Structured Abstract
Purpose of the Review
To provide a state-of-the-art knowledge on the implications of microRNA (miRNA) dys-regulation in lymphoid malignancies.
Recent Findings
Several new studies have broadened our understanding of how aberrancies of the miRNome contribute to the development of a malignant lymphoid phenotype. Recently, a new pathogenetic model involving miRNAs and protein coding genes (such as TP53 and ZAP-70) has been identified and as the prognostic implications of the most recurrent chromosomal abnormalities in human B-chronic lymphocytic leukemia. Moreover, new recent studies have addressed the role of miRNAs in human lymphomas and acute lymphoblastic leukemias.
Summary
The recent advances in our understanding of the role of miRNAs in lymphoid malignancies demonstrate that miRNAs can effectively be used as tumoral biomarkers with diagnostic, prognostic and predictive-of-response-to-therapy implications.
Keywords: microRNAs, lymphoma, leukemia, biomarkers
Introduction
MicroRNAs (miRNAs) are non-coding RNAs (ncRNAs) that regulate gene expression and affect several biological processes, spanning from development, differentiation, cell cycle regulation to senescence and metabolism (1-5).
The biogenesis of miRNAs has been described in several excellent reviews (6, 7). The full spectrum of miRNAs expressed in a specific cell type (the miRNome) varies between normal and pathologic tissues (8), and specific signatures of deregulated miRNAs harbor diagnostic and prognostic implications (9, 10). The first link between miRNAs and cancer came from the discovery of loss of miR-15a and miR-16-1 in chronic lymphocytic leukemia (11) and from the discovery that these ncRNAs are frequently located in cancer associated genomic regions (CAGRs), which include minimal regions of amplification, loss of heterozygosity, common breakpoint regions in or near oncogenes (OGs) and tumor suppressor genes (TSGs), and fragile sites (preferential sites of chromatid exchange, deletion, translocation, amplification or integration of plasmid DNA and tumor-associated viruses) (12). Since then, a plethora of studies have investigated the aberrancies of the miRNome in almost all types of human cancer, both solid tumors and hematological malignancies (for reviews see (10, 13-18)). In particular, while some miRNAs act mainly as TSGs, others exert a predominantly oncogenic function, although labeling a miRNA as exclusively a TSG or an OG is inappropriate, given that the overall function of any given miRNA seems to depend on its cellular context. The present review will focus on the most recent discoveries in the field of miRNAs and lymphoid malignancies, which led to the identification of new pathogenetic molecular networks with prognostic significance and potential therapeutic implications.
microRNAs in Lymphoid Leukemias
Specific signatures of de-regulated miRNAs have been described in both acute and chronic lymphocytic leukemias (ALL and CLL, respectively). Table 1 summarizes the main de-regulated miRNAs in human lymphoid malignancies further discussed in this review. High levels of miR-128b have been described in ALL (19), and high expression of miR-128a and miR-128b can distinguish between ALL and AML (acute myeloid leukemia) (20), with an accuracy of 98% (20), making these two miRNAs diagnostic biomarkers for acute leukemia. More recently, Zhang et al., have identified a miRNA signature in children with ALL complicated by central nervous system (CNS) relapse. High levels of miR-7, -198 and -663, and low expression of miR-126, -345, -222, and -551a associate with a higher risk of CNS relapse (21). Intriguingly, some of the predicted targets of this “miRNA cascade” include neuron function- and neurotransmitter- related genes, although an experimental validation of this targeting is still needed. The miR-17-92 cluster has been correlated with the development of mixed lineage leukemia (MLL)-rearranged acute leukemia (22). Up-regulation of this cluster was observed not only in MLL-associated AML, but also in ALL, and is possibly due to both DNA copy number amplification at 13q31, and to direct up-regulation by MLL fusions (22). Kaddar et al., have investigate the prognostic significance of miR-16 in childhood ALL and its relationships to normal and malignant lymphocyte proliferation (23). Overall, they found that low levels of miR-16 are associated with a better outcome, an unexpected discovery, since it has been shown that miR-16 directly targets several anti-apoptotic genes (such as BCL2, MCL1 and CDK6 (24)), therefore harboring proapoptotic effects. Interestingly, we have recently demonstrated that in addition to anti-apoptotic genes, the miR-15a/16-1 cluster also directly targets the tumor suppressor gene TP53, in chronic lymphocytic leukemia (CLL) (25), suggesting that the cluster has a dual role, both as an OG and as a TSG. It will be interesting to investigate whether miR-16 also targets TP53 in ALL, which could provide a molecular explanation for the positive prognostic implications of this miRNA in pediatric ALL. MiRNAs are also involved in viral-induced leukemias. The human T-cell leukemia virus type-1 (HTLV-1) is responsible for adult T-cell leukemia, a very aggressive CD4+ malignancy. It has been demonstrated that several miRNAs are de-regulated in HTLV-1-infected T-lymphocytes when compared to uninfected cells. Among them, miR-146a is strongly up-regulated by the viral infection (26). In particular, the virus encoded trans-activator protein tax is able to induce miR-146a, which in turn affects the expression of several target genes (such as IRAK1, TRAF-6, IL-8, CXCR4, and MMP9) (26). How the tax-miR-146a trans-activation leads to a proliferating effect on infected lymphocytes still needs to be established but this interaction might cast a new light on the mechanism of HTLV-1-mediated leukemogenesis.
Table 1. List of most frequently de-regulated miRNAs in lymphoid malignancies.
| Disease | miRNA | Expression | Biomarker | Comment | Reference |
|---|---|---|---|---|---|
| ALL | miR-128b | High | D | 19 | |
| miR-128a and miR-128b | High | D | Differentiate ALL from AML | 20 | |
| miR-7, miR-198, miR-663 | High | P | Higher risk of CNS relapse | 21 | |
| miR-126, miR-345, miR-222, miR-551a | Low | P | Higher risk of CNS relapse | 21 | |
| miR-17-92 cluster | High | D | 22 | ||
| miR-16 | Low | P | Better prognosis in childhood ALL | 23 | |
| CLL | miR-15a/16-1 cluster | Low | D, P | Down-regulated in 13q deleted CLL; Associates with indolent CLL, by targeting TP53 | 11, 25 |
| miR-34b/34c cluster | Low | P | In 11q deleted CLL, aggressive disease, high levels of ZAP-70 | 25 | |
| miR-29b and miR-181b | Low | P | Down-regulated in aggressive CLL, characterized by high levels of their direct target TCL1 | 35 | |
| miR-29c and miR-223 | Low | P | Associates with TFS and OS | 37 | |
| miR-155 | High | P | Aggressive CLL | 9, 41 | |
| NHLs | miR-155 | High | D, P | Induces ALL/high grade lymphoma in transgenic mouse models; Distinguishes ABC-DLBCL and GBC-DLBCL | 43, 48, 49 |
| miR-21 and miR-221 | High | D, P | High expression in ABC-DLBCL | 49 | |
| miR-21 | High | D | Key miRNA in B-lymphomagenesis | 50 | |
| miR-330, miR-17-5p, miR-106a, and miR-210 | High/Low | D | Distinguish DLBCL, FL and reactive lymph nodes | 51 | |
| miR-17-92 cluster | High | D, PR | High expression in 65% of B-cell lymphomas; Associated with resistance to radiotherapy | 53, 56 | |
| miR-34a | High | PR | Confers drug resistance in B-lymphocytes over-expressing Myc | 58 | |
| miR-143 and miR-145 | Low | D | In BL and other NHL | 60 | |
| HL | miR-9 and let-7a | High | D | 63 | |
| miR-96, miR-128a, and miR-128b | Down | D | Selectively down-regulated in HL cells infected with EBV | 64 | |
| miR-150 | Low | D | 65 | ||
| miR-155 | High | D | 65 | ||
| miR-135a | Low | P | Higher relapse risk and shorter DFS | 67 | |
| SS | miR-21 | High | D | 61 | |
| MF | miR-155, miR-92a and miR-93 | High | D | In MF versus SS | 62 |
ALL= Acute Lymphocytic Leukemia; AML= Acute Myeloid Leukemia; CLL= Chronic Lymphocytic Leukemia; NHLs= Non-Hodgkin Lymphomas; HL= Hodgkin Lymphoma; SS= Sézary Syndrome; MF= Mycosis Fungoides. D= Diagnostic Biomarker; P= Prognostic Biomarker; PR= Predictive-of-response-to-treatment Biomarker; CNS= Central Nervous System; TFS= Treatment Free Survival; OS= Overall Survival; DLBCL= Diffuse large B-cell lymphoma; ABC-DLBCL= Activated B-cell variant of DLBCL; GBC-DLBCL= Germinal center B-cell-like variant of DLBCL; FL= Follicular Lymphoma; BL= Burkitt Lymphoma; EBV= Epstein Barr Virus; DFS= Disease Free Survival.
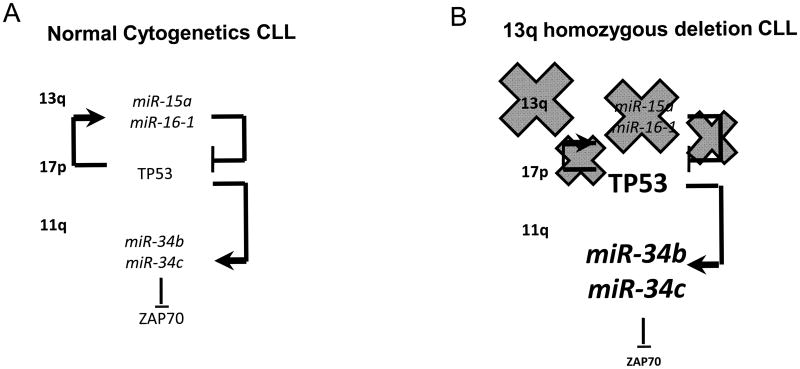
CLL is characterized by common and recurrent chromosomal aberrations (27). The most frequent of these abnormalities is the 13q14 deletion. Based on the analysis of a large number of CLL cases with monoallelic 13q14 deletion, a minimal deleted region (MDR) has been defined. This MDR includes a long non-coding RNA, called DLEU2 (deleted in leukemia 2), strongly conserved among vertebrates, and the first exon of the DLEU1 gene, another non-coding RNA (28, 29). The miR-15a/16-1 cluster is located within intron 4 of DLEU2, and genetic alterations affecting DLEU2 mRNA expression would also affect miR-15a/16-1 cluster expression (11). Therefore, the expression of miR-15a/16-1 is reduced in the majority of CLL patients carrying the 13q deletion (11). One of the main molecular signatures of B-CLL is the increased expression of the anti-apoptotic BCL2 in the mostly non-dividing malignant B lymphocytes, and we first demonstrated that BCL2 is a direct target of miR-15a/16-1 (24), explaining the indolent course of the disease in patients who carry the 13q deletion. In fact, restoration of miR-15a/16-1 expression induces apoptosis in leukemic cell lines both in vitro and in vivo (24, 30). Interestingly, we also identified a signature of de-regulated miRNAs that distinguishes between the indolent and the aggressive form of CLL (9). CLL is characterized by an extremely variable clinical course and the available clinical staging systems are relatively poor predictors of disease behavior. MiRNAs can effectively stratify CLL patients based on the biological aggressiveness of their disease. Surprisingly, high levels of miR-15a/16-1 (and of the paralogous cluster miR-15b/16-2 on chromosome 3), are associated with the de novo aggressive CLL phenotype (9). This finding prompted us to investigate other possible molecular mechanisms, able to explain this apparent paradox. We identified a new pathogenetic network linking the three most common chromosomal abnormalities in CLL (the 13q, the 17p and the 11q deletions), to their prognostic implications (good prognosis for CLLs with the 13q deletion, bad prognosis for CLLs with the 17p and 11q deletion alone or in association with the 13q deletion). We demonstrated that the miR-15a/16-1 cluster (on chromosome 13q), the tumor suppressor TP53 gene (on chromosome 17p), the miR-34b/34c cluster (on chromosome 11q) and ZAP-70, a surrogate prognostic marker for CLL, are the molecular effectors involved in this network. In particular, miR-15a/16-1 directly target TP53 and are also trans-activated by TP53. TP53 also trans-activates the miR-34b/34c cluster, which in turn directly targets ZAP-70. Overall, we have outlined a new model of CLL pathogenesis that accounts for the prognostic implications of three of the most frequent recurrent chromosomal abnormalities in B-CLL (25) (Figure 1).
Figure 1. A network involving miRNAs and Protein Coding Genes is involved in B-CLL pathogenesis and can explain the prognostic implications of the most recurrent chromosomal abnormalities in B-CLL.
A. In CLL with normal cytogenetics, the miR-15a/16-1 cluster (located at 13q) is transactivated by TP53 (on chromosome 17p) and directly silences this tumor suppressor gene. TP53 can also transactivate the miR-34b/34c cluster (located at 11q), which directly target the surrogate prognostic marker ZAP-70.
B. In CLL carrying the 13q deletion (the most common chromosomal abnormality), the miR-15a/16-1 cluster is absent because of the deletion, which releases TP53 from the inhibitory control by the cluster. High levels of TP53, activate the expression of the miR-34b/34c cluster, which targets ZAP-70 and reduces the expression of this tumoral marker.
Recently, Klein et al. investigated the causal involvement of 13q14-MDR-encoded tumor suppressor genes in CLL pathogenesis. They generated two groups of transgenic mice models: one mimicking the MDR, the other containing a specific deletion of the miR-15a/16-1 cluster. Both groups developed indolent B-clonal lymphoproliferations, with low penetrance (40% MDR, 25% miR-15a/16-1 deletion) (31). Although the same spectrum of clonal lymphoproliferative disorders was observed in both animal models, the disease was more aggressive in the MDR group than in the miR-15a/16-1 group, suggesting that additional genetic elements in the 13q14 region may affect the severity of the disease. Another well known CLL mouse model is the New Zealand black (NZB) strain that spontaneously develops a CD5-positive lymphoproliferation at an advanced age. This strain carries a point mutation close to the stem-loop of miR-16-1 (32). This mutation leads to abnormal processing of miR-16-1 and reduced expression of the mature miR-16. Although NZB mice develop CLL later than miR-15a/16-1 deleted mice (31, 32), suggesting a milder phenotype, a germline mutation that has been described in a small fraction of human CLL cases (9), has been associated with cases of familial CLL.
The oncogene TCL1 (T-cell leukemia/lymphoma 1A) is a marker of aggressive CLL and associates with other markers of poor prognisis such as high levels of ZAP-70 and unmutated IgVH status (33, 34). Pekarsky et al., have demonstrated that TCL1 is also regulated by two miRNAs, namely miR-29b and miR-181b (35). FurthermoremiR-181a directly targets BCL2 (36), suggesting a molecular concerted targeting action of members of the miR-181 family and of the miR-15a/16-1 cluster in the regulation of BCL2 expression. In a cohort of 110 CLL patients, with a median follow up of 72 months,
Stamatopoulos et al., found that downregulation of miR-29c and miR-223 are predictive of treatment free survival (TFS) and overall survival (OS) (37). Low expression of miR-223, -29b, -29c, and miR-181 family are associated with disease progression in CLL cases harboring the 17p deletion, whereas patients carrying the trisomy 12 abnormality and high expression of miR-181a experience a more aggressive variant of CLL (38). Interestingly, miR-29 family has been demonstrated to control key epigenetic mechanisms (such as the expression of all three main DNA methyltranferases) both in solid tumors and in hematological malignancies (39, 40). Therefore further studies to interpret the prognostic significance of miR-29 de-regulation based on the epigenetic modifications induced by this miRNA family are warranted. Another miRNA frequently up-regulated in several types of cancer is miR-155, expression of which is higher in aggressive CLL versus normal CD19+ B cells and indolent CLLs (9, 41). Recently, it has been demonstrated that the miR-155 host gene in the long non-coding RNA BIC (B-cell integration cluster) is regulated by MYB (42), providing new evidence for a potential role for this transcription factor in aggressive CLLs.
miRNAs in Lymphomas
De-regulation of miRNAs has been described in both Hodgkin (HL) and non-Hodgkin lymphomas (NHL).
One of the most commonly up-regulated miRNAs in both lymphoma groups is miR-155. In a transgenic mouse model with conditional miR-155 over-expression in B-lymphocytes, a polyclonal pre-leukemic pre-B-cell proliferation followed by full blown B-cell malignancy was described (43). The chain of events leading to the accumulation of large pre-B cells and acute lymphoblastic leukemia/high-grade lymphoma in these mice is initiated by miR-155 targeting of SHIP (Src homology 2 domain-containing inositol-5-phosphatase), and C/EBPbeta (CCAAT enhancer-binding protein beta), two key regulators of the interleukin-6 signaling pathway (44). In miR-155 knock-out mice models, the observed phenotype consisted mainly in a defect in cytokine production that interfered with TH2 lymphocyte differentiation, and compromised dendritic cell function (45, 46). In a subtype of NHL, the diffuse large B-cell lymphoma (DLBCL), miR-155 also carries prognostic implications. The 5-year survival rates for the activated B-cell variant (ABC-DLBCL), and the germinal center B-cell-like variant (GBC-DLBCL) are 30% and 59% respectively (47), and high levels of miR-155 are characteristic of ABC-DLBCL (48, 49). This suggests that miRNA is a biomarker of poor prognosis for DLBCL. Similarly, higher levels of miR-21, and miR-221 are associated with the variant of DLBCL with the worst outcome (49).
Recently, Medina et al. have provided the first in vivo evidence that miR-21 acts as a key oncogene in B-lymphomagenesis (50). The authors generated mice conditionally expressing miR-21 and observed that this miRNA drives a pre-B malignant lymphoid-like phenotype, which completely regresses when miR-21 is inactivated. Interestingly, this study also demonstrates that tumors can become addicted to oncogenic miRNAs, and supports efforts to target these miRNAs pharmalogically as a strategy for cancer treatment.
In addition to prognostic biomarkers, miRNAs can also be used effectively for differential diagnosis of NHLs. A group of four miRNAs (composed of miR-330, -17-5p, -106a, and -210) is able to distinguish DLBCL, follicular lymphoma and reactive lymph nodes, with an accuracy of 98% (51). By investigating genome-wide miRNA expression and copy number in 86 DLBCLs (59 primary tumors and 27 cell lines), Li et al identified a signature of miRNAs that segregated DLBCLs into three groups with different prognosis and markedly different MYC transcriptional activity (52). These groups were not related to the cell of origin, or to the extent of T-cell infiltrate or to the tumor site, but represent newly identified, miRNA-driven prognostic groups. Up-regulation of the miR-17-92 cluster occurs in about 65% of B-cell lymphomas (53). When this cluster is over-expressed in murine multipotent progenitor cells of MYC-transgenic mice,n increased lymphomagenesis was observed (53). Moreover, miR-17-92 transgenic mice models havet a higher rate of lymphoproliferative disorders, autoimmunity and premature death (54) and these effects are mediated by the direct targeting of two tumor suppressor genes: PTEN and BIM (54). Interestingly, two members of the miR-17-92 cluster (miR-17-5p and miR-20a) are transactivated by the transcription factor c-MYC (55), and in turn the cluster directly targets E2F1, a c-MYC transactivated transcription factor promoting cell-cycle progression (55). Therefore, the miR-17-92 cluster functions as a fine tuner of c-MYC regulatory function on cell-cycle progression. Recently, it has been demonstrated that the miR-17-92 cluster also significantly enhances resistance to radiotherapy in human mantle cell lymphoma cells (56), revealing a role for this cluster as a predictive biomarker of response to treatment. Another effect of c-MYC on the miRNome is miR-34a downregulation (57). Recently, Sotillo et al., showed that in B-lymphoid cells over-expressing Myc, miR-34a confers drug resistance, by inhibiting Tp53-dependent bortezomib-induced apoptosis (58). This result was unexpected, since upregulation of miR-34a by Tp53 is thought to enhance its acetylation and contribute to the pro-apoptotic effects of this tumor suppressor (59). This study casts a new light on the role of miR-34a in B-malignancies and confirms that a miRNA can act both as an OG and as a TSG, depending on the cellular environment. Reduced expression of miR-143 and miR-145 has also been documented in B-cell malignancies (60). Re-expression of these miRNAs in a Burkitt lymphoma cell line demonstrated a dose-dependent growth inhibitory effect, mediated in part by miRNA induced down-regulation of the oncogene ERK5 (60).
Recently, two studies have investigated the miRNA profile in cutaneous lymphomas. In a recent study, van der Fits et all have demonstrated high levels of miR-21 in Sézary Syndrome (SS), a cutaneous T-cell lymphoma with CD4+ tumor cells (Sézary cells) present in the skin, lymph nodes, and peripheral blood (61). They also showed that STAT3, a transcription factor induced by IL-21, directly activates miR-21, and that this mechanism is involved in miR-21 de-regulation in SS. The same group also published the first profile of de-regulated miRNA in mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma. High levels of miR-155, miR-92a, and miR-93 were associated specifically with MF, and not with SS (62), suggesting a possible role for these three miRNAs in the differential diagnosis of these two cutaneous lymphomas.
In Hodgkin Lymphoma (HL), high levels of miR-9 and let-7a were described, paralleling low expression of their target PRDM1/Blimp-1 (63). In a study conducted in 49 HL primary samples, Navarro et al. demonstrated that a signature of 25 miRNAs could distinguish HL from reactive lymph nodes, and another 36 miRNAs were able to differentiate between nodular sclerosis and the mixed cellularity subtype of HL (64). Interestingly, miR-96, -128a and -128b are selectively downregulated in HL cells with Epstein Barr virus (EBV) infection, but only one of these miRNAs is part of the signature of 25 de-regulated miRNAs in HL versus reactive lymph nodes, suggesting that EBV might not be relevant in driving HL pathogenesis. In HL cell lines, down-regulation of miR-150 and up-regulation of miR-155 are frequent (65). Since HL has its origin in the germinal center (GC), and high levels of miR-155 have also been described in GC during normal lymphopoiesis, this suggest that the observed up-regulation of miR-155 in HL might be the result of an abnormal block to lymphocyte differentiation at the GC level. In another study, Van Vlierberghe et al., compared the miRNome of microdissected Reed Sternberg cells and Hodgkin cell lines versus CD77+ B-cells, and found a signature of 12 up- and 3 down-regulated miRNAs (66). This signature overlaps only in part with the one observed in HL patients (64), and this may be due to the fact that a microdissection procedure was used in only one of the two studies. Finally, miRNAs can also have prognostic significance in HL. Low expression of miR-135a is associated with a higher relapse risk and a shorter disease free survival (67). These effects are in part mediated by miR-135a direct targeting of JAK2, a cytoplasmic tyrosine kinase involved in a specific subset of cytokine receptor signaling pathways. By targeting JAK2, miR-135a induces down-regulation of the antiapoptotic BCL-XL, therefore leading to apoptosis and decreased cell growth (67).
Conclusion
The involvement of miRNAs in the pathogenesis of lymphoid malignancies is now well established. The identification of the molecular effectors mediating the effects of the miRNome de-regulation on the development of malignancy is undergoing, and suggesting potential new strategies to overcome such aberrations. In addition to new promising therapeutic implications, these discoveries have clearly demonstrated that miRNAs can also serve as molecular biomarkers of cancer with prognostic implications and as predictive biomarkers of response to treatment. It is likely that in the next few years, new miRNA-based diagnostic, prognostic and predictive kits will be available to clinicians and that miRNA-derived treatments will begin their clinical trial journey towards the development of new clinical agents. In addition it is likely that in the next few years miRNAs and anti-miRNAs will themselves be used as drugs.
Key Points.
miRNA de-regulation is implicated in the pathogenesis of lymphoid malignancies.
Restoration of normal expression levels of miRNAs can lead to anti-cancer effects.
miRNAs can effectively be used as tumoral biomarkers with diagnostic, prognostic implications, and may also predict response to therapy.
Acknowledgments
Dr. Fabbri is supported by a 2009 Kimmel Scholar Award.
Dr. Croce is supported by several NIH grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev. 2005;15:200–5. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15(4):410–5. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Carleton M, Cleary MA, Linsley PS. Cell cycle. 17. Vol. 6. Georgetown, Tex: 2007. Sep 1, MicroRNAs and cell cycle regulation; pp. 2127–32. [DOI] [PubMed] [Google Scholar]
- 5.Boehm M, Slack FJ. Cell cycle. 8. Vol. 5. Georgetown, Tex: 2006. Apr, MicroRNA control of lifespan and metabolism; pp. 837–40. [DOI] [PubMed] [Google Scholar]
- 6.Winter J, Diederichs S. MicroRNA biogenesis and cancer. Methods Mol Biol. 2011;676:3–22. doi: 10.1007/978-1-60761-863-8_1. [DOI] [PubMed] [Google Scholar]
- 7.Davis-Dusenbery BN, Hata A. Mechanisms of control of microRNA biogenesis. J Biochem. 2010 Oct;148(4):381–92. doi: 10.1093/jb/mvq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevignani C, Calin GA, Siracusa LD, Croce CM. Mammalian microRNAs: a small world for fine-tuning gene expression. Mamm Genome. 2006;17(3):189–202. doi: 10.1007/s00335-005-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005 Oct 27;353(17):1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 10.Fabbri M, Garzon R, Andreeff M, Kantarjian HM, Garcia-Manero G, Calin GA. MicroRNAs and noncoding RNAs in hematological malignancies: molecular, clinical and therapeutic implications. Leukemia. 2008 Jun;22(6):1095–105. doi: 10.1038/leu.2008.30. [DOI] [PubMed] [Google Scholar]
- 11.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002 Nov 26;99(24):15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004 Mar 2;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson KM, Weiss GJ. MicroRNAs and cancer: past, present, and potential future. Mol Cancer Ther. 2008 Dec;7(12):3655–60. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 14.Fabbri M, Croce CM, Calin GA. MicroRNAs in the ontogeny of leukemias and lymphomas. Leuk Lymphoma. 2009 Feb;50(2):160–70. doi: 10.1080/10428190802535114. [DOI] [PubMed] [Google Scholar]
- 15.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008 Jan-Feb;14(1):1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 16.Deng S, Calin GA, Croce CM, Coukos G, Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell cycle. 2008 Sep 1;7(17):2643–6. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 17.Garzon R, Croce CM. MicroRNAs in normal and malignant hematopoiesis. Curr Opin Hematol. 2008 Jul;15(4):352–8. doi: 10.1097/MOH.0b013e328303e15d. [DOI] [PubMed] [Google Scholar]
- 18.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009 Oct;10(10):704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva WA, Jr, et al. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007 Nov;40(11):1435–40. doi: 10.1590/s0100-879x2007001100003. [DOI] [PubMed] [Google Scholar]
- 20.Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly MB, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA. 2007 Dec 11;104(50):19971–6. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Luo XQ, Zhang P, Huang LB, Zheng YS, Wu J, et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS ONE. 2009;4(11):e7826. doi: 10.1371/journal.pone.0007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mi S, Li Z, Chen P, He C, Cao D, Elkahloun A, et al. Aberrant overexpression and function of the miR-17-92 cluster in MLL-rearranged acute leukemia. Proc Natl Acad Sci USA. 2010 Feb 23;107(8):3710–5. doi: 10.1073/pnas.0914900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaddar T, Chien WW, Bertrand Y, Pages MP, Rouault JP, Salles G, et al. Prognostic value of miR-16 expression in childhood acute lymphoblastic leukemia relationships to normal and malignant lymphocyte proliferation. Leuk Res. 2009 Sep;33(9):1217–23. doi: 10.1016/j.leukres.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005 Sep 27;102(39):13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Fabbri M, Bottoni A, Shimizu M, Spizzo R, Nicoloso MS, Rossi S, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011 Jan 5;305(1):59–67. doi: 10.1001/jama.2010.1919. The first manuscript to identify a new pathogenetic network involving miRNAs and protein coding genes (such as TP53 and ZAP-70), able to explain the prognostic implications of the most recurrent chromosomal aberrations in human CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita M, Tanaka Y, Mori N. MicroRNA miR-146a is induced by HTLV-1 tax and increases the growth of HTLV-1-infected T-cells. Int J Cancer. 2009 Dec 16; doi: 10.1002/ijc.25115. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005 Feb 24;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Corcoran M, Rasool O, Ivanova G, Ibbotson R, Grander D, et al. Cloning of two candidate tumor suppressor genes within a 10 kb region on chromosome 13q14, frequently deleted in chronic lymphocytic leukemia. Oncogene. 1997 Nov 13;15(20):2463–73. doi: 10.1038/sj.onc.1201643. [DOI] [PubMed] [Google Scholar]
- 29.Migliazza A, Bosch F, Komatsu H, Cayanis E, Martinotti S, Toniato E, et al. Nucleotide sequence, transcription map, and mutation analysis of the 13q14 chromosomal region deleted in B-cell chronic lymphocytic leukemia. Blood. 2001 Apr 1;97(7):2098–104. doi: 10.1182/blood.v97.7.2098. [DOI] [PubMed] [Google Scholar]
- 30.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA. 2008 Apr 1;105(13):5166–71. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer cell. 2010 Jan 19;17(1):28–40. doi: 10.1016/j.ccr.2009.11.019. The first mouse model showing that the deletion of miR-15a/16-1 cluster triggers leukemia. [DOI] [PubMed] [Google Scholar]
- 32.Raveche ES, Salerno E, Scaglione BJ, Manohar V, Abbasi F, Lin YC, et al. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007 Jun 15;109(12):5079–86. doi: 10.1182/blood-2007-02-071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan XJ, Albesiano E, Zanesi N, Yancopoulos S, Sawyer A, Romano E, et al. B cell receptors in TCL1 transgenic mice resemble those of aggressive, treatment-resistant human chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2006 Aug 1;103(31):11713–8. doi: 10.1073/pnas.0604564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herling M, Patel KA, Khalili J, Schlette E, Kobayashi R, Medeiros LJ, et al. TCL1 shows a regulated expression pattern in chronic lymphocytic leukemia that correlates with molecular subtypes and proliferative state. Leukemia. 2006 Feb;20(2):280–5. doi: 10.1038/sj.leu.2404017. [DOI] [PubMed] [Google Scholar]
- 35.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006 Dec 15;66(24):11590–3. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 36.Neilson JR, Zheng GX, Burge CB, Sharp PA. Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 2007 Mar 1;21(5):578–89. doi: 10.1101/gad.1522907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatopoulos B, Meuleman N, Haibe-Kains B, Saussoy P, Van den Neste E, Michaux L, et al. MicroRNA-29c and microRNA-223 downregulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009 Jan 14; doi: 10.1182/blood-2008-11-189407. [DOI] [PubMed] [Google Scholar]
- 38.Visone R, Rassenti LZ, Veronese A, Taccioli C, Costinean S, Aguda BD, et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood. 2009 Oct 29;114(18):3872–9. doi: 10.1182/blood-2009-06-229211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007 Oct 2;104(40):15805–10. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, et al. MicroRNA -29b induces global DNA hypomethylation and tumor suppressor gene re-expression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009 Feb 11; doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marton S, Garcia MR, Robello C, Persson H, Trajtenberg F, Pritsch O, et al. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008 Feb;22(2):330–8. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]
- 42*.Vargova K, Curik N, Burda P, Basova P, Kulvait V, Pospisil V, et al. MYB transcriptionally regulates the miR-155 host gene in chronic lymphocytic leukemia. Blood. 2011 Feb 4; doi: 10.1182/blood-2010-05-285064. The first evidence of an involvement of MYB in the de-regulation of miRNAs in CLL. [DOI] [PubMed] [Google Scholar]
- 43.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci USA. 2006 May 2;103(18):7024–9. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costinean S, Sandhu SK, Pedersen IM, Tili E, Trotta R, Perrotti D, et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood. 2009 Aug 13;114(7):1374–82. doi: 10.1182/blood-2009-05-220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, et al. Regulation of the germinal center response by microRNA-155. Science. 2007 Apr 27;316(5824):604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007 Apr 27;316(5824):608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003 Sep 15;198(6):851–62. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci. 2005 Mar 8;102(10):3627–32. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007 Sep 1;121(5):1156–61. doi: 10.1002/ijc.22800. [DOI] [PubMed] [Google Scholar]
- 50.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010 Sep 2;467(7311):86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 51.Roehle A, Hoefig KP, Repsilber D, Thorns C, Ziepert M, Wesche KO, et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol of haematology. 2008 Sep;142(5):732–44. doi: 10.1111/j.1365-2141.2008.07237.x. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Kim SW, Rai D, Bolla AR, Adhvaryu S, Kinney MC, et al. Copy number abnormalities, MYC activity and the genetic fingerprint of normal B-cells mechanistically define the microRNA profile of DLBCL. Blood. 2009 Mar 10; doi: 10.1182/blood-2009-01-202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004 May 1;64(9):3087–95. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 54.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008 Apr;9(4):405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005 Jun 9;435(7043):839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 56.Jiang P, Rao EY, Meng N, Zhao Y, Wang JJ. MicroRNA-17-92 significantly enhances radioresistance in human mantle cell lymphoma cells. Radiat Oncol. 2010;5:100. doi: 10.1186/1748-717X-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008 Jan;40(1):43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.Sotillo E, Laver T, Mellert H, Schelter JM, Cleary MA, McMahon S, et al. Myc overexpression brings out unexpected antiapoptotic effects of miR-34a. Oncogene. 2011 Feb 7; doi: 10.1038/onc.2010.634. The first manuscript showing that miR-34a effects vary upon Myc expression in cancer cell, and this affects drug resistance of malignant cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008 Sep 9;105(36):13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akao Y, Nakagawa Y, Kitade Y, Kinoshita T, Naoe T. Downregulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci. 2007 Dec;98(12):1914–20. doi: 10.1111/j.1349-7006.2007.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Fits L, van Kester MS, Qin Y, Out-Luiting JJ, Smit F, Zoutman WH, et al. MicroRNA-21 Expression in CD4+ T Cells Is Regulated by STAT3 and Is Pathologically Involved in Sezary Syndrome. J Invest Dermatol. 2011 Mar;131(3):762–8. doi: 10.1038/jid.2010.349. [DOI] [PubMed] [Google Scholar]
- 62*.van Kester MS, Ballabio E, Benner MF, Chen XH, van der Fits L, et al. miRNA expression profiling of mycosis fungoides. Mol Oncol. 2011 Feb 24; doi: 10.1016/j.molonc.2011.02.003. The first report on miRNA expression in mycosis fungoides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nie K, Gomez M, Landgraf P, Garcia JF, Liu Y, Tan LH, et al. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008 Jul;173(1):242–52. doi: 10.2353/ajpath.2008.080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navarro A, Gaya A, Martinez A, Urbano-Ispizua A, Pons A, Balague O, et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008 Mar 1;111(5):2825–32. doi: 10.1182/blood-2007-06-096784. [DOI] [PubMed] [Google Scholar]
- 65.Gibcus JH, Tan LP, Harms G, Schakel RN, de Jong D, Blokzijl T, et al. Neoplasia. 2. Vol. 11. New York, NY: 2009. Feb, Hodgkin lymphoma cell lines are characterized by a specific miRNA expression profile; pp. 167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Vlierberghe P, De Weer A, Mestdagh P, Feys T, De Preter K, De Paepe P, et al. Comparison of miRNA profiles of microdissected Hodgkin/Reed-Sternberg cells and Hodgkin cell lines versus CD77+ B-cells reveals a distinct subset of differentially expressed miRNAs. Br J Haematol. 2009 Dec;147(5):686–90. doi: 10.1111/j.1365-2141.2009.07909.x. [DOI] [PubMed] [Google Scholar]
- 67.Navarro A, Diaz T, Martinez A, Gaya A, Pons A, Gel B, et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood. 2009 Oct 1;114(14):2945–51. doi: 10.1182/blood-2009-02-204842. [DOI] [PubMed] [Google Scholar]