Abstract
The leukocyte response in inflammation is characterized by an initial recruitment of polymorphonuclear leukocytes (PMN) preceding a second wave of monocytes to the site of injury or infection. In the mouse, 2 populations of monocytes have been identified, Gr1−CCR2−CX3CR1hi resident monocytes and Gr1+CCR2+CX3CR1lo inflammatory monocytes. Here, intravital microscopy of the musculus cremaster and a subcutaneous air pouch model were used to investigate a possible link between PMN extravasation and the subsequent emigration of inflammatory monocytes in response to local stimulation with PAF. In mice that were made neutropenic by injection of a PMN-depleting antibody, the extravasation of inflammatory monocytes, but not resident monocytes, was markedly reduced compared with mice with intact white blood cell count but was restored by local treatment with secretion of activated PMN. Components of the PMN secretion were found to directly activate inflammatory monocytes and further examination revealed PMN-derived LL-37 and heparin-binding protein (HBP/CAP37/azurocidin) as primary mediators of the recruitment of inflammatory monocytes via activation of formyl-peptide receptors. These data show that LL-37 and HBP specifically stimulate mobilization of inflammatory monocytes. This cellular cross-talk functionally results in enhanced cytokine levels and increased bacterial clearance, thus boosting the early immune response.
Introduction
Polymorphonuclear leukocytes (PMN) dominate the initial leukocyte influx to sites of acute infection and inflammation.1 This first wave of PMN extravasation precedes a second wave of monocyte extravasation. Recruited PMN are thought to trigger this cellular switch by releasing soluble factors that initiate monocyte recruitment,2–4 much of which may be mediated by ready-made PMN granule proteins deposited at the site of inflammation.5,6 Indeed, supernatants of activated PMN from patients with specific granule deficiency lacking proteins in their primary, secondary, and tertiary granules show a reduced capacity to attract monocytes despite normal monocyte chemotaxis in vitro to other stimuli.7 After this initial observation, several PMN-derived granule proteins with monocyte-chemotactic activity were identified, among them LL-37, cathepsin G, human neutrophil peptide 1-3 (HNP1-3, α-defensins), and heparin-binding protein (HBP, also known as CAP37 and azurocidin).8–11 Their action was found to be pertussis toxin (PTx)-sensitive and several receptors were suggested to mediate the chemotactic effect.11–13
Peripheral blood monocytes constitute a heterogeneous population of circulating leukocytes in both humans14 and mice.15 In the murine blood, 2 monocyte subsets can be distinguished based on their expression of CX3CR1, CCR2, and Gr1. Whereas resident monocytes (Gr1−CCR2−CX3CR1hi) home to noninflamed tissues, inflammatory monocytes (Gr1+CCR2+CX3CR1lo) are predominantly recruited to sites of inflammation by mechanisms involving CCR2.15 These inflammatory monocytes were recently shown to be of critical importance in diverse inflammatory and infectious diseases.16–19
In this study, we investigated the significance of the initial PMN efflux for the subsequent extravasation of monocytes. Our results demonstrate that PMN seed granule proteins in the tissue which contribute to mobilization specifically of inflammatory monocytes. Functionally, the PMN-dependent invasion of inflammatory monocytes results in a more vigorous immune response as shown by enhanced cytokine release and bacterial clearance at the site of inflammation.
Methods
Animals
Wild-type C57BL/6 and gene-targeted CCR2−/−, CX3CR1eGFP/+, CX3CR1eGFP/eGFP, and DPPI−/− mice were used for in vivo experiments. All genetically modified animals were on C57BL/6 background. PMN depletion was performed by intraperitoneal injection of monoclonal antibody (mAb) RB6-8C5 (100 μg/mouse) or mAb 1A8 (10 μg/mouse) 8 hours before injection of the stimuli. All animal experiments were approved by the ethical committee for animal experimentation in Stockholm (N149/06, N277/07, N199/07).
Intravital microscopy of the cremaster muscle
Monocyte adhesion and extravasation in the musculus cremaster was observed by intravital microscopy in CX3CR1eGFP/+ mice or in C57BL/6 mice in which inflammatory monocytes were tracked after taking up fluorescently labeled latex beads.18,20 Mice received intrascrotal injections of platelet-activating factor (PAF; 10−6 M, 300 μL; Sigma-Aldrich, St Louis, MO) and the musculus cremaster was exteriorized for microscopic observation 12h later. Intravital microscopy of leukocyte adhesion and extravasation in the musculus cremaster was performed as described previously.21
Fluorescence intensity of monocytes extravasated into the cremaster muscle was quantified by ImageJ software (http://rsb.info.nih.gov/ij) subtracting the background fluorescence from the monocyte fluorescence. Arbitrary fluorescence units were plotted versus the cell count, revealing 2 populations.
For analysis of monocyte migration, the muscle of neutropenic CX3CR1eGFP/+ mice was exposed 12 hours after local injection of PAF. The migration of fluorescent cells was tracked for 30 minutes by time-lapse video microscopy. Thereafter, the cremaster muscle was superfused with PMN secretion (PMN-sec) and the same cells were tracked for another 30 minutes.
Subcutaneous air pouch
A subcutaneous air pouch was created as described previously.22 In brief, mice were subcutaneously injected in the back at days 0 and 4 with 5 mL sterile air. At day 7, 1 mL sterile phosphate-buffered saline (PBS) containing PAF (10−6 mol/L) was injected into the pouch. Twelve hours later, the pouch was lavaged with 5 mmol/L ethylenediaminetetraacetic acid (EDTA) in PBS. Mice receiving PMN-sec were injected with PAF in 500 μL PBS plus 500 μL PMN-sec. In separate experiments, the air pouch was injected with HNP1-3 (5 μg, purified from human PMN23 [a gift from O. E. Sorensen, Lund University, Lund, Sweden]), LL-37, cathelin-related antimicrobial peptide (CRAMP; 10 μg each; both Innovagen, Lund, Sweden [gifts from B. Agerberth, Karolinska Institut, Stockholm, Sweden]), cathepsin G (10 μg; Sigma-Aldrich), or HBP (10 μg; produced as described previously24 [a gift from Hans Flodgaard, Leukotech, Copenhagen, Denmark]). PTx (4 μg; Invitrogen, Carlsbad, CA) and butyloxycarbonyl l-phenylalanyl-l-leucyl-l-phenylalanine (BOC-PLPLP; 200 nM in 100 μL PBS; GenScript, Piscataway, NJ) were injected intravenously 1 hour before instillation of PAF. After harvesting, cells in the lavage fluid were spun down, counted manually, and differentiated in the fluorescence-activated cell sorting (FACS) using anti-Gr1 phycoerythrin (PE)-Cy5 (BioLegend, San Diego, CA) and anti-F4/80 fluorescein isothiocyanate (Serotec, Oxford, UK). Some mice were injected with Listeria monocytogenes (106/pouch), and the air pouch was lavaged 24 hours later. In these mice, the liver was also removed and homogenized. Tissue homogenate was plated on brain heart infusion agar (Sigma-Aldrich), and the colonies were quantified.
Concentrations of monocyte chemoattractant protein (MCP-1; eBioscience, San Diego, CA), MCP-3 (Bender MedSystems, Vienna, Austria), interleukin-6 receptor (IL-6R; R&D Systems, Minneapolis, MN), tumor necrosis factor (TNF), and IL-6 (both eBioscience) were analyzed in the air pouch lavage by enzyme-linked immunosorbent assay (ELISA). Myeloperoxidase (MPO) was analyzed spectrophotometrically.25 Matrix metalloproteinase-9 (MMP-9) and MCP-1 in the air pouch was analyzed by Western blot.26
For analysis of phagocytic capacity, mice were injected with IgG-opsonized fluorescent Staphylococcus aureus bacteria (Invitrogen) 1 hour before lavage of the air pouch. Harvested cells were spun down, and monocytes were separated from PMN by density centrifugation. Monocytes were then plated on a 96-well plate (50 000/well), and the fluorescence was analyzed in a plate reader (Fluoroskan Ascent; Thermo Fisher Scientific, Waltham, MA). In addition, the number of bacteria in the lavage fluid was manually quantified by fluorescence microscopy and expressed as percentage of total number of injected bacteria.
Intracellular Ca2+ mobilization
Whole blood from CCR2−/− and C57BL/6 mice was harvested by cardiac puncture. After lysis of red blood cells, the leukocytes were stained with anti-Gr1 and anti-F4/80. In addition, leukocytes were incubated with the Ca2+-sensitive fluorochrome X-rhod-1 (Invitrogen). Mobilization of intracellular Ca2+ was analyzed by FACS in inflammatory monocytes (Gr1+F4/80+) and resident monocytes (Gr1−F4/80+) after stimulation with medium, ionomycin (1 μmol/L), or PMN-sec. Fluorescence was measured 60 seconds before and every 30 seconds after stimulation up to 150 seconds.
Preparation of PMN secretion and immunodepletion of granule proteins
PMN degranulation was induced in freshly isolated PMN through cross-linking of CD18.27 PMN were sedimented by centrifugation, and the cell-free supernatant containing PMN-sec was stored at −70°C. Granule release was confirmed by Western blot and MPO analyses.25
Monoclonal antibodies to HBP (a gift from H. Flodgaard), cathepsin G (Calbiochem, San Diego, CA), or LL-37 (a gift from B. Agerberth) were coupled to protein A Sepharose (GE Healthcare, Chalfont St Giles, UK). The antibody/protein A Sepharose complexes were incubated with the PMN-sec under gentle rotation overnight at 4°C. The Sepharose beads were spun down, and the efficacy and specificity of immunoadsorption was verified with Western blot using polyclonal antibodies to HBP, LL-37, or cathepsin G.
PMN subcellular fractionation
Subcellular fractionation of PMN was performed by density centrifugation on Percoll gradients, as described by Kjeldsen et al.28 To get rid of the Percoll, fractions were centrifuged for 90 minutes at 45 000 rpm in an ultracentrifuge (Beckman Coulter, Fullerton, CA) at 4°C. To determine which fraction corresponded to which granule compartment, each individual fraction was analyzed for the presence of specific markers of the respective granule as described previously (O.S., H.H., L.L., manuscript submitted April 2008).
Analysis of formyl peptide receptor expression
Whole blood from C57BL/6 mice were subjected to red cell lysis and reacted with Gr1-PercP-Cy5.5, CD45-APC-Cy7 (BD Biosciences, San Jose, CA), and CD115-PE (eBiosciences) for 30 minutes. High-pressure sorting with purity precision mode was performed in a FACSAria sorter (BD Biosciences) to separate and collect CD115+Gr1+CD45+ inflammatory monocytes. Total RNA was isolated from inflammatory monocytes and reverse-transcribed into cDNA by Moloney murine leukemia virus RT (Invitrogen). Polymerase chain reactions (PCRs) were performed with 20 ng cDNA using Taq DNA polymerase (Promega) for 38 cycles (30 seconds at 95°C, 40 seconds at 60°C, and 1 minute at 72°C) and specific primers (aldolase: 5′-AGCTGTCTGACATCGCTCACCG-3′, 5′-CACATACTGGCAGCGCTTCAAG-3′; formyl peptide receptor 2 [fpr2]: 5′-GTAAGAAGGAGACCTCAGCTG-3′, 5′-CCCTCTAGCATCTCTAACTGTA GTC-3′; and fpr1: 5′-CTGTAGATCTGTCCAGAGC TGTTG-3′, 5′-CAATGTAAGAAGAAA TGCACAAATC-3′).
Statistics
Data are expressed as means plus or minus SD. The statistical calculations were done using Statistica software (StatSoft, Tulsa, OK). Ca2+ measurements were analyzed using one-way analyzes of variance followed by a Tukey honestly significant difference test. All other data were analyzed with t test for independent samples. A P value less than .05 was considered significant.
Results
Neutrophil extravasation precedes monocyte recruitment
Local application of PAF induces rapid infiltration of PMN into the musculus cremaster of CX3CR1eGFP/+ mice as determined by intravital microscopy (Figure S1A; available on the Blood website; see the Supplemental Materials link at the top of the online article). Similar results were obtained in a subcutaneous air pouch of C57BL/6 mice after injection of PAF and differential cell quantification of the lavaged cells (Figure S1B). Before stimulation, monocytes were absent in the cremaster muscle, whereas a small number of monocytes was detected in the air pouch. The number of monocytes remained stable for the first 2 hours after injection of PAF and increased significantly above baseline only after 4 hours of treatment in both models. Thereafter, the number of infiltrating monocytes continued to increase, whereas the count of emigrated PMN started to decline after 8 to 12 hours in the cremaster and the air pouch, respectively.
Neutrophil secretion products mediate early monocyte extravasation
To investigate the causal link between PMN extravasation and subsequent efflux of monocytes, we depleted mice of PMN by injection of mAb RB6-8C5.5,29,30 To determine whether treatment with RB6-8C5 also affects the number of circulating monocytes, we injected the antibody into CX3CR1eGFP/+ mice and analyzed blood samples at several consecutive time points. FACS analysis revealed a prompt decrease of the number of circulating PMN, whereas neither the Gr1+ nor the Gr1− monocyte subpopulation was affected at the dose used in the study (Figure S2A,B). Moreover, expression of CD62L and CD11b on the Gr1+ monocyte subset was not altered by treatment with RB6-8C5 (data not shown), indicating that there is no activation of monocytes by the antibody. In agreement with this observation, the serum levels of TNF and IL-6 were not increased after treatment with the antibody (data not shown).
Monocyte recruitment was studied in neutropenic mice in response to PAF stimulation. Depletion of PMN significantly reduced the number of extravasated monocytes (Figure 1A,B). Intravital microscopy of the cremaster muscle in CX3CR1eGFP/+ mice revealed that not only monocyte extravasation but also monocyte adhesion was significantly impaired in neutropenic mice (Figure 1A). Similar results were also found when we used the Ly-6G specific mAb 1A8 to deplete PMN. The pattern of monocyte extravasation observed in these mice (Figure S3B) was identical to that seen in mice treated with RB6-8C5.
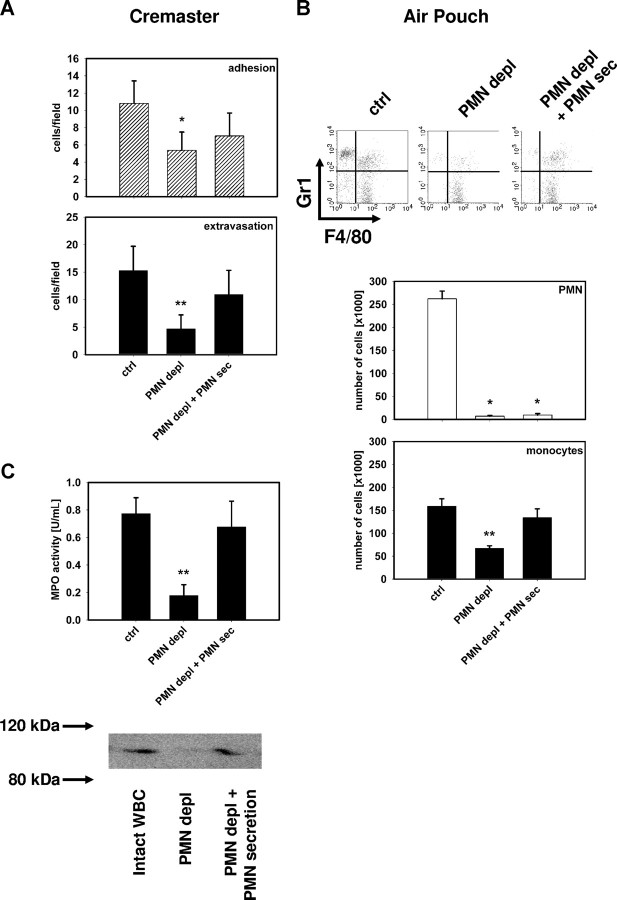
Figure 1.
PMN secretion products mediate monocyte recruitment. (A) Number of adherent and extravasated fluorescent cells in the musculus cremaster quantified 12 hours after intrascrotal application of PAF (10−6 M) in CX3CR1eGFP/+ mice with intact WBC (ctrl), in neutropenic mice (PMN depl), and in neutropenic mice receiving intrascrotal injection of PMN secretion (PMN depl + PMN-sec). (B) Representative FACS plots showing Gr1 versus F4/80 staining and number of PMN and monocytes in the subcutaneous air pouch of C57BL/6 mice 12 hours after injection of PAF (10−6 M) in mice with intact WBC (ctrl), in neutropenic mice (PMN depl), and in neutropenic mice where PMN secretion was injected into the pouch (PMN depl + PMN-sec). (C) Analysis of MPO activity (top) and detection of MMP-9 (bottom) in the air pouch lavage fluid of C57BL/6 mice (ctrl), neutropenic mice (PMN depl), and neutropenic mice injected with PMN secretion (PMN depl + PMN-sec). Data are expressed as means (± SD), n = 4-6 for each bar. * indicates significant difference compared with ctrl; ** indicates significant difference compared both to ctrl and PMN depl + PMN-sec.
To investigate whether the relationship between PMN and monocyte recruitment also exists in a tissue where resident macrophages are present, we studied monocyte extravasation in a peritonitis model. Indeed, PMN depletion reduced the number of extravasated monocytes in thioglycollate-induced peritonitis by 56% (± 12%) compared with mice with intact WBC (not shown).
We further aimed at determining whether the monocyte-attracting activity was due to the release of a soluble factor from PMN. For this purpose, we injected PMN secretion (PMN-sec) locally into neutropenic mice together with PAF and analyzed the subsequent recruitment of monocytes. PMN-sec was harvested from freshly isolated human PMN activated by cross-linking of CD18.25,27,31,32,33 Previous work has shown that PMN-sec obtained by this approach contains proteins from primary, secondary, and tertiary granules, as well as secretory vesicles.25 However, it is devoid of TNF, interferon γ (IFNγ), IL-8, MCP-1, and reactive oxygen species.33 Local application of PMN-sec restored extravasation of monocytes in the cremaster muscle (Figure 1A) and in the air pouch (Figure 1B) of neutropenic mice to levels not different from the numbers found in mice with intact WBC. Analysis of the air pouch lavage fluid revealed that the recruitment of PMN was associated with the deposition of substantial amounts of the PMN granule proteins MPO and MMP-9 in mice with intact WBC in contrast to PMN-depleted mice where these proteins were lacking (Figure 1C). However, the two proteins could be restored by injection of the PMN-sec.
PMN granule contents specifically mobilize inflammatory monocytes
Inflammatory monocytes have significant functions in the early immune response during infection and injury.16–19 To specifically address the extravasation of this monocyte subset versus resident monocytes in the cremaster model, 2 approaches were chosen to distinguish between the 2 monocyte subsets. First, in CX3CR1eGFP/+ mice, resident and inflammatory monocytes exhibit distinct differences in their fluorescence, with inflammatory monocytes displaying a lower intensity.15 The fluorescence intensity of extravasated monocytes was quantified off-line in recorded images of 30 different cremaster muscles in total in mice with intact WBC, neutropenic mice, and neutropenic mice treated with PMN-sec. By plotting fluorescence intensity versus cell number in the 3 groups combined, 2 different populations were distinguished (Figure 2A). Examining the 3 groups individually, we found that PMN depletion predominantly reduces the extravasation of inflammatory monocytes, the majority of which could be restored by local administration of PMN-sec (Figure 2A). In a second approach, we labeled inflammatory monocytes in C57BL/6 mice with fluorescent latex beads18,20 and followed their extravasation. Again, intrascrotal application of PMN-sec significantly enhanced the number of extravasated inflammatory monocytes in the musculus cremaster of neutropenic mice (Figure 2B). In contrast to neutropenic CX3CR1eGFP/+ mice (Figure 1A), we also found a significant increase in the number of adherent latex bead-positive inflammatory monocytes in response to PMN-sec, which might be because specifically only inflammatory monocytes are quantified in this model, whereas in CX3CR1eGFP/+ mice, also resident monocytes, which are not affected by PMN-sec, are included in the analysis.
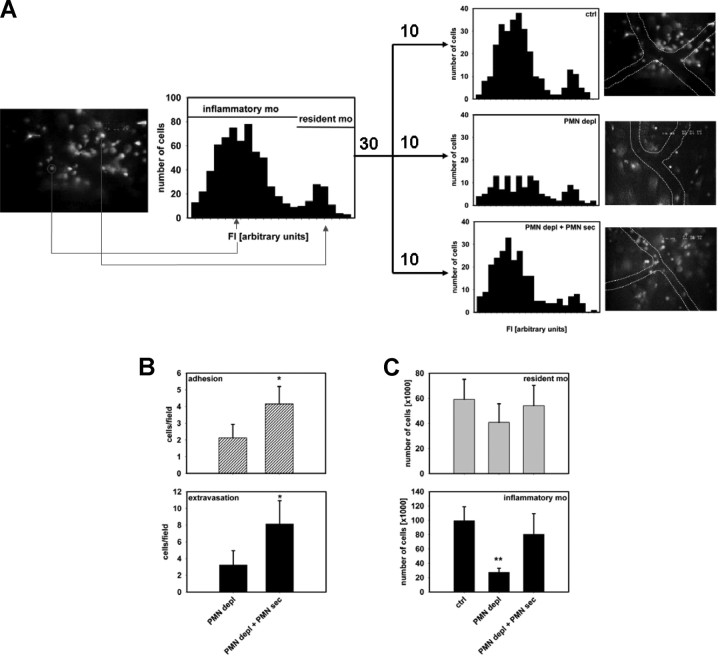
Figure 2.
PMN secretion specifically mobilizes inflammatory monocytes. (A) Fluorescence intensity of extravasated monocytes in the musculus cremaster of CX3CR1eGFP/+ mice after intrascrotal injection of PAF (10−6 M). High-fluorescent cells represent the resident monocytes, whereas low-fluorescent cells constitute the inflammatory monocytes. Analyses were made in mice with intact WBC count (top right), in neutropenic mice (middle right), and in neutropenic mice treated intrascrotally with PMN secretion (bottom right). Data are based on analyses in 10 cremaster muscles in each group. Representative images are shown to the right of each histogram. (B) Adhesion and extravasation of inflammatory monocytes in the musculus cremaster 12 hours after intrascrotal injection of PAF (10−6 M). Analyses were made in neutropenic C57BL/6 mice with or without local treatment with PMN secretion. Ingestion of fluorescent latex beads allowed visualization of the inflammatory monocyte subset. * indicates significant difference compared with PMN depl. (C) Recruitment of resident and inflammatory monocytes in the air pouch of C57BL/6 mice 12 hours after stimulation with PAF (10−6 M). The monocytes were differentiated by FACS based on their Gr1 expression. Analyses were made in mice with intact WBC count (ctrl), neutropenic mice (PMN depl), and neutropenic mice treated with PMN secretion (PMN depl + PMN-sec). ** indicates significant difference compared with both ctrl and PMN depl + PMN-sec.
In the air pouch lavage fluid from mice with intact WBC, we found that approximately 65% of monocytes were Gr1+ indicating an inflammatory phenotype. Depletion of PMN reduced extravasation exclusively of this subset, whereas Gr1− monocytes were not affected (Figure 2C). Injection of PMN-sec restored the number of extravasated Gr1+ monocytes. Similar results were obtained in mice that were treated with mAb 1A8 instead of the RB6-8C5 clone (Figure S3C). These results were also reproduced in CX3CR1eGFP/+ mice, where specifically the recruitment of eGFPloGr1+ monocytes was affected by the presence of PMN and their secretion products (Figure S4).
Further evidence for the importance of PMN and their secretion products was gathered from experiments with mice lacking dipeptidyl peptidase I (DPPI), which is a lysosomal cysteine protease critical for the processing of neutrophil serine proteases. These mice have impaired PMN recruitment and lack granule constituents in their primary granules, thereby reflecting the murine homolog of the Papillon-Lefèvre syndrome.34 In the air pouch model, we confirmed the reduced accumulation of PMN in response to PAF compared with wild-type mice (Figure S5). This was associated with a reduction in the extravasation of inflammatory monocytes, which was not further lowered by depletion of PMN. It is noteworthy, however, that treatment of neutropenic DPPI−/− mice with PMN-sec significantly increased the accumulation of inflam-matory monocytes to levels also seen in wild-type mice receiving the same treatment (Figure S5).
PMN secretion specifically activates inflammatory monocytes
Having shown that PMN-sec specifically induces the mobilization of inflammatory monocytes, we sought to determine whether this is due to direct activation of these cells by PMN granule constituents. First, we studied the migration distance of monocytes in the cremaster muscle in CX3CR1eGFP/+ mice. PAF was injected intrascrotally in neutropenic mice and 12 hours later, the cremaster muscle was exposed. The migration distance of inflammatory monocytes (Fl < 70) and resident monocytes (Fl > 70) over a 30-minute period before and after superfusion with PMN-sec was calculated. While no difference in migration distance between the 2 monocyte subsets before superfusion was observed (Figure 3A), PMN secretion gave rise to a more profound increase in migration velocity of inflammatory monocytes than of resident monocytes.
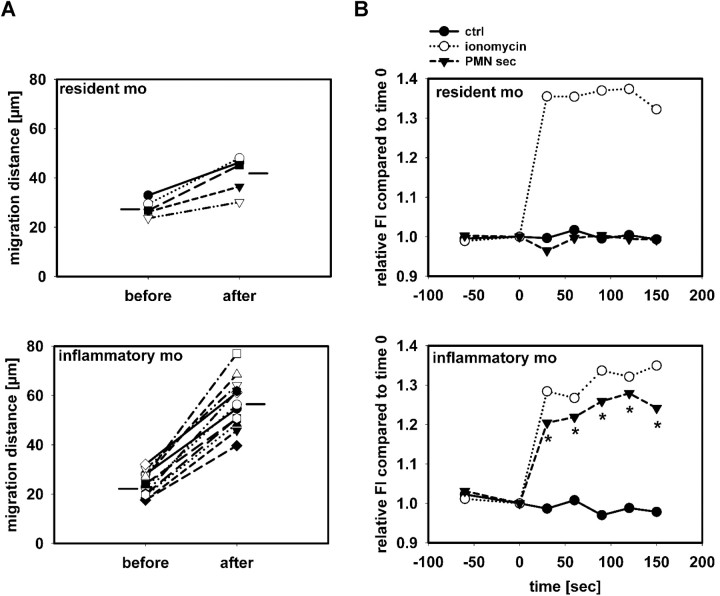
Figure 3.
PMN secretion specifically activates inflammatory monocytes. (A) Migration distance of monocytes in 3 cremaster muscles of CX3CR1eGFP/+ mice over a 30-minute period before and after superfusion with PMN secretion. Distinction between resident and inflammatory monocytes was based on their fluorescence intensity. Horizontal lines indicate group average. The difference in number of cells included in the analysis reflects the different efficacy in recruitment between the 2 monocyte subsets. (B) Leukocytes from C57BL/6 mice were harvested by cardiac puncture, and intracellular Ca2+ mobilization was measured in resident monocytes (Gr1−, F4/80+, top) and inflammatory monocytes (Gr1+, F4/80+, bottom) after stimulation with medium (ctrl), ionomycin, or PMN secretion (PMN-sec). Data were acquired before and at 30-second intervals after stimulation (time = 0) and presented as average of 4 to 6 analyses for each data point. * indicates significant difference between treatment with PMN secretion and control.
In a second approach, we analyzed the intracellular Ca2+ mobilization in inflammatory and resident monocytes after stimulation with PMN-sec. While the calcium ionophore ionomycin induced intracellular Ca2+ mobilization in both populations, only inflammatory monocytes responded with a Ca2+ increase after stimulation with PMN-sec (Figure 3B).
Recruitment of inflammatory monocytes by PMN secretion is CCR2-dependent
The chemokine receptor CCR2 and recently also CX3CR1 were reported to be critically involved in the recruitment of inflammatory monocytes.18 To investigate their contribution to the recruitment of inflammatory monocytes by PMN-sec, we performed air pouch experiments in CX3CR1eGFP/eGFP and in CCR2−/− mice (Figure 4A). In CX3CR1eGFP/eGFP mice, lacking a functional fractalkine receptor, accumulation of inflammatory monocytes was reduced by depletion of PMN. As in heterozygous CX3CR1eGFP/+ mice, the monocyte recruitment was restored by local administration of PMN-sec. In contrast to the CX3CR1-deficient mice, the basal recruitment in CCR2−/− mice was reduced and depletion of PMN did not affect the extravasation of inflammatory monocytes. More importantly, although we could restore the reduced extravasation of inflammatory monocytes in neutropenic mice lacking CX3CR1 by PMN-sec, we were unable to do so in CCR2−/− mice. All together, this indicates that CCR2 is critically involved in the PMN-mediated monocyte recruitment. To investigate whether PMN-sec activates monocytes through direct interaction with CCR2, we analyzed the Ca2+ mobilization in monocytes of CCR2−/− mice in response to stimulation with PMN-sec (Figure 4B). However, we found no difference in the release pattern of intracellular Ca2+ compared with wild-type mice (Figure 3B) indicating that CCR2 is not critically involved in Ca2+ mobilization triggered by PMN-sec.
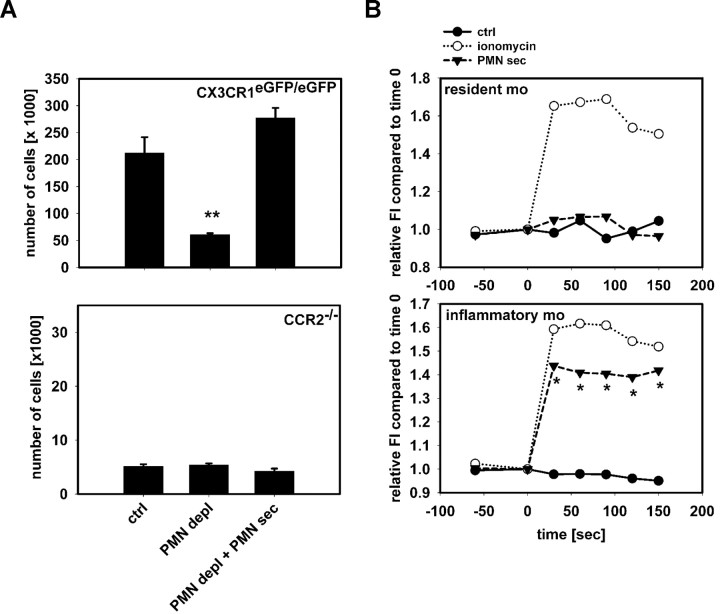
Figure 4.
Recruitment of inflammatory monocytes by PMN secretion is CCR2-dependent. (A) Recruitment of Gr1+ inflammatory monocytes in the air pouch of CX3CR1eGFP/eGFP mice (top) and CCR2−/− (bottom) mice 12 hours after stimulation with PAF (10−6 M). Analyses were made in mice with intact WBC count (ctrl), neutropenic mice (PMN depl), and neutropenic mice treated with PMN secretion (PMN depl + PMN-sec). Note the difference in scale between the charts. ** indicates significant difference compared with both ctrl and PMN depl + PMN-sec. (B) Leukocytes from CCR2−/− mice were harvested by cardiac puncture and intracellular Ca2+ mobilization was measured in resident monocytes (Gr1−, F4/80+, top) and inflammatory monocytes (Gr1+, F4/80+, bottom) after stimulation with medium (ctrl), ionomycin, or PMN secretion (PMN-sec). Data were acquired before and at 30-second intervals after stimulation (time = 0) and presented as average of 4 to 6 analyses for each data point. * indicates significant difference between treatment with PMN secretion and control.
The PMN-dependent recruitment of inflammatory monocytes yields a more efficient immune response
Because we find a selective mobilization of inflammatory monocytes by PMN, we aimed at identifying functional consequences of this response. Hence, we analyzed cytokine concentrations in the air pouch lavage, phagocytic capacity of monocytes in the air pouch as well as dissemination of L monocytogenes. Diminished extravasation of inflammatory monocytes into the air pouch in neutropenic mice was associated with lower concentrations of TNF and IL-6 in the lavage fluid compared with mice with intact WBC (Figure 5A). Substitution with PMN-sec in neutropenic mice restored cytokine concentrations in the air pouch lavage to the baseline value. A similar pattern was seen when analyzing bacterial clearance and phagocytosis. Here, IgG-opsonized fluorescent S aureus bacteria were injected into the air pouch 1 hour before harvesting the leukocytes. PMN depletion led to a substantial increase in the total number of bacteria in the air pouch fluid concomitant with a reduced number of phagocytosed bacteria per monocyte, together signifying a reduced bacterial clearance (Figure 5B). Although the first observation may be due to a contribution of PMN to the phagocytosis, the latter is an indicator of the phagocytic potency of inflammatory monocytes. Furthermore, injection of PMN-sec into the air pouch of neutropenic mice not only decreased the number of bacteria in the pouch but also enhanced the uptake of bacteria per monocyte (Figure 5B).
Figure 5.
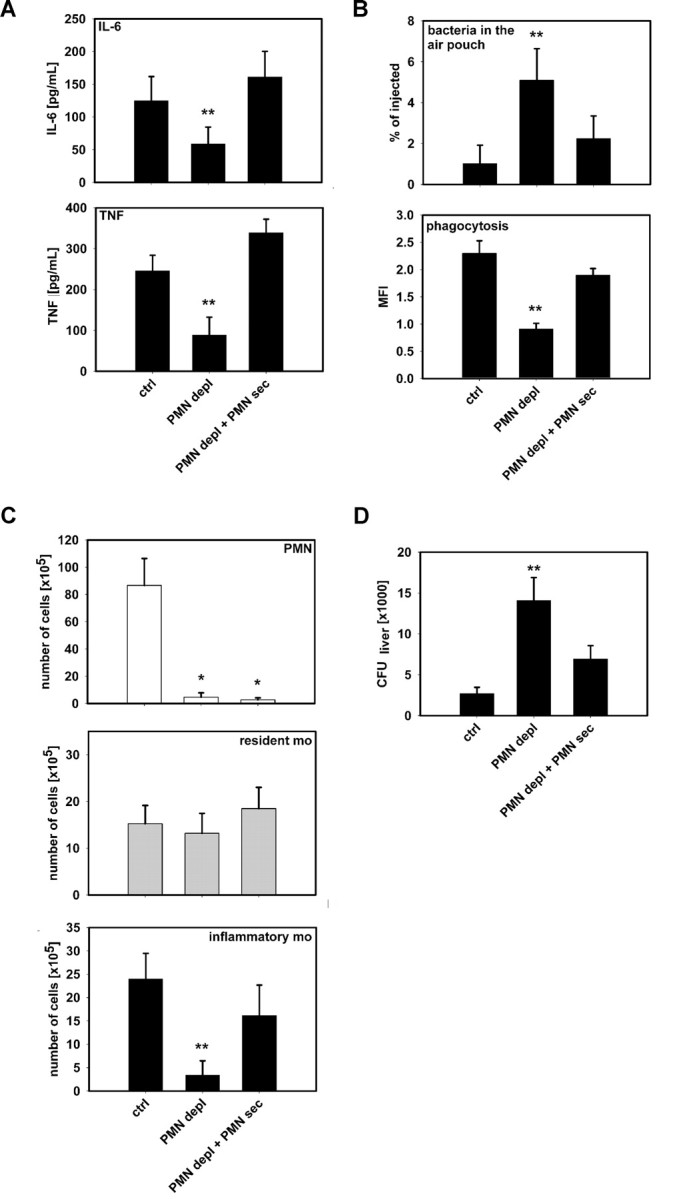
PMN secretion stimulates cytokine release and bacterial phagocytosis by monocytes. (A) Concentrations of IL-6 (top) and TNF (bottom) in air pouch fluid of mice with intact WBC count (ctrl), neutropenic mice (PMN depl), and neutropenic mice treated with PMN secretion (PMN depl + PMN-sec). (B) Leukocyte recruitment in the air pouch was induced by PAF and fluorescent IgG-opsonized S aureus bacteria were injected 1 hour before the cells were harvested. Upper panel shows the number of bacteria retrieved from the air pouch fluid expressed in percentage of total amount of injected bacteria. Bottom panel shows fluorescence intensity of monocytes as a measure of bacterial phagocytosis. (C) Recruitment of PMN (top, Gr1+, F4/80−), resident monocytes (middle, Gr1−, F4/80+), and inflammatory monocytes (bottom, Gr1+, F4/80+) in the air pouch after injection of L monocytogenes (106). (D) Dissemination of L monocytogenes 24 hours after inoculation into the air pouch. Data show colony-forming units (CFU) in liver homogenates from mice with intact WBC, neutropenic mice, and neutropenic mice treated with PMN secretion. Data are expressed as means (± SD); n = 4-6 for each bar. * indicates significant difference compared with ctrl; ** indicates significant difference compared with both ctrl and PMN depl + PMN-sec.
We then investigated the monocyte recruitment in response to local infection with L monocytogenes and spreading of the bacteria from the air pouch cavity as an integrative measure of the functional significance of the recruitment of inflammatory monocytes. Injection of L monocytogenes into the air pouch induced a strong infiltration of both monocytes and PMN (Figure 5C). The ratio between inflammatory monocytes and resident monocytes was similar to what was observed with PAF treatment. Rendering mice neutropenic not only abolished PMN recruitment but also selectively reduced the number of extravasated inflammatory monocytes. Much of this defect was compensated by injection of PMN-sec. In the same mice, we investigated colonization of bacteria in the liver. There we found a significant increase in CFUs in neutropenic mice (Figure 5D), although part of this increase likely can be attributed to the loss of the PMN phagocytic capacity. Local instillation of PMN-sec reduced the amount of CFUs from the liver homogenate.
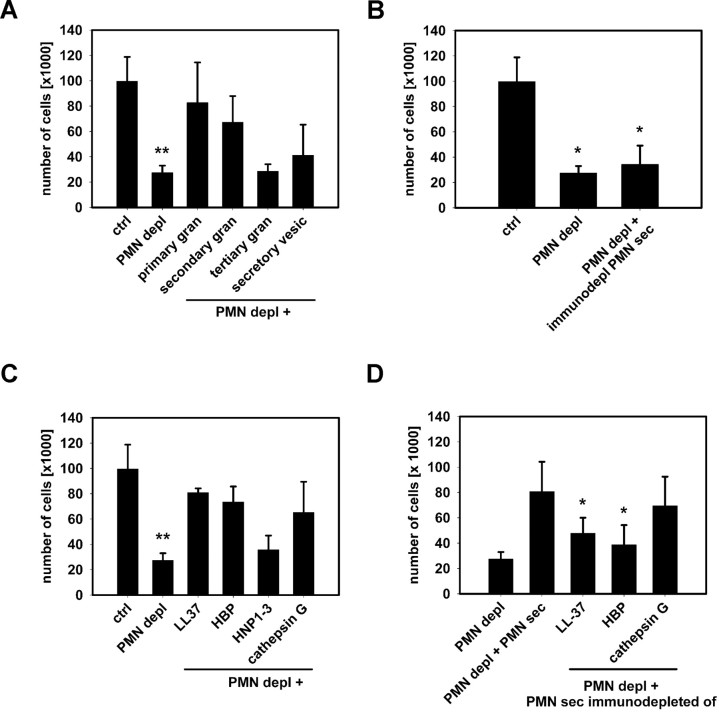
PMN-dependent extravasation of inflammatory monocytes is predominantly mediated by LL-37 and HBP
In further experiments, we aimed at identifying the active components mediating the selective recruitment of inflammatory monocytes. IL-6R,35 chemokines,36,37 and neutrophil granule7–13 proteins were previously reported to mediate PMN-dependent monocyte recruitment. Levels of IL-6R in the air pouch lavage fluid were not altered by PMN depletion or application of PMN-sec (Table S1), making a role of IL-6R in this early recruitment of inflammatory monocytes unlikely. In addition, concentrations of MCP-1 or MCP-3 were not affected by depletion of PMN or injection of PMN-sec suggesting that PMN granule proteins do not act via a secondary chemokine production (Table S1). Moreover, injection of PMN-sec in the absence of PAF induced a profound influx of Gr1+ monocyte into the air pouch (data not shown), further supporting the direct monocyte-activating properties of PMN granule components. Heat-inactivation of PMN-sec before injection or treatment of the mice with PTx completely abolished the response to PMN-sec (data not shown) indicating that (1) a protein rather than, for example, a lipid mediator in the PMN-sec mediates the recruitment of inflammatory monocytes and (2) a Gi-dependent receptor is involved in monocyte activation. Subcellular fractionation of the PMN allows not only to localize granule proteins in a subset of granules but also to pinpoint a functional response to a certain granule subclass (O.S., H.H., L.L., manuscript submitted April 2008). We injected individual granule fractions into the air pouch of neutropenic mice and analyzed the subsequent recruitment of inflammatory monocytes. Administration of either the primary or secondary granule fraction significantly increased the recruitment of inflammatory monocytes, whereas the tertiary granule and the secretory vesicle fraction were without effect (Figure 6A). LL-37, HBP, HNP1-3, and cathepsin G stored in primary or secondary granules, were previously shown to have chemotactic effect on monocytes.8–10 These effects were found to be PTx-sensitive and associated with a Ca2+ response in the target cell. The concordance between these observations and our results led us to immunodeplete the PMN-sec of these 4 polypeptides and to test the remaining activity of the secretion. Selective removal of these candidate proteins rendered the secretion nonfunctional with regard to chemotactic activity (Figure 6B). When testing the efficiency of recombinant/isolated forms of these proteins in the air pouch model, we found a significant increase of recruited inflammatory monocytes for stimulation with HBP, LL-37, and cathepsin G but not HNP1-3 (Figure 6C). Similar results were also found by treatment with CRAMP, the murine homologue of LL-37 (data not shown). To assess whether the concentrations at which the recombinant and isolated proteins were used are relevant, we quantified the concentrations of HBP, LL-37, cathepsin G, and HNP1-3 in the PMN-sec. We also quantified the concentration of HBP, LL-37, and cathepsin G by ELISA and of HNP1-3 in the PMN-sec by quantitative dot blot in the secretion and found concentrations of 3.4 μg/mL HBP, 3.1 μg/mL LL-37, 2.1 μg/mL cathepsin G, and 2.5 μg/mL HNP1-3. We thus conclude that concentrations of 5 and 10 μg/mL as used in Figure 6 are indeed relevant. In additional experiments, we immunodepleted individually the PMN-sec of LL-37, HBP, and cathepsin G. When testing the efficiency of respective immunodepleted PMN-sec in the air pouch model of neutropenic mice, we found that secretion depleted of LL-37 or HBP showed a significantly lower activity compared with crude secretion (Figure 6D).
Figure 6.
Identification of HBP and LL-37 as principal mediators of PMN-induced mobilization of inflammatory monocytes. (A) Extravasation of Gr1+ inflammatory monocytes in the air pouch of neutropenic mice after stimulation with PAF and individual PMN granule fractions. ** indicates significant difference compared with both ctrl and PMN depl + primary or secondary granule fraction. (B) Immunodepletion of LL-37, HBP, HNP1-3, and cathepsin G from the PMN secretion, renders the secretion nonfunctional. * indicates significant difference compared with ctrl. (C) Extravasation of Gr1+ inflammatory monocytes in the air pouch of neutropenic mice after stimulation with PAF combined with recombinant/isolated forms of LL-37 (10 μg/mL), HBP (10 μg/mL), HNP1-3 (5 μg/mL), or cathepsin G (10 μg/mL). ** indicates significant difference compared with both ctrl and PMN depl + LL37/HBP/cathepsin G. (D) Extravasation of Gr1+ inflammatory monocytes in the air pouch of neutropenic mice after stimulation with PAF combined with crude PMN secretion or PMN secretion immunodepleted of LL-37, HBP, and cathepsin G, respectively. * indicates significant difference compared with PMN depl + PMN-sec. Data are expressed as means (± SD) n = 5 for each bar.
Activation of inflammatory monocytes by PMN secretion is in part mediated by formyl peptide receptors
LL-37 and cathepsin G were previously shown to mediate their monocyte chemoattracting activity via formyl peptide receptors (fpr), and we therefore investigated the involvement of these receptors in activation and recruitment of inflammatory monocytes in response to PMN-sec. The expression of fpr1 and fpr2 by inflammatory monocytes was confirmed by PCR (Figure S6). The extravasation of inflammatory monocytes into the air pouch of mice with intact WBC after stimulation with PAF was significantly reduced in the presence of the fpr antagonist BOC-PLPLP (Figure 7A). Moreover, the antagonist attenuated the effect of the PMN-sec and of LL-37 and HBP on the recruitment of inflammatory monocytes to a similar extent (Figure 7A). We further analyzed the influence of the fpr antagonist on the intracellular Ca2+ mobilization in inflammatory monocytes induced by these stimuli. BOC-PLPLP significantly reduced the Ca2+ response in inflammatory monocytes induced by PMN-sec (Figure 7B). Of the 3 granule proteins tested, HBP had the strongest effect on Ca2+ mobilization in inflammatory monocytes, whereas cathepsin G was the weakest inducer. Pretreatment with BOC-PLPLP significantly attenuated the Ca2+ mobilization induced by LL-37, whereas the antagonizing effect was less pronounced when stimulation was with HBP or cathepsin G. These data indicate the contribution of fpr in the recruitment of inflammatory monocytes in response to PMN granule proteins.
Figure 7.
Involvement of FPRs in the PMN-induced mobilization of inflammatory monocytes. (A) Extravasation of Gr1+ inflammatory monocytes in the air pouch of neutropenic mice (PMN depl) after stimulation with PAF combined with PMN secretion or individual granule proteins. ▨ indicate pretreatment with the FPR antagonist BOC-PLPLP. * indicates significant difference compared with respective treatment in the absence of the fpr antagonist. (B) Ca2+ mobilization in inflammatory monocytes in response to PMN secretion or individual granule proteins. Ca2+ mobilization was measured in the FACS in the presence or absence of the FPR antagonist BOC-PLPLP. * indicates significant difference compared with respective treatment in the absence of the fpr antagonist.
Discussion
After identification of monocyte subsets in the mouse,15 the functional characterization of these cells has gained intensive attention. Taking advantage of 2 different recruitment models in vivo, we demonstrate that extravasating PMN release their granule contents which directly and specifically activate inflammatory monocytes, thereby stimulating early recruitment of these cells. Selective removal of specific components of the PMN-sec and the use of isolated/recombinant forms of PMN granule proteins draw attention to HBP and LL-37 as principal messengers in the PMN-monocyte cross-talk.
Murine inflammatory monocytes were defined as CX3CR1loCCR2+GR1+15. This population of murine monocytes shares morphologic characteristics and chemokine receptor expression patterns with the classical human CD14hiCD16− monocytes, whereas murine resident monocytes are thought to correspond to human CD14+CD16+ nonclassical monocytes.38 Classical human monocytes are potent phagocytes39 and produce higher amounts of cytokines like IL-6 and TNF.40,41 In contrast, nonclassical monocytes are potent antigen-presenting cells.42 Such distinct differences have not yet been described for the murine equivalent. We demonstrate here that the number of Gr1+ monocytes in the air pouch fluid is closely correlated with the concentration of IL-6 and TNF in the air pouch fluid, thereby suggesting that the inflammatory monocytes are the predominant source of cytokines. In addition, we show that Gr1+ monocytes phagocytose bacteria more effectively, yielding a powerful contribution to pathogen clearance. Increased cytokine release and bacterial phagocytosis, however, may also be related to the activation of monocytes by PMN-sec products. In this respect, several PMN granule proteins have been shown to induce cytokine release and phagocytosis.25,43,44 The concerted action of PMN granule proteins to mobilize inflammatory monocytes and to directly enhance their antimicrobial activity stands out as an important mechanism boosting the early immune response.
The PMN granule proteins HBP, LL-37, and cathepsin G are potent multifunctional mediators of immune reactions.13,45,46 All 3 proteins were previously shown to have monocyte-activating properties. We have earlier demonstrated that HBP activates monocytes via β2-integrins, leading to Ca2+ mobilization,47 monocyte adhesion,27 and the release of cytokines (O.S., H.H., L.L., manuscript submitted April 2008). However, the chemotactic effect of HBP is Gi-protein-dependent and therefore unlikely to be mediated via CD11/CD18. Cathepsin G and HBP share high homology,48 and cathepsin G mediates its chemotactic effect via FPR1,11 whose murine ortholog is fpr1. The formyl peptide receptors constitutes a promiscuous subfamily of the G-protein–coupled receptors. They are characterized by activation through a large variety of ligands and redundancy with other chemotactic receptors.49 The functional relevance of fpr in the recruitment of monocytes has earlier been shown by fpr-blockage, resulting in attenuated monocyte infiltration into the lung of mice infected with Streptococcus pneumoniae.4 Moreover, mice lacking fpr1 exhibit higher susceptibility for L monocytogenes infections.50 However, monocyte recruitment in fpr1-deficient mice has not yet been investigated, nor are data available for the recently described mice lacking fpr2.51 Here we show that the chemotactic effect of cathepsin G and HBP is primarily mediated via fpr. LL-37 and the murine homologue CRAMP were shown to exert their monocyte-attracting activity via FPRL1 in the human and fpr2 in the mouse, respectively.11,12 Data from our study indicate that HBP and LL-37 act in concert to initiate extravasation of inflammatory monocytes, much of which is mediated via fpr. Human FPR and FPRL1 are expressed on PMN as well as monocytes.49 Here we show the expression of their murine orthologs fpr1 and fpr2 on inflammatory monocytes. Taken together, our data indicate the involvement of fpr in the switch from PMN to monocyte infiltration with selectivity for inflammatory monocytes.
Accumulation of inflammatory monocytes in atherosclerosis has recently been demonstrated to depend on CCR2 and CX3CR1.18 However, mice lacking CX3CR1 do not have a distinct phenotype, and monocyte recruitment in thioglycollate-induced peritonitis is not impaired. Moreover, CX3CR1 is not required for responses to contact sensitizers, peripheral nerve injury, or challenge with Toxoplasma gondii.52 In our study, recruitment of inflammatory monocytes in response to PMN granule proteins is dependent on CCR2 but not CX3CR1. The difference between our observations and the results from Tacke et al18 may be related to atherosclerosis as a chronic inflammation as opposed to the models of acute inflammation used in our study. We suspected a direct interaction between PMN granule proteins and CCR2 as a possible mechanism for the selective recruitment of inflammatory monocytes. However, given the conserved Ca2+-mobilization in inflammatory CCR2−/− monocytes in response to PMN-sec this seems less likely. At this stage, it is unclear how CCR2 contributes to PMN-mediated recruitment of inflammatory monocytes. However, our data indicate that both CCR2 and the PMN granule proteins LL-37 and HBP are indispensable and sequentially used in the early recruitment of inflammatory monocytes. Our observation on the lack of involvement of CX3CR1 in recruitment of inflammatory monocytes is supported by recent data showing that CX3CR1 is used by resident monocytes patrolling healthy tissue to emigrate at sites of injury.53 In the very same study,53 and in an earlier study by Henderson et al,54 it is suggested that certain monocyte subsets emigrate independently of PMN and their granule proteins. However, both studies rely on observations in a peritonitis model where the presence of resident macrophages may allow for mechanisms that bypass the PMN as a door opener for monocytes.
Disorders with neutrophil dysfunction are usually characterized by recurrent infections. These are not only attributable to the lack of PMN but also a secondary defect in monocyte function. In the case of the specific granule deficiency and the Papillon-Lefèvre syndrome, PMN lack certain granule proteins and are deficient in accumulating at sites of inflammation.7,34,55,56 For both syndromes, a reduction in monocyte accumulation at the site of inflammation has been reported in human and in mice. In addition, our data using DPPI−/− mice indicate that recruitment of inflammatory monocytes is impaired in these mice. This may be due in part to the reduced PMN extravasation and the lack of primary granule constituents in PMN of these mice. Moreover, the active form of the secondary granule protein LL-37 (or its murine homologue CRAMP) may not be processed in these animals because of the lack of proteinase-3, which is needed to liberate LL-37 from its proform.57 This may contribute to the reduced recruitment of inflammatory monocytes in DPPI−/− mice with intact WBC. Taken together, these data further emphasize the importance of PMN and their granule proteins in regulating the immune response in a clinical situation.
Our study suggests an essential role of the primary PMN extravasation for the subsequent recruitment of inflammatory monocytes. We demonstrate that the early recruitment of inflammatory monocytes by PMN is linked to the rapid discharge and deposition of PMN granule proteins LL-37 and HBP. At later time points, other mechanisms, such as release of IL-6R35 or chemokines,36,37 may come into play. The granule proteins HBP and LL-37 use monocytic fprs, thereby initiating recruitment of these cells to the inflamed locus. Functionally, the proposed PMN-monocyte axis boosts the immune response and yields a more powerful pathogen clearance.
Acknowledgments
We thank Dr F. Tacke for advice with monocyte labeling and M. Heidenholm for excellent technical assistance. We would like to acknowledge Dr S. Jung and Dr D. R. Littman for CX3CR1eGFP/eGFP mice and Drs B. Agerberth, H. Flodgaard, and O. E. Sorensen for generous gifts of reagents.
This study was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, the AFA Health Fund, the Torsten and Ragnar Söderbergs Foundations, the Deutsche Forschungsgemeinschaft (FOR809, WE1913/7-2+10-1, ZE827/1-1) and the Lars Hierta Memorial Fund. O.S. is a recipient of a postdoctoral grant from the Deutsche Forschungsgemeinschaft (SO 876/1-1).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: O.S., A.Z., H.H., K.B., and M.E.R. performed experiments. O.S., A.Z., and L.L. analyzed results. O.S., E.E.E, C.W., and L.L. designed the study and wrote the manuscript. E.E.E., A.G.R., A.Z., and C.T.P. contributed mouse strains and essential reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oliver Soehnlein, Department of Physiology and Pharmacology, Karolinska Institute, S-171 77 Stockholm, Sweden; e-mail: oliver.sohnlein@ki.se.
References
- 1.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 2.Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest. 1988;59:200–213. [PubMed] [Google Scholar]
- 3.Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–1576. doi: 10.2741/1904. [DOI] [PubMed] [Google Scholar]
- 4.Fillion I, Ouellet N, Simard M, et al. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J Immunol. 2001;166:7353–7361. doi: 10.4049/jimmunol.166.12.7353. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Stohlman SA, Hinton DR, Marten NW. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J Immunol. 2003;170:3331–3336. doi: 10.4049/jimmunol.170.6.3331. [DOI] [PubMed] [Google Scholar]
- 6.Antony VB, Sahn SA, Antony AC, Repine JE. Bacillus Calmette-Guerin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest. 1985;76:1514–1521. doi: 10.1172/JCI112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallin JI, Fletcher MP, Seligmann BE, et al. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59:1317–1329. [PubMed] [Google Scholar]
- 8.Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 11.Sun R, Iribarren P, Zhang N, et al. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J Leukoc Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 14.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacke F, Ginhoux F, Jakubzick C, et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- 22.Werr J, Johansson J, Eriksson EE, et al. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- 23.Faurschou M, Sørensen OE, Johnsen AH, Askaa J, Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim Biophys Acta. 2002;1591:29–35. doi: 10.1016/s0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen PB, Bjørn S, Hastrup S, et al. Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett. 1996;390:109–112. doi: 10.1016/0014-5793(96)00639-4. [DOI] [PubMed] [Google Scholar]
- 25.Soehnlein O, Kenne E, Rotzius P, Eriksson EE, Lindbom L. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin Exp Immunol. 2008;151:139–145. doi: 10.1111/j.1365-2249.2007.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmeisser A, Soehnlein O, Illmer T, et al. ACE inhibition lowers angiotensin II-induced chemokine expression by reduction of NF-kappaB activity and AT1 receptor expression. Biochem Biophys Res Commun. 2004;325:532–540. doi: 10.1016/j.bbrc.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Soehnlein O, Xie X, Ulbrich H, et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–6405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 28.Kjeldsen L, Sengelov H, Borregaard N. Subcellular fractionation of human neutrophils on Percoll density gradients. J Immunol Methods. 1999;232:131–143. doi: 10.1016/s0022-1759(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 29.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 31.Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta(2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med. 2000;191:1829–1839. doi: 10.1084/jem.191.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walzog B, Seifert R, Zakrzewicz A, Gaehtgens P, Ley K. Cross-linking of CD18 in human neutrophils induces an increase of intracellular free Ca2+, exocytosis of azurophilic granules, quantitative up-regulation of CD18, shedding of L-selectin, and actin polymerization. J Leukoc Biol. 1994;56:625–635. doi: 10.1002/jlb.56.5.625. [DOI] [PubMed] [Google Scholar]
- 33.Soehnlein O, Oehmcke S, Ma X, et al. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J. doi: 10.1183/09031936.00173207. In press. [DOI] [PubMed] [Google Scholar]
- 34.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalaris A, Rabe B, Paliga K, et al. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 36.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 37.Weber C, Koenen RR. Fine-tuning leukocyte responses: towards a chemokine ‘interactome’. Trends Immunol. 2006;27:268–273. doi: 10.1016/j.it.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 39.Grage-Griebenow E, Flad HD, Ernst M, et al. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology. 2000;202:42–50. doi: 10.1016/S0171-2985(00)80051-0. [DOI] [PubMed] [Google Scholar]
- 40.Szabo G, Miller-Graziano CL, Wu JY, Takayama T, Kodys K. Differential tumor necrosis factor production by human monocyte subsets. J Leukoc Biol. 1990;47:206–216. doi: 10.1002/jlb.47.3.206. [DOI] [PubMed] [Google Scholar]
- 41.Schinkel C, Sendtner R, Zimmer S, Faist E. Functional analysis of monocyte subsets in surgical sepsis. J Trauma. 1998;44:743–748. doi: 10.1097/00005373-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Grage-Griebenow E, Lorenzen D, Fetting R, Flad HD, Ernst M. Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol. 1993;23:3126–3135. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- 43.Chaly YV, Paleolog EM, Kolesnikova TS, et al. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 44.Heinzelmann M, Platz A, Flodgaard H, Polk HC, Jr, Miller FN. Endocytosis of heparin-binding protein (CAP37) is essential for the enhancement of lipopolysaccharide-induced TNF-alpha production in human monocytes. J Immunol. 1999;162:4240–4245. [PubMed] [Google Scholar]
- 45.Pereira HA. CAP37, a neutrophil-derived multifunctional inflammatory mediator. J Leukoc Biol. 1995;57:805–812. doi: 10.1002/jlb.57.6.805. [DOI] [PubMed] [Google Scholar]
- 46.Chertov O, Yang D, Howard OM, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68–78. doi: 10.1034/j.1600-065x.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- 47.Påhlman LI, Mörgelin M, Eckert J, et al. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177:1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- 48.Wilde CG, Snable JL, Griffith JE, Scott RW. Characterization of two azurphil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990;265:2038–2041. [PubMed] [Google Scholar]
- 49.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao JL, Guillabert A, Hu J, et al. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J Immunol. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 52.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 54.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 55.Gombart AF, Koeffler HP. Neutrophil specific granule deficiency and mutations in the gene encoding transcription factor C/EBP(epsilon). Curr Opin Hematol. 2002;9:36–42. doi: 10.1097/00062752-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Verbeek W, Lekstrom-Himes J, Park DJ, et al. Myeloid transcription factor C/EBPepsilon is involved in the positive regulation of lactoferrin gene expression in neutrophils. Blood. 1999;94:3141–3150. [PubMed] [Google Scholar]
- 57.Sørensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.