Interest is reviving in the role of antibodies in autoimmunity. Over the last two decades, T cells occupied center stage for several reasons: They predominate within tissues undergoing autoimmune attack, they can transfer autoimmune disease in animal models, and diseases such as rheumatoid arthritis and insulin-dependent diabetes mellitus associate with particular MHC class II alleles (1–3). It is true that therapy aimed specifically at T cells has proved disappointing (1, 2), but this disappointment has been made up for by the impressive success of clinical trials in rheumatoid arthritis of inhibitors of tumor necrosis factor (4). Tumor necrosis factor is a proinflammatory cytokine that is produced by macrophages under the influence of T cells.
The antibodies that characterize autoimmune diseases have long been the bread-and-butter of clinical immunology as disease markers. They also have received limited support over the years as candidate causal agents (5, 6). The revival of interest in B cells as contributors to the cause of autoimmunity stems from recent progress concerning their mechanism of activation, the genetics of autoimmunity, and the application of anti-B-cell therapy. Thus, the membrane protein complex CD19/CD21 has been found to couple the innate immune recognition of microbial antigens by the complement system to the activation of B cells (7). CD21 binds the C3d fragment of activated C3 that becomes covalently attached to targets of complement activation, and CD19 costimulates signaling through the antigen receptor, membrane Ig. CD21 also is expressed by follicular dendritic cells and mediates the long-term retention of antigen that is required for the maintenance of memory B cells. C1q deficiency is a strong predisposing factor for systemic lupus erythematosus, and studies on knockout mice indicate that this major component of complement may be required not only to help mop up leaked autoantigens (as has long been thought) but also to regulate apoptosis (8). The genetics of systemic lupus erythematosus also implicate the Fc-γ receptor type IIA (9).
B cells clearly are needed for development of collagen-induced arthritis in mice: For instance, denatured collagen does not induce the disease. Crosses with naturally C5-deficient mice indicate that an intact C5 gene is required for the disease to develop (10). Furthermore, substitution in the MHC of a class II allele that suppresses the early burst of IL-4 production partially protects against the disease, in accordance with the need for this early burst as demonstrated by treatment of mice with anti-IL-4 monoclonal antibody (11, 12).
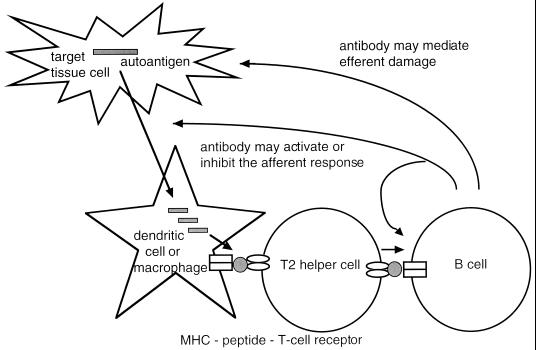
All of this new work implicates B cells in the afferent phase of the autoimmune response, as shown in Fig. 1. The question remains whether they also participate in the effector phase, as they have long been known to do in the transplantation response (13). A striking example of effector activity recently has been described in a mouse strain transgenic for a T cell receptor that recognizes a ubiquitously expressed self-antigen (14). In this system, the Mathis group (14) found that mice develop an arthritis that is driven almost entirely by immunoglobulins. The target of both the initiating T cells and the pathogenic immunoglobulins was identified as glucose 6-phosphate isomerase, a glycolytic enzyme.
Figure 1.
Antibody may activate the afferent arm of the immune response and also may mediate efferent tissue damage. A representative autoantigen and a peptide epitope derived from it are shaded gray.
A third line of evidence emerges from the introduction of a humanized anti-CD20 monoclonal antibody for therapeutic use (15). In one published instance (16) and in several others (J. C. Edwards, personal communication), treatment with this reagent markedly reduced the symptoms of rheumatoid arthritis.
The two papers that appear in this issue of PNAS elegantly substantiate this view. Nagaraju et al. (17) investigate the self-sustaining autoimmune myositis that develops on conditional up-regulation of an MHC class I gene in skeletal muscle. This development is accompanied by autoantibodies, including, in some mice, antibodies to histidyl-tRNA synthetase as is characteristic of myositis in man. As Nagaraju et al. point out, this finding parallels the system of Mathis and colleagues (14) referred to above and again shows that a nonspecific stimulus can give rise to a highly specific pattern of autoimmune disease. What has yet to be evaluated is the role of these anti-HTR antibodies in pathogenesis. They may turn out to be yet another example of an antibody as a marker rather than as a causal agent, particularly because myositis in man may be driven primarily by CD8 T cells (18).
Ditzel et al. (19) describe an antibody/antigen pair, again citing the Mathis and colleagues' system. The points of interest in their study lie not so much in the possibility—at present only circumstantial—that the antibody may mediate pathogenesis, but rather in the approach that they have taken to making the human monoclonal antibody, and the way in which it has been used to identify the antigen. In addition, the disease with which the antibody is associated, Felty's syndrome, is an extra-articular manifestation of rheumatoid arthritis that has several immunological features of interest. They prepared an antibody phage display library from RNA taken from patients' bone marrow lymphocytes and screened it by panning on fresh neutrophils and on a fixed cell line (antibodies to neutrophils are characteristic of the disease). The resulting antibody was then screened on a phage cDNA library, and the eukaryotic elongation factor eEF1A-1 thus was identified as the target antigen. The majority of patients with Felty's syndrome proved to have elevated levels of such antibodies.
Clearly the exact role in pathogenesis of such antibodies remains to be established. It is likely that B cell ablating therapies, such as that referred to above, will play an important part in this quest. If these and other approaches look promising, the time may come to reopen the long dormant possibility of inducing peripheral B cell tolerance (20).
Acknowledgments
Our laboratories are supported by the Wellcome Trust.
Footnotes
References
- 1.McDevitt H O. Curr Opin Immunol. 1998;10:677–681. doi: 10.1016/s0952-7915(98)80088-5. [DOI] [PubMed] [Google Scholar]
- 2.Panayi G S. Rheumatology (Oxford) 1999;38, Suppl. 2:8–10. [PubMed] [Google Scholar]
- 3.Kadowaki K M, Matsuno H, Tsuji H, Tunru I. Clin Exp Immunol. 1994;97:212–218. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maini R N, Taylor P C. Annu Rev Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- 5.Roitt I M, Sumar N. Clin Exp Rheumatol. 1990;8, Suppl. 5:89–91. [PubMed] [Google Scholar]
- 6.Outschoorn I, Rowley M J, Cook A D, Mackay I R. Clin Immunol Immunopathol. 1993;66:59–66. doi: 10.1006/clin.1993.1008. [DOI] [PubMed] [Google Scholar]
- 7.Fearon D T, Carroll M C. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 8.Walport M J, Davies K A, Botto M. Immunobiology. 1998;199:265–285. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 9.Norsworthy P, Theodoridis E, Botto M, Athanassiou P, Beynon H, Gordon C, Isenberg D, Walport M J, Davies K A. Arthritis Rheum. 1999;42:1828–1832. doi: 10.1002/1529-0131(199909)42:9<1828::AID-ANR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Mori L, de Libero G. Arthritis Rheum. 1998;41:256–262. doi: 10.1002/1529-0131(199802)41:2<256::AID-ART9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Brunner M, Larsen S, Sette A, Mitchison A. Eur J Immunol. 1995;25:3285–3289. doi: 10.1002/eji.1830251213. [DOI] [PubMed] [Google Scholar]
- 12.Hesse M, Bayrak S, Mitchison A. Eur J Immunol. 1996;26:3234–3237. doi: 10.1002/eji.1830261259. [DOI] [PubMed] [Google Scholar]
- 13.Grauhan O, Muller J, v. Baeyer H, Volk H D, Fietze E, Cohnert T, Meyer R, Pfitzmann R, Mansfeld H, Siniawski H, et al. J Heart Lung Transplant. 1998;17:1184–1194. [PubMed] [Google Scholar]
- 14.Matsumoto I, Staub A, Benoist C, Mathis D. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 15.Gopal A K, Press O W. J Lab Clin Med. 1999;134:445–450. doi: 10.1016/s0022-2143(99)90164-6. [DOI] [PubMed] [Google Scholar]
- 16.Protheroe A, Edwards J C, Simmons A, Maclennan K, Selby P. Rheumatology (Oxford) 1999;38:1150–1152. doi: 10.1093/rheumatology/38.11.1150. [DOI] [PubMed] [Google Scholar]
- 17.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon P J, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, et al. Proc Natl Acad Sci USA. 2000;97:9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohlfeld R, Engel A G, Goebels N, Behrens L. Curr Opin Rheumatol. 1997;9:520–526. doi: 10.1097/00002281-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ditzel H J, Masaki Y, Nielsen H, Farnaes L, Burton D R. Proc Natl Acad Sci USA. 2000;97:9234–9239. doi: 10.1073/pnas.97.16.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard J G, Mitchison N A. Prog Allergy. 1975;18:43–96. doi: 10.1159/000395256. [DOI] [PubMed] [Google Scholar]