Abstract
Cervical cancer is the second most common malignancy among women worldwide and is highly radioresistant, often resulting in local treatment failure. For locally advanced disease, radiation is combined with low-dose chemotherapy; however, this modality often leads to severe toxicity. Curcumin, a polyphenol extracted from rhizomes of the plant Curcuma longa, is a widely studied chemopreventive agent that was shown to have a low toxicity profile in three human clinical trials. Here, we show that pretreatment of two cervical carcinoma cell lines, HeLa and SiHa, with curcumin before ionizing radiation (IR) resulted in significant dose-dependent radiosensitization of these cells. It is noteworthy that curcumin failed to radiosensitize normal human diploid fibroblasts. Although in tumor cells, curcumin did not significantly affect IR-induced activation of AKT and nuclear factor-κB, we found that it caused a significant increase in the production of reactive oxygen species, which further led to sustained extracellular signal-regulated kinase (ERK) 1/2 activation. The antioxidant compound N-acetylcysteine blocked the curcumin-induced increased reactive oxygen species (ROS), sustained activation of ERK1/2, and decreased survival after IR in HeLa cells, implicating a ROS-dependent mechanism for curcumin radiosensitivity. Moreover, PD98059 (2′-amino-3′-methoxyflavone)-, PD184352- [2-(2-chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide], and U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophynylthio)butadiene]-specific inhibitors of mitogen-activated protein kinase kinase 1/2 (MEK1/2) blocked curcumin-mediated radiosensitization, demonstrating that the sustained ERK1/2 activation resulting from ROS generation leads to curcumin-mediated radiosensitization. Together, these results suggest a novel mechanism for curcumin-mediated radiosensitization involving increased ROS and ERK1/2 activation and suggest that curcumin application (either systemically or topically) may be an effective radiation modifying modality in the treatment of cervical cancer.
Radiation therapy plays a critical role in the local and regional control of malignant tumors. Recent attempts to enhance the efficacy of radiation therapy have focused on using conventional chemotherapeutic agents as biological response modifiers (Candelaria et al., 2006). Although this approach has in some cases resulted in better therapeutic outcome (Seiwert et al., 2007), its efficacy can be limited by a number of factors, including increased toxicity, normal tissue injury, and increased side effects. Future improvements in the therapeutic index for radiotherapy depend on increasing the sensitivity of tumor cells to radiation and reducing the effects of radiation on normal tissues. One way to circumvent this problem is to use compounds with relatively safe toxicity profiles and test their use as potential radiosensitizers.
Chemoradiotherapy involving cisplatin and 5-fluorouracil is the current standard of care for patients with stage IIA to IVA cervical cancer (Morris et al., 1999). Several agents and drug combinations including carboplatin, cisplatin, 5-fluorouracil, ifosfamide, etoposide, and most recently taxanes have been used as radiation sensitizers (Candelaria et al., 2006). Although concurrent chemoradiotherapy improves survival compared with radiotherapy alone (Morris et al., 1999), the combination is associated with an increased likelihood of dose-limiting toxicities like gastrointestinal and hematological toxicities (Kirwan et al., 2003). Considering the toxic side effects of chemotherapy, achieving radiosensitization with minimal toxicity could be beneficial for patients with locally advanced cervical cancer.
Curcumin is the yellow pigment of turmeric, a natural product with diverse biological activities (Duvoix et al., 2005). Turmeric, which contains primarily curcumin and other curcuminoid compounds, is obtained from the roots of the plant Curcuma longa, which grows in tropical regions. Based on epidemiological data that showed a reduced rate for colon carcinogenesis in populations whose diet is rich in curcumin, studies on the beneficial and chemopreventive activity of curcumin were initiated (Gescher et al., 2001). Several of the preclinical studies, carried out over the last 20 years, have confirmed potent antitumorigenic activity of curcumin in the initiation and promotion phases in two stage mouse tumor models (Howells et al., 2007). Phase I clinical trials on curcumin showed that it is safe to humans up to 12,000 mg/day when taken orally (Cheng et al., 2001; Sharma et al., 2001; Lao et al., 2006) and caused histological improvement of precancerous lesions in some patients, suggesting that it is biologically active at these doses (Cheng et al., 2001). Previous reports have indicated that curcumin confers radiosensitizing effects in prostate cancer cell lines (Chendil et al., 2004) and squamous cell carcinoma lines (Khafif et al., 2005).
The ability of curcumin to alter the redox status of transformed cells (Scapagnini et al., 2002) and its desirable safety profile prompted us to investigate whether it can also alter their radiation sensitivity. We report that pretreatment with curcumin radiosensitized cervical carcinoma cells in vitro in a time- and dose-dependent manner but had no radiosensitizing effect on the radiation responses of the normal diploid fibroblasts. This radiosensitization occurred in an AKT- and NF-κB-independent and reactive oxygen species (ROS)-ERK1/2-dependent mechanism. These results, together with published reports indicating that curcumin is a potent sensitizer of other tumor cell lines and a protector of normal tissue from radiation toxicity, suggest that this natural product may be an effective radiosensitizer or radioenhancer in the management of patients with advanced cervical cancer.
Methods and Materials
Cell Culture
All cell lines were incubated at 37°C with 5% CO2. HeLa and SiHa cell lines were obtained from the American Type Culture Collection (Manassas, VA). MRC-5 diploid fibroblasts were a gift from Dr. Suzy Torti (Wake Forest University, Winston-Salem, NC). All the cell lines were grown in Dulbecco’s modified Eagle’s medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal calf serum, and L-glutamine.
Reagents
Curcumin (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO to obtain a 10 mM stock solution. The PI-3 kinase inhibitor LY294002, the MEK1 inhibitor PD98059, and staurosporine were obtained from Cell Signaling Technology Inc. (Danvers, MA), and stock solutions were prepared by dissolving in DMSO. N-Acetylcysteine (NAC) (Sigma-Aldrich) was dissolved in Dulbecco’s modified Eagle’s medium and adjusted to pH 7 with 10 M sodium hydroxide. AG-1478 and U0126 were obtained from Calbiochem (San Diego, CA), and stock solutions were prepared in DMSO. PD184352 was a generous gift from Dr. Paul Dent (Virginia Commonwealth University, Richmond, VA).
Clonogenic Survival Assays
Low-Density Clonogenic Assay
Cells were plated in 60-mm culture dishes at densities of 100, 300, and 1000 cells per plate and allowed to attach overnight. Curcumin was added for indicated times and doses, then cells were irradiated. For analysis of oxidative stress, 5 mM NAC or 10 U/ml Catalase was added 6 h before irradiation. For clonogenic survival assays with PD98059, PD184352, or U0126, we added indicated doses of drug during the last 2 h of curcumin treatment. The drugs were removed 1 h after radiation. Cells were incubated for 10 to 14 days after irradiation and then fixed with 10% methanol/10% acetic acid and stained with 0.4% crystal violet. Colonies containing more than 50 cells were counted. The plating efficiencies were determined for each treatment and normalized to controls. The curves were fitted using a second order polynomial function. The average normalized surviving fraction from three independent experiments and the S.E.M. were reported.
High-Density Clonogenic Assay
Cells were plated at 1 × 106 cells/100-mm plates, allowed to reach 80% confluence, and irradiated or pretreated with curcumin before radiation. One hour after radiation, the cells were trypsinized and plated into 60-mm dishes at lower numbers. These plates were incubated for 10 to 14 days and stained as described above.
Clonogenic Assay under Hypoxia
Cells were plated at 5 × 105 cells/60-mm preconditioned glass Petri dishes and allowed to reach 80% confluence. The cells were treated with DMSO or 10 μM curcumin for 8 h. During the last 1 h, the medium was replaced by 1 ml of fresh medium (minimal essential medium with 10% fetal calf serum and 1% penicillin/streptomycin, 2 mM glucose, 20 mM HEPES, without pyruvate) containing DMSO or curcumin. The plates were sealed into aluminum chambers and allowed to acclimatize for 30 min. For hypoxic treatments, the percentage O2 in the gas phases was decreased to 0.01% by a series of precision evacuations of the chamber followed by replacement with nitrogen (gas exchanges). After warming, the chambers were shaken continuously at 37°C to ensure that the O2 in the gas phase was in equilibrium with the O2 in the culture media. The aluminum chambers were directly placed in the Cs137 irradiator, and the cells were irradiated at 2, 4, and 6 Gy for aerobic treatments and 5, 10, or 15 Gy for the hypoxic treatments. The chambers were shaken continuously at 37°C for 1 h, and then the plates were trypsinized and replated at low densities as described above.
Cell Proliferation Assay (MTT Assay)
MTT assays were performed using a kit obtained from Roche Applied Science (Indianapolis, IN). Cells were plated onto 24-well plates at a density of 500 (HeLa) or 3000 (MRC-5) cells per well, treated with indicated doses of curcumin for 8 h, and then irradiated. The drug was removed 1 h after irradiation. Cell viability was assessed according to the manufacturer’s protocol when the controls were confluent (~7 days). Two hundred microliter aliquots from each well were transferred into a 96-well plate, and absorbance values were measured at 560 nm with an automated plate reader (Molecular Devices, Sunnyvale, CA).
Analysis of ROS Levels
HeLa cells were plated at 4 × 104 cells/well, allowed to attach overnight, and treated with DMSO or 10 μM curcumin for 8 h. Cells were then washed with PBS+ solution (0.14 g of calcium chloride and 0.1 g of magnesium chloride in 1 liter of PBS). The cells were then incubated with 10 μM 2′,7′-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich) or a control carboxy-DCF-DA probe (Invitrogen, Carlsbad, CA) for 30 min at 37°C before irradiation. Relevant plates were irradiated at 10 Gy, and the fluorescence was measured for all the plates using a fluorometer at an absorption wavelength of 485 nm and emission wavelength of 520 nm. The results were expressed as relative fluorescence, normalized to unirradiated control. Error bars represent S.E. from three independent experiments.
Immunoblotting
Cells were harvested after corresponding treatments, as described previously (Koumenis et al., 2002). For detection of AKT, ERK1/2, and β-actin, whole-cell lysates (20–40 μg of total protein) were resolved on 10% SDS-polyacrylamide gel electrophoresis gels, transferred to polyvinylidene difluoride membrane, blocked for 30 min at room temperature in Tris-buffered saline-Tween solution containing 5% milk, and incubated for 1 to 2 h at room temperature with the following antibodies in Tris-buffered saline-Tween solution containing 1% milk at the following dilutions: anti-phospho-AKT antibody (1:1000; Cell Signaling Technology Inc.), anti-AKT (1:1000; Cell Signaling Technology Inc.), anti-phospho-ERK1/2 (1:5000; Cell Signaling Technology Inc.), anti-ERK1/2 (1:2000; Zymed Laboratories, South San Francisco, CA), and anti-β-actin (1:50,000; Sigma-Aldrich). All membranes were incubated with horseradish peroxidase-conjugated anti-mouse (1:2000) or anti-rabbit (1:2000) secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and immunoreactive bands were detected using ECL Plus chemiluminescence (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
For detection of p65 and Lamin A/C, cytoplasmic and nuclear fractions were separated. In brief, cells were washed with cold PBS and suspended in 0.4 ml of lysis buffer (10 mM HEPES, pH 7.9, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2.0 mg/ml aprotinin, and 0.5 mg/ml benzamidine). The cells were allowed to swell on ice for 5 min followed by centrifugation at 1500 rpm for 10 min. The nuclear pellet was resuspended in 100 μl of ice-cold nuclear extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 2.0 mg/ml leupeptin, and 2.0 mg/ml aprotinin), and the tube was incubated on ice for 15 min with intermittent vortexing. This nuclear extract was then centrifuged for 15 min in a Microfuge at 4°C. Ten micrograms of protein was resolved on 10% SDS-polyacrylamide gel electrophoresis gels, transferred to polyvinylidene difluoride membrane, and immunoblotted with specific antibodies at the following dilutions: anti-p65 (SC-109 at 1:1000; Santa Cruz Biotechnology, Inc.) and Lamin A/C (no. 612162 at 1:2000; BD Biosciences, San Jose, CA).
Electromobility Shift Assay
Nuclear extracts from irradiated cells that were pretreated with either DMSO or curcumin were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (Promega, Madison, WI) for 30 min at 37°C, and the DNA-protein complex formed was separated from free oligonucleotide on 10% native polyacrylamide gels. The specificity of binding was examined by competition with 3-fold excess of unlabeled oligonucleotide. For supershift analysis, nuclear extracts prepared from irradiated cells were incubated with an antibody against p65 subunit (Santa Cruz Biotechnology, Inc.) of NF-κB for 30 min at room temperature before the complex was analyzed by electrophoretic mobility shift assay. The dried gels were visualized and radioactive bands were quantified by PhosphorImager (GE Healthcare) with the use of ImageQuant software (GE Healthcare).
Cell Cycle Analysis
HeLa cells were trypsinized and collected in 2 ml of PBS/1% FBS at 5000 rpm in a swinging bucket centrifuge. The cells were resuspended in 0.5 ml of PBS and fixed in 5 ml of cold (4°C) ethanol overnight at −20°C. Subsequently, cells were washed in 2 ml of PBS/1% FBS and resuspended in 1 ml of PBS, and 0.5 ml of phosphate-citric acid buffer (192 ml of 0.2 M Na2HPO4 + 8 ml of 0.1 M citric acid, pH 7.8) and let stand at room temperature for 5 min. The cells were further resuspended in 300 μl of PI/RNase staining solution (300 μl of PBS with 1% FBS + 30 μl of PI + 8 μl of RNase), incubated at 37°C for 15 min, and analyzed by flow cytometry (FACSCalibur and CellQuest Pro; BD Biosciences).
Results
Curcumin Radiosensitized Cervical Carcinoma Cells in Vitro
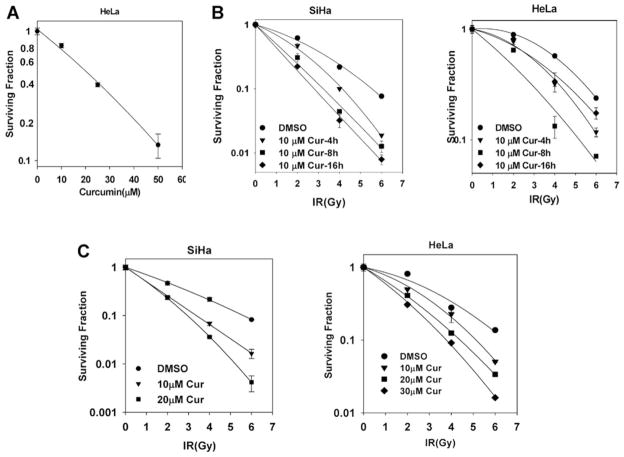
To examine the effects of curcumin on human tumor cell radiosensitivity, we chose two cervical tumor cell lines, HeLa and SiHa. These cell lines are HPV 18 and HPV 16 positive, respectively, and both have been previously used in radiosensitivity assays (Zoberia et al., 2002). We initially determined the toxicity of curcumin treatment alone on these cells. Treatment with curcumin demonstrated a dose-dependent decrease in clonogenic cell survival (Fig. 1A). Using the concentration of curcumin that showed a 20% decrease in cell survival (10 μM) after 8-h pretreatment, we further investigated the effects of curcumin pretreatment on radiation-induced cell kill. Curcumin reduced clonogenic survival in both cell lines at each of the radiation doses we tested (Fig. 1B). In SiHa cells, curcumin induced a time-dependent increase in sensitization, but in HeLa cells, radiosensitization was significantly higher after an 8-h pretreatment, compared with 16 h, probably reflecting an increased rate of metabolic inactivation of curcumin in these cells. The dose enhancement ratio (ratio of radiation dose without to dose of radiation with the radiosensitizer to achieve the same amount of cell kill) was 1.6 for SiHa and 1.4 for HeLa at 0.1 surviving fraction. Based on these results, we chose a curcumin dose of 10 μM for 8 h as the pretreatment condition used in further molecular studies. To more closely reproduce the cellular conditions used in molecular analysis studies (see below), we also performed the clonogenic survival assays under high density as described under Materials and Methods. The radiosensitization achieved by curcumin was similar to that seen when the clonogenic survival assays were performed at low density (Fig. 1C). Taken together, these results clearly demonstrate that curcumin treatment sensitizes these tumor cell lines to ionizing radiation.
Fig. 1.
Curcumin sensitizes cervical tumor cell lines to ionizing radiation. A, HeLa cells were treated with DMSO or 10, 25, and 50 μM curcumin for 8 h. Survival was assessed by low-density clonogenic survival assay as described under Materials and Methods. B, SiHa and HeLa cells were treated with DMSO or 10 μM curcumin for 4, 8, and 16 h followed by 0, 2, 4, or 6 Gy ionizing radiation. Survival was assessed by low-density clonogenic survival assay as described under Materials and Methods. Results represent the averages of three independent experiments (±S.E.). C, HeLa and SiHa cells were treated with DMSO or 10 or 20 μM curcumin for 8 h followed by 0, 2, 4, or 6 Gy ionizing radiation. One hour after radiation, cells were trypsinized and replated for clonogenic survival assays. Results represent the averages of three independent experiments (±S.E.).
Differential Effects of Curcumin and Radiation in Tumor versus Normal Cells
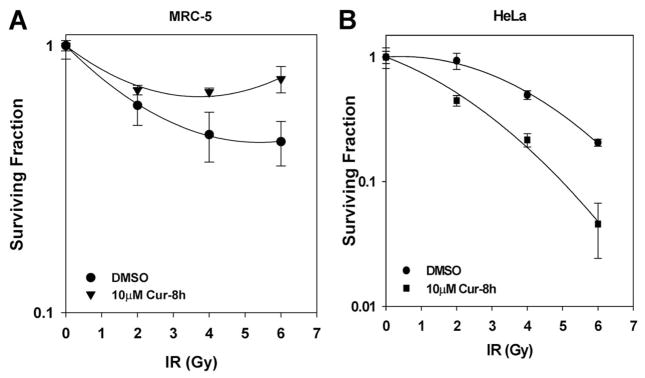
For a radiosensitizer to be a clinically effective, it is critical that it has the ability to selectively affect tumor cells but have minimal or no radiosensitizing effects on normal, untransformed cells (Hall, 2000). Although curcumin has been shown to preferentially induce apoptosis in transformed versus normal cells (Syng-Ai et al., 2004), its potential radiation-modifying effects on normal cells are largely unexplored. We therefore assessed the long-term survival of normal diploid fibroblasts after treatments with curcumin and IR at doses that were used to assess the clonogenic survival of tumor cells. Because these normal cells are poorly clonogenic, we used a modified, long-term MTT assay (see Materials and Methods) to assess the radiosensitivity of the normal cells. Treatment with curcumin before radiation did not radiosensitize normal cells; in fact, it appeared to confer a radioprotective effect to these cells (Fig. 2A). In contrast, under the same MTT conditions, HeLa tumor cells responded in a manner similar to the clonogenic assay showing significant radiosensitization in the presence of curcumin (Fig. 2B).
Fig. 2.
Curcumin does not sensitize normal cells to ionizing radiation. A, MRC-5 diploid fibroblasts (3000 cells) were treated with DMSO or 10 μM curcumin for 8 h followed by irradiation at 2-, 4-, or 6-Gy dose of IR. MTT assay was performed, when the controls were confluent, as described under Materials and Methods. Results represent the averages of four independent experiments (±S.E.). B, HeLa cells (500 cells) were treated with DMSO or 10 μM curcumin for 8 h followed by irradiation at 2-, 4-, or 6-Gy dose of IR. MTT assay was performed, when the controls were confluent, as described under Materials and Methods. Results represent the averages of four independent experiments (±S.E.).
Effects of Curcumin on Radiation-Induced NF-κB Activation
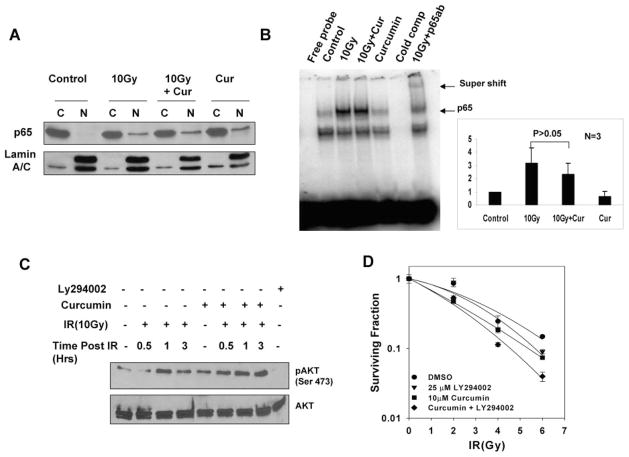
Ionizing radiation induces cell death after DNA damage (Hall, 2000). However, cellular defense mechanisms are also triggered to prevent radiation-induced death. One such prosurvival pathway is the activation of NF-κB cascade, which has been hypothesized to lead to increased radiation resistance in tumors (Baldwin, 2001). Moreover, NF-κB has been reported to be a direct target of curcumin in various tumor cells lines (Singh and Aggarwal, 1995; Shishodia et al., 2005). To determine the effects of curcumin on IR-induced NF-κB activation, we analyzed the levels of the p65 subunit of NF-κB in the cytoplasmic and nuclear fractions from mock-irradiated and irradiated cells that were either pretreated with curcumin or vehicle control. As seen in Fig. 3A, IR induced an increase in p65 translocation to the nucleus. However, neither treatment with curcumin alone nor pretreatment with curcumin before radiation had an inhibitory effect on translocation of p65 subunit of NF-κB in to the nucleus. We also investigated the effects of curcumin on radiation-induced NF-κB DNA binding activity. IR caused a significant increase in NF-κB DNA binding ability. However, curcumin pretreatment had no significant effect on IR-induced NF-κB DNA binding ability (Fig. 3B). Quantitation from three independent experiments indicated a modest inhibition of IR-induced NF-κB activation upon curcumin pretreatment, but this effect was not statistically significant (Fig. 3B, right). Taken together, these results suggest that curcumin-mediated radiosensitization is not likely dependent on its effects on NF-κB activity.
Fig. 3.
Curcumin induced radiosensitization is not dependent on inhibition of NF-κB or AKT. A, HeLa cells were serum starved for 24 h, treated either with DMSO or 10 μM for 8 h, followed by mock irradiation or 10-Gy dose of IR. The cells were harvested 3 h after irradiation. Ten micrograms of the cytoplasmic and nuclear lysates was analyzed by Western blot using antibodies against the p65 subunit of NF-κB. Lamin A/C was used as a loading control for nuclear fractions. B, HeLa cells were treated either with DMSO or 10 μM for 8 h, followed by mock irradiation or 10-Gy dose of IR. Five micrograms of nuclear lysates was incubated with P32-labeled oligonucleotides. For competitive binding, 3-fold excess unlabeled oligo probes were used. For supershift analysis, nuclear extracts from irradiated samples were incubated with antibody against the p65 subunit of NF-κB for 30 min. All the samples were examined for NF-κB by DNA binding by electrophoretic mobility shift assay. Results were quantified from three independent experiments. C, SiHa cells treated either with DMSO or 10 μM for 8 h, followed by mock irradiation or 10-Gy dose of IR. Treatment with 25 μM LY294002 for 3 h before radiation was used as a negative control for phosphorylation of AKT. Forty micrograms of the whole-cell lysates was analyzed by Western blot using antibodies against pAKT (Ser473) and AKT. D, HeLa cells treated with DMSO, 10 μM curcumin (for 8 h), and 25 μM LY294002 (for 3 h) or 3 h of LY294002 treatment during the last 3 h of curcumin treatment. The cells were irradiated at 2, 4, or 6 Gy and replated for a clonogenic survival assay after 1 h of IR. Results represent the averages of three independent experiments (±S.E.).
Curcumin-Mediated Radiosensitization Was Not Mediated by Inhibition of IR-Induced AKT Activation
Various studies using clinically relevant doses and fractionation schemes of IR suggest a role for EGFR activation in tumor radioresistance and recurrence (Schmidt-Ullrich et al., 1997; Sartor, 2003). We investigated the EGFR signaling pathway as a potential target of curcumin to mediate the radiosensitization. We initially focused on the AKT proto-oncogene that plays a central role in several cell signaling processes that lead to increased cell proliferation, resistance to apoptotic stimuli, and antiangiogenesis (Zhan and Han, 2004). To test whether curcumin inhibited radiation-induced AKT activation, we analyzed cell lysates from mock-irradiated and irradiated cells that were either pretreated with curcumin or DMSO. IR activates AKT (determined by Ser473 phosphorylation) within minutes of radiation, and this activation starts to decrease by 3 h after radiation. Curcumin pretreatment did not inhibit the basal level or radiation-induced activation of the prosurvival factor AKT even 3 h after radiation. To further examine the possibility that curcumin-mediated sensitization was dependent on AKT activation, we performed clonogenic assays using LY294002, a PI-3 kinase inhibitor that inhibits AKT activation. LY294002 pretreatment did sensitize tumor cells to IR as previously reported (Kim et al., 2006). However, LY294002 enhanced the radiosensitizing effect of curcumin (Fig. 3D). This result strongly suggests that curcumin and LY294002 act through distinct pathways to induce radiosensitization; therefore, Akt signaling is not involved in curcumin-induced radiosensitization.
Curcumin Prolonged Radiation-Induced ERK1/2 Activation
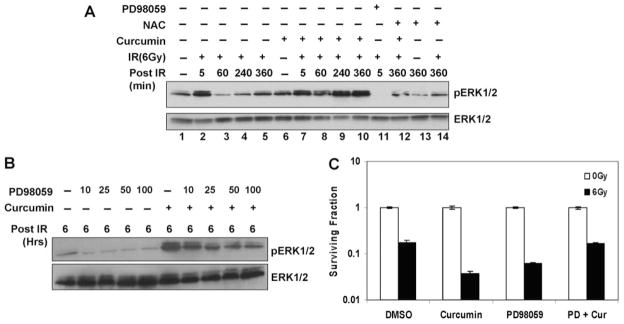
To identify the molecular targets involved in curcumin-mediated radiosensitization, we turned our focus to another EGFR signaling cascade, which involves activation of mitogen-activated protein kinases (MAPKs). Exposure of cells to IR or other toxic stresses simultaneously induces compensatory activation of MAPK pathways, which play critical roles in controlling cell survival and repopulation effects after irradiation (Dent et al., 2003). We determined the effects of curcumin on radiation-induced ERK1/2 activation with cell lysates from irradiated cells that were either pre-treated with curcumin or DMSO. As shown in Fig. 4A, irradiated cells showed a transient increase in ERK1/2 activation that decreased to below the basal levels within an hour after radiation. Curcumin pretreatment alone did not significantly affect ERK1/2 activation. We were surprised to find that pretreatment with curcumin prolonged IR-induced ERK activation, and elevated levels of phosphorylated ERK1/2 were evident even 6 h after radiation (Figs. 4B and 6A).
Fig. 4.
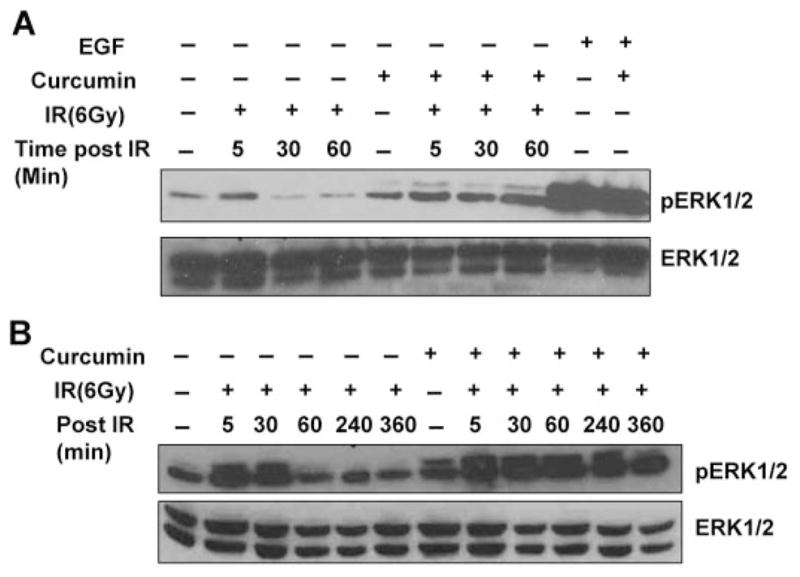
Effect of curcumin on radiation-induced ERK1/2 activation. A, HeLa cells were serum starved for 24 h. The cells were pretreated with DMSO or 10 μM curcumin for 8 h followed by mock irradiation or irradiated 6-Gy dose of IR. Treatments with epidermal growth factor (EGF) were used as a control for ERK1/2 phosphorylation. Whole-cell lysates were analyzed by Western blot using antibodies against p-ERK1/2 and total ERK1/2. B, SiHa cells were serum starved for 24 h. The cells were pretreated with DMSO or 10 μM curcumin for 8 h followed by mock irradiation or irradiated 6-Gy dose of IR. Whole-cell lysates were analyzed by Western blot using antibodies against p-ERK1/2 and total ERK1/2.
Fig. 6.
Curcumin-mediated radiosensitization occurs as a result of sustained ERK1/2 activation, which is further dependent on increased ROS production. A, HeLa cells were serum starved for 24 h, pretreated with DMSO, 10 μM curcumin, N-acetylcysteine, or a combination of NAC and curcumin, followed by mock irradiation or irradiation with a 6-Gy radiation dose. Pretreatment with PD98059 was used as a control for ERK1/2 phosphorylation. Cell lysates were analyzed by Western blot using antibodies against pERK1/2 and ERK1/2. B, HeLa cells were serum starved for 24 h, cotreated with increasing doses of PD98059 and DMSO or 10 μM curcumin for 8 h, followed by irradiation at 6 Gy, and the cells were harvested 6 h after radiation. Cell lysates analyzed by Western blot using antibodies against pERK1/2 and ERK1/2. C, HeLa cells treated with DMSO, 10 μM curcumin (8 h), 50 μM PD98059 (2 h), or PD98059 treatment during the last 2 h of curcumin treatment. The cells were mock-irradiated or irradiated at 6 Gy. The drugs were washed off 1 h after radiation and replated for clonogenic survival assays. Results represent the averages of three independent experiments (±S.E.).
Curcumin Potentiated Radiation-Induced ROS Generation in Tumor Cells
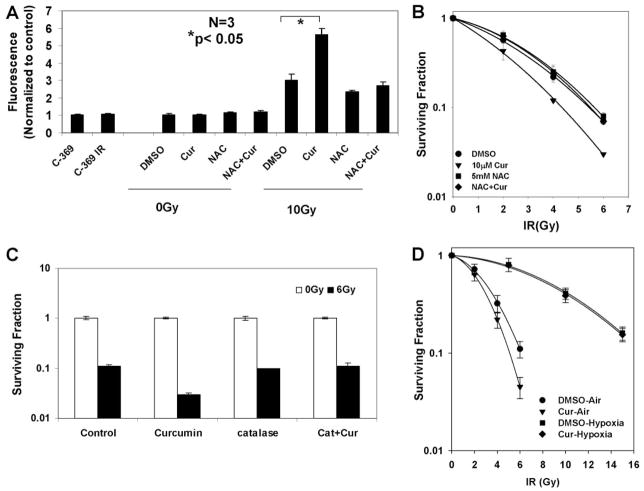
Although the antioxidant properties appear to be critical for the chemopreventive effects of curcumin, it is well documented that like most polyphenols, curcumin also acts as a pro-oxidant under certain conditions (Bhaumik et al., 1999; Galati et al., 2002) by producing ROS. Moreover, the sustained MAPK activation has been linked to increased production of ROS by chemotherapeutic agents (Wang et al., 2000; Kim et al., 2005). To test the ability of curcumin to increase ROS in tumor cells and to investigate the effect of combined treatments with curcumin and IR in this process, we used a redox-sensitive dye, DCF-DA, whose increase in fluorescence is a marker for increase in ROS (Brandt and Keston, 1965). Curcumin treatment alone did not cause a significant increase in production of ROS, but its treatment before radiation dramatically increased ROS levels (~6-fold) compared with radiation alone (~3-fold). Cotreatment of the antioxidant and free-radical scavenger NAC with curcumin before IR blocked the increase in ROS production to levels produced by IR alone, supporting the hypothesis that curcumin in the presence of radiation acts as a potent pro-oxidant. We further hypothesized that if the pro-oxidant effect of curcumin is required for its radiosensitizing properties, then addition of an antioxidant should also compromise its radiosensitizing effects. As shown in Fig. 5B, inclusion of NAC alone in the medium did not alter the radiosensitivity of the cells to IR. Pre-treatment with curcumin produced a substantial increase in radiosensitivity. However, when cells were treated with both curcumin and NAC, the radiosensitizing effect of curcumin was nearly abolished. Similar results were obtained by using another antioxidant catalase (Fig. 5C). These results strongly suggest that curcumin is indeed exerting most of its radiosensitizing effect through a mechanism that involves an increase in ROS.
Fig. 5.
Curcumin potentiates IR-induced ROS generation and ERK activation, which is compromised by the addition of antioxidants. A, HeLa cells were treated with DMSO or 10 μM curcumin for 8 h, followed by mock irradiation or 10-Gy dose of IR. For treatments with NAC, cells were treated with 5 mM NAC for the last 6 h of treatments with DMSO or curcumin followed by radiation. Thirty minutes before IR, the cells were loaded with 10 μM dihydro-dichlorofluorescein-diacetate. Carboxy-dihydro-dichlorofluorescein-diacetate, a nonoxidizable analog of DCF-DA, was used as a control for probe uptake. Results are represented as percentage increase in fluorescence normalized to control, DMSO-treated cells. B, HeLa cells were treated with DMSO, curcumin (10 μM) only, NAC (5 mM) only or with curcumin, and NAC for 8 h before irradiation with a dose of 2, 4, or 6 Gy. Control cells were mock-irradiated (0 Gy). Standard clonogenic assays were used to assay cell survival after irradiation. Results represent the averages of three independent experiments (±S.E.). C, HeLa cells treated with DMSO, 10 μM curcumin (8 h), catalase (10 units/ml for 2 h), or catalase treatment during the last 2 h of curcumin treatment. The cells were mock-irradiated or irradiated at 6 Gy. The drugs were washed off 1 h after radiation. Survival was assessed by low-density clonogenic survival. D, HeLa cells were treated with DMSO, curcumin (10 μM) under air or hypoxia before irradiation with a dose of 2, 4, or 6 Gy (air) or 5, 10, or 15 Gy (hypoxia). Control cells were mock-irradiated (0 Gy). Standard clonogenic assays were used to assay cell survival after irradiation. Results represent the averages of six independent experiments (±S.E.).
During these experiments, we did observe a slight decrease in curcumin uptake in the presence of NAC. To determine whether this effect was responsible for the observed inhibitory effects of NAC on curcumin radiosensitization, we performed clonogenic survival assays on cells treated with NAC under varying concentrations of curcumin. NAC pretreatment completely inhibited curcumin-mediated radiosensitization at all doses of curcumin, suggesting that the effects of NAC we observed were not artifacts of inhibition of curcumin uptake (data not shown).
To further examine the role of ROS in curcumin-mediated radiosensitization, we performed clonogenic survival assays under hypoxia. We reasoned that if ROS plays a significant role in curcumin-mediated radiosensitization, then the presence of oxygen should be required to generate the ROS and fix the DNA damage. To test this hypothesis, we performed clonogenic assays with cells treated with DMSO (control) or curcumin under normoxic (21% oxygen) or hypoxic (0.01% oxygen) conditions. As shown in Fig. 5D, when cells were irradiated in air, there was a significant radiosensitization by curcumin, as previously observed. However, when the cells were irradiated under hypoxia, we did not observe any radiosensitization in curcumin-pretreated cells. These results further support a role for ROS in curcumin-mediated radiosensitization of cervical carcinoma cells.
Effects of Increased ROS on the ERK1/2 Signaling Pathway
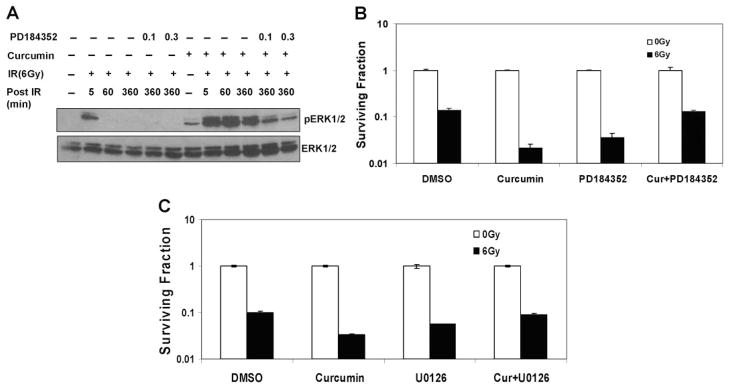
Because oxidative stress is also known to activate MAPKs (McCubrey et al., 2006), we examined the effects of antioxidants on the sustained ERK1/2 activation induced by curcumin after IR. Curcumin pretreatment led to a sustained ERK1/2 activation after IR. NAC alone or NAC treatments before IR had no significant effects on ERK1/2 activation. However, treatment of cells with NAC (for the last 6 h) along with curcumin reduced the ERK1/2 phosphorylation to near control levels (Fig. 6A). This result indicates that increase in oxidative stress after curcumin and IR leads to the sustained ERK1/2 signaling. We reasoned that if curcumin-mediated sensitization was dependent on the prolonged ERK1/2 activation, then inhibition of this phosphorylation should also compromise curcumin-mediated radiosensitization. Using a concentration of MEK1/2 inhibitors (Figs. 6B and 7A) that reduced the ERK1/2 levels in curcumin- and IR-treated cells to those observed with irradiation alone, we performed clonogenic survival assays on irradiated cells pretreated with vehicle control, curcumin, or curcumin together with MEK1/2 inhibitors. Pretreatment of cells with PD98059 compromised curcumin-mediated radiosensitization, demonstrating the involvement of the ERK1/2 pathway in the radiosensitization mechanism. Similar results were obtained with more specific inhibitors of MEK1/2-PD184352 (Fig. 7B) and U0126 (Fig. 7C). Note that PD98059, PD184352, or U0126 alone, which decreased ERK1/2 levels to a greater extent, sensitized the cells to IR, suggesting that both suboptimal and sustained levels of ERK1/2 phosphorylation contribute to tumor cell radiosensitivity (Valerie et al., 2007, and refs. therein).
Fig. 7.
Effect of ERK1/2 inhibition on curcumin-mediated radiosensitization. A, HeLa cells were serum starved for 24 h, cotreated with 0.1 or 0.3 μM PD184352 and DMSO or 10 μM curcumin for 8 h, followed by irradiation at 6 Gy, and the cells were harvested 6 h after radiation. Cell lysates were analyzed by Western blot using antibodies against pERK1/2 and ERK1/2. B, HeLa cells treated with DMSO, 10 μM curcumin (8 h), 0.3 μM PD184352 (2 h), or PD184352 treatment during the last 2 h of curcumin treatment. The cells were mock-irradiated or irradiated at 6 Gy. The drugs were washed off 1 h after radiation. Survival was assessed by low-density clonogenic survival assay. Results represent the averages of three independent experiments (±S.E.). C, HeLa cells treated with DMSO, 10 μM curcumin (8 h), 1 μM U0126 (2 h), or U0126 treatment during the last 2 h of curcumin treatment. The cells were mock-irradiated or irradiated at 6 Gy. The drugs were washed off 1 h after radiation. Survival was assessed by low-density clonogenic survival assay. Results represent the averages of three independent experiments (±S.E.).
Curcumin Does Not Increase Apoptosis in Irradiated Cells
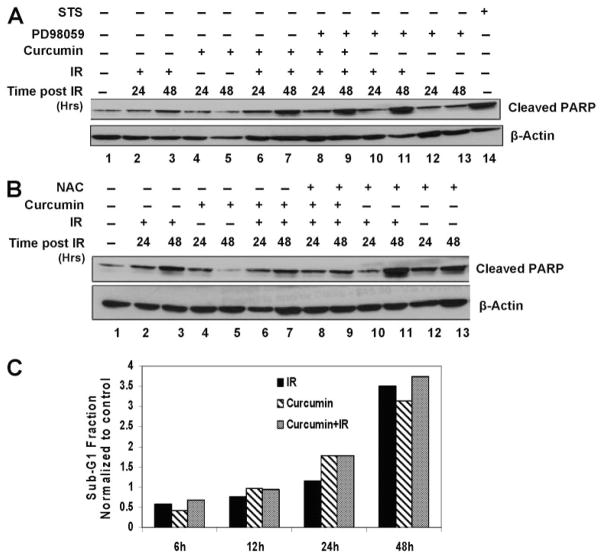
Because sustained ERK1/2 activation after ROS has been demonstrated to cause apoptosis (Wang et al., 2000; Schweyer et al., 2004), we examined the effect of curcumin and IR treatments on apoptosis of the cervical tumor cells. There was a modest increase in apoptosis at 48 h after irradiation or curcumin treatments, as determined by PARP cleavage. This PARP cleavage in combined curcumin and IR treatment was not significantly different from IR or curcumin treatments alone (Fig. 8A). Furthermore, inhibition of ROS by the antioxidant NAC (Fig. 8B, lanes 8 and 9) or ERK1/2 activation by PD98059 (Fig. 8A, lanes 8 and 9) did not have any further effect on PARP cleavage of curcumin- and IR-treated cells. Likewise, analysis of the sub-G1 fraction of cells as a measure of apoptosis by flow cytometry, we also failed to observe any significant difference in the apoptosis in curcumin- and IR-treated cells compared with IR treatments alone (Fig. 8C). Taken together, these results indicate that apoptosis does not play a significant role in cell death caused by curcumin and IR treatments. However, in these cell lines other mechanisms of cell death after IR (e.g., mitotic catastrophe or cellular senescence) may be responsible for curcumin-induced radiosensitization, and we are currently testing these possibilities.
Fig. 8.
Curcumin induced apoptosis is through IR-induced ROS and prolonged ERK activation. A, HeLa cells were pretreated with DMSO, 10 μM curcumin (8 h), 5 mM NAC (6 h), or NAC treatment during the last 6 h of curcumin treatment. The cells were irradiated at 6 Gy and harvested 24 or 48 h after IR. Whole-cell lysates were analyzed by Western blot using antibodies against cleaved PARP and β-Actin. B, HeLa cells were pre-treated with DMSO, 10 μM curcumin (8 h), 50 μM PD98059 (2 h), or PD98059 treatment during the last 2 h of curcumin treatment. The cells were irradiated at 6 Gy and harvested 24 or 48 h after IR. Whole-cell lysates were analyzed by Western blot using antibodies against cleaved PARP and β-Actin. Lysate from cells treated with 1 μM staurosporine for 2 h was used a positive control for cleaved PARP. C, HeLa cells were treated with DMSO or curcumin followed by mock irradiation or 6-Gy dose of IR. The cells were fixed and treated with PI/RNase containing solution as described under Materials and Methods and subsequently analyzed by flow cytometry. Results represent the values of sub-G1 fractions normalized to un-irradiated controls.
Discussion
Our results demonstrate that curcumin is a potent radiosensitizer of human cervical tumor cells. We have provided evidence supporting a central role for increased oxidative stress that leads to the activation of ERK1/2 signaling in two different cervical tumor cell lines as a mechanism for radiosensitization (Fig. 9).
Fig. 9.
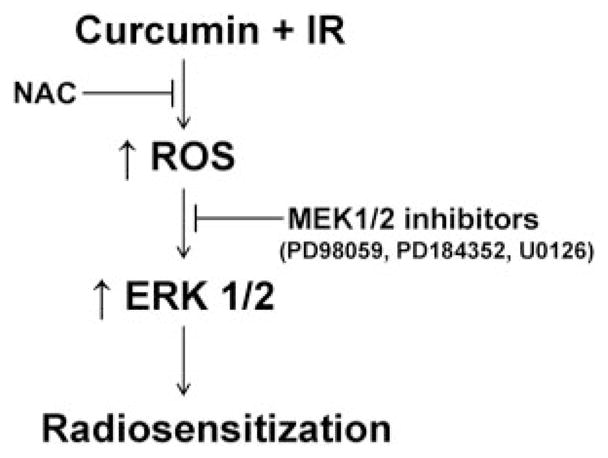
Model of curcumin-mediated radiosensitization. Curcumin pre-treatment leads to an increase in reactive oxygen species after ionizing radiation. The increased ROS signals for the activation of ERK1/2, which in turn sensitizes the cells to radiation.
NF-κB, a proinflammatory transcription factor, is overexpressed in a variety of solid tumors and is thought to contribute to the aggressive phenotype of these tumors by increasing apoptotic resistance and resistance to radiation and chemotherapy treatments (Aggarwal, 2004). However, the role of NF-κB in the survival of solid tumor cells in response to IR is controversial. Although some studies have shown that cells with compromised NF-κB activity are more radiosensitive than cells with an intact NF-κB pathway (Jung et al., 1995), others have failed to show such a correlation (Ashburner et al., 1999; Pajonk et al., 1999). Thus, although an antiapoptotic role of NF-κB in tumor cell lines has been firmly established, its role in radiosensitivity and long-term cell survival remains unclear. Various studies have suggested that curcumin inhibits tumor cell survival via inhibition of the transcription factor NF-κB (Chendil et al., 2004; Sharma et al., 2006). Our results in cervical carcinoma cells show that NF-κB is unlikely to play a significant role in curcumin-mediated radiosensitization in this cell type. We believe that cell type-specific effects may contribute to these differences. Likewise, AKT activation is implicated in the radioresistance of cervical cancer (Kim et al., 2006). Consistent with previous reports, we saw an increase in AKT activation after IR (Fig. 3C); however, our results indicate that curcumin does not inhibit the basal or IR-induced AKT activation. We believe that the effects of curcumin on Akt activation are also cell-type dependent because we observed curcumin-induced inhibition of AKT activation in other cell lines, including colorectal and prostate cells (C. Naczki and C. Koumenis, unpublished data).
Growing evidence suggests that cancer cells produce high levels of ROS and are constantly under oxidative stress (Toyokuni et al., 1995). Maintenance of an appropriate level of intracellular ROS is important in keeping redox balance and signaling cellular proliferation (Martin and Barrett, 2002); however, overproduction of ROS or cellular ability to suppress ROS would result in a significant increase of intracellular oxidative stress, leading to cellular damage and ultimately cause cell death. Recent reports indicate the possibility of using agents that promote cellular ROS accumulation to effectively kill cancer cells in vitro (Huang et al., 2000; Pelicano et al., 2003). Although curcumin is a naturally occurring antioxidant, like most polyphenols, it can also exhibit pro-oxidant properties under certain conditions. For example, high concentrations of curcumin (>50 μM) were found to promote ROS generation in published reports (Bhaumik et al., 1999; Galati et al., 2002), whereas low doses of curcumin (such as at 10 μM) usually decreases ROS generation (Chan et al., 2003). Note that both the antioxidant and pro-oxidant activities are believed to be involved in the anticancer activity of curcumin (Hadi et al., 2000). Our results are consistent with these findings. At concentrations where we observed the radiosensitizing effects of curcumin, we did not observe an increased ROS production with curcumin treatment alone; however, curcumin pretreatment potentiated IR-induced generation of ROS in these cells. Two possible models that could explain this action of curcumin include either: 1) scavenging of the antioxidant enzymes more effectively and hence magnifying the oxidative stress on cells when treated by IR or 2) participation of curcumin in Fenton-type chemical reactions that are initiated by the effects of ionizing radiation and hence generating substantial amounts of ROS in the cells pushing them toward cell death. We are currently testing these possibilities.
The ERK pathway plays a major role in regulating cell growth and differentiation, being highly induced in response to growth factors, cytokines, phorbol esters, and oxidant injury (Strnisková et al., 2002). Some studies have shown that curcumin represses ERK1/2 activation (Woo et al., 2005), whereas others have found no effect of curcumin on ERK activation (Collett and Campbell, 2004). Because curcumin modulates many pathways, its detailed mechanisms may vary depending on the cancer cell type and/or the concentrations used. Increasing evidence links sustained ERK activation to cell death induced by ROS (Wang et al., 2000; Ramachandiran et al., 2002). Our results indicate that although curcumin had no significant effects on ROS production or ERK activation by itself, it potentiated radiation-induced ROS generation that led to a sustained ERK1/2 activation. In addition, decreasing the ERK1/2 activation to its basal level with PD98059, PD184352, or U0126 compromised curcumin-mediated radiosensitization, demonstrating that prolonged ERK activation was necessary for the radiosensitization. Note that the EGFR inhibitor AG-1478 also caused a dose-dependent decrease in ERK1/2 phosphorylation after curcumin and IR treatments (data not shown). These results suggest that curcumin-mediated radiosensitization may also be mediated by effects on EGFR signaling pathway and downstream MEK1/2 phosphorylation of ERK1/2 (Hagan et al., 2007). The effects of autocrine and paracrine regulation of EGFR-ERK1/2 signaling mediated by TGF-α on tumor cell radiosensitivity have been well summarized in a recent review (Valerie et al., 2007). Together, these results lead us to propose a model in which moderate levels of ERK1/2 activation are required for cell survival, whereas either complete knock-down or sustained activation of ERK1/2 appear to be detrimental to the cell (Wang et al., 2000, 2007). Although sustained ERK activation is linked to cell death by apoptosis, curcumin-mediated radiosensitization does not appear to be due to cell death induced by apoptosis because we did not observe activation of general apoptosis markers. Other potential mechanisms (e.g., mitotic catastrophe/necrosis) could be involved. A recent report has indicated that sustained ERK1/2 causes cellular senescence (Cozzi et al., 2006), and we are currently testing this possibility.
In a recent comprehensive review on physiological relevance of phytochemical chemopreventive agents, Howells et al. (2007) summarized the in vitro and in vivo studies and clinical trials on curcumin. The clinical trials indicated that the concentrations of curcumin that were achievable in the plasma of patients were only at a lower micromolar range; hence, they have suggested that the in vitro studies with curcumin in the 10 μM range are of physiological relevance. The significant radiosensitization achieved by the low dose of curcumin (10 μM) at clinically relevant doses (2–6 Gy) has promising implications for improving radiation therapy, especially in radioresistant tumors such as the tumors of the uterine cervix. The potential radiosensitizing effect of curcumin could offer a better therapeutic outcome by either increasing the fraction of lethally damaged tumor cells or lowering the required radiation dose required to produce the same therapeutic outcome (and thus reducing potential side-effects). This potential benefit could be augmented by the demonstrated protection conferred by curcumin against damage of normal tissue (Okunieff et al., 2006). Because the bioavailability of curcumin is low outside the gastrointestinal tract (Howells et al., 2007), it is conceivable that curcumin delivered as a topical application could substantially improve the cytotoxic effect of the concurrent chemoradiation therapy. Animal tumor data from the work here will create the basis for human patient studies to study the safety and efficacy of curcumin in therapeutic modalities in conjunction with radiation therapy.
Acknowledgments
We thank Meixia Bi, Lori Hart, Diane Fels, Jiangbin Ye, Christine Naczki, Racquel Collins-Underwood, and Mitra Kooshki for guidance and expert technical assistance.
This study was supported by grant R01-CA104922 from the National Cancer Institute (to C.K.).
ABBREVIATIONS
- NF
nuclear factor
- ROS
reactive oxygen species
- ERK
extracellular signal-regulated kinase
- DMSO
dimethyl sulfoxide
- PI
propidium iodide
- LY294002
2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
- MEK
mitogen-activated protein kinase kinase
- PD98059
2′-amino-3′-methoxyflavone
- NAC
N-acetylcysteine
- AG-1478
4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(2-aminophynyltio)butadiene
- Gy
gray
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- PBS
phosphate-buffered saline
- DCF-DA
2′,7′-dichlorofluorescein diacetate
- FBS
fetal bovine serum
- IR
ionizing radiation
- EGFR
epidermal growth factor receptor
- MAPK
mitogen-activated protein kinase
- PARP
poly(ADP-ribose) polymerase
References
- Aggarwal BB. Nuclear factor-kappa B: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ashburner BP, Shackelford RE, Baldwin AS, Jr, Paules RS. Lack of involvement of ataxia telangiectasia mutated (ATM) in regulation of nuclear factor-kappaB (NF-kappaB) in human diploid fibroblasts. Cancer Res. 1999;59:5456–5460. [PubMed] [Google Scholar]
- Baldwin AS., Jr Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BVV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- Brandt R, Keston AS. Synthesis of dichlorodihydrofluorescin: a stable reagent for fluorometric analysis. Anal Biochem. 1965;11:6–9. doi: 10.1016/0003-2697(65)90035-7. [DOI] [PubMed] [Google Scholar]
- Candelaria M, Garcia-Arias A, Cetina L, Duenas-Gonzalez Radiosensitizers in cervical cancer: cisplatin and beyond. Radiat Oncol. 2006;1:15. doi: 10.1186/1748-717X-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WH, Wu CC, Yu JS. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J Cell Biochem. 2003;90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Collett GP, Campbell FC. curcumin induced c-jun N terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- Cozzi SJ, Parsons PG, Ogbourne SM, Pedley J, Boyle GM. Induction of senescence in diterpene ester-treated melanoma cells via protein kinase C-dependent hyperactivation of the mitogen-activated protein kinase pathway. Cancer Res. 2006;66:10083–10091. doi: 10.1158/0008-5472.CAN-06-0348. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Galati G, Sabzevari O, Wilson JX, O’Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- Gescher AJ, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: a tale of failure and promise. Lancet Oncol. 2001;2:371–379. doi: 10.1016/S1470-2045(00)00392-2. [DOI] [PubMed] [Google Scholar]
- Hadi SM, Asad SF, Singh S, Ahmad A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life. 2000;50:167–171. doi: 10.1080/152165400300001471. [DOI] [PubMed] [Google Scholar]
- Hagan MP, Yacoub A, Dent P. Radiation-induced PARP activation is enhanced through EGFR-ERK signaling. J Cell Biochem. 2007;101:1384–1393. doi: 10.1002/jcb.21253. [DOI] [PubMed] [Google Scholar]
- Hall EJ. Radiobiology for the Radiologist. 5. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, Hudson EA, Manson MM. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28:1274–1304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- Jung M, Zhang Y, Lee S, Dritschilo A. Correction of radiation sensitivity in ataxia telangiectasia cells by a truncated I kappa B-alpha. Science. 1995;268:1619–1621. doi: 10.1126/science.7777860. [DOI] [PubMed] [Google Scholar]
- Khafif A, Hurst R, Kyker K, Fliss DM, Gil Z, Medina JE. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:317–321. doi: 10.1016/j.otohns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Lee JW, Song SY, Choi JJ, Choi CH, Kim BG, Lee JH, Bae DS. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006;94:1678–1682. doi: 10.1038/sj.bjc.6603180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, Kim JM. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol. 2005;25:374–382. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
- Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68:217–226. doi: 10.1016/s0167-8140(03)00197-x. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao CD, Ruffin MT, 4th, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Alternat Med. 2006 Mar 17;:6–10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65:890–898. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Pajonk F, Pajonk K, McBride WH. Inhibition of NF-κB, clonogenicity, and radiosensitivity of human cancer cells. J Natl Cancer Inst. 1999;91:1956–1960. doi: 10.1093/jnci/91.22.1956. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ. Mitogen activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol. 2002;15:1635–1642. doi: 10.1021/tx0200663. [DOI] [PubMed] [Google Scholar]
- Sartor CI. Epidermal growth factor family receptors and inhibitors: radiation response modulators. Semin Radiat Oncol. 2003;13:22–30. doi: 10.1053/srao.2003.50003. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, Helen P, Schlott T, Radzun HJ, Fayyazi A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer. 2004;91:589–598. doi: 10.1038/sj.bjc.6601919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm-general principles. Nat Clin Pract Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- Sharma C, Kaur J, Shishodia S, Aggarwal BB, Ralhan R. Curcumin down regulates smokeless tobacco-induced NF-kappaB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology. 2006;228:1–15. doi: 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Strnisková M, Barancik M, Ravingerova T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen Physiol Biophys. 2002;21:231–255. [PubMed] [Google Scholar]
- Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–1108. [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Holbrook J. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim HS. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKA to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- Zhan M, Han ZC. Phosphatidylinositide 3-kinase/AKT in radiation responses. Histol Histopathol. 2004;19:915–923. doi: 10.14670/HH-19.915. [DOI] [PubMed] [Google Scholar]
- Zoberia IC, Bradbury MB, Currya HA, Bishtb KS, Goswamic PC, Roti Rotia JL, Gius D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Letters. 2002;175:165–173. doi: 10.1016/s0304-3835(01)00719-4. [DOI] [PubMed] [Google Scholar]