Abstract
Background
Knowledge about adverse symptoms over time from fiber supplementation is lacking.
Purpose
To compare the severity of adverse gastrointestinal (GI) symptoms during supplementation with dietary fiber or placebo over time in adults with fecal incontinence. Secondary aims were to determine the relationship between symptom severity and emotional upset and their association with study attrition and reducing fiber dose.
Methods
Subjects (N=189, 77% female, 92% white, (age = 58 years, SD = 14) with fecal incontinence were randomly assigned to placebo or a supplement of 16g total dietary fiber/day from one of three sources: gum arabic, psyllium, or carboxymethylcellulose. They reported GI symptoms daily during baseline (14 days), incremental fiber dosing (6 days), and two segments of steady full fiber dose (32 days total).
Results
Severity of symptoms in all groups was minimal. Adjusting for study segment and day, a greater feeling of fullness in the psyllium group was the only symptom that differed from symptoms in the placebo group. Odds of having greater severity of flatus, belching, fullness, and bloating were 1.2–2.0 times greater in the steady dose segment compared to baseline. There was a positive association between symptom severity and emotional upset. Subjects with a greater feeling of fullness or bloating or higher scores for total symptom severity or emotional upset were more likely to withdraw from the study sooner or reduce fiber dose.
Conclusions
Persons with fecal incontinence experience a variety of GI symptoms over time. Symptom severity and emotional upset appear to influence fiber tolerance and study attrition. Supplements seemed well tolerated.
Keywords: fiber, diet, fecal incontinence, gastrointestinal symptoms, time
Tolerance of an intervention influences retention in a clinical trial as well as sustainability of the intervention in the long-term. Adverse gastrointestinal (GI) symptoms are one of the main measures of intolerance of a dietary intervention, including dietary fiber supplementation (Livesey, 2001). Dietary fiber has been administered in a variety of preparations and doses to manage a spectrum of clinical disorders, including diabetes (Flammang, Kendall, Baumgartner, Slagle, & Choe, 2006) hypercholesterolemia and heart disease risk (Smith, Queenan, Thomas, Fulcher, & Slavin, 2008), irritable bowel syndrome (Parisi et al., 2005), and obesity (Pittler & Ernst, 2004), as well as to increase fiber intake in healthy persons (Vuksan et al., 2008). The analyses of most past studies of dietary fiber typically used a statistic of central tendency and spread (such as a mean and standard deviation) to summarize scores rating the severity of adverse GI symptoms across study periods. Knowledge of the course of adverse symptoms over time from dietary fiber supplementation is lacking. The development and refinement of statistical theory, methods, and software to analyze symptom data collected repeatedly over time has made it possible to more readily describe the pattern of symptom development associated with fiber supplementation during the study. This knowledge can be useful information to include in the informed consent process and to guide the design of the intervention protocol; for example, there is little evidence whether lengthening protocol duration to include a period of incremental dosing of dietary fiber lessens the severity of symptoms and attrition.
Although measures of intensity and amount and/or frequency are the main components of evaluations of GI symptom tolerance, the degree of emotional upset about the symptom is rarely reported. Hence, the influence of emotional upset about symptoms on study retention is understudied. Data about the severity of GI symptoms and how emotionally upsetting the symptoms were to subjects collected over time can be used in analyzing their association with study withdrawal and supplement reduction.
As part of a randomized clinical trial investigating the effects of three dietary fiber sources (gum arabic, psyllium, or carboxymethlycellulose (CMC)) or placebo in managing fecal incontinence in community-living adults, GI symptoms were examined over time. Monitoring a group receiving a placebo also enabled description of GI symptoms in fecal incontinence, which has not been characterized. Defecation characteristics (stool frequency, consistency, amount, and incontinence) are not included as adverse symptoms in this secondary analysis as they were among the primary outcomes. The purpose of this analysis was to determine whether there were differences in symptom severity among the four supplement groups over the course of the study. Secondary aims were to determine the relationship between symptom severity and emotional upset and their association with study attrition and reduction of fiber dose.
Review of Literature
Dietary supplementation with gum arabic and psyllium fiber in previous clinical studies have been associated with few and minor adverse GI symptoms. Flatus, bloating, and abdominal/stomach cramping were the symptoms most often observed. No clinical studies using CMC were found. Flatus was observed after supplementation with both gum arabic, which is highly fermented by colonic bacteria, and psyllium, which is moderately fermented (Bliss et al., 2001). Flatus occurred in 50% of 16 chronic renal failure patients who received a daily supplement of 50 g of a gum arabic source to increase nitrogen excretion in stool; flatus was observed to subside after two weeks (Bliss, Stein, Schleifer, & Settle, 1996). In a pilot study to the current trial, the average severity of flatus ranged from 0.8 to 1.3 (rating scale: 1 = small, 2 = medium, 3 = large) when gum arabic, psyllium, or placebo was consumed as well as during the usual baseline diet of 39 community living subjects with fecal incontinence (Bliss et al., 2001). The supplements consisted of 23.3 g total fiber from gum arabic (in 25 g source of gum arabic), 7.1 g total fiber from psyllium (in 25 g source), and 1 g of a pectin source for placebo. There was no significant difference in the average severity of flatus among groups during the supplement period controlling for baseline flatus severity. Flatus was also reported by 15 healthy subjects when consuming 8 g of total fiber from psyllium (in a 15 g source from psyllium seed) compared to defined, low fiber diet (about 12 g fiber per day) (Fischer et al., 2004).
Others have also reported no difference in flatus during psyllium supplementation compared to a control diet (Jenkins et al., 2002; Vuksan et al., 2008). Studies differ in reports of other GI symptoms, however. Healthy subjects on one of four high-fiber diets (25–28.7 g of fiber per day in one of four cereals) for 3 weeks each had significantly more bloating compared to a low fiber control diet (12 g fiber per day) (Vuksan et al., 2008). Most symptoms had low mean scores (< 1 on a scale of 0–5). Flatulence had the highest mean score (ranging from 1.4 – 2.3 among the diets) but was not different among groups. Approximately two and a half times as many elderly subjects using psyllium as a laxative or stool softener (21%) reported abdominal pain than those who did not use the supplement (8%, p < .0001) (Stewart, Hale, Moore, May, & Marks, 1991). The amount of the psyllium source ranged from 1–40 g per day with the majority ingesting between 0 and 19 g per day. Several GI symptoms, including abdominal or stomach cramping, heartburn, nausea, or vomiting, were experienced by 40 of 54 (74%) patients with hypercholesterolemia and constipation when consuming a supplement of 10.5 g of psyllium per day for 3 weeks (Uehleke, Ortiz, & Stange, 2008). The mean total symptom score (frequency and intensity using a 5-point scale) decreased from week 1 to 3 (M = 27.7, SD = 29.8) to (M = 24.1, SD = 32.2). On the other hand, Jenkins et al. (2002) reported no difference in bloating, abdominal pain, or flatus in 68 hyperlipidemic adults on a high fiber diet or a control low-fat, low-cholesterol diet for 3 weeks each. The high-fiber diet included 4 servings of self-selected foods containing psyllium (7.2 g per day on average) and β-glucan (0.75 g per day on average). The severity of the symptoms, however, was not reported. Attrition rates ranged from 3% to 20%. Although Anderson et al. (2000) did not report findings about adverse symptoms, they reported an attrition rate of 13% in a group of a 161 hypercholesteremic adults who supplemented their diet with 10. 6 of psyllium per day for 26 weeks compared to 23% in the placebo group.
There is a paucity of information about the emotional upset from adverse gastrointestinal symptoms associated with interventions involving dietary fiber. The International Consultation on Incontinence (Abrams, Cardozo, Khoury, & Wein, 2009) recommended that the “subjective impact or bother” (p. 1812) of symptoms be included among the observations in clinical incontinence research.
In summary, GI symptoms associated with gum arabic and psyllium supplementation tend to be small on average. Flatus was reported after gum arabic ingestion, and psyllium was associated with both upper (e.g., bloating) and lower (e.g., abdominal cramping) GI symptoms. Differences in symptoms may be due in part to differences in the preparations, amounts, and duration of administration of psyllium and patient characteristics. Data characterizing these symptoms over time and emotional upset with them are lacking. The relationship of symptoms and emotional upset to study attrition and reduction of supplement dose has not been systematically examined. Symptoms associated with CMC supplementation in clinical studies need study.
The specific aims of this analysis were to: (a) compare ratings of the severity (determined from frequency and amount) of GI symptoms that occurred during baseline and steady dose segments and in the three dietary fiber and placebo groups over time; (b) describe the types and severity of GI symptoms during the segment when fiber administration was gradually increased; (c) determine the relationship between symptom severity and emotional upset; (d) determine the influence of symptom severity and emotional upset on time to study withdrawal and reduction of supplement dose; and (e) compare symptom severity and emotional upset of subjects who withdrew from the study during supplementation and those who completed the study.
Methods
Eligibility and Sample
Subjects were recruited from a large health maintenance organization and the practice of a University-affiliated group of colon and rectal surgeons in Minnesota. Subjects met the following inclusion criteria: at least 18 years of age, cognitively intact (Mini Mental State Examination score > 24) (Folstein, Folstein, & McHugh, 1975), community-living and not residing in a nursing home, normal continuity of the gastrointestinal (GI) tract, toilet independently, not be tube-fed or have swallowing problems, and report usually having at least two episodes of fecal incontinence of unformed and loose or liquid stool consistency within two weeks. Subjects who did not have two episodes of fecal incontinence or could not perform the study procedures during the baseline period were not eligible to be randomly assigned to receive a supplement. Exclusion criteria were known allergy to any of the fibers, inflammatory bowel disease (e.g., ulcerative colitis), receiving abdominal radiation or chemotherapy, a malabsorption disorder, or temporary incontinence, for example, due to pregnancy or impaction.
Dietary Fibers and Supplements
The supplements administered consisted of one of three dietary fibers or a placebo. The dietary fibers were gum arabic, psyllium, and CMC. The compositions of the fibers are described in Table 1. The fibers were selected because they represent three levels of fermentation (low, medium and high) by fecal bacteria. A total of 16 g of total dietary fiber was administered daily. The amounts of the fiber sources needed (17.3 g gum arabic, 16.8 g psyllium, and 21.5 g CMC) were determined from the initial analysis of their fiber content. The supplements were prepared as a fruit juice mixture and a small muffin, each taken twice daily at the morning and evening meals. Subjects were able to select a preferred juice flavor and muffin spice from a set of offered choices. Each of the two juice mixtures contained 270 ml (9 oz.) of juice diluted to half the concentration recommended on the can. The two juices together provided a total of 7 g of total fiber that was divided approximately equally between them. The two muffins provided 9 g of total fiber, approximately 4.5 g of total fiber in each. The placebo juices contained half-strength juice only and the placebo muffins contained the ingredients of the basic recipe only. Subjects and data collectors were blinded to the type of fiber administered and its distribution in the juice and muffin.
Table 1.
Dietary Fibers Used in Supplements
| Dietary Fiber and Source | Description | Postulated Degree of Fermentation by Bacteria and Solubility |
|---|---|---|
| Carboxymethylcellulose (Gallipot, Inc., St. Paul, MN) | Linear polymer of glucose residues with synthetic substitutions of carboxymethyl groups on the glucose molecules that make it highly soluble | Low |
| Psyllium (Gallipot, Inc., St. Paul, MN) | Primarily an arabinoxylan form of hemicellulose with limited solubility extracted from Plantago ovata seed husks | Medium |
| Gum arabic (gum acacia) (TIC Gums, Inc., Belcamp, MD) | Complex branched-chain heteropolysaccharide of galactose, arabinose, rhamnose and glucuronic acids with uronic acids mainly on the periphery of its structure; highly soluble* | High |
Description of gum Arabic was adapted from Adiotomore, Eastwood, Edwards, & Brydon (1990).
Participants were instructed to drink 5 oz. of fluid per day with each muffin in addition to the 9 oz. juice mixture and their usual fluid intake; during the baseline segment they were to drink an additional 28 oz. of fluid per day as this amount was provided with the supplements. Subjects could reduce their intake of dietary fiber once during the study if they reported that they were not tolerating the supplements, and the reduced amount was maintained for the remainder of the study. The reduced amount of fiber was 10 g of total fiber per day and was distributed in the juice and muffin proportionally to the original supplement.
Design and Procedures
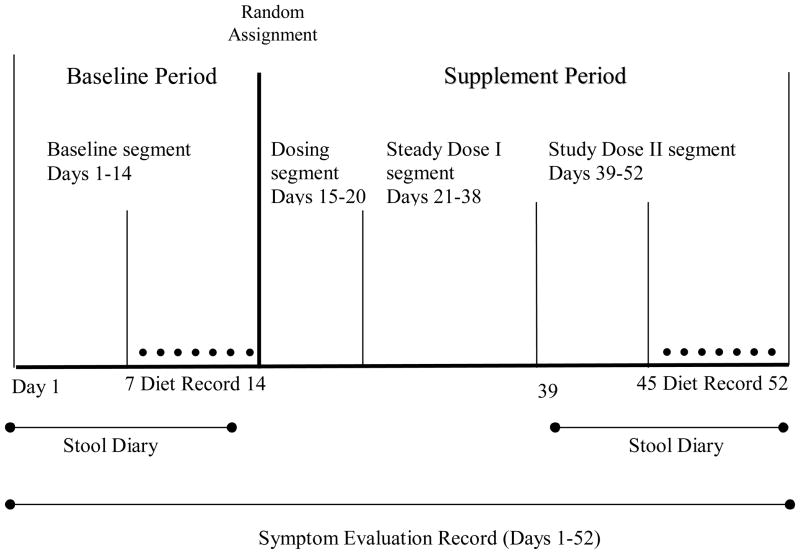
This study had a longitudinal, pre-post, among-groups design. The protocol of the parent study was 52 days in length and divided into the following segments: a 14-day baseline segment, a 6-day incremental dosing segment, an 18-day steady dose 1 segment, and a 14-day steady dose 2 segment (Figure 1). During the incremental dosing segment, the amount of fiber administered was gradually increased by one-third every two days to the total of 16 g of total fiber per day. Participants consumed 16 g of total fiber per day during the steady dose 1 and steady dose 2 segments unless a subject required a reduction in the amount of dietary fiber in the supplement (as explained above). During the steady dose 2 segment, the same type of data was collected as during the baseline segment. In determining the length of the segments of the parent study, past research and subject burden were considered. In a previous study, clinical effects from dietary fibers were seen in approximately two weeks of taking a steady dose of fiber. Advancing the amounts of dietary fiber over six days seemed to promote tolerance of any flatus that occurred (Bliss et al., 2001). Past experience informed suitable lengths of data collection for planned analyses and overall participation that promoted completion of the study by subjects.
Figure 1.
Segments of the study design
Participants reported on a set of GI and obfuscating symptoms daily throughout the study using an instrument that was a modification of the one used by Zumarraga, Levitt, and Suarez (1997). The GI symptoms are listed in Table 2, and the two obfuscating symptoms were headache and sleepiness. Subjects reported the amount of symptoms and also the number of times that flatus occurred in a day. The symptom severity measure incorporated both frequency and amount. Subjects also rated the level of emotional upset with each symptom (Table 2).
Table 2.
Dependent Variable Definitions and Measurement
| Variable Definition | Instrument/Method of Measurement | Type of Data |
|---|---|---|
| Fecal Incontinence | Stool Diary completed daily for 14 days at start and end of study | Dichotomous |
| Involuntary/accidental leakage of feces | ||
| Fecal Incontince Severity | Calculated using frequency, consistency, and amount of fecal incontinence data reported on Stool Diary | Interval |
| Extent of frequency, consistency, and amount of involuntary/accidental fecal leakage | ||
| Adverse Symptoms | Symptom Evaluation Record completed daily for 52 days throughout study Gastrointestinal symptoms were flatus belching, bloating, abdominal cramping, nausea, a feeling of fullness, and stomach upset |
5-point ordinal scale for amount of symptoms: none, minimal, small, medium, and large; the number of times of flatus |
| Selected physiological sensations or feelings | ||
| Symptom Severity | Calculated using frequency and amount data reported on the Symptom Evaluation Record | Interval |
| Extent of frequency and amount of symptom | ||
| Emotional Upset Associated with Adverse Symptoms | Symptom Evaluation Record completed daily for 52 days throughout study | 4-point ordinal scale: 0 = not at all 1 = a little 2 = some 3 = a lot 4 = extremely |
| Level of being emotionally bothered or disturbed by symptoms | ||
| Demographic data | Demographics Form completed at start of study | Interval and categorical |
| Description of subjects’ characteristics or background | ||
| Height and Weight | Measured using tape measure and scale at start of study | Interval |
| Dietary Fiber Intake | Diet Record of type, amount, and method of preparation of each food and beverage was completed daily for 7 days at start and end of study then a computer-based analysis of total and soluble dietary fiber was done | Interval |
| Amount of dietary fiber consumed each day |
Participants were instructed to maintain their normal activities and eat their usual diet throughout the study. They answered questions about demographic characteristics and their height and weight were measured at the start of the study (Table 2). To estimate usual fiber intake, subjects completed a daily diet record for the last 7 days of the baseline and steady dose 2 segments (Table 2). The data collector reviewed the diet records with the subject and collected them daily. Data were entered into Nutritionist Pro software1 (Axxya Systems, Stafford, TX) and analyzed for fiber content.
Statistical Analyses
Descriptive statistics were generated for all measures, frequencies for categorical data and means with standard deviations or medians with ranges for interval data, depending on the normality of the distribution. Demographics and diet composition were compared between groups using an analysis of variance (ANOVA). As might be expected, measures of symptom severity and emotional upset were skewed. The first assessment was on the aggregate data with comparisons of median and range of symptoms seen over the whole baseline versus supplement segments. These summative medians were compared using a Kruskal-Wallis ANOVA. Post hoc comparisons used pair-wise Mann-Whitney U tests with a Bonferroni adjustment to control the overall alpha level.
To assess the measures of symptom severity and emotional upset over time between groups, mixed models using general estimating equations (GEE) were used. GEE produces a marginal model, also referred to as population average model; that is, regression coefficients in marginal models are interpretable as the effect on the mean response in the study population rather than the effect on an individual response as in linear mixed-modeling, also referred to as subject specific (Hardin & Hilbe, 2003). Population averaged models parameterize the marginal distributions of the population whereas subject specific models fully parameterize the distribution regression weights in the population. Which approach to use depends in part on the question one is trying to answer. The population average model was chosen for this analysis because the description of symptoms over time was a secondary aim of the parent study; this model was pursued to assess whether on average subjects in one of the fiber groups had more symptoms than subjects in another fiber group.
There are three elements that need to be decided in order to perform any GEE analysis: (a) distribution of the outcome (b) best link function, and (c) likely covariance structure. Reports of the number of times of having flatus each day were counted. The first choice for the distribution used to model was Poisson; however, this model exhibited over-dispersion (deviance great than 1 which means the true variance is greater than the mean). In a Poisson distribution, the mean and variance should be close to the same. In agreement with this, the mean = 7.7 while the variance = 91. With these indications, a negative binomial distribution with a log link function was chosen. The deviance for this model was very close to 1. Reports of all other symptoms were ordered multinomial, thus a multinomial distribution was used in modeling. The appropriate link function for this distribution is the cumulative logit link which constrains the parameters to monotonically increase (Fitzmaurice, Davidian, Verbeke, & Molenberghs, 2009; Ballinger, 2004). The covariance form chosen was independent, as this is the only available option for the multinomial distribution in SAS, used for this analysis. Various authors have commented that misspecification of the working correlation matrix may affect the efficiency of the parameter estimates but not the consistency, especially in models dealing only with time-independent covariates (Pan & Connett, 2002). Each symptom was modeled individually with a covariate controlling for supplement group, study segment, and day within segment to predict the log odds of a symptom’s measures being greater by supplement group and study segment. The k group multinomial model also generates k-1 intercept estimates which are the log odds of being in a higher symptom category compared to the reference category, none. These estimates can be converted to the odds of being in a higher symptom category, compared to none, by using the formula OR = eβX where x = 0 for the reference group and x = 1 for the comparison group in the single regression problem. For the multiple regression problem, βX represents a matrix representing β0 + β1X1 + ………+ βnXn.. These estimates for the five symptom models are presented in Table 3. The proportional odds assumption was tested by estimating the slopes for each response category, β1, β2, β3, β4 and assessing for β1= β2 = β3 = β4. The assumption of equal slopes appeared reasonable for all five symptoms. β estimates for the covariates are interpretable as the log odds of increasing symptom category by an increase of 1 in the covariate. These log odds are used to estimate odds ratios using the formula: OR = eβ. A conservative Bonferroni correction was made to the significance level for pair-wise comparisons of fiber groups (each, p < .008) and periods (p < .017) to preserve an overall type I error rate of .05.
Table 3.
Intercepts for Multinomial Models and the Odds of Being in Higher Categories of Symptom Amount Compared with No Symptoms
| Symptom | Severity | β (95% CI) | Odds Ratio (95% CI) |
|---|---|---|---|
| Flatus | minimal | 1.41 (1.08, 1.74) | 4.1 (2.9, 5.7) |
| small | −.41 (−.72, −.10) | .66 (.48, .90) | |
| medium | −1.36 (−1.69, −1.04) | .26 (.18, .35) | |
| large | −2.88 (−3.24, −2.52) | .06 (.04, .08) | |
| Belching | minimal | −.49 (−.93, −.04) | .61 (.39, .96) |
| small | −1.98 (−2.46, −1.49) | .14 (.09, .23) | |
| medium | −3.02 (−3.54, −2.5) | .05 (.03, .08) | |
| large | −5.03 (−5.65, −4.4) | .01 (.004, .012) | |
| Bloating | minimal | −1.14 (−1.59, −.68) | .32 (.20, .51) |
| small | −1.91 (−2.37, −1.45) | .15 (.09, .23) | |
| medium | −2.61 (−3.09, −2.14) | .07 (.05, .12) | |
| large | −4.23 (−4.77, −3.69) | .01 (.008, .02) | |
| Fullness | minimal | −1.53 (−2.04, −1.01) | .22 (.13, .36) |
| small | −2.19 (−2.73, −1.66) | .11 (.07, .19) | |
| medium | −2.88 (−3.43, −2.33) | .06 (.03, .10) | |
| large | −4.91 (−4.8, −3.55) | .02 (.01, .03) | |
| Cramps | minimal | −1.43 (−1.9, −.96) | .24 (.185, .38) |
| small | −2.33 (−2.8, −1.85) | .10 (.06, .16) | |
| medium | −3.28 (−3.81, −2.76) | .04 (.02, .06) | |
| large | −4.89 (−5.58, −4.2) | .01 (.004, .014) |
CI = confidence interval
The issue of missing data was addressed by plotting the number of missing data points by day by fiber group for the 189 subjects who completed the study. The proportions of missing values did not exceed a total of 4% on any one day, and the pattern of missing appeared randomly scattered between subjects and fiber group. No individual subject missed more than 2% of data collection days. Study staff collected and reviewed diet records with subjects daily and symptom forms weekly as part of the protocol. Therefore, missingness was not considered an issue in this analysis.
Time to withdrawal and time to reduction of fiber by group were first compared using the method of Kaplan-Meier. The effects of symptom severity and emotional upset on time to withdrawal and time to reduction of fiber dose were assessed using a proportional hazards regression analysis. Each symptom’s severity and upset measure, then their total scores were assessed in separate models. A model assessing both the effects of symptoms and fiber group on the time to withdrawing from the study or reducing fiber dose was not possible because fiber group and symptoms were highly confounded (i.e., different fiber groups had different levels of symptom severity). Hence, only symptoms were used in these “time to” models.
Results
Group Demographics and Self-Reported Usual Fiber Intake
There were four supplement groups of adults (N = 189) who completed the study protocol. The groups were the (a) placebo group (n =47), (b) CMC group (n = 47), (c) gum arabic group (n = 49), and (d) psyllium group (n = 46). Groups did not differ significantly in their demographic characteristics (Table 4). The majority of subjects in each group were female, white, non-Hispanic, and middle-aged. Approximately one-quarter in each group were employed and had secondary education. The body mass index of the groups showed they were overweight (25.5 – 29.9) or obese (>30) (Classification of overweight and obesity. n. d.). There was no significant difference in the self-reported usual total or soluble fiber intake among the groups in the Baseline or Steady Dose 2 segments (Table 4).
Table 4.
Demographics and Usual Fiber Intake
| N (%)a | Placebo | CMC | Gum Arabic | Psyllium | p |
|---|---|---|---|---|---|
| Years of age, Mean (SD) | 59 (13) | 59 (13) | 55 (15) | 60 (14) | .37 |
| Sex | .07 | ||||
| Male | 16 (34) | 12 (26) | 11 (22) | 5 (11) | |
| Female | 31 (66) | 35 (75) | 38 (78) | 41 (89) | |
| Race/Ethnicity | .80 | ||||
| White | 42 (89) | 42 (89) | 47 (96) | 43 (94) | |
| Black or African American | 2 (4) | 2 (4) | 2 (4) | 1 (2) | |
| American Indian/Alaska Native | 0 | 1 (2%) | 0 | 0 | |
| Asian | 1 (2) | 0 | 0 | 1 (2) | |
| More than one race | 2 (4) | 2 (4) | 0 | 1 (2) | |
| Hispanic | 0 | 1 (2) | 1 (2) | 1 (2) | .80 |
| Education | .32 | ||||
| High school graduate or equivalent | 4 (9) | 9 (20) | 1 (2) | 6 (13) | |
| College graduate | 24 (51) | 17 (37) | 27 (56) | 21 (46) | |
| Employment | |||||
| Employed | 25 (23) | 27 (25) | 33 (30) | 24 (22) | .42 |
| Retired | 17 (36) | 15 (32) | 16 (33) | 21 (46) | .50 |
| BMI, M (SD) | 29 (6) | 31 (7) | 29 (8) | 30 (8) | .51 |
| Self-Reported Dietary Fiber Intake g/1000 kcal/d M (SD) | |||||
| Soluble fiber | |||||
| Baseline | .23 (.21) | .18 (.22) | .21 (.24) | .18 (.16) | .95* |
| Steady 2 | .18 (.14) | .11 (.13) | .10 (.13) | .10 (.16) | |
| Total fiber | |||||
| Baseline | 10.2 (3.0) | 8.9 (2.7) | 9.6 (2.6) | 9.2 (3.1) | .20* |
| Steady 2 | 8.2 (2.5) | 7.6 (2.1) | 7.7 (1.9) | 7.1 (2.0) | |
Unless indicated otherwise
The means during the Steady 2 Period are adjusted for the baseline means for soluable and total fiber.
BMI = Body mass index
GI Symptom Severity over Time
Baseline and steady dose segments
A total of 9,591 daily symptom evaluation records from those who completed the study were analyzed. Participants experienced a median of 3 (range = 0–8) GI symptoms in all the study segments. The severity of each GI symptom was between minor and small in the baseline as well as the steady dose 1 and 2 segments for each supplement group. The median severity was 0 for the symptoms of nausea (range = 0 – 3), belching, feeling of fullness, bloating, and abdominal cramping (range for the latter 4 symptoms = 0 – 4). The severity of flatus was a median of 1 (range = 0 – 4) for the placebo, CMC and psyllium groups and 1.5 (0 – 4) for the gum arabic group in the baseline segment. Flatus severity was 1 (0 – 4) for the placebo and gum arabic groups and 2 (0 – 4) for CMC and psyllium groups in the steady dose 1 and 2 segments. The median number of times of flatus in the four supplement groups was 3 to 4.5 (range = 0 to 75) in the baseline segment and 5 – 6 (range = 0 – 93) in steady dose 1 and 2. There was no statistical difference in the severity of any symptom among the groups or between the baseline and steady dose segments. There was also no statistical difference in the median number of flatus episodes that occurred among groups or study segments.
Symptom Type and Severity over Time
Mixed modeling analyses, which used the daily ratings of symptom severity and adjusted for study day, segment, and group, showed that there were differences in the severity of specific symptoms over time (Table 5). The odds of having greater severity of flatus, belching, fullness, and bloating in the steady dose 1 segment were 1.5 – 1.9 times higher than in the baseline segment; the odds in the steady dose 2 segment were 1.4 – 1.7 times higher than baseline. The likelihood of greater belching was higher in the steady dose 1 segment compared to the steady dose 2 segment while the likelihood of a greater feeling of fullness was higher in the steady dose 2 segment. Even though there was a greater likelihood of having an increased feeling of fullness in the latter study segments, during a segment, the odds of feeling full decreased .01 per day as each day of a segment passed. The odds of greater abdominal cramping were 20% higher in the steady dose 1 segment than the steady dose 2 segment.
Table 5.
Severity of Gastrointestinal Symptoms before and during Steady Intake of a Fiber Supplement or Placebo over Time
| Adjusted Symptom Severity per Day | OR** | 95% CI | p |
|---|---|---|---|
| Number of Times of Flatus | |||
| Steady Dose 1 vs. Baseline | 1.4 | 1.3 – 1.6 | <.001 |
| Steady Dose 2 vs. Baseline | 1.4 | 1.3 – 1.6 | <.001 |
| Flatus Severity | |||
| Steady Dose 1 vs. Baseline | 1.5 | 1.3 – 1.8 | < .001 |
| Steady Dose 2 vs. Baseline | 1.4 | 1.2–1.7 | < .001 |
| Belching Severity | |||
| Steady Dose 1 vs. Baseline | 1.2 | 1.02 – 1.5 | .008 |
| Steady Dose 1 vs. Steady Dose 2 | 1.2 | 1.05 – 1.4 | .001 |
| Belching by day | .99 | .98 – 1.00 | .05 |
| Bloating Severity | |||
| Steady Dose 1 vs. Baseline | 1.8 | 1.4 – 2.3 | < .001 |
| Steady Dose 2 vs. Baseline | 1.7 | 1.3 – 2.2 | < .001 |
| Feeling of Fullness Severity | |||
| Steady Dose 1 vs. Baseline | 1.95 | 1.5 – 2.5 | < .001 |
| Steady Dose 2 vs. Baseline | 1.7 | 1.3 – 2.2 | < .001 |
| Steady Dose 2 vs. Steady Dose 1 | 1.2 | 1.01 – 1.4 | .01 |
| Psyllium vs. Placebo | 2.4 | 1.1 – 5.2 | .004 |
| Feeling of Fullness by day | .99 | .975 – .999 | .02 |
| Abdominal Cramping Severity | |||
| Steady Dose 2 vs. Steady Dose 1 | 1.2 | .09 – 1.4 | .01 |
Adjusted for supplement group, study segment, and day of segment
Odds Ratio = eβ
Note. Symptom severity was adjusted for supplement group, study segment, and segment day.
OR = Odds ratio
CI = Confidence interval
Only one symptom had a greater likelihood of occurring in one of the dietary fiber groups than the placebo group. A greater feeling of fullness was about twice as likely to occur in the psyllium group compared to the placebo group. There was no significant difference in the severity of other symptoms among fiber groups.
Symptoms during Incremental Dosing of Dietary Fiber
In a second model, symptoms that occurred during the Incremental Dosing segment were compared among supplement groups. Adjusting for study segment and day, there was no difference in symptom severity among the groups. Controlling for group and study segment, the likelihood of a greater feeling of fullness (OR = 1.05, 95% CI = 1.009 – 1.100, p < .019) and bloating (OR = 1.06, CI = 1.016 – 1.116, p < .009) increased by about .05% each day compared to the previous day in a segment.
Symptom Severity and Emotional Upset
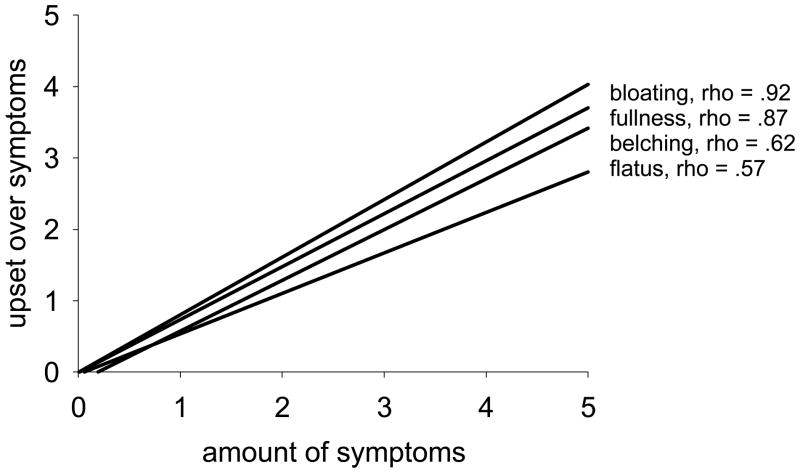
There was a significant association between the severity of a specific symptom and emotional upset about that symptom. Figure 2 illustrates this relationship using four of the more common symptoms. There was a strong and positive relationship between emotional upset with the symptom and the severity of bloating or feeling of fullness; the relationship was moderate for flatus and belching (Figure 2).
Figure 2.
Correlation between severity of adverse gastrointestinal symptoms and level of emotional upset with symptoms in individuals with fecal incontinence
Symptom Severity and Time to Withdrawal
Seventeen subjects (8%) withdrew from the study after consuming a supplement; there was no significant difference in time to withdrawal from the study among the supplement groups (p = .08). Symptom evaluation forms (total n = 84 forms) from the baseline segment through study withdrawal were returned by 11 subjects: 5 in the psyllium group and 6 in the CMC group. The characteristics of these 11 participants are as follows: age = 65 (13.2) years, 64% female, 91% white.
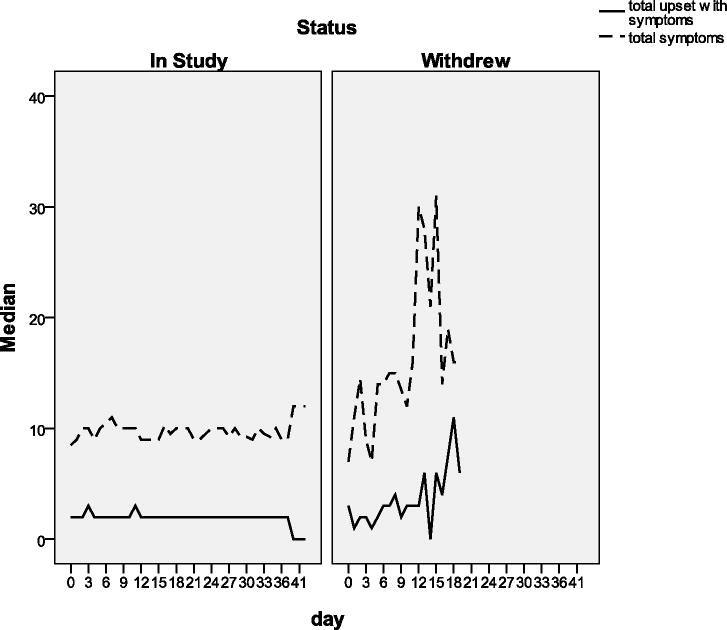
Participants who withdrew from the study had a greater overall score for total symptom severity (median = 11, range = 5 – 60) than study completers (median = 10, range = 0.5 – 75) (Figure 3). The total score for emotional upset with symptoms was almost twice as high for those who withdrew (median = 4.8, range = 0 – 12) versus those who completed (median = 2.6, range = 0 – 15) (Figure 3). All 17 participants who withdrew indicated intolerance of the supplements as one of the reasons for leaving the study. Three participants reported more than one reason for withdrawal, including a change in general health, perceiving the study was too much of a time commitment, and not wanting/being able to continue with data collection procedures.
Figure 3.
The total severity of adverse gastrointestinal symptoms by day of subjects with fecal incontinence who completed the study (left) and those who started supplementation but withdrew from the study early (right)
In examining each individual symptom, participants with a greater feeling of fullness were 2.2 times as likely to withdrawal from the study and withdraw sooner than those without this feeling (95% CI = 1.6 – 3.0, p < .001). Participants who were more emotionally upset about a feeling of nausea, were 2.2 times more likely to withdrawal from the study sooner than those less emotional upset (CI = 1.5 – 3.20, p < .001). Participants were 1.03 times as likely to leave the study sooner with every additional point increase in the total score for symptom severity (CI = 1.006 – 1.05, p < .01). They were 1.1 times more likely to leave sooner for every point increase in their emotional upset score (CI = 1.05 – 1.2, p < .001).
Symptoms, Emotional Upset, and Fiber Dose Reduction
Twenty-five participants reduced the amount of dietary fiber in their supplement. Those who reduced their fiber dose were 84% female, 96% White (M = 58 yrs, SD = 10), 60% employed and 32 % retired, 12% with a high school diploma, and 36% with a college degree. There was no difference in their demographic characteristics compared to those who did not reduce their fiber amount. No participant in the placebo group requested a reduction, and one (2%) participant in the gum arabic group, nine (20%) in the psyllium group, and 15 (32%) in the CMC group reduced their dose of fiber. Participants in the CMC and psyllium groups reduced their fiber doses sooner than the gum arabic group (p < .001). Emotional upset with symptoms had a significant association with reducing the amount of fiber in the supplement. For every increase of one in their total emotional upset score, subjects were 1.08 times more likely to reduce their fiber dose (CI = 1.03 – 1.1, p < .002). The only individual symptom to be associated with reduction of fiber dose was a greater feeling of fullness (OR = 1.3, CI = 1.06 – 1.6, p < .01).
Discussion
The findings of this study add new knowledge about the course of GI symptoms during supplementation with soluble dietary fiber or placebo in adults with fecal incontinence. The severity of individual symptoms associated with all of the fibers or placebo were small in each study segment. Minor symptom severity has been previously reported when administering gum arabic or psyllium in various doses to other groups (Bliss et al., 2001; Jenkins et al., 2002; Vuksan et al., 2008). The amount of fiber in the usual diet of subjects is similar to that of others on a Western diet or with fecal incontinence (Bliss et al., 2000; Elmadfa & Freisling, 2009; Vuksan et al., 2008). The current U.S. Dietary Guidelines for Americans recommend consumption of a total dietary fiber intake of 14 g/1000 kcal (U.S. Department of Health and Human Services & U.S. Department of Agriculture, 2005). The amount of supplemented fiber increased subject’s total fiber intake, which was generally below recommended levels, to meet the guideline.
Our findings show that the likelihood of having greater severity of several GI symptoms increased over time in individuals with fecal incontinence whether they ingested a fiber supplement or not. These symptoms included flatus, belching, a feeling of fullness, and bloating. Flatus and bloating are lower GI symptoms while belching and a feeling of fullness are upper GI symptoms. The absence of a significant difference in symptoms between the placebo and fiber groups suggests that individuals with fecal incontinence (and no additional fiber intake) have adverse GI symptoms similar to those associated with dietary fiber intake or experience a placebo effect.
Only a feeling of fullness was significantly greater in the psyllium group compared to the placebo group. Psyllium supplementation has been shown to slow gastric emptying, increase the feeling of satiety and reduce hunger (Bergmann et al., 1992), and flatten the postprandial serum glucose, insulin, and triglyceride curves suggesting a delay in absorption of some substrates (Rigaud, Paycha, Meulemans, Merrouche, & Mignon, 1998). Also, most of the dietary fiber in psyllium is insoluble fiber as contrasted with gum arabic and CMC which are primarily soluble fiber sources. These effects may play a role in the feeling of fullness associated with psyllium supplementation.
The severity of other GI symptoms reported during the incremental dosing or steady supplementation with one of the soluble dietary fibers in this study were not significantly different than when a placebo was taken. Uehleke et al. (2008) also showed an increase in several symptoms over time during psyllium ingestion. However, without a control group for comparison, Uehleke et al. were unable to show whether similar symptoms may have occurred without fiber supplementation. In this study, there was little difference in symptoms between the groups receiving placebo or fiber. Using a cross-over design, Jenkins et al. (2002) also found no difference in three GI symptoms during supplementation with a lower dose of psyllium (7.2 g per day) compared to a control diet; they did not, however, report about a feeling of fullness. Subjects receiving either a fiber supplement or placebo reported having several GI symptoms at the same time. Findings suggest that a broad range of relevant symptoms should be included in investigations of fiber supplementation for fecal incontinence. The findings of the placebo group in this study provide some of the first data characterizing the course of GI symptoms in individuals with fecal incontinence; it is not known whether similar symptom patterns would occur in individuals with normal bowel patterns.
The severity of symptoms was associated with study attrition. Regardless of the fiber source, subjects who withdrew from the study experienced greater symptom severity. The significant association of emotional upset and reduction of fiber dose and study withdrawal suggests there is an emotional component to tolerance of GI symptoms. We found no previous studies of fiber that reported emotional upset with symptoms and this measure seems an important factor to be included in future studies.
There are limitations to this study. Although self-report of dietary intake is a common and practical method in clinical studies and validity and reliability improve with training and review, findings of usual fiber intake must be considered estimates compared to laboratory analysis of intake or providing a controlled diet in a metabolic unit. The scales to report symptoms and emotional upset lacked testing of validity and reliability. Generalizability of results is limited because subjects have fecal incontinence, and their symptoms in response to a dietary fiber supplement may not be the same as those of individuals with a normal bowel pattern.
Using a longitudinal study design and the appropriate modeling using generalized estimating equations and Cox proportional hazards regression analyses allowed for addressing a gap in knowledge about symptoms associated with intake of dietary fiber supplements. Rather than collapsing all daily information into summary pre and post supplement measures, the patterns of symptom severity could be assessed, as well as emotional upset over time, and whether these differed significantly among groups.
Persons with fecal incontinence experienced a variety of GI symptoms over time. Knowledge of symptoms associated with fecal incontinence over time may be of interest to patients newly experiencing the problem, lower uncertainty and anxiety when symptoms occur, and assist with coping. Informing patients about the course of symptoms that may be expected during therapies such as fiber supplementation might facilitate acceptance of the therapy and adherence. The fiber supplements used in this study appear well tolerated in terms of GI side effects, which is advantageous for clinical use. Results inform the development of designs and interventions involving dietary fiber supplements for future studies; for example, an incremental dosing period may not be necessary as GI symptoms in groups receiving fiber did not differ from those on placebo. Investigators should anticipate that symptom severity and emotional upset can influence tolerance to a diet intervention and study attrition and develop a plan to address these responses.
Acknowledgments
This study was funded by the National Institute of Nursing Research, NIH, R01-R07756 “Impact of Fiber Fermentation on Fecal Incontinence.”
Footnotes
Mention of a proprietary product does not constitute a recommendation or warranty of the product by the University of Minnesota or the USDA-ARS and does not imply its approval to the exclusion of other suitable products.
References
- Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4. Paris, France: Health Publication Ltd; 2009. [Google Scholar]
- Adiotomore J, Eastwood MA, Edwards CA, Brydon WG. Dietary fiber: In vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition. 1990;52:128–134. doi: 10.1093/ajcn/52.1.128. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Davidson MH, Blonde L, Brown WV, Howard WJ, Ginsberg H, et al. Long-term cholesterol-lowering effects of psyllium as an adjunct to diet therapy in the treatment of hypercholesterolemia. The American Journal of Clinical Nutrition. 2000;71:1433–1438. doi: 10.1093/ajcn/71.6.1433. [DOI] [PubMed] [Google Scholar]
- Bergmann JF, Chassany O, Petit A, Triki R, Caulin C, Segrestaa JM. Correlation between echographic gastric emptying and appetite: Influence of psyllium. Gut. 1992;33:1042–1043. doi: 10.1136/gut.33.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss DZ, Jung HJ, Savik K, Lowry A, LeMoine M, Jensen L, et al. Supplementation with dietary fiber improves fecal incontinence. Nursing Research. 2001;50:203–213. doi: 10.1097/00006199-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Bliss DZ, McLaughlin J, Jung HJ, Lowry A, Savik K, Jensen L. Comparison of the nutritional composition of diets of persons with fecal incontinence and that of age-and gender-matched controls. Journal of Wound, Ostomy, and Continence Nursing. 2000;27:90–97. doi: 10.1016/s1071-5754(00)90075-5. [DOI] [PubMed] [Google Scholar]
- Classification of overweight and obesity. Retrieved on November 9, 2009, from http://www.nhlbi.nih.gov/guidelines/obesity/e_txtbk/txgd/414.htm.
- Elmadfa I, Freisling H. Nutritional status in Europe: Methods and results. Nutrition Reviews. 2009;67(Suppl 1):S130–S134. doi: 10.1111/j.1753-4887.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA. The gel-forming polysaccharide of psyllium husk (plantago ovata forsk) Carbohydrate Research. 2004;339:2009–2017. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Flammang AM, Kendall DM, Baumgartner CJ, Slagle TD, Choe YS. Effect of a viscous fiber bar on postprandial glycemia in subjects with type 2 diabetes. Journal of the American College of Nutrition. 2006;25:409–414. doi: 10.1080/07315724.2006.10719553. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Vuksan V, Vidgen E, Parker T, Faulkner D, et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. The American Journal of Clinical Nutrition. 2002;75:834–839. doi: 10.1093/ajcn/75.5.834. [DOI] [PubMed] [Google Scholar]
- Livesey G. Tolerance of low-digestible carbohydrates: A general view. The British Journal of Nutrition. 2001;85(Suppl 1):S7–16. doi: 10.1079/bjn2000257. [DOI] [PubMed] [Google Scholar]
- Parisi G, Bottona E, Carrara M, Cardin F, Faedo A, Goldin D, et al. Treatment effects of partially hydrolyzed guar gum on symptoms and quality of life of patients with irritable bowel syndrome. A multicenter randomized open trial. Digestive Diseases and Sciences. 2005;50:1107–1112. doi: 10.1007/s10620-005-2713-7. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Dietary supplements for body-weight reduction: A systematic review. The American Journal of Clinical Nutrition. 2004;79:529–536. doi: 10.1093/ajcn/79.4.529. [DOI] [PubMed] [Google Scholar]
- Rigaud D, Paycha F, Meulemans A, Merrouche M, Mignon M. Effect of psyllium on gastric emptying, hunger feeling and food intake in normal volunteers: A double blind study. European Journal of Clinical Nutrition. 1998;52:239–245. doi: 10.1038/sj.ejcn.1600518. [DOI] [PubMed] [Google Scholar]
- Smith KN, Queenan KM, Thomas W, Fulcher RG, Slavin JL. Physiological effects of concentrated barley beta-glucan in mildly hypercholesterolemic adults. Journal of the American College of Nutrition. 2008;27:434–440. doi: 10.1080/07315724.2008.10719722. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Hale WE, Moore MT, May FE, Marks RG. Effect of psyllium hydrophilic mucilloid on serum cholesterol in the elderly. Digestive Diseases and Sciences. 1991;36:329–334. doi: 10.1007/BF01318205. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, & U.S. Department of Agriculture. Dietary guidelines for Americans. Washington, DC: U. S. Government Printing Office; 2005. [Google Scholar]
- Uehleke B, Ortiz M, Stange R. Cholesterol reduction using psyllium husks - do gastrointestinal adverse effects limit compliance? Results of a specific observational study. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2008;15:153–159. doi: 10.1016/j.phymed.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Jenkins AL, Jenkins DJ, Rogovik AL, Sievenpiper JL, Jovanovski E. Using cereal to increase dietary fiber intake to the recommended level and the effect of fiber on bowel function in healthy persons consuming North American diets. The American Journal of Clinical Nutrition. 2008;88:1256–1262. doi: 10.3945/ajcn.2008.25956. [DOI] [PubMed] [Google Scholar]
- Zumarraga L, Levitt MD, Suarez F. Absence of gaseous symptoms during ingestion of commercial fibre preparations. Alimentary Pharmacology & Therapeutics. 1997;11:1067–1072. doi: 10.1046/j.1365-2036.1997.00250.x. [DOI] [PubMed] [Google Scholar]