Abstract
The presence of oligoclonal bands of IgG (OCB) in cerebrospinal fluid (CSF) is used to establish a diagnosis of multiple sclerosis (MS), but their specificity has remained an enigma since its first description over forty years ago. We now report that the use of lipid arrays identifies heteromeric complexes of myelin derived lipids as a prominent target for this intrathecal B cell response.
Keywords: Multiple sclerosis, Oligoclonal bands, Cerebrospinal fluid, Lipids, Antibodies
1. Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system (CNS) in which repeated inflammatory episodes of demyelination and axonal loss result in chronic disability (Compston and Coles, 2002). There is increasing evidence that MS pathogenesis cannot be attributed solely to T cell dependent mechanisms, but also involves a significant contribution from the B cell compartment (Lassmann et al., 2001; Lucchinetti et al., 2000; Hauser et al., 2008). The most obvious indication that MS is associated with an abnormal humoral response is an increased intrathecal synthesis of immunoglobulin G (IgG) that manifests itself as discrete oligoclonal bands (OCBs) of IgG in cerebrospinal fluid (Meinl et al., 2006; Antel and Bar-Or, 2006; Owens et al., 2006a,b; Awad et al., 2010). OCBs are important diagnostic and prognostic laboratory markers for MS (Tintore et al., 2008; Cross and Wu, 2010). They are visualized as prominent, distinct Ig bands in isoelectric focusing (IEF) gels of CSF samples. OCBs are products of clonally expanded B cells. They are mature antibodies, mostly of the complement-activating IgG1 isotype and contain numerous somatic hypermutations. This suggests that OCBs are produced in the context of sustained antigen stimulation (Qin et al., 1998; Owens et al., 1998; Baranzini et al., 1999; Ritchie et al., 2004). In spite of intensive research, the target antigen(s) recognized by individual OCBs in MS have remained elusive (Meinl et al., 2006; Awad et al., 2010).
Human autoantibodies directed against heterodimeric lipid complexes were recently described in Guillain–Barré syndrome, a spectrum of acute antibody-mediated demyelinating and axonal diseases affecting the peripheral nervous system (Kusunoki et al., 2008). Combinatorial lipid arrays reveal that epitope recognition by such autoantibodies is dependent upon cis interactions with other lipids that form heterogenous microdomains in plasma membranes (Rinaldi et al., 2009). We have now used this approach to investigate antibody specificities in serum and CSF from Scottish patients withMS (n = 40) and with other neurological disease (OND, n = 40) (see Table 1). This demonstrated that responses directed against myelin lipids were preferentially sequestered in the CSF compartment. However CSF contains a mixture of immunoglobulins derived from OCBs and antibodies derived from the periphery that generate a polyclonal background. We therefore used recombinant antibodies derived from single B- and plasma cells selected from the CSF of American MS patients and controls to investigate OCB specificity (Owens et al., 2009). This approach is feasible as CSF-resident B cells have been shown to be responsible for the production of OCBs (Obermeier et al., 2008). We investigated 73 recombinant antibodies from MS patients and 27 from patients with other neurological diseases (OND) for lipid antigen specificities.
Table 1.
Clinical data. Demographics of patients included in lipid array screening with whole CSF samples.
| Variable | MS | OND |
|---|---|---|
| Number | 40 | 40 |
| Age | ||
| Median | 38.5 | 44.5 |
| Range | 25–64 | 16–69 |
| Sex | ||
| F:M | 24:16 | 28:12 |
| CSF IEF | ||
| Isolated OCB | 37 | 0 |
| No bands | 1 | 36 |
| Othera | 2 | 4 |
Isolated CSF anti-lipid antibodies were not identified in any MS patients without OCB.
Polyclonal or monoclonal CSF IgG or paired bands in CSF & serum.
Our results demonstrate that the use of lipid arrays identifies heteromeric complexes of myelin derived lipids as a prominent target for the intrathecal B cell response in multiple sclerosis.
2. Materials and methods
2.1. Patients and CSF samples
Serum and CSF were collected from patients with MS (n = 40) and other neurological diseases (OND, n = 40) (Table 1). In the group ofMS patients 24 had relapsing–remitting MS (RRMS) and 16 had primary progressive MS (PPMS). All MS patients met the McDonald diagnostic criteria for MS (Polman et al., 2005). The group of 40 patients with OND had the following diagnoses: neuropathy (9 including: 1 GBS, 1 MMN and 2 chronic demyelinating neuropathies); migraine (4); idiopathic intracranial hypertension (4); non-specific headaches (2); negative investigations for non-specific neurological symptoms (11); Parkinson's Disease (2); stroke (2); cerebral venous sinus thrombosis (1); viral meningitis (1); cervical spondylosis (1); dementia (1); progressive ataxia (1); pancreatic cancer (1). Case notes were retrospectively reviewed for demographic data. The study was carried out in accordance with the local ethics committee (South Glasgow and Clyde REC).
2.2. Preparation of bivalent recombinant antibodies from single CSF B cells
Bivalent IgG1 human recombinant antibodies (rAbs) were generated from expanded single CSF plasma blast clones. Briefly, HEK 293-EBNA cells were transiently co-transfected with selected full-length heavy and light-chain constructs cloned into pCEP4 expressions vector as previously described (Owens et al., 2009), and IgG was purified from culture supernatants by Protein-A sepharose chromatography.
2.3. Lipid arrays
All stock solutions of lipids were prepared at 0.1 µg/ml in methanol. Heterodimeric complexes were created by combining stock solutions that had been sonicated for 2 min immediately prior to use at a ratio of 1:1 (vol:vol). Lipid arrays were spotted onto polyvinyl-difluoride (PVDF) membranes (Invitrogen) affixed to microscope slides (VWR, Darmstadt, Germany) using a Camag TLC Autosampler to create a combinatorial array containing: sulfatide (Sul), galactocerebroside (GalC), ceramide (Cer), cardiolipin (Card), sphingosine (SS), sphingomyelin (SM), digalactosyl diglyceride (DGG), monogalactosyl diglyceride (MGG), phosphatidylcholine (PC) and all possible 1:1 combinations, a total of 55 potential targets.
Membranes were blocked for 2% BSA/PBS and then incubated for 1 h at 4 °C with primary sample diluted in 0.1% BSA/PBS. CSF and serum samples were probed at 1:10 and 1:100 dilutions respectively, uncorrected for total IgG concentrations. Monoclonal antibodies were probed at a concentration of 10 µg/ml unless otherwise stated. After a wash cycle, specific antibody binding was detected by a horseradish peroxidase-conjugated polyclonal rabbit anti-human IgG (Dakocytomation, Hamburg, Germany) diluted in 0.1% BSA/PBS.
After a further wash cycle detection was via an ECL plus (Amersham/GE Healthcare, UK) chemiluminescent reaction, rendered on radiographic film after 1 min exposure. Films were digitalized by flatbed scanning and the images quantified by ImageQuant TL software (Amersham Biosciences, Little Chalfont, UK). We have previously reported inter- (n = 5) and intra-assay (n = 9) coefficients of variation at 4.1% and 8.6% respectively (Rinaldi et al., 2009). Arrays were validated using monoclonal antibodies which bound sulfatide and cholesterol.
2.4. Immunocytology of myelinating cultures
In vitro myelinating cultures were established as described previously (Thomson et al., 2008; Sorensen et al., 2008). Briefly, a single cell suspension was prepared from E15.5 rat spinal cord (Sprague Dawley) and plated on to a confluent monolayer of neurosphere derived astrocytes in plating media (50%DMEM, 25% heat inactivated horse serum, 25%HBSS with Ca2+ and Mg2+ and 2 mMl-glutamine (Invitrogen, Paisley, UK)) at a density of 150,000 cells/200 µl/ 13 mm diameter cover slip. Cells were left to attach for 2 h at 37 °C after which an additional 300 µl of plating media and 500 µl of differentiation medium (DMEM (4500 mg/ml glucose)), 10 ng/ml biotin, 0.5% N1 hormone mixture (1 mg/ml apotransferrin, 20 mM putrescine, 4 µM progesterone, and 6 µM selenium) 50 nM hydrocortisone, and 0.5 mg/ml insulin (Sigma, Dorset, UK) were added. Cultures were maintained at 37 °C/7% CO2 and fed three times a week by replacing half the culture medium with fresh differentiation media. After twelve days in vitro insulin was omitted from the culture medium to promote myelination.
Immunofluorescence microscopy was performed after 28 days in vitro using CSF-derived rAbs 4, 17, 33, 37, 73, 76, 80, and 97 (Table 2), the murine sulfatide reactive mAb O4 (Sommer and Schachner, 1981) and mAb Z2 specific for myelin oligodendrocyte glycoprotein (MOG; all at 10 µg/ml) for 30 min on ice. After washing in ice cold DMEM the cultures were fixed in 4% paraformaldehyde for 15 min at room temperature. Bound antibody was then detected using appropriate secondary antibodies (Alexa Fluor®, Invitrogen). Unbound secondary antibody was removed by washing with PBS followed by distilled H20 and the cultures mounted in Vectashield (Vector Laboratories, Peterborough, UK).
Table 2.
Features of recombinant antibodies (rAbs) derived from MS and OND CSF.
| Patient | rAb | Diagnosis | No of rAbs produced |
No of rAbs reactive to lipid |
|---|---|---|---|---|
| MS 02-19 | 1, 2, 19, 20, 57, 58, 59, 60, 61 | PPMS | 9 | 0 |
| MS 03-1a | 3, *4, 5, 6, 69, 70, 71, 72, *73, 74, 75, *76, 77 | RRMS | 13 | 7 |
| MS 04-2 | 7, 8, 9, 62, 63, 64, 65 | RPMS | 7 | 3 |
| MS 03-7 | 66, 67 | RRMS | 2 | 0 |
| MS 04-3a | 68 | RRMS | 1 | 0 |
| ON 03-5a | 27, 28, 29 30 | RRMS | 4 | 0 |
| ON 03-3a | 21, 22, 23, 24, 25, 26 | RRMS | 6 | 0 |
| ON 04-7a | 31, 32, *33, 34, 35, 36, *37 | RRMS | 7 | 2 |
| ON 04-8a | 38, 39, 40, 41 | RRMS | 4 | 0 |
| ON 07-7a | 51, 52, 53, 54, 55, 56 | RRMS | 6 | 1 |
| MS 05-3 | 10, 11, 12, 13, 14, 15, 16, *17, 18, 78, 79, *80, 81, 82, | RRMS | 14 | 7 |
| IC 05-2b | 87, 88, 89, 90, 91, 92 | Chronic meningitis | 6 | 0 |
| IC 06-1c | 83, 84, 85, 86 | SSPE | 4 | 2 |
| SSPE 83d | 93, 94 | SSPE | 2 | 1 |
| IC 04-4 | 99, 100 | Cryptococcal meningitis | 2 | 0 |
| ON 07-5e | 42, 43, 44, 45, 46, 47, 48, 49, 50 | NMO | 9 | 4 |
| IC 08-5f | 95, 96, *97, 98 | VZV radiculopathy | 4 | 3 |
Asterisk denotes those particular rAb in whom reactivity to myelinating CNS cultures was assessed.
Single-cell repertoire analysis was performed on CSF obtained during their first clinical event. Each CIS patient subsequently developed relapsing–remitting MS within a 6 month period.
Single-cell repertoire analysis was performed on CSF obtained from a patient with chronic meningitis. The cause of the disease and the specificity of the rAbs are unknown.
Single-cell repertoire analysis was performed on CSF obtained from a patient with subacute sclerosing panencephalitis (SSPE). All the rAbs have been demonstrated to bind measles virus (MV) proteins (Owens et al., 2006b).
Single-cell repertoire analysis was performed on plasma cells microdissected from SSPE brain tissue by laser capture. Both rAbs bind the MV nucleocapsid protein (Burgoon et al., 1999, 2006).
Single-cell repertoire analysis was performed on CSF obtained during an initial event of optic neuritis. The patient was subsequently diagnosed with neuromyelitis optica. 6 of these rAbs recognize the aquaporin-4 water channel (Bennett et al., 2009).
Single-cell repertoire analysis was performed on CSF obtained from a patient with VZV radiculomyelitis. The specificity of the rAbs is unknown.
3. Results
3.1. Analysis of lipid reactivity in CSF from MS patients and controls
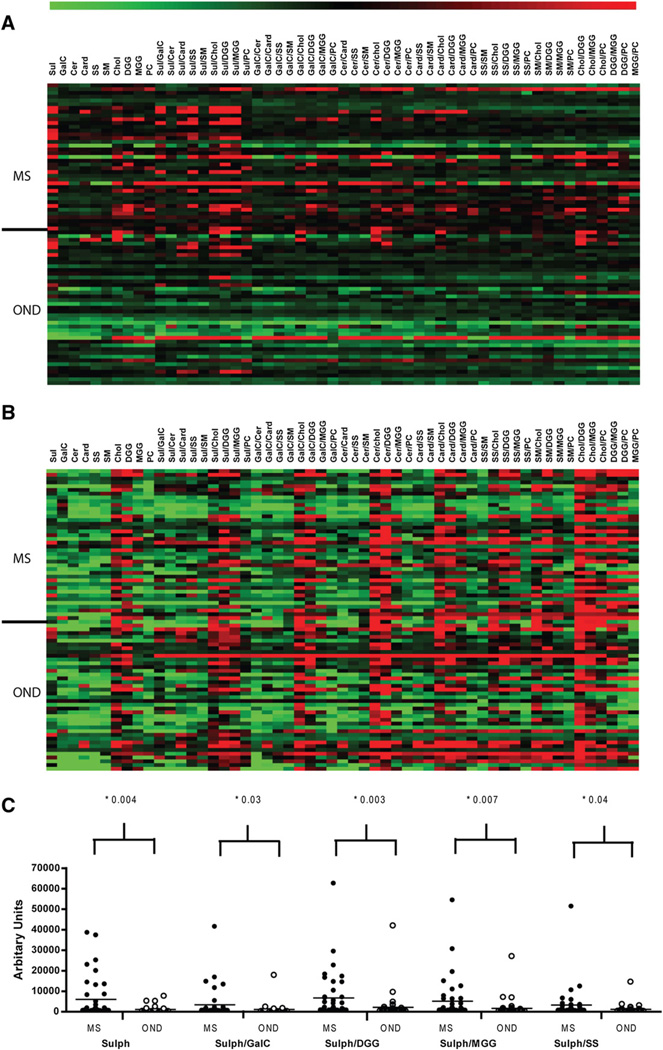
In a first series of experiments we investigated the specificity of anti-lipid antibodies in paired serum and CSF from patients with MS and OND. This confirmed previous reports that MS CSF is enriched in antibodies with specificities for myelin-derived lipids (Ilyas et al., 2003; Kanter et al., 2006), but identified many additional complex-specific responses. Heat map analysis identified a disease-associated lipid-specific CSF antibody response in MS (Fig. 1A, B) that preferentially targeted sulfatide (column 1, ANOVA with Bonferroni p-value adjusted for multiple testing; p = 0.004) and heterodimeric complexes of sulfatide with other lipids, in particular galactocerebroside (p = 0.03) (Fig. 1C). Within individual CSF samples it was evident that antibody binding was modulated by specific lipid partnerships (Fig. 2A–D). In particular within MS CSF, binding to sulfatide was inhibited by combination with sphingomyelin (p <0.01, 1 way ANOVA with Dunnett's correction). (Fig. 1A) Lipid reactive antibodies were detected in 60% of MS and 25% OND CSF. The majority (75%) of the lipid reactive MS CSF samples recognized sulfatide and/or sulfatide containing complexes. This CSF sulfatide binding was mostly unaccompanied (77%) by a corresponding serum response to sulfatide or sulfatide containing complexes indicating the response was intrathecal (p = 0.007, Fisher's exact test compared to OND CSF/serum pairs) and may therefore represent OCB specificities.
Fig. 1.
Array analyses of CSF and serum. Heat maps created using logarithmic transformations of the mean intensities recorded for each lipid antigen depict CSF (A) and serum (B) IgG reactivity in MS and OND patients. Lipids are displayed as column headings and each row represents an individual patient. (C) Anti-sulfatide/sulfatide complexes in CSF of patients with MS and OND. Closed circles denote MS CSF and open circles denote OND CSF. Reactivities to sulfatide and particular combinations of sulfatide and other lipids were observed more frequently within MS CSF (p values were obtained by ANOVA with Bonferroni correction).
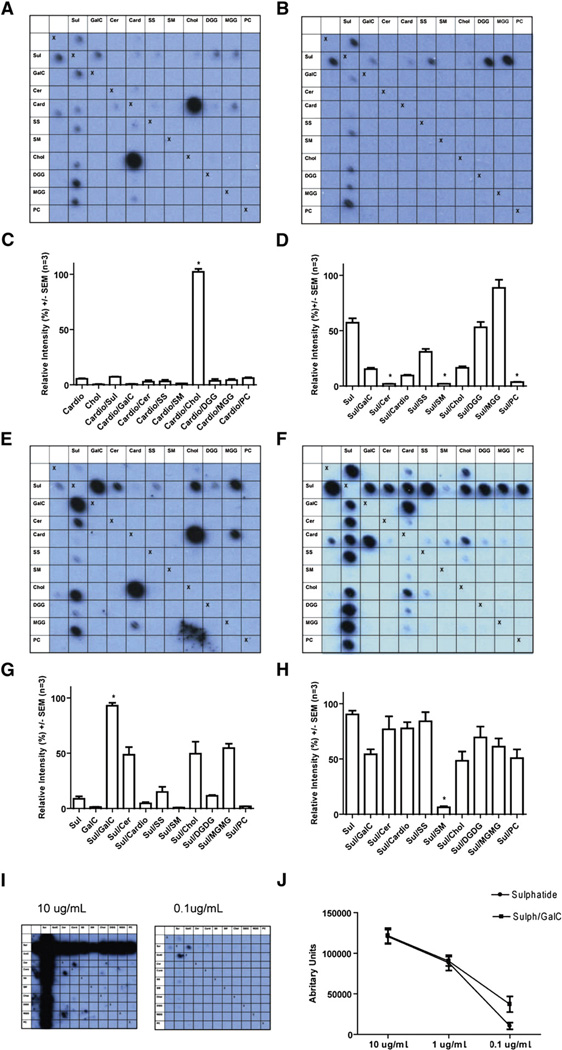
Fig. 2.
Illustrative blots and their quantitative analysis. Data were obtained using MS CSF (A–D) and recombinant antibodies 3, 73, 17 (Table 2) cloned from a single CSF-resident plasma cell (E–J). Row and column headings reveal the complex at each location. We used the following lipids either alone or in combination: sulfatide (Sul), galactocerebroside (GalC), ceramide (Cer), cardiolipin (Card), sphingosine (SS), sphingomyelin (SM), cholesterol (Chol), digalactosyl diglyceride (DGG), monogalactosyl diglyceride (MGG), phosphatidylcholine (PC). “Xs” represent the negative controls (methanol) which act as a line of symmetry for duplicate spots on the same membrane. (A, C) PPMS CSF blot and quantitative data, for binding to the complex formed by cholesterol and cardiolipin is increased by 96.3% compared to the sum of the intensities for the individual lipids (p = 0.0004, paired t test n=3). (B, D) Complex attenuation in a CSF MS blot, where binding to sulfatide yields a mean relative intensity signal of 57.1%, yet the intensity for the complexes of sulfatide created with the lipids ceramide, sphingomyelin and phosphatidylcholine is almost eliminated at between 1.96% and 3.66% (p<0.0001, GLM ANOVA with Tukey n = 3). (E, G) Blot and quantification from MS rAb 3 with anti-myelin lipid complex reactivity. Binding to the complex of sulfatide and galactocerebroside is increased by 82.84% (p = 0.004 paired t test, n = 3) compared to the sum of the mean intensities recorded from the individual lipids. (F, H) Blot and quantification from MS rAb 73 with binding to sulfatide that is inhibited when sulfatide is complexed with the lipid sphingomyelin (p<0.0001 GLM ANOVA with Tukey, n = 3). (I, J) MS rAb 17 assayed by serial dilution. The pattern of binding seen at higher rAb concentrations when signals are saturated appears largely complex independent. At a lower concentration of 0.1 µg/ml binding to the complex created by sulfatide and galactocerebroside can be seen to be enhanced (p = 0.0143 paired t test, n=3).
3.2. Lipid reactivity of recombinant antibodies from single CSF B cells
Having demonstrated that the array was able to detect anti-lipid antibody profiles in MS and control CSF, we next investigated 100 IgG1 recombinant antibodies (rAb) derived from clonally expanded CSF B cells/plasma blasts (Owens et al., 2009) obtained from patients with MS (n = 11; 73 rAb) and other CNS inflammatory diseases (ONID; n = 6; 27 rAb) (Table 2) (Owens et al., 2006a,b; Burgoon et al., 1999, 2006; Bennett et al., 2009). It is known that CSF-resident B cells may produce OCBs (Obermeier et al., 2008).
Overall, lipid specific rAb were identified in 45% of MS patients and accounted for 27% (20/73) of the rAb of which 19% (14/73) bound sulfatide and/or sulfatide containing complexes while the remaining 8% (6/73) recognized cholesterol (Fig. 3A). Antibody binding was modulated strongly by interactions with other lipids resulting in different patterns of reactivity. These included both complex-enhanced recognition, in which antibody binding to a heterodimeric complex is greater than either individual component (Fig. 2E), and complex-inhibited, in which certain lipids inhibit antibody binding (Fig. 2F). The pattern of binding at high rAb concentrations often appeared largely complex independent, but at low concentrations it could be observed that they preferentially recognized specific heteromeric complexes (Fig. 2I, J). The use of combinatorial lipid arrays also revealed that individual rAb could also bind structures formed by pairs of structurally unrelated lipids, for example rAb 3 bound to Sulfatide/GalC and cholesterol/cardiolipin (Fig. 2E). This suggests that either the same or a very similar antigenic surface can be generated by complexes of structurally unrelated lipids, or that certain antibodies are able to bind multiple antigens.
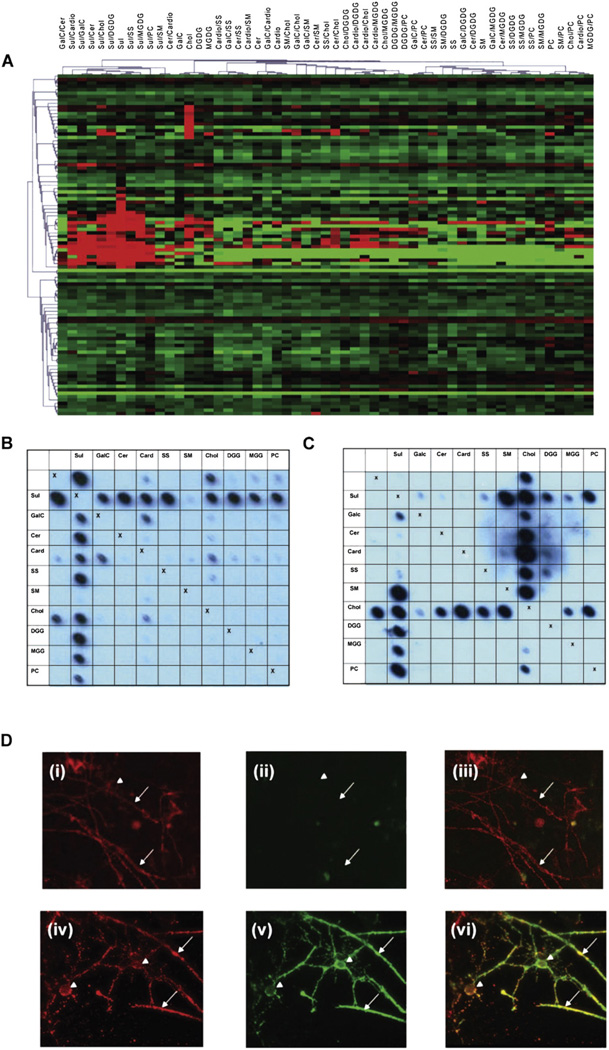
Fig. 3.
Array analyses for rAbs. (A) Heat map depicting reactivity of rAbs derived from MS and OND patient CSF. The lipid antigens are displayed as column headings and each row represents an individual rAb. Table 2 lists the sources of MS and control rAbs and more detailed analyses of individual rAbs can be found in Owens et al. (2009) and Bennett et al. (2009). Covariance clustering of all rAb reactivities has been performed revealing two populations of lipid reactive antibodies; one directed against sulfatide or sulfatide/lipid complexes and the other against cholesterol. MS rAbs do not segregate with unique binding profiles, as lipid reactivities are also seen in OND rAbs (19% V 37%). (B) Blot of the MS derived rAb 76. Note antibody binding to sulfatide is virtually abolished in the presence of sphingomyelin. (C) Blot of the mAb O4. In contrast to rAb 76, binding to sulfatide is enhanced not inhibited by sphingomyelin. (D) Unfixed myelinating cultures derived from embryonic rat spinal cord were stained at 4 °C with either (D) (i)–(iii) MS derived rAb 76 (10 µg/ml) or (D) (iv)–(vi) mAb O4 (10 µg/ml) to visualize sulfatide (green) and the MOG-specific mAb Z2 (10 µg/ml) to identify oligodendrocytes and myelin sheaths (red). After fixation with 4% paraformaldehyde bound antibody were detected using species-specific secondary reagents (anti-human — green; anti-mouse — red). Control cultures were stained with polyclonal human IgG, mouse myeloma proteins and/or secondary antibodies alone. In no case did immunoreactivity for MOG (red) at the surface of oligodendrocytes (arrow head) or myelinated internodes (small arrows) co-localize with bound human rAb ((ii), green) as demonstrated in the merged image (iii). Staining obtained using this and other rAbs was restricted to a diffuse background indistinguishable from that observed when cultures were stained using polyclonal human IgG pooled from multiple donors. In contrast the sulfatide reactive mAb O4 binds the surface of oligodendrocytes and myelin sheaths where it co-localizes with the MOG-specific mAb Z2 (iv)–(vi). This marked difference in immunoreactivity of sulfatide reactive rAbs and mAb O4 is associated with marked differences in recognition of lipid complexes using lipid arrays.
The array patterns obtained using individual rAb reproduced many features observed when arrays were probed with CSF from unrelated MS patients (Fig. 2A, B) indicating these anti-lipid antibodies represent common components of the intrathecal response. Although these specificities are increased in MS CSF (Figs. 1, 2A–D) they were not restricted to MS as lipid reactive rAb with similar specificities were generated from CSF B cells isolated from OND patients (Fig. 3A; 10/27 rAb, 37%). This suggests MS does not differ from OND with respect to the initial recruitment/expansion of B cells with these specificities, but in their retention and activation that enables them to make a sustained contribution to the intrathecal antibody response. Interestingly several lipid-reactive OND rAbs also bind defined protein antigens (Table 2). This is not a unique phenomenon as demonstrated by the specificity of neutralizing anti-HIV antibodies which can recognize complex epitopes formed by HIV gp41 and phospholipids such as cardiolipin (Alam et al., 2007). This suggests similar multi or polyreactivity may also occur with the MS associated OCB repertoire, although we have not yet identified any putative protein partners.
3.3. Immunocytological analysis of recombinant antibody binding to primary myelinating cultures
In order to determine whether the ability of rAbs to bind sulfatide containing complexes in the arrays equated with the recognition of this lipid in a biological context, we investigated the ability of eight rAbs to bind to membrane surfaces in myelinating CNS cultures (Thomson et al., 2008; Sorensen et al., 2008) using the sulfatide-reactive mAb O4 as a positive control. None of the rAbs investigated bound to the surface of any membrane in these myelinating cultures. This is in contrast to the intense surface reactivity of the sulfatide-specific mAb O4 that co-localizes with MOG immunoreactivity, a late stage marker for myelination (Fig. 3D). This indicates that the sulfatide-containing complexes recognized by the rAbs are not normally accessible within the plane of the live myelin, and are therefore unlikely to provide targets for primary antibody-mediated demyelination. A possible explanation for the marked difference in the ability of the rAbs and O4 to bind to this biological surface is that they differ in their fine specificity. MAb O4 fails to bind sulfatide as a single lipid, and only binds strongly to sulfatide when in complex with sphingomyelin on the arrays (Fig. 3C), in striking contrast to the typical rAb behavior, in which sulfatide binding is inhibited by sphingomyelin (Fig. 3B).
4. Discussion
The observation that MS is associated with sustained intrathecal antibody synthesis was first made over forty years ago, but the specificity and pathological significance of this response has remained an enigma. We now report that a significant proportion of this response is directed against lipid antigens, in particular sulfatide and heteromeric complexes containing sulfatide, a glycolipid highly enriched in the myelin membrane. While some MS-associated antibodies bind sulfatide as a single lipid, others only bind when sulfatide is complexed with other lipids. The complex assay thereby increases the overall detection frequency of lipid-reactive antibodies.
Several previous studies reported the presence of lipid-reactive antibodies in the CSF of patients with MS including identification of sulfatide-specific IgGs using ELISA (Ilyas et al., 2003) and lipid arrays (Kanter et al., 2006). Our data investigating the specificity of anti-lipid antibodies in paired serum and CSF from MS patients indicate that the majority of MS patients demonstrate reactivity to lipids (60%), in particular to the lipid sulfatide. This sulfatide reactivity was mostly CSF specific in the majority (p = 0.007, Fisher's exact test compared to OND CSF/serum pairs). Interestingly lipid-reactive IgM OCBs have been reported to serve as a prognostic marker for a severe disease course (Villar et al., 2005). However, studies have been unable to allocate these specificities unambiguously to the intrathecal B cell response. To address this issue we examined recombinant antibodies derived from clonally expanded CSF B cells. Since clonally expanded B cells in the CSF are strongly considered to contribute to OCB production (Obermeier et al., 2008), many of these antibodies will represent OCBs, although this is clearly a complex and incompletely resolved area of study. With this caveat, we found that 27% (20/73) of the antibodies bound lipid complexes, the majority of which (19%; 14/73) bound sulfatide and/or sulfatide containing complexes. This suggests that intrathecal sulfatide-reactive B cell responses are a generalized feature of MS. The observation that sulfatide reactivity is a major component of the anti-lipid repertoire in MS suggests this intrathecal B cell response may provide an autoimmune counterpart to infectious CNS diseases where the intrathecal B cell response is directed predominantly against the causative pathogen.
Identification of myelin-associated lipids as a major target of the intrathecal B cell responses associated with MS raises a number of important questions, in particular the origin of this response and how might it contribute to disease pathogenesis. The reagents used in this study were all derived from B cells encoding mature IgGs that have undergone extensive somatic hypermutation and class switching. This is indicative of sustained antigen-driven selection and provision of T cell help. Although we currently have no clues about the precise mechanisms driving the expansion and differentiation of glycolipid-reactive B cells, improper tolerance induction by receptor editing in post-germinal B cells might be involved (Rice et al., 2005), while the initiating trigger may be myelin damaged through other disease mechanisms or molecular mimicry with microbial agents. Molecular mimicry is an established mechanism to break B cell tolerance to glycosphingolipids, as demonstrated in patients with Guillain–Barré syndrome in which bacterial lipopolysaccharides can induce pathogenic autoantibody responses to specific gangliosides. Alternatively, it may reflect a poly-specific response derived from circulating IgG+ memory B cells. Recent studies demonstrating a large component of this repertoire has the potential to produce non-pathogenic, poly-specific autoantibodies (Witsch et al., 2006; Mietzner et al., 2008; Mouquet et al., 2010) that provide a link between antibodies derived from the IgG+ memory B cell compartment in healthy individuals and those obtained from clonally expanded B cell populations in the CSF. In both cases the immunoglobulin sequences have undergone significant somatic hypermutation, they do not appear to mediate any primary pathology and there is evidence of polyspecificity. In the case of the CSF B cell derived antibodies investigated in this study, two of four lipid reactive antibodies derived from a patient with NMO also bind aquaporin IV, while all three obtained from patients with SSPE bind measles virus antigens (Table 2). From a structural point of view this is not surprising, because the area of the combining site of an antibody is about 1600 Å2 (Padlan, 1996), whereas a carbohydrate such as sulfatide occupies about 30 Å2. Therefore several ligands may bind simultaneously to different regions of a combining site. Whether the CSF lipid-reactive repertoire in patients with MS is also polyspecific is still unknown, as the MS derived antibodies investigated in this study have not yet been shown to bind a defined protein antigen. The presence of clonally expanded lipid-specific B cells in the CSF of patients with infectious diseases indicates that this phenomenon is not specific to MS, but may be a relatively common response in inflammatory brain diseases particularly, but not exclusively in those with extensive white matter involvement and myelin damage. We speculate that it is the subsequent expansion and maintenance of B cell clones with these specificities that differentiate MS from OND.
The pathological significance of this intrathecal IgG response to myelin-associated lipids remains a matter of speculation. In Guillain–Barré syndrome, clear evidence indicates that different anti-glycolipid and glycolipid complex antibodies directly contribute to the pathogenesis of the disease subtypes (Kusunoki et al., 2008; Plomp and Willison, 2009; Kaida et al., 2009). Furthermore in GBS, we have recently detected antibodies to sulfatide and sulfatide containing complexes, including GalC (Rinaldi et al., unpublished results). With respect to sulfatide, it has been shown to provide a target for demyelinating antibodies in experimental models of MS (Kanter et al., 2006; Rosenbluth et al., 2003) suggesting that the response observed in MS patients might contribute to demyelination and enhance disease severity (Kanter et al., 2006). However our data do not support this view. Efficient recognition of antigen at the surface of the myelin oligodendrocyte continuum is a pre-requisite for an antibody to mediate primary demyelination in vivo (Linington et al., 1988). We found earlier that only a few antibodies showed weak staining of granules in the cytoplasm of some cells, whereas most antibodies failed to react with MS or control brain (Owens et al., 2009), and show here that none of the sulfatide-reactive rAbs studied bound to the surface of either live CNS myelin or cells of the oligodendrocyte lineage indicating that they are unlikely to mediate demyelination in vivo (Fig. 3). This is not due to the absence per se of sulfatide at the membrane surface, as demonstrated by the binding of the sulfatide reactive mAb O4 (Sommer and Schachner, 1981), but rather the precise configuration of sulfatide or sulfatide-containing complexes in live membranes limits their recognition by CSF derived rAbs. Thus from our array data, the mAb O4 appears unable to bind sulfatide in isolation, requiring an accessory lipid such as sphingomyelin to do so. The presumption is therefore that sulfatide and sphingomyelin also form an sulfatide/sphingomyelin complex in myelin membranes that allows O4 to bind effectively, but the same complex may inhibit binding of other sulfatide reactive mAbs to sulfatide through epitope masking by the adjacent lipid.
While the supramolecular structure of lipid molecules in a physiological bilayer is relatively well understood, their geometry and packing when bound to a PVDF membrane is not known. Clearly, even minor differences in the accessibility of certain regions of the molecules might alter the recognition patterns of antibodies. We currently assume that sulfatide-containing complexes are presumed to become available to drive B cell selection and maintain the intrathecal B cell response during immune-mediated demyelination. However we cannot yet exclude the possibility that components of the intrathecal repertoire play a direct role in lesion formation, although we favor indirect effects of glycolipid-reactive Abs such as opsonization of myelin debris to promote its clearance by phagocytes, or BCR mediated uptake of myelin debris and subsequent presentation of myelin-derived epitopes to T cells.
While the biological significance of this intrathecal B cell response remains unclear, its established link with individual CSF oligoclonal bands (Obermeier et al., 2008) has allowed us to define the specificity of a discrete component of this response, resolving a question that was first raised over 40 years ago. The lipid array used here was generated using only 10 lipid species and their heteromeric pairings, yet identified specificities associated with around 30% of the intrathecal response. We anticipate that increasing array complexity and altering alpha-hydroxylation, acyl chain length and saturation, will identify additional lipid targets.
Acknowledgments
Dr William Robinson is thanked for technical discussions on lipid array methodology.
This study is supported by grants from the Wellcome Trust (077041/Z/05/Z, to HJW), the Multiple Sclerosis Society of Great Britain and Northern Ireland (CL and AA), The RS Macdonald Charitable Trust (CL and HW) and NIH/NINDS (PO1-NS32623-19 for GPO). SR holds a Wellcome Trust Clinical Training Fellowship. KB and CO'L were supported by unrestricted educational grants from Biogen Idec and Merck Serono. FG-H is in receipt of a Lord Kelvin and Adam Smith PhD scholarship. JLB is supported by a research grant (RG4320) from the National Multiple Sclerosis Society (USA) and a research grant from the Guthy-Jackson Foundation.
References
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2 F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 2007;178(7):4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antel J, Bar-Or A. Roles of immunoglobulins and B cells in multiple sclerosis: from pathogenesis to treatment. J. Neuroimmunol. 2006;180(1–2):3–8. doi: 10.1016/j.jneuroim.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Awad A, Hemmer B, Hartung HP, Kieseier B, Bennett JL, Stuve O. Analyses of cerebrospinal fluid in the diagnosis and monitoring of multiple sclerosis. J. Neuroimmunol. 2010;219(1–2):1–7. doi: 10.1016/j.jneuroim.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, Oksenberg JR. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol. 1999;163(9):5133–5144. [PubMed] [Google Scholar]
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 2009;66(5):617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Owens GP, Smith-Jensen T, Walker D, Gilden DH. Cloning the antibody response in humans with inflammatory central nervous system disease: analysis of the expressed IgG repertoire in subacute sclerosing panencephalitis brain reveals disease-relevant antibodies that recognize specific measles virus antigens. J. Immunol. 1999;163(6):3496–3502. [PubMed] [Google Scholar]
- Burgoon MP, Caldas YA, Keays KM, Yu X, Gilden DH, Owens GP. Recombinant antibodies generated from both clonal and less abundant plasma cell immunoglobulin G sequences in subacute sclerosing panencephalitis brain are directed against measles virus. J. Neurovirol. 2006;12(5):398–402. doi: 10.1080/13550280600957414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Cross AH, Wu GF. Multiple sclerosis: oligoclonal bands still yield clues about multiple sclerosis. Nat. Rev. Neurosci. 2010;6(11):588–589. doi: 10.1038/nrneurol.2010.142. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, HERMES Trial G. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Ilyas AA, Chen ZW, Cook SD. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J. Neuroimmunol. 2003;139(1–2):76–80. doi: 10.1016/s0165-5728(03)00131-0. [DOI] [PubMed] [Google Scholar]
- Kaida K, Ariga T, Yu RK. Antiganglioside antibodies and their pathophysiological effects on Guillain–Barre syndrome and related disorders—a review. Glycobiology. 2009;19(7):676–692. doi: 10.1093/glycob/cwp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L, Robinson WH. Lipid microarrays identify key mediators of autoimmune brain inflammation. J. Nat. Med. 2006;12(1):138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- Kusunoki S, Kaida K, Ueda M. Antibodies against gangliosides and ganglioside complexes in Guillain–Barre syndrome: new aspects of research. Biochim. Biophys. Acta. 2008;1780(3):441–444. doi: 10.1016/j.bbagen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 2001;7(3):115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 1988;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006;59(6):880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc. Natl. Acad. Sci. U.S.A. 2008;105(28):9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467(7315):591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Mentele R, Malotka J, Kellermann J, Kumpfel T, Wekerle H, Lottspeich F, Hohlfeld R, Dornmair K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat. Med. 2008;14(6):688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, Gilden DH. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann. Neurol. 1998;43(2):236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Gilden DH, Burgoon MP. The B cell response in multiple sclerosis. Neurol. Res. 2006a;28(3):236–244. doi: 10.1179/016164106X98099. [DOI] [PubMed] [Google Scholar]
- Owens GP, Shearer AJ, Yu X, Ritchie AM, Keays KM, Bennett JL, Gilden DH, Burgoon MP. Screening random peptide libraries with subacute sclerosing panencephalitis brain-derived recombinant antibodies identifies multiple epitopes in the C-terminal region of the measles virus nucleocapsid protein. J. Virol. 2006b;80(24):12121–12130. doi: 10.1128/JVI.01704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Lassmann H, O'Connor KC, Ritchie AM, Shearer A, Lam C, Yu X, Birlea M, DuPree C, Williamson RA, Hafler DA, Burgoon MP, Gilden D. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann. Neurol. 2009;65(6):639–649. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlan EA. X-ray crystallography of antibodies. Adv. Protein Chem. 1996;49:57–133. doi: 10.1016/s0065-3233(08)60488-x. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Willison HJ. Pathophysiological actions of neuropathy-related anti-ganglioside antibodies at the neuromuscular junction. J. Physiol. 2009;587(Pt 16):3979–3999. doi: 10.1113/jphysiol.2009.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J. Clin. Invest. 1998;102(5):1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc. Natl. Acad. Sci. U.S.A. 2005;102(5):1608–1613. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S, Brennan KM, Goodyear CS, O'Leary C, Schiavo G, Crocker PR, Willison HJ. Analysis of lectin binding to glycolipid complexes using combinatorial glycoarrays. Glycobiology. 2009;19(7):789–796. doi: 10.1093/glycob/cwp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, Owens GP. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol. 2004;173(1):649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J, Schiff R, Liang WL, Dou W. Antibody-mediated CNS demyelination II. Focal spinal cord lesions induced by implantation of an IgM antisulfatide-secreting hybridoma. J. Neurocytol. 2003;32(3):265–276. doi: 10.1023/B:NEUR.0000010085.91976.a6. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 1981;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Sorensen A, Moffat K, Thomson C, Barnett SC. Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro. Glia. 2008;56(7):750–763. doi: 10.1002/glia.20650. [DOI] [PubMed] [Google Scholar]
- Thomson CE, McCulloch M, Sorenson A, Barnett SC, Seed BV, Griffiths IR, McLaughlin M. Myelinated, synapsing cultures of murine spinal cord—validation as an in vitro model of the central nervous system. Eur. J. Neurosci. 2008;28(8):1518–1535. doi: 10.1111/j.1460-9568.2008.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintore M, Rovira A, Rio J, Tur C, Pelayo R, Nos C, Tellez N, Perkal H, Comabella M, Sastre-Garriga J, Montalban X. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology. 2008;70(13 Pt 2):1079–1083. doi: 10.1212/01.wnl.0000280576.73609.c6. [DOI] [PubMed] [Google Scholar]
- Villar LM, Sadaba MC, Roldan E, Masjuan J, Gonzalez-Porque P, Villarrubia N, Espino M, Garcia-Trujillo JA, Bootello A, Alvarez-Cermeno JC. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J. Clin. Invest. 2005;115(1):187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch EJ, Cao H, Fukuyama H, Weigert M. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J. Exp. Med. 2006;203(7):1761–1772. doi: 10.1084/jem.20060075. [DOI] [PMC free article] [PubMed] [Google Scholar]