
Figure 3.
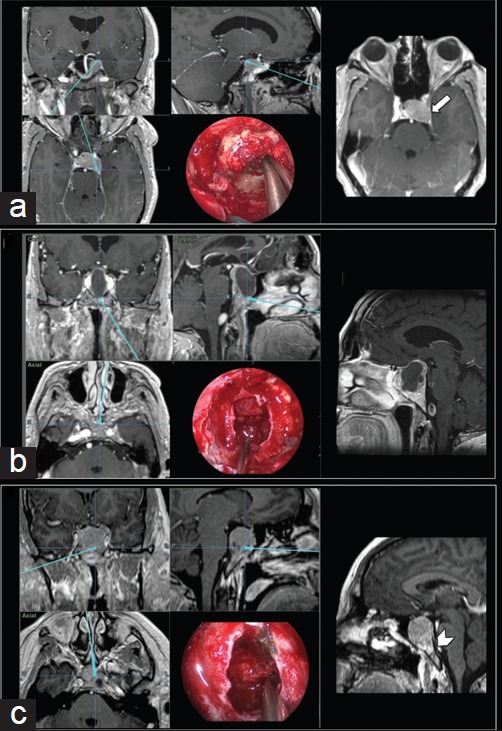
Intraoperative neuronavigation images and each respective endoscopic endonasal intraoperative view. (a) Pituitary adenoma extending laterally and posteriorly to the left cavernous sinus and Meckel's cave – detail: T1-weighted contrast-enhanced axial MR image showing tumor invasion of the left cavernous sinus (arrow). (b) Large macroadenoma with a suprasellar extension and superior displacement of the optic chiasm. (c) Upper clivus invasion by a macroadenoma
Due to the deleterious effects of carotid artery damage, any surgeon performing transsphenoidal procedures should remain acutely aware and respectful of the ICA throughout the entire procedure.[9] Accordingly, patients presenting with tumors closely related to the ICA should routinely be treated with the assistance of IGNS, as the technology not only assists in localization in the sagittal plane (to identify the midline), but also allows the surgeon to localize the ICAs and safeguard against inadvertent injury[3] [Figure 4].
Figure 4.
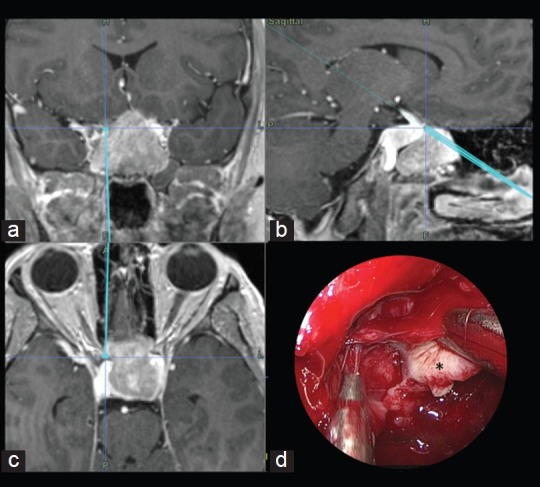
Illustrative case: A 30-year-old male presented with headaches and progressive visual loss. Endocrine evaluation showed increased Thyrotropin (TSH) levels. MR imaging was performed and a macroadenoma, partially embracing the right carotid artery, was diagnosed. The neuronavigation system was used to assist the approach and to evaluate tumor proximity to the right carotid artery. Coronal (a), Sagittal (b) and Axial (c) intraoperative neuronavigation images of a transsphenoidal approach for a macroadenoma. (d) Endoscopic endonasal surgical view of left ICA localization during tumor (*) removal
IGNS is also very helpful when addressing suprasellar tumors expanding along the anterior cranial base, when an expanded endonasal transellar transplanum approach needs to be formed. In this approach, the tuberculum sellae and planum sphenoidale are removed to provide increased access and ensure proper visualization. IGNS can assist the bone removal to the lateral limits of the carotid arteries and the anterior limit of the tumor, avoiding entering into the cribriform plate and facilitating the identification of the anatomical landmarks and identifying the extrasellar components of the tumor[1] [Figure 5].
Figure 5.

Sagittal (a), axial (b), and coronal (c and d) T1-weighted, contrast-enhanced MR images of a macroadenoma extending to the anterior fossa. (e) Endoscopic view of tumor resection during an endoscopic endonasal transellar transplanum approach. ACA: anterior cerebral artery, OCh: optic chiasm, T: tumor
Computer-based neuronavigation system may also contribute to increase surgeon proficiency. Surgeons with a not so broad experience in transsphenoidal approaches can be more confident about planning and executing the procedure, when assisted by this sort of technology. Computer-based image guidance technology is also helpful for training purposes, since visualization in different sectional planes augments the understanding of normal and pathological anatomy. Some studies report that it enhances surgical efficiency and improves the learning curve of residents.[4,5]
DISCUSSION
Advances in medical imaging technology along with the implementation of minimally invasive procedures have contributed greatly to contemporary neurosurgical practice. Today, the use of IGNS is commonplace in neurosurgery, as it is extremely useful as both a pre-surgical and intraoperative device that improves accuracy in defining anatomical structures.[2,3,5,8,10]
During pituitary surgery, IGNS may help the surgeon deal with anatomical variances and discrimination of tumor from normal tissue when the lesion extends into the extrasellar space. Without question, a thorough knowledge of the anatomy of the nose, sinuses, and anterior skull base remains the most important foundation to perform safe transsphenoidal surgeries.[4] However, previous operations, anatomical variations, and invasion of surrounding structures may critically interfere with intraoperative orientation, thus exposing the patient to greater risk.[6]
In contrast to traditional craniotomy approaches, where shifting of brain and intracranial structures may reduce intraoperative accuracy of intraoperative navigation, transsphenoidal surgery is particularly suited to the use of IGNSs, as the landmarks are generally bony and not prone to shifting [Table 1].
Table 1.
Advantages and limitations of the image-guided navigation systems for pituitary surgery
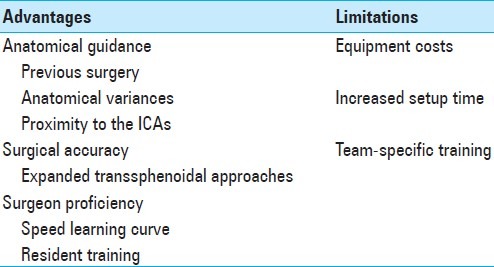
Disadvantages related to the use of IGNSs exist and should be considered. One limitation to the widespread of this technology is the cost added to the surgical procedure.[1,8] In addition to the increased direct costs associated with using the technology, indirect costs may be incurred if the setup is onerous (especially if the use of the IGNS does not translate to decreased actual operative time). It should be noted, however, that the use of IGNS has shown to add little time to surgical cases in experienced hands. Accordingly, proper training is necessary for the personnel in the operating room.[6] Furthermore, the technology may necessitate a larger operating room and rearrangement of other equipments in the room. This is especially salient when working with optical systems which require a clear line of sight between the infrared camera, reference arch, and pointer instruments, or with the magnet system which requires a clear magnetic field free of other metals that can cause interference.[8,13,17] Another limitation we have encountered in magnetic systems is related to the artifact that it may generate in neurophysiologic monitoring. The magnetic field interferes with the current generated in the electrodes and may make wave interpretation unfeasible.
CONCLUSION
Major innovations in medical imaging have played a key role in decreasing morbidity and mortality once associated with pituitary surgery. Intraoperative IGNS provides real-time stereotactic feedback, increasing surgical accuracy and enabling safe and effective minimally invasive interventions.[17] However, the use of intraoperative neuronavigation is not a replacement for surgical experience and a systematic knowledge of regional anatomy.[4] It must be recognized as a tool by which the neurosurgeon can reduce the risk associated with surgical access and treatment of pituitary pathologies.
Publication of this manuscript has been made possible by an educational grant from
 BRAINLAB
BRAINLAB
Footnotes
Disclaimer: The authors of this paper have received no outside funding, and have nothing to disclose.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/3/73/95418
Contributor Information
Danielle de Lara, Email: danielle.delara@osumc.edu.
Leo F. S. Ditzel Filho, Email: leo.ditzel@osumc.edu.
Daniel M. Prevedello, Email: Daniel.prevedello@osumc.edu.
Bradley A. Otto, Email: brad.otto@osumc.edu.
Ricardo L. Carrau, Email: ricardo.carrau@osumc.edu.
REFERENCES
- 1.Asthagiri AR, Laws ER, Jr, Jane JA., Jr Image guidance in pituitary surgery. Front Horm Res. 2006;34:46–63. doi: 10.1159/000091571. [DOI] [PubMed] [Google Scholar]
- 2.Barillot C, Coupe P, El Ganaoui O, Gibaud B, Hellier P, Jannin P, et al. Image guidance in neurosurgical procedures, the “VisAGeS” point of view. 2007 4th IEEE International Symposium on Biomedical Imaging: Macro to Nano. 2007;1-3:1056–9. [Google Scholar]
- 3.Charalampaki P, Reisch R, Ayad A, Welschehold S, Conrad J, Wuster C. Image-guided endonasal transsphenoidal microsurgical treatment of recurrent microadenomas of the pituitary gland. Minim Invasive Neurosurg. 2006;49:93–7. doi: 10.1055/s-2006-932170. [DOI] [PubMed] [Google Scholar]
- 4.Eliashar R, Sichel JY, Gross M, Hocwald E, Dano I, Biron A, et al. Image guided navigation system--A new technology for complex endoscopic endonasal surgery. Postgrad Med J. 2003;79:686–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Gong J, Mohr G, Vezina JL. Endoscopic pituitary surgery with and without image guidance: An experimental comparison. Surg Neurol. 2007;67:572–8. doi: 10.1016/j.surneu.2006.08.083. discussion 578. [DOI] [PubMed] [Google Scholar]
- 6.Heermann R, Schwab B, Issing PR, Haupt C, Hempel C, Lenarz T. Image-guided surgery of the anterior skull base. Acta Otolaryngol. 2001;121:973–8. [PubMed] [Google Scholar]
- 7.Jagannathan J, Prevedello DM, Ayer VS, Dumont AS, Jane JA, Jr, Laws ER. Computer-assisted frameless stereotaxy in transsphenoidal surgery at a single institution: Review of 176 cases. Neurosurg Focus. 2006;20:E9. doi: 10.3171/foc.2006.20.2.10. [DOI] [PubMed] [Google Scholar]
- 8.Kucharczyk W, Bernstein M. Do the benefits of image guidance in neurosurgery justify the costs? From stereotaxy to intraoperative MR. Am J Neuroradiol. 1997;18:1855–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroki A, Kayama T. Endoscopic approach to the pituitary lesions: Contemporary method and review of the literature. Biomed Pharmacother. 2002;56(Suppl 1):158s–64. doi: 10.1016/s0753-3322(02)00228-7. [DOI] [PubMed] [Google Scholar]
- 10.Mayberg MR, LaPresto E, Cunningham EJ. Image-guided endoscopy: Description of technique and potential applications. Neurosurg Focus. 2005;19:E10. doi: 10.3171/foc.2005.19.1.11. [DOI] [PubMed] [Google Scholar]
- 11.Metson R, Gliklich RE, Cosenza M. A comparison of image guidance systems for sinus surgery. Laryngoscope. 1998;108:1164–70. doi: 10.1097/00005537-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Prevedello DM, Doglietto F, Jane JA, Jr, Jagannathan J, Han J, Laws ER., Jr History of endoscopic skull base surgery: Its evolution and current reality. J Neurosurg. 2007;107:206–13. doi: 10.3171/JNS-07/07/0206. [DOI] [PubMed] [Google Scholar]
- 13.Smouha EE, Shapiro AW, Davis RP, Shindo ML, Sobol LL, Acker DE. Image-guided surgery of the skull base using a novel miniature position sensor. Skull Base Surg. 1999;9:101–7. doi: 10.1055/s-2008-1058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyderman C, Zimmer LA, Kassam A. Sources of registration error with image guidance systems during endoscopic anterior cranial base surgery. Otolaryngol Head Neck Surg. 2004;131:145–9. doi: 10.1016/j.otohns.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Suess O, Kombos T, Kurth R, Suess S, Mularski S, Hammersen S, et al. Intracranial image-guided neurosurgery: Experience with a new electromagnetic navigation system. Acta Neurochir (Wien) 2001;143:927–34. doi: 10.1007/s007010170023. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Asai J, Nagashima G, Itokawa H, Chang CW, Noda M, et al. Transcranial echo-guided transsphenoidal surgical approach for the removal of large macroadenomas. J Neurosurg. 2004;100:68–72. doi: 10.3171/jns.2004.100.1.0068. [DOI] [PubMed] [Google Scholar]
- 17.Wiltfang J, Rupprecht S, Ganslandt O, Nimsky C, Kessler P, Schultze-Mosgau S, et al. Intraoperative image-guided surgery of the lateral and anterior skull base in patients with tumors or trauma. Skull Base. 2003;13:21–9. doi: 10.1055/s-2003-820554. [DOI] [PMC free article] [PubMed] [Google Scholar]


