Identifying and cataloging all gene sequences and their corresponding protein high-resolution structures will serve only as a prelude to the real challenge of future biological inquiry—the deciphering of the cell circuitry and its detailed time-dependent responses to various stimuli. Most of the central functions of the cell are performed by highly organized self-assembled cellular machines that are controlled and regulated by a complex set of dynamic interactions between multiple molecular building blocks. Analytical tools will need to be developed to identify and measure these molecular interactions. Alongside the traditional methods of molecular biology, electron and x-ray microscopies, crystallography, immunocytochemistry, and many others, optical microscopy, and in particular fluorescence microscopy, will play a vital role in this endeavor.
Fluorescence microscopy offers many advantages for probing live cells. It is noninvasive; it provides imaging in three dimensions; it has high sensitivity down to the single molecule level, and it allows the observation of molecular- and organelle-specific signals. The developments of green (and other color) fluorescent proteins as specific genetically manipulated molecular markers and of other sophisticated fluorescence indicators together with improvements in imaging techniques have revolutionized fluorescence imaging of live cells. The application of novel spectroscopic methods and advanced image analysis techniques now provides quantitative analytical tools for the study of cellular dynamics. However, conventional optical microscopy lacks the required nanometer resolution needed for the task described above. As was shown by Abbe over 100 years ago, the wave nature of light imposes a fundamental constraint on the attainable spatial resolution known as the “diffraction limit of light” (1). For commonly used dyes and high numerical aperture oil immersion objectives, this resolution limit is on the order of 250–300 nm. In recent years, we have witnessed, in response to this challenge, inventive solutions that successfully break the diffraction limit of light. These include surface-specific two-dimensional aperture (2) and aperture-less (3) near-field scanning optical microscopy, wide-field three-dimensional image restoration by computational methods (4) and three-dimensional nonuniform periodic excitation (and emission) patterns that contain high spatial frequency components as in 4PI (5), incoherent interference illumination image interference (6), standing-wave total-internal-reflection fluorescence (7), and harmonic excitation light (8) microscopies. In a previous issue of PNAS, Klar et al. demonstrated a novel and clever approach for superresolution imaging that is quite different from previous approaches, holding great promise for future application (9).
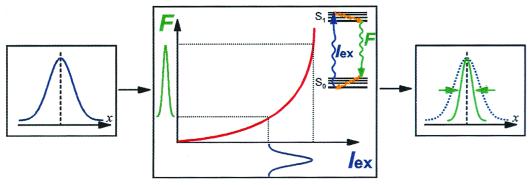
Although the most recent superresolution methods listed above rely only on the volumetric shaping of the excitation light (through a near-field aperture/tip or periodic light gradients generated by interference), attempts have also been made to enhance resolution further by using a nonlinear relationship between the excitation and the fluorescence emission (10, 11). The combination of strong light gradients and strong nonlinear response can further shrink the spatial extent of the point-spread function (PSF), as schematically shown in Fig. 1. Unfortunately, commonly used nonlinear two- and multiphoton excitations suffer from two main drawbacks. Efficient detection requires fluorophores that emit in the visible. Multiphoton excitation of visible fluorophores calls for a longer wavelength (doubled or tripled) fundamental. Because the diffraction limit of light scales linearly with the wavelength, this results in the doubling or tripling of the extent of the excitation spot. In fact, the shrinking of the PSF gained by the nonlinear response cannot even fully compensate for the swelling of the PSF resulting from the longer excitation wavelength. Secondly, because multiphoton excitations are higher-order nonresonant processes, their absorption cross sections are many orders of magnitude smaller than that of the linear one-photon process. Intense short laser pulses are needed for efficient excitation. However, such pulses are phototoxic to the cell because they accelerate radical production, thereby limiting the available observation time before cell damage.
Figure 1.
A nonlinear relationship between the fluorescence emission (F) and the excitation light (Iex) “shrinks” the emission PSF. An excitation “input” PSF (blue, Left) will be converted into an emission “output” PSF (green, Right) via a “nonlinear fluorescence box” (Center). The Jablonski diagram at the top right of the “fluorescence box” describes an arbitrary nonlinear excitation process (although only one wavy arrow is shown for Iex, it can describe a multiphoton process). The output PSF is normalized and compared with the input PSF.
Realizing the drawbacks of multiphoton excitation for superresolution imaging, the Hell group has been searching for alternative “smart” nonlinearities that use visible rather than infrared excitation and have large effective cross sections. Although previous approaches treated fluorescence as a linear process, the Hell group has been looking for ways to exploit the spectroscopic properties of fluorophores to produce such nonlinearities. They proposed and theoretically investigated the possibility of inhibition of fluorescence resonance energy transfer under donor saturation conditions for a donor-acceptor conjugated dye pair (12, 13). More recently, they proposed a scheme that relies on repeated excitation by a short-pulse pump probe to effectively prolong the fluorescence lifetime of molecules at the center of the PSF (compared with molecules at its outskirts) (14). Both proposals still await experimental verification. Their third and earlier proposal (15) has now been experimentally demonstrated (9, 16).
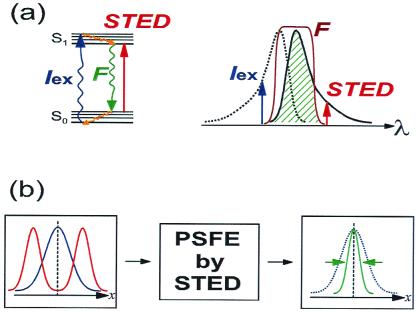
The concept, dubbed “point-spread-function engineering (PSFE) by stimulated emission depletion (STED)” relies on two main ingredients: (i) fluorophores that act as effective four-level systems and display very efficient stimulated emission (as good laser dyes do), and (ii) a nondegenerate (different wavelength) pump-probe scheme that excites the fluorophores with one beam and quenches them with the other. A short (200-fs) visible pulse is focused to a diffraction-limited focal spot and excites all fluorophores in this volume. A second near-infrared and longer pulse (40 ps) stimulates the emission from molecules at the outer part of the focus, forcing them into the ground state immediately after they are excited. The longer duration of this second pulse guarantees full quenching without reabsorption. The beauty of this approach is in taking advantage of two constructive effects: first, the quenching efficiency scales exponentially with the STED intensity (as opposed to the power law dependence in multiphoton processes) and thus requires only moderate powers. Second, the cross section for stimulated emission is large and comparable to the one-photon excitation cross section. The carving and reshaping of the PSF is achieved by a phase mask that is inserted into the STED beam path. The phase mask redistributes the STED energy, yielding a null intensity (node) at the exact focus but preserving the wings of the STED beam. These wings are the “carving knife” that quenches the fluorescence and shrinks the detected (emission) PSF. Fig. 2 illustrates the essentials of the technique. Fig. 2a shows a Jablonski (energy level) diagram, the absorption and emission spectra of the fluorophore and the spectral alignment of the excitation and the STED pulses. Fig. 2b shows how the extent of the PSF is shrunk in one dimension by using this method (the illustration in one dimension is for demonstration only; shrinking is effective in all three dimensions).
Figure 2.
PSFE by STED. (a Left) A Jablonski diagram showing the ground (S0) and the first excited singlet (S1) states of a fluorophore with the corresponding vibrational manifolds. The fluorophore acts as an effective four-level system because the vibrational relaxations of S1 and S0 are fast compared with the fluorescence lifetime. A short pulse (Iex, 200 fs) excites the fluorophore into a high vibrational state of S1. The slow STED pulse (STED, 40 ps) stimulates emission from the lowest S1 vibrational state into a high vibrational state of S0. The stimulated emission depletes S1 and quenches the fluorescence. (Right) The equivalent wavelength diagram showing the absorption curve (dotted black line), the emission curve (solid black line), and the positions of Iex, STED and the filter (F). The filter blocks the two lasers and passes the signal (hatched green). (b) The combination of the excitation PSF (blue) with the STED engineered intensity distribution (red) acts as an input to the PSFE by STED “nonlinear box.” The output PSF (green) is shrunk.
By using a very simple nonoptimized phase mask, Klar et al. were able to break the diffraction limit and shrink the PSF by a factor of six along the optical axis and by a factor of two in the radial direction, reducing the volume of the confocal spot by a factor of 2 × 2 × 6 ≈ 18 (the math is slightly complicated by the reshaping effect!). Very importantly, they showed that their superresolution method is compatible with live-cell imaging (9). The fundamental limitations of the technique are not yet clear. In principle, as the intensity of the STED beam is increased, the extent of the PSF should be further decreased. However, photophysical properties of fluorophores (quantum yields, intersystem crossing rates, triplet state lifetimes, nonradiative decay rates, saturation intensities, photobleaching rates) and other physical factors, such as probe concentration in the focal volume, imperfection of filters, and leakage of the excitation and the STED beams into the detection band, will determine the technique's resolution limits. Theoretical and experimental studies addressing these questions will be needed to assess the ultimate capabilities of the technique. Regardless of the outcome of these studies, it is quite clear that many improvements can be envisioned.
Combining PSFE by STED with one or more of the other superresolution techniques is likely to follow. Three-dimensional image restoration by deconvolution is obviously compatible. STED combined with nonuniform periodic excitation schemes will provide sophisticated control of the light-producing nanometric excitation patterns in three dimensions. A hybrid approach combining two-photon excitation with one-photon STED might offer improvements in signal to noise (at the expense of higher intensities). Furthermore, it might be possible to use adaptive optics techniques to improve the shape of the STED beam. A computer-controlled spatial light modulator or a deformable mirror could replace the fixed phase mask. Adaptive algorithms such as genetic evolution (17) might allow the control and optimization of the STED pulse's amplitude, phase, and spatial distributions across the focal spot, affording control, manipulation, and optimization of the shape of the superresolution spot.
The development of optimized probes for PSFE by STED will greatly benefit future applications. The technique will not be easily expanded to multicolor probes, because different wavelength STED pulses will be needed. Nevertheless, it should be possible to multiplex several signals by using probes with the same color but different lifetimes. Because the technique relies on pulsed excitation, fluorescence lifetime imaging (FLIM) (18) by time-gated detection or by time-correlated single-photon counting can be simultaneously implemented. Combining FLIM with PSFE by STED will allow the dynamic colocalization of cellular factors with superresolution accuracy in the live cell. Another interesting area for future exploration is the combination of PSFE by STED with fluorescence resonance energy transfer inhibition (13). Last, the reduced volume of the PSF should improve single-molecule experiments, merging ultrahigh resolution with ultrasensitive detection.
The work by Klar et al. has the potential to transform the fluorescence microscopy “Renaissance” we are currently experiencing into an “Enlightenment Millennium.” Our perception of the ideas put forward by Abbe over a century ago will certainly be transformed. The powerful concept of wedding nonlinear microscopies with tailored fluorophores' photophysical properties will continue to produce innovative methodologies and open novel windows into cellular dynamics. The few predictions listed above are only a small subset of what is to follow, and this beautiful story is not going to end here: the technique has even more to offer beyond live-cell imaging and biology. Spatial and temporal manipulations of the amplitude and phase of short pulses could provide coherent control of chemical reactions on the nanometer scale. Such capabilities could find uses, for example, in controlling photoresist chemistry for ultrahigh-resolution lithography, electrooptics, and magnetooptics data storage and many other nanotechnology applications. Keep your eyes open!
Acknowledgments
I thank Jennifer Glass, Thilo Lacoste, Ted Laurence, and Xavier Michalet for critical reading and insightful comments. The research from this laboratory is supported by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory and the Director, Office of Science, Office of Biological and Environmental Research, of the U. S. Department of Energy under contract no. DE-AC03–76SF00098 and the Office of Naval Research contract N0001498F0402.
Footnotes
See companion article on page 8206 in issue 15 of volume 97.
References
- 1.Abbe E. Archiv für Mikroskopische Anat Entwicklungsmech. 1873;9:413–468. [Google Scholar]
- 2.Dunn R C. Chem Rev. 1999;99:2891–2927. doi: 10.1021/cr980130e. [DOI] [PubMed] [Google Scholar]
- 3.Inouye Y, Kawata S. Opt Lett. 1994;19:159–161. doi: 10.1364/ol.19.000159. [DOI] [PubMed] [Google Scholar]
- 4.Carrington W A, Lynch R M, Moore E D W, Isenberg G, Fogarty K E, Fay F S. Science. 1995;268:1483–1487. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- 5.Hell S, Stelzer E H K. J Opt Soc Am A. 1992;9:2159–2166. [Google Scholar]
- 6.Gustafsson M G L, Agard D A, Sedat J W. J Microsc. 1999;195:10–16. doi: 10.1046/j.1365-2818.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 7.Cragg G E, So P T C. Opt Lett. 2000;25:46–48. doi: 10.1364/ol.25.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohn J T, Knapp H F, Stemmer A. Proc Natl Acad Sci USA. 2000;97:7232–7236. doi: 10.1073/pnas.130181797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klar T A, Jakobs S, Dyba M, Enger A, Hell S W. Proc Natl Acad Sci USA. 2000;97:8206–8210. doi: 10.1073/pnas.97.15.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppard C J R, Gu M. Optik (Stuttgart) 1990;86:104–106. [Google Scholar]
- 11.Hell S, Stelzer E H K. Opt Commun. 1992;93:277–282. [Google Scholar]
- 12.Hanninen P E, Lehtela L, Hell S W. Opt Commun. 1996;130:29–33. [Google Scholar]
- 13.Schonle A, Hanninen P E, Hell S W. Ann Phys (Leipzig) 1999;8:115–133. [Google Scholar]
- 14.Schonle A, Hell S W. Eur Phys J D. 1999;6:283–290. [Google Scholar]
- 15.Hell S W, Wichmann J. Opt Lett. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- 16.Klar T A, Hell S W. Opt Lett. 1999;24:954–956. doi: 10.1364/ol.24.000954. [DOI] [PubMed] [Google Scholar]
- 17.Zeek E, Bartels R, Murnane M M, Kapteyn H C, Backus S, Vdovin G. Opt Lett. 2000;25:587–589. doi: 10.1364/ol.25.000587. [DOI] [PubMed] [Google Scholar]
- 18.Gadella T W J, Jovin T M, Clegg R M. Biophys Chem. 1993;48:221–239. [Google Scholar]