Abstract
Background
Bacteria play critical roles in marine nutrient cycles by incorporating and redistributing dissolved organic matter (DOM) and inorganic nutrients in the ocean. TonB-dependent transporter (TBDT) proteins allow Gram-negative bacteria to take up scarce resources from nutrient-limiting environments as well as siderophores, heme, vitamin B12, and recently identified carbohydrates. Thus, the characterization of TBDT distribution and functions is essential to better understand the contribution TBDT to DOM assimilation and its consequences on nutrient cycling in the environment.
Methodology/Principal Findings
This study presents the distribution of encoded known and putative TBDT proteins in the genomes of microorganisms and from the Global Ocean Survey data. Using a Lek clustering algorithm and substrate specificities, the TBDT sequences were mainly classified into the following three groups: (1) DOM transporters; (2) Siderophores/Vitamins transporters; and (3) Heme/Hemophores/Iron(heme)-binding protein transporters. Diverse TBDTs were found in the genomes of oligotroph Citromicrobium bathyomarinum JL354 and Citromicrobium sp JLT1363 and were highly expressed in the stationary phase of bacterial growth. The results show that the Gammaproteobacteria and the Cytophaga-Flavobacterium-Bacteroides (CFB) group bacteria accounted for the majority of the TBDT gene pool in marine surface waters.
Conclusions/Significance
The results of this study confirm the ecological importance of TBDTs in DOM assimilation for bacteria in marine environments owing to a wide range of substrate utilization potential in the ubiquitous Gammaproteobacteria and CFB group bacteria.
Introduction
Bacteria play important roles in ocean carbon and nutrient cycling by incorporating and redistributing dissolved organic matter (DOM) and inorganic nutrients [1], [2]. Transport proteins are a primary mechanism in inorganic nutrient uptake and DOM assimilation in microbial cells. Therefore, the identification and characterization of transport proteins may be important towards understanding a broad range of DOM molecules available for assimilation and utilization by microbes.
Marine bacteria require various efficient transport systems to successfully sequester essential nutrients from ocean environments with limited resources. Bacterial transporters are frequently observed in microbial metatranscriptome and metaproteome data [3]–[8]. Majority of the transporters detected in marine environments include ATP-binding cassette (ABC) transporters [3]–[5], tripartite ATP-independent periplasmic (TRAP) transporters [4], phosphotransferase system (PTS) transporters [4], and TonB-dependent transporters (TBDTs) [6]–[8]. ABC transporters are ubiquitous in bacteria and function in the import of growth substrates or factors, including carbohydrates, amino acids, polypeptides, vitamins, and metal-chelate complexes [9]. ABC transporters are considered as good indicators for bacterial DOM utilization patterns [10]. PTS is another important mechanism used by bacteria for carbohydrate uptake [11], and TRAP transporters transport C4-dicarboxylates malate, succinate, and fumarate [12]. Moreover, TBDT in the bacterial outer membrane often promotes the transport of rare nutrients and is known for its high-affinity uptake of iron complexes (siderophores, citrate, heme, hemoglobin, transferrin, and lactoferrin) [13], [14] as well as of vitamin B12 [15], vitamin B1 [16], and metals (nickel) [17]. In addition, a TBDT can combine with a TonB-ExbB-ExbD system and a specific ABC transporter system to complete the transfer of siderophores or vitamin B12 across the cytoplasmic membrane [18], [19]. Interestingly, several TBDTs are well characterized and are able to access a wide variety of substrates [19]–[24]. Experimental data reveal that carbohydrates, amino acid, and organic acid are TonB-dependent substrates [20]–[24]. For example, TBDTs were proposed to participate in the transport of macromolecules, such as alginate and N-acetyl-chitinoligosaccharides, as observed for Xanthomonas bacteria [20], [21], Sphingomonas sp. strain A1 [22], and Caulobacter crescentus [23]. Sphingomonadales organisms are well known for their growth in oligotrophic marine waters and for their ability to degrade aromatic compounds [25]. Sphingomonas wittichii RW1 affiliated with Sphingomonadales currently contains the most TBDTs among all microorganisms [25]. These TBDTs are used by bacteria for uptake of diverse substrates, including aromatic compounds [25]. The statements above clearly indicate that TBDTs are involved in DOM uptake.
TBDT sequences exhibited low sequence similarity with one another, indicating an unexpectedly high diversity within TBDT sequences. The accounts of the TBDT system in different bacteria were summarized based on bioinformatic analyses [14], [26], [27], and previous research identified the functions of few TBDTs in cyanobacteria and nitrogen-fixing nodulating bacteria by using CLANS [28]. The siderophores-related TBDTs in marine bacteria and metagenomics have recently been analyzed using OrthoMCL [29]. However, previous attempts on the genomic analysis of TBDT were mainly focused on a rather narrow group of species [26], [27] or on a particular subfamily of TBDTs, such as siderophore transporters [14]. A complete functional characterization of TBDT has not been performed to date.
This study performs a comprehensive survey of TBDT in all available bacterial genomes and in the Global Ocean Survey (GOS) metagenome [30]. The predicted TBDTs were classified into groups according to their pair-wise sequence similarities and substrate type. The prediction of substrates for the selected TBDTs was conducted using gene context analysis. Majority of the TBDT sequences in the metagenomic data sets originated from the Gammaproteobacteria and Cytophaga-Flavobacterium-Bacteroides (CFB) group bacteria. TBDTs for siderophores and DOM transportation were present in the Sphingomonadales organisms as well. The genomes of two marine bacteria, namely, the oligotroph Citromicrobium bathyomarinum JL354 [31] and Citromicrobium sp. JLT1363 [32] from the order Sphingomonadales, were sequenced and annotated, and TBDTs were found to be abundant in their genomes. Moreover, the protein expression profiles of cells show that TBDTs were involved in coping with the low levels of nutrients during the stationary phase. The overall findings have advanced the understanding of the role of TBDTs in oceanic organic matter and nutrient cycles.
Results
Distribution of Transporters in Bacterial Genomes
TBDT genes are distributed among a variety of divergent bacterial taxa, including Proteobacteria, Cyanobacteria, Verrucomicrobia, and Spirochetes. Overall, approximately 36% of the draft and completed genomes from NCBI (3,274 genomes) contain TBDTs. The number of TBDT genes in various bacteria is presented in Table S1. This study primarily focuses on TBDTs in marine bacteria, and thus, species were included such that all the major groups of bacteria are covered. As described in Table S2, 68% of the 174 analyzed genomes contained 1–15 TBDTs and 14% contained more than 30 TBDTs. The other transporters of the representatives of the major bacterial groups, including members of the Sphingomonadales, SAR11, the Roseobacter clade, Gammaproteobacteria, CFB and cyanobacteria, which are distributed in the surface marine waters [33], were investigated further (Table 1).
Table 1. Comparison of substrate dependent transporters among selected marine bacteria.
| Organism | Taxonomic affiliation | Genome Size (Mb) | Total number of ORFs | TBDT* | ABC transporter† | PTS | TRAP | Trophic strategy‡ |
| Citromicrobium bathyomarinum JL354 | Alphaproteobacteria-Sphingomonadales | 3.27 | 3235 | 27 (8) | 22(7) | 1 | 0 | N/A |
| Citromicrobium sp. JLT1363 | Alphaproteobacteria-Sphingomonadales | 3.12 | 3003 | 31(10) | 25 (8) | 1 | 1 | N/A |
| Erythrobacter litoralis HTCC2594 | Alphaproteobacteria-Sphingomonadales | 3.05 | 3011 | 18 (6) | 23 (8) | 3 | 0 | extreme oligotrophs |
| Sphingomonas wittichii RW1 | Alphaproteobacteria-Sphingomonadales | 5.92 | 4850 | 134 (23) | 34 (6) | 4 | 0 | extreme oligotrophs |
| Roseobacter denitrificans OCh 114 | Alphaproteobacteria-Roseobacter clade | 4.32 | 4129 | 1 (0) | 110 (25) | 2 | 22 | extreme oligotrophs |
| Dinoroseobacter shibae DFL 12 | Alphaproteobacteria-Roseobacter clade | 4.42 | 4192 | 3 (1) | 86 (19) | 4 | 28 | extreme oligotrophs |
| Ruegeria pomeroyi DSS-3 | Alphaproteobacteria-Roseobacter clade | 4.6 | 4252 | 0 (0) | 102 (22) | 4 | 26 | moderate copiotrophs |
| Candidatus Pelagibacter ubique HTCC1062 | Alphaproteobacteria-SAR11 clade | 1.31 | 1354 | 0 (0) | 24 (18) | 0 | 3 | extreme oligotrophs |
| Candidatus Pelagibacter ubique HTCC1002 | Alphaproteobacteria-SAR11 clade | 1.33 | 1393 | 0 (0) | 23 (17) | 0 | 2 | extreme oligotrophs |
| Rhodopseudomonas palustris CGA009 | Alphaproteobacteria | 5.46 | 4820 | 17 (3) | 117 (21) | 5 | 8 | moderate oligotrophs |
| Prochlorococcus marinus str. MIT 9202 | Cyanobacteria | 1.69 | 1890 | 1 (1) | 40 (24) | 0 | 0 | extreme oligotrophs |
| Prochlorococcus marinus str. MIT 9301 | Cyanobacteria | 1.64 | 1906 | 0 (0) | 33 (20) | 0 | 0 | extreme oligotrophs |
| Synechococcus sp. WH 7803 | Cyanobacteria | 2.37 | 2533 | 0 (0) | 47 (20) | 0 | 0 | extreme oligotrophs |
| Synechococcus sp. WH 8102 | Cyanobacteria | 2.43 | 2519 | 0 (0) | 50 (21) | 0 | 0 | extreme oligotrophs |
| Synechococcus sp. PCC 7002 | Cyanobacteria | 3.41 | 3187 | 6 (2) | 78 (23) | 0 | 0 | extreme oligotrophs |
| Cytophaga hutchinsonii ATCC 33406 | CFB group bacteria | 4.43 | 3785 | 5 (1) | 42 (9) | 0 | 0 | moderate copiotrophs |
| Flavobacteria bacterium BBFL7 | CFB group bacteria | 3.08 | 2592 | 10 (3) | 32 (10) | 0 | 1 | moderate copiotrophs |
| Flavobacterium johnsoniae UW101 | CFB group bacteria | 6.1 | 5017 | 47 (8) | 41 (7) | 0 | 0 | moderate copiotrophs |
| Flavobacteria bacterium MS024-3C | CFB group bacteria | 1.52 | 1384 | 7(5) | 16(11) | 0 | 0 | N/A |
| Polaribacter sp. MED152 | CFB group bacteria | 2.97 | 2611 | 10(3) | 22(7) | 0 | 0 | N/A |
| Salinibacter ruber DSM 13855 | CFB group bacteria | 3.59 | 2801 | 16 (4) | 59 (16) | 0 | 3 | moderate copiotrophs |
| gamma proteobacterium HTCC2207 | Gammaproteobacteria-SAR92 clade | 2.62 | 2388 | 17 (6) | 21 (8) | 2 | 1 | extreme oligotrophs |
| gamma proteobacterium NOR5-3 | Gammaproteobacteria-NOR5/OM60 clade | 4.20 | 3671 | 27 (6) | 37 (9) | 2 | 2 | extreme oligotrophs |
| gamma proteobacterium HTCC2143 | Gammaproteobacteria-BD1-7 clade | 3.93 | 3662 | 22 (6) | 39 (10) | 2 | 2 | extreme oligotrophs |
| gamma proteobacterium HTCC2148 | Gammaproteobacteria-NOR5/OM60 clade | 4.31 | 3827 | 37 (9) | 49 (11) | 2 | 2 | extreme oligotrophs |
| gamma proteobacterium HTCC2080 | Gammaproteobacteria-NOR5/OM60 clade | 3.58 | 3185 | 28 (8) | 17 (5) | 2 | 1 | extreme oligotrophs |
| Congregibacter litoralis KT71 | Gammaproteobacteria-NOR5/OM60 clade | 4.33 | 3941 | 26 (6) | 27 (6) | 2 | 2 | extreme oligotrophs |
| Pseudoalteromonas haloplanktis TAC125 | Gammaproteobacteria-Alteromonadales | 3.85 | 3485 | 34 (9) | 29 (8) | 4 | 0 | moderate copiotrophs |
| Idiomarina loihiensis L2TR | Gammaproteobacteria-Alteromonadales | 2.84 | 2628 | 28 (10) | 29 (10) | 4 | 0 | moderate copiotrophs |
| Shewanella baltica OS185 | Gammaproteobacteria-Alteromonadales | 5.31 | 4394 | 33 (6) | 53 (10) | 7 | 3 | extreme copiotrophs |
| Alteromonadales bacterium TW-7 | Gammaproteobacteria-Alteromonadales | 4.10 | 3783 | 32 (8) | 42 (10) | 4 | 0 | N/A |
| gamma proteobacterium NOR51-B | Gammaproteobacteria | 3.26 | 2930 | 32(10) | 44(13) | 4 | 3 | N/A |
| Nitrosococcus oceani ATCC19707 | Gammaproteobacteria | 3.52 | 3017 | 7 (2) | 34 (10) | 5 | 1 | moderate oligotrophs |
| Vibrio vulnificus YJ016 | Gammaproteobacteria | 5.21 | 5098 | 7 (1) | 95 (18) | 24 | 9 | extreme copiotrophs |
| Photobacterium profundum SS9 | Gammaproteobacteria | 6.40 | 5489 | 7 (1) | 84 (13) | 5 | 10 | extreme copiotrophs |
| Acinetobacter baumannii ATCC17978 | Gammaproteobacteria-Acinetobacter | 4.00 | 3367 | 22(6) | 34(9) | 2 | 0 | moderate oligotrophs |
| Acinetobacter sp. ADP1 | Gammaproteobacteria-Acinetobacter | 3.60 | 3307 | 22(6) | 38(11) | 3 | 0 | moderate oligotrophs |
| Nitrosomonas europaea ATCC 19718 | Betaproteobacteria | 2.81 | 2461 | 30 (11) | 43 (15) | 4 | 0 | moderate oligotrophs |
| Ralstonia eutropha H16 | Betaproteobacteria | 4.05 | 3651 | 17 (4) | 65 (16) | 6 | 5 | moderate copiotrophs |
Abbreviations of transporters are: TBDT, TonB-dependent transporter; PTS, phosphotransferase system; TRAP, tripartite ATP-independent periplasmic protein.
The number of transporters per Mbp of each genome is shown in bracket(s).
The ABC transporters related multiple domains encode as one polypeptide.
Information from reference [34].
N/A means no data.
TBDT sequences appeared to be highly prevalent in the orders Sphingomonadales, Alteromonadales, in the CFB group bacteria, and in the oligotrophic marine Gammaproteobacteria (OMG) group, which includes members of the BD1-7, SAR92, and OM60/NOR5 clades (Table 1). Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363 contain 27 and 31 TBDT sequences in their genomes. Except for strain Ruegeria pomeroyi DSS-3, which lacked TBDT sequences in the genome, all the other analyzed Roseobacter strains carried one to five TBDT sequences (Table S1). The genes for TBDT were also present in several cyanobacterial strains, and not all marine bacteria contained TBDT. For example, Candidatus Pelagibacter ubique, which is the most abundant prokaryote in the SAR11 clade, did not contain TBDT.
However, ABC transporters were recognized in all the analyzed genomes, indicating that they are absolutely essential for substrate transport in bacteria. The number of ABC transporter genes ranged from 22 to 117 genes per genome (Table 1), and the number of ABC transporters was higher than that of TBDTs in most bacteria. No clear correlation between the number of transporters and bacterial trophic strategy [34] or genome size was found because many copiotrophic or oligotrophic bacteria had an unusually high number of genes that encode TBDTs or ABC transporters in their genomes. However, the number of ABC transporters per Mbp of each genome was distinctly higher in some bacteria with few TBDTs. For example, a specific enrichment in the number of ABC transporters was present in the Roseobacter clade, and SAR11 and cyanobacteria had abundant ABC transporters (Table 1). A significant negative correlation between the number of ABC transporters and TBDTs per Mbp of each genome was found (Spearman rank correlation: r s = −0.677; N = 39; P<0.0001). The Roseobacter clade and Vibrio had an unusually high number of genes that encode TRAP transporters and PTS systems in their genomes, respectively. The distribution of transporter genes in bacterial genomes suggests that the use of the transporter system among bacteria had considerable differences. However, the presence of a reasonable number of transporters enabled the bacteria to use a broad range of substrates for growth.
Distribution of Transporters in Aquatic Systems
The GOS dataset used in this study contained genomic sequences derived from 57 samples from the open ocean to the coast across temperate and tropical regions as well as few non-marine aquatic samples [30], [35]. A total of 44073 putative TBDT homologs that varied in number from 65 to 3,946 were found in the samples (Table S3). The frequency of the TBDTs in the total open reading frames (ORFs) provided a rough estimate of the prevalence of TBDT in ocean waters, ranging from a low of approximately 0.1% (GS4) in the North American East Coast to a high of approximately 2% (GS18) in the Caribbean Sea Coastal (Table S3). To compare the distribution of TBDTs in diverse environmental samples, the frequency of TBDT was determined by comparing the average number of control single-copy gene hits for each site as an indicator to be used for the estimation of the total genome equivalents (see Materials and Methods ). The Caribbean Sea Coastal GS18, which has extremely high frequency, was the most abundant station. The Sargasso Sea data sets (GSb, GSc, and GS1a) and the data sets from the Galapagos Islands (warm seep GS30 and GS31) also exhibited high hits, but with moderate frequency. Moreover, the estimations of frequency and number of genes suggested that TBDTs were less common in the Indian Ocean, with the exception of GS117, GS110b, and GS122b, as well as in the coastal and estuarine stations of the North American East Coast (GS4, GS11 and GS12) and reef stations (GS49 and GS51) (Table S3). No significant relationships between the environmental factors (chlorophyll a, temperature, and salinity) and TBDT distributions were found. Moreover, a greater number of ABC transporters with extremely high frequency were found at hypersaline site GS33 than at the other sites (Table S3). The relatively high number and frequency of ABC transporter gene hits were identified in the warm seeps GS30 and GS31. The open ocean sites (Sargasso Sea GS1a, Indian Ocean GS110b, and GS112b) as well as reef station GS108b appeared to show relatively low frequency. No clear trend was observed in the type of environment in which the TBDT genes or ABC transporter genes were observed frequently. A significant positive correlation between the amounts of TBDT and ABC transporter homologs (Spearman rank correlation: r s = 0.791; N = 57; P<0.0001) seemed to exist. Most of the observed frequencies of the ABC transporter homologs in the total ORFs along the GOS transect were approximately 1% (Table S3).
Functional Characterization of TBDTs in Marine Bacterial Genomes
In this study, the Lek clustering algorithm was used to cluster the GOS sequences and NCBI sequences that contain TBDT sequences of known functions and investigate any sequence homology between TBDTs with known functions and the various putative ones (Table 2). Notably, the clustering results were not influenced by the removal of the GOS sequences.
Table 2. Functional classification of the TBDT sequences extracted from NR and the GOS datasets.
| Function categories | Lek Cluster number | TBDT* | Substrates† | NCBI sequences | GOS peptides |
| Group I: DOM transporters | Cluster 3090 | Sden_2708(91794059), CPS_1021(71281574), PSPTO_3242(28870407), XCC2385(21231822), MalA(220964479), XCC2469(21231904), MalA(16126526), NagA(13421615), XCC2828(21232259), SO_3514(24375018), CC_0446(16124701), XCC0120(21229598), XCC2944(21232375), XCC4120(21233542) XCC2887(21232318) | Chito-oligosaccharides, phytate, maltodextrin, maltose, chitin, xylan, xylose, pectin | 816 | 3107 |
| Cluster 720 | RagA(110636966, 110636973),SusC(29349110), CsuF(29348741) | Digested proteins, starch/malto-oligo-saccharides, chondroitin sulfate/hyaluronic acid | 3565 | 2060 | |
| Cluster 427 | XCC4222(21233639) | Arabinose | 945 | 5748 | |
| Cluster 952 | SuxA(21232787), Sfri_3988(114565138) | Sucrose | 53 | 4 | |
| Group II: Siderophores/Vitamins transporters | Cluster 3303 | FecA(729471, 16132112) | Ferric-citrate | 288 | 946 |
| Cluster 410 | FmtA(53719389), PupA(45723), FatA(132510), FcuA(1169655), ViuA(267356), RhtA(6685883), BfrI(33592999), Bcep18194_b2436(78063283), PupB(585759), IutA(84060860), SftP(6019468), XCC0674(21230149), CPS_0067(71281279), FhuE(16129065), PbuA(1172035), FpvA(12230910), FauA(4589285), FhuE(2507465), FptA(1169730), SO_2715(24374256), PhuR(3044098), PrhA(17549099), IutA(1170593),FctA(871032), BfrZ(6850914), FyuA(517234),BauA(49175779), Fiu(170080464), FhuA(16128143), VciA(147673813), PiuA_Fiu(115587765), FoxA(1169726), FegA(1518696),FhuA(2507464), OptS(116050410), IrpC(17380443), OrbA(76810798) | Aerobactin, alcaligin, anguibactin, catecholates, chrysobactin, coprogen, ferrioxamine B, rhodoturolic acid, desferrioxamine, ferric malleobactin, ferric ornibactin, ferrichrome, hexylsulfate, pseudobactin A, pseudobactin M114, pyochelin, pyoverdine, rhizobactin 1021, thiamin, vibriobactin, yersiniabactin | 4576 | 9462 | |
| Cluster 973 | XCC3067(21232497), PA1271(15596468), RS02718(17547119), VC0156(15640186), BtuB(416728), CC1750(109897435), BPSL0976(53718618), Saro0693(23107401), CirA(2507462,16130093), FepA(16128567), PirA(2981053), PfeA(548479), FepA(2507463), IroN(2738252), CfrA(112360090),IrgA(12644182), BfrA(1314835), BfeA(538279), RSP_2402(77462960) | Vitamin B12, catecholates,enterobactin, 2,3-dihydroxybenzoylserine(DHBS), | 1677 | 1082 | |
| Cluster 325 | SO_0815(24372404) | Vitamin B12 | 168 | 91 | |
| Cluster 180 | BF1991(53713281), BF0615(53711906), PG1899(34541505) | Fibronectin, thiamin | 113 | 1690 | |
| Cluster 2835 | GOX1347(58039791) | Thiamin | 100 | 6 | |
| Group III: Heme/Hemophores/Iron(heme)-binding transporters | Cluster 1609 | HumA(53829495) | Heme | 413 | 1432 |
| Cluster 1856 | HxuC(1170441), PfhR(4838477), HgbA(28194090), HemR(3646475,6016198), HgbA(33152990), HpuB(11386826), HasR(34787214), HmbR(687640), HmuR(2501236), ChuA(1763009), ShuA(1655877), VctA(18476494), ShuA(82778670), TbpA(150361), FrpB4(15646121), HuvA(12697532), TdhA(33151615), BLL7076(27382187), LbpA(915278), HutA(529727, 148292078), HutR(147671724) | Heme | 1244 | 515 | |
| Group IV: Metal transporters | Cluster 767 | NosA(151399), OprC(1498191,15598985), | Copper, Copper chelate | 184 | 110 |
| Cluster 987 | Bll6948(27382059), Daro_3944(71909555), RPA_4757(39937815),pHCG3_081(47177035), Daro_1684(71907314) | Nickel, Cobalt | 64 | 0 | |
| Total number of sequences clustered together with the identified substrate dependent TBDTs | 14206 | 26253 | |||
| Total number of TBDTs | 15905 | 44073 |
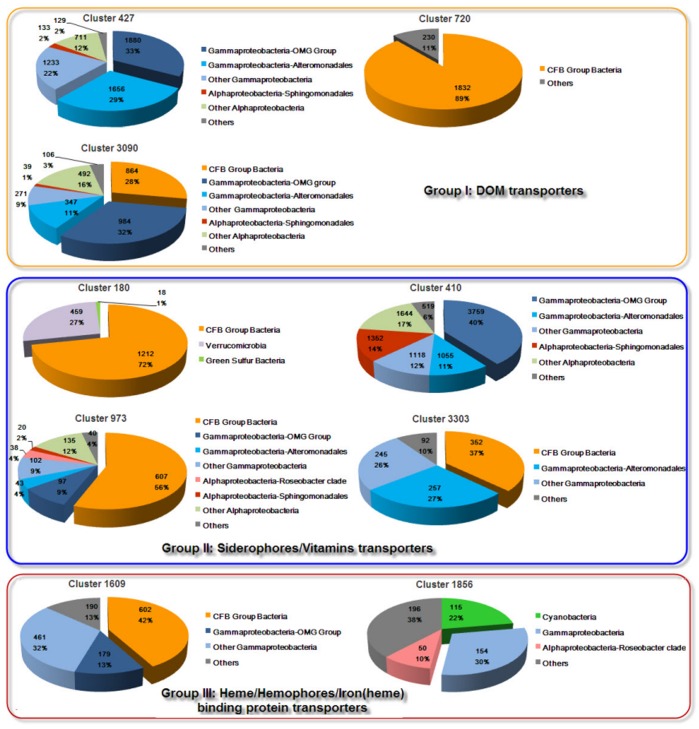
The results found 3,343 clusters of TBDTs in the bacterial and environmental sequences, in which 17 Lek clusters that contain the experimentally determined TBDT sequences were found (Table 2). The Lek clusters were subsequently classified into several groups (I to IV) according to the known substrate types (“DOM,” “siderophores/vitamins,” “heme/hemophores/iron(heme)-binding proteins,” and “metals”). Each group contained sequences from two or more Lek clusters (Table 2). Group I, which was recognized in the current analysis, consisted of novel transporters for various types of DOM, including carbohydrates, amino acids, lipids, organic acid, and protein degradation products. Members of Group II include siderophores, vitamin B1, and vitamin B12. Group III consisted of TBDTs that transport iron from heme or iron proteins with high affinity. Nickel and cobalt were the predicted substrates in Group V. The amino acid sequences of clustered siderophores-related transporters were not significantly similar to the TBDTs involved in DOM uptake. However, some of siderophores-related transporters shared some sequence homologies with the vitamin B12/vitamin B1 transporter proteins and were thus clustered together (Table 2).
TBDTs in selected marine bacterial genomes mainly functioned as transporters with diverse substrates, as summarized in Table S2. Members of clusters 427 and 3090 (DOM transporters) were mainly found in Gammaproteobacteria, including Alteromonadales (such as Alteromonadales bacterium TW-7) and the OMG group of bacteria (such as gamma proteobacterium HTCC2207), as well as in Sphingomonadales (such as Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363). Cluster 720 (DOM transporters) was specific in its distribution to species of the CFB group of bacteria. One or two corresponding genes were identified in most of the analyzed CFB species. As many as 46 and 49 genes were found in Pedobacter heparinus DSM 2366 and Pedobacter sp. BAL39, respectively (Table S2).
Members of cluster 410 (siderophore transporters) were found in the genomes of most of the species analyzed using TBDT. Majority of the siderophore transporters came from the gamma- and alpha-proteobacteria (such as the Alteromonadales, the OMG group of bacteria, and the Sphingomonadales). As many as 117 genes that encode siderophore transporters were found in Sphingomonas wittichii RW1 (Table S2).
Clusters 1609 and 1856 contained TBDT that transports iron from heme or iron-binding protein as an alternative iron source for the bacteria. The corresponding genes were identified in most of the analyzed species, and their number ranged from one to four genes per genome (Table S2). TBDTs in the Roseobacter clade and cyanobacteria were mainly distributed in clusters 410 and 1856, suggesting that they were responsible for iron acquisition in the bacteria. The members of the last group (clusters 767 and 987) remained as few sequences, indicating that nickel- or cobalt-specific TBDTs were not common among the bacteria (Table S2).
Functional Characterization of TBDTs in Metagenomes
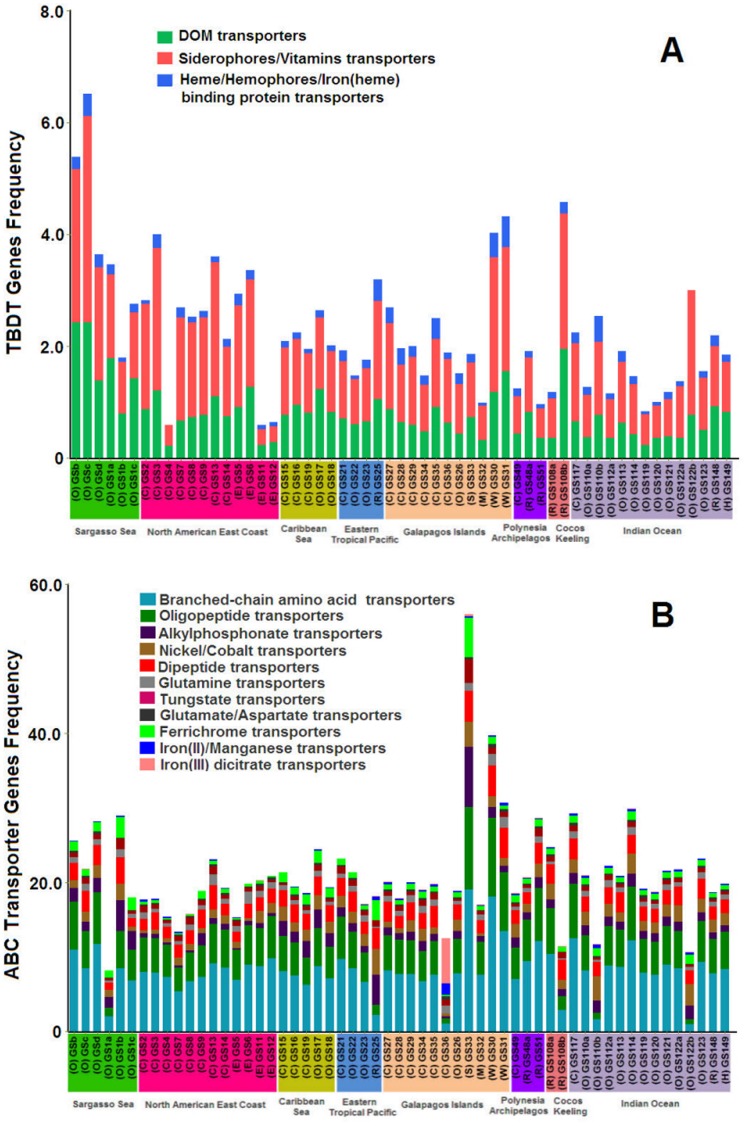
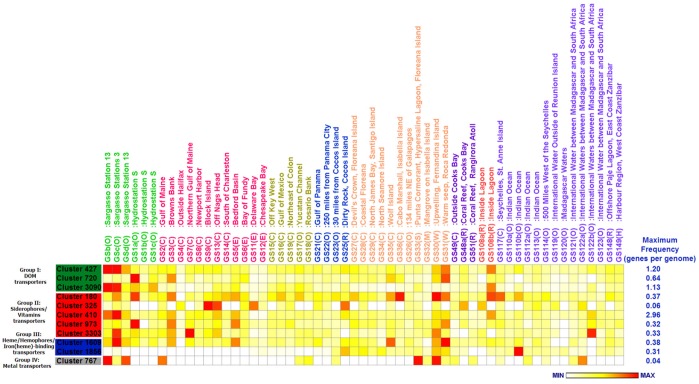
Similarly, the TBDT genes with varying frequencies in all metagenomic datasets were mainly for siderophores/vitamin transporters and DOM-related transporters, followed by heme/hemophores/iron(heme)-binding protein transporters (Figure 1A). Nickel and cobalt TBDTs appeared to be uncommon in the surface ocean. Overall, DOM and siderophore transporters were particularly prevalent across 57 GOS sampling sites. High-frequency DOM-related TBDTs from Gammaproteobacteria (clusters 427 and 3090) and siderophores-related TBDTs (cluster 410) can be found in the Sargasso Sea (GSb and GSc), as shown in Figure 2. The relatively high-frequency DOM-related TBDTs from the CFB group (cluster 720) were distributed in GS1a, GS3, GS31, and GS35 (Figure 2). In addition, GS31 and GS35 contained heme transporters (cluster 1609) at relatively high frequency and siderophore transporters at relatively moderate frequency, suggesting complementary pathways for acquiring iron (Figure 2).
Figure 1. Frequencies of TBDT (A) and ABC transporter (B) genes in GOS samples at different locations.
The number of transporters from GOS sites was normalized as described in the Methods section. The frequency is relative to the average number of six single-copy gene hits for each site. A color scheme is used to show the functional groups. Color (bottom axis) represents different sampling areas and each habitat type (n) is indicated (O: open sea, C: coastal, E: estuary, R: reef, W: warm seep, S: hypersaline, H: harbor, M: mangrove). Samples 01a, 01b and 01c were obtained using different size fractions from the same station: sample 01a, 20-3 µm; sample 01b, 3-0.8 µm; sample 01c, 0.8-0.1 µm.
Figure 2. Heat map comparison of frequencies each cluster in different GOS samples.
The frequency value each GOS site was assigned a color relative to the maximum value seen in each cluster. The maximum value of frequency is shown at the end of its corresponding row. The colors represent 0 (white) to MAX (red). The different GOS sampling areas are highlighted using colored text.
Predicted substrates for the frequently abundant ABC transporters in marine environments branched into chain amino acids, oligopeptides, alkylphosphonates, dipeptides, tungstates, glutamates, and aspartates (Figure 1B). An overlap between the TonB-dependent substrates and the ABC transporter substrates, such as nickel, cobalt, and iron complex, was observed. The ABC transporter specificity for various amino acids and peptides was notably prevalent across marine environments, suggesting that the ABC transporters performed essential roles as nitrogen resources in the bacterial uptake of amino acids or peptides. The Fe3+ ABC transporter genes for ferrichrome and iron dicitrate were more abundant than the Fe2+ transporter genes in the metagenomes (Figure 1B), as also indicated in previous studies [14]. However, the overall frequency of the identified siderophores-related TBDT genes (estimated average frequency of approximately 0.8) was close to that of the Fe3+ ABC transporter genes (estimated average frequency of approximately 1.1). Thus, the Fe3+ TonB-dependent transportation strategy may be more prevalent than originally believed [14].
The retrieved GOS sequences were dominated by TBDT genes that were closely related to Gammaproteobacteria (primary members of the Alteromonadales and the OMG group of bacteria) with moderate contributions from species in the CFB phyla (Figure 3). Sequences related to the order Sphingomonadales were generally rare, but were also contributors to siderophores- and DOM-related transporters in the metagenomic data (Figure 3). Taxonomic distribution of the retrieved GOS sequences in the major protein families was similar to the TBDT distribution pattern in the bacterial genome.
Figure 3. Best BLAST matches in GenBank of TBDT sequences along the GOS transect and their taxonomic affiliation.
Colors are used to represent the major phylogroups.
The stringent E-value cutoff values for the BLASTP search [36] and the Lek clustering [37], [38] were chosen to ensure that the functionally relevant TBDTs are present in the same cluster when the predicted clusters were too high. Approximately 11% of the TBDTs from the non-redundant (NR) database and 40% of the TBDTs from the GOS were categorized in the clusters without function-known genes (Table 2). For example, many sequences from GS18 were in the clusters that only contained GOS sequences, although the highest number of TBDTs was observed in GS18 (Table S3). The GOS sequences possibly came from uncultured marine bacteria, which showed relatively low similarity to TBDT sequences from the bacterial genome. On the other hand, TBDT subfamilies that only included GOS sequences can be considered likely to be as-yet undiscovered TBDT subfamilies. The current data supported the notion that the number and variety of TonB-dependent substrates were underestimated [19] and especially that the experimental studies for identifying TBDT substrates in marine samples were extremely limited. The large diversity and structural complexity of DOM in the marine environment make it a potential substrate for TBDTs.
Genomics-based Prediction of Substrates for TBDTs in the Genomes of Two Citromicrobium Strains
The genomic localizations of the genes of the identified TBDTs were analyzed to predict their substrate preferences. Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363 are closely related with each other phylogenetically, and both strains have similar functional distribution of TBDT. However, the genomic organization and gene context surrounding the TBDT gene in the bacteria were often not conserved, suggesting the diversity of the substrates for TBDT (Figures S1 and S2). Most of the coding genes for TBDT were not randomly distributed across the genome, but were located in a physiologically meaningful genomic context.
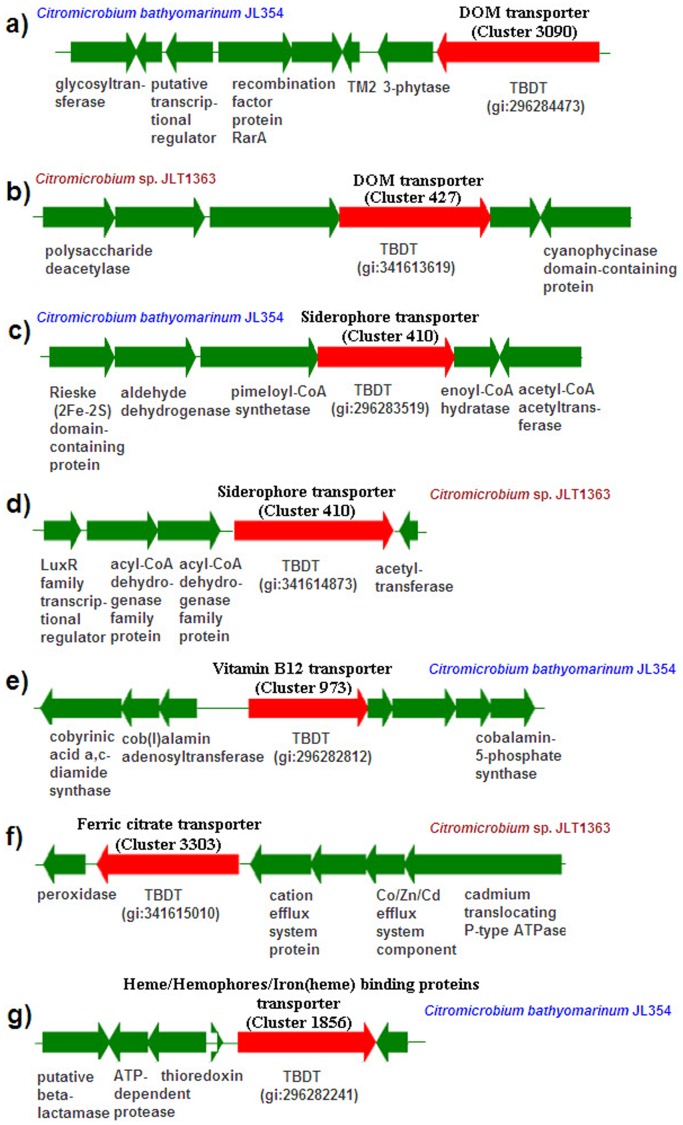
Based on the clustering analysis (Figures S1 and S2), majority of TBDTs were found to transport siderophores and DOM. Interestingly, some TBDT genes resided within operons predicted to code for enzymes that transfer fatty acyl substitution; two examples are shown in Figures 4a and 4b. These genes may have essential functions in the production and attachment of the siderophore fatty acyl chain [39]. The siderophore contains a fatty acyl chain or an α-hydroxy carboxylic acid moiety that dominates the marine siderophores [40]. The present findings suggest that Citromicrobium bacteria with a fatty acyl side chain are capable of transporting siderophores. Figure 4c and 4d show the other iron transporters. Aside from putative TBDT for heme and siderophores uptake, both strains carried TBDT sequences that may be used for DOM and vitamin B12 import (Table S2). For example, a TBDT gene in Citromicrobium bathyomarinum JL354 was adjacent to a phytase gene, suggesting that phytic acid is a substrate for TBDT (Figure 4e). A TBDT in Citromicrobium sp. JLT1363 was located in close proximity to a gene-encoding cyanophycinase that degrades the amino acid polymer cyanophycin, which is an important intracellular nitrogen-storer of cyanobacteria (Figure 4f). Citromicrobium bathyomarinum JL354 had a gene encoding TBDT proximity to genes associated with vitamin B12 biosynthesis, allowing it to be annotated as a vitamin B12 transporter (Figure 4g).
Figure 4. Open reading frames identified in the genome of Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363 adjacent to the TBDT gene (marked in red) and predicted to be related to substrate utilization.
The arrows indicate the direction of transcription.
Genomics-based Prediction of Substrates for Selected TBDTs from Metagenomes
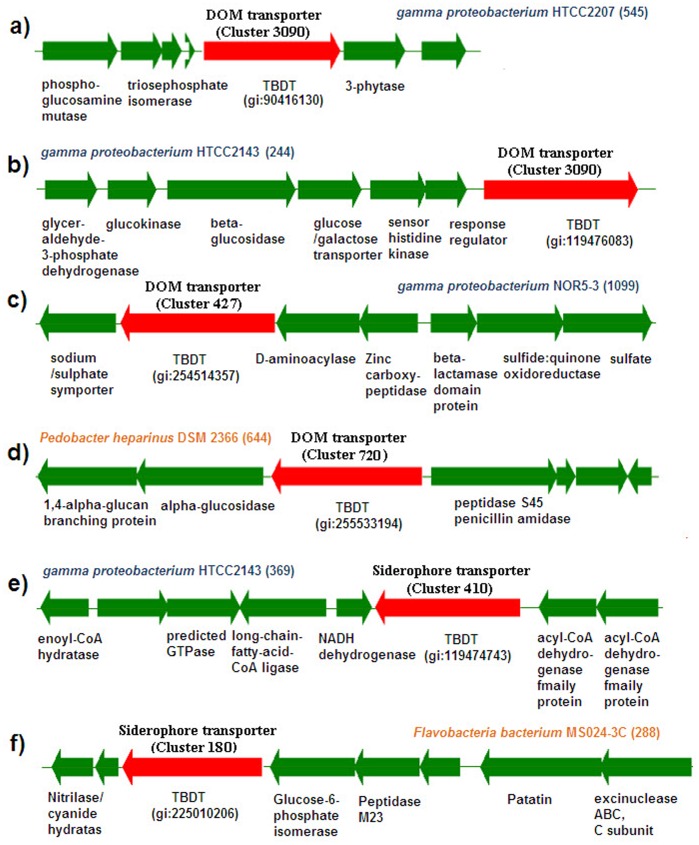
Overall, the environmental TBDT sequences in the major clusters that were most abundant were best matches to the Gammaproteobacteria or CFB group bacteria. Among the species, the gamma proteobacterium HTCC2207, gamma proteobacterium HTCC2143, gamma proteobacterium NOR5-3, Pedobacter heparinus DSM 2366, and Flavobacteria bacterium MS024-3C species contained the greatest number and greatest identity of recruited GOS sequences in major clusters.
Figure 5 shows the recruitment sequences observed in large amounts and similar to TBDT genes. Many environmental TBDT sequences in cluster 3090 showed greatest similarity to a phytatic acid transporter from gamma proteobacterium HTCC2207 (Figure 5a) or a TBDT gene in gamma proteobacterium HTCC2143 located upstream of the galactose utilization genes (Figure 5b). Environmental TBDT sequences in cluster 427 showed the greatest similarity to a TBDT gene in gamma proteobacterium NOR5-3 located adjacent to a D-aminoacylase gene that catalyses N-acyl-D-amino acid derivates (Figure 5c). Homologs of a peptidoglycan transporter in Pedobacter heparinus DSM 2366 were detected frequently in the environment (Figure 5d). In cluster 180, numerous sequences were homologous to a TBDT in gamma proteobacterium NOR5-3 located near a predicted gene encoding cyanide hydratase (Figure 5e). A previous study indicated that a putative siderophore component was involved in cyanide utilization [41]. Gammaproteobacterium HTCC2143 had a siderophore transporter gene residing within an operon that was predicted to code for enzymes involved in fatty acid metabolism, similar to the siderophore transporter gene in Citromicrobium bathyomarinum JL354 (Figure 5f).
Figure 5. Genomic context of the representative gene clusters containing TBDT (marked in red) from Gammaproteobacteria and CFB best matches by environmental sequences in large mounts.
The number of the best hits for this gene in GOS is indicated in a bracket.
In this study, the results of the genome context analysis suggest that the substrates for TBDTs included siderophores, ferric citrate, vitamin B12, heme, phytic acid, galactose, cyanophycin, N-acyl-D-amino acid derivates, peptidoglycan, and cyanide (Figures 4 and 5). To further detail the DOM utilization patterns, further study must be undertaken to identify more substrates for TBDT, especially from a representative set of bacteria (Gammaproteobacteria and the CFB group).
Proteomic Analysis of Citromicrobium Strains
Proteomic views of Citromicrobium bathyomarinum JL354 and Citromicrobium sp JLT1363 during the stationary phase of culture are presented in Table S4 and Table S5, respectively. Many known abundant bacterial core proteins [42], such as ATP synthase, elongation factors, chaperonin, transporter systems, and ribosomal proteins, contain abundant proteins. The functional categories in the identified proteins are shown in Figures S3 and S4. Transporter activity in terms of numbers was one of the most represented biological process or molecular function gene ontology (GO). Both strains were shown to produce a large number of proteins involved in the TonB-dependent transport system, which comprises a considerable part of the total periplasmic proteome (Table 3). Eight TBDTs and three ABC transporter subunits were detected in a total of 18 periplasmic proteomes from Citromicrobium bathyomarinum JL354 (Table 3). Fifteen TBDTs and six ABC transporter subunits were found in a total of 31 periplasmic proteomes from Citromicrobium sp JLT1363 (Table 3). These TBDTs might be involved as DOMs, siderophores, or thiamin transporters. Moreover, remarkably high expressions of various TBDTs were observed for Sphingomonas [43] and Pseudoalteromonas haloplanktis TAC125 [44], which contained a high number of TBDT genes in their genomes. ABC transporters were absolutely required for all bacterial growth. For instance, the ABC transporters were among the most highly detected proteins from the stationary phases of Ruegeria pomeroyi DSS-3 [45] and Candidatus Pelagibacter ubique [46]. The enrichment of TBDTs in the proteome indicated that TBDT proteins might indeed contribute to bacterial growth. More importantly, TonB-dependent transporter systems may partially compensate for a low number of ABC transporters in marine microorganisms.
Table 3. Most abundant transporter proteins detected in in Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363, respectively.
| Strain | Accession number | Description | Predicted substrate* |
| Citromicrobium bathyomarinum JL354 | TBDT | ||
| gi|296282297 | TonB-dependent receptor-like protein | Thiamin | |
| gi|296282571 | putative outer membrane receptor for iron transport | Siderophore | |
| gi|296282704 | TonB-dependent receptor | DOM | |
| gi|296283519 | TonB-dependent receptor | Siderophore | |
| gi|296284195 | TonB-dependent receptor | Siderophore | |
| gi|296284565 | hypothetical protein CbatJ_13101 | DOM | |
| gi|296284567 | TonB-dependent receptor | DOM | |
| gi|296284689 | TonB-dependent receptor | Siderophore | |
| ABC transporters | |||
| gi|296283772 | ABC-type transport system | N/A | |
| gi|296284204 | putative ABC transporter ATP-binding protein | N/A | |
| gi|296284590 | ABC-type transport system periplasmic component | N/A | |
| Citromicrobium sp. JLT1363 | TBDT | ||
| gi|341613545 | TonB-dependent receptor | Hemin/Hemophores/Iron(Heme) binding proteins | |
| gi|341613619 | TonB-dependent receptor | DOM | |
| gi|341613721 | TonB-dependent receptor | Siderophore | |
| gi|341614223 | TonB-dependent receptor, plug | Siderophore | |
| gi|341614279 | TonB-dependent receptor | Siderophore | |
| gi|341614547 | TonB-dependent receptor | Siderophore | |
| gi|341614682 | TonB-dependent receptor | DOM | |
| gi|341614873 | TonB-dependent receptor | Siderophore | |
| gi|341615010 | putative outer membrane receptor protein | Ferric citrate | |
| gi|341615076 | TonB-dependent receptor | DOM | |
| gi|341615079 | TonB-dependent siderophore receptor | Siderophore | |
| gi|341615951 | putative TonB-dependent receptor | Siderophore | |
| gi|341615953 | hemin receptor | Hemin/Hemophores/Iron(Heme) binding proteins | |
| gi|341616139 | TonB-dependent receptor | Siderophore | |
| gi|341616145 | Outer membrane protein | Siderophore | |
| ABC transporters | |||
| gi|341613400 | ABC transporter, ATP-binding protein | N/A | |
| gi|341614549 | iron compound ABC transporter, permease protein | Siderophore | |
| gi|341614550 | ferrichrome ABC transporter ATP-binding protein | Siderophore | |
| gi|341614655 | ABC-type transport system periplasmic component | N/A | |
| gi|341615504 | phosphate transporter ATP-binding protein | phosphate | |
| gi|341615777 | putative ABC transporter ATP-binding protein | N/A |
Predicted substrate based on the functional classification of the TBDT and gene context analysis.
Discussion
The results of the analysis of the genetic contexts near the TBDT show that some TBDT genes were closely associated with the carbohydrate, amino acid, or other substrate metabolism enzymes in the operons (Figures 4 and 5), suggesting that these genes are functionally linked and may therefore play important roles in diverse DOM uptake for marine bacteria, but are not limited to iron and vitamin B12 uptake only.
Marine organic matter was thought to originate primarily from phytoplankton production [47], [48]. Carbohydrates produced by phytoplankton form an important fraction of the DOM in the ocean [48]. For the Gammaproteobacteria and the CFB group of bacteria, TBDTs located in the gene cluster related to carbohydrate metabolism might play a major role in utilizing labile substrates, such as xylose, arabinose, and galactose derived from phytoplankton. In the surface ocean, the ability of monosaccharide incorporation by bacteria depends on ABC transporters because of the abundance and omnipresence of the bacterioplankton population (such as SAR11 and the Roseobacter clade) in the marine ecosystem [10]. Carbohydrate utilization of bacterial population is further enhanced in the presence of TBDTs from Gammaproteobacteria and CFB bacteria.
Polysaccharides released by phytoplankton were identified as the major constituents of naturally occurring marine high-molecular-weight DOM [49]. Complex molecules that contain N-acetylglucosamine (GlcNA), such as chito-oligosaccharides, are substrates for TBDT [21], [23]. For instance, some CFB can transport and consume chitin and GlcNA within the DOM pool, and thus, it should be a relevant part of chitin-like DOM degradation in marine systems [50]. The N-acyl-D-amino acid derivates may also be used as a putative substrate for TBDT from Gammaproteobacteria. Other polysaccharides, such as pectin and alginates, which were the dominant components in diatom and brown algae cell walls, respectively, were also identified as potential substrates for TBDTs [26]. These data suggest that members of the CFB and Gammaproteobacteria not only contained polysaccharide hydrolases, but also hydrolysate specific-TBDTs with a selective advantage over other heterotrophic bacteria in a marine environment enriched with polysaccharides, such as in biofilm. The sea surface microlayer has commonly been thought to be a gelatinous biofilm and has been shown to be dominated by Gammaproteobacteria and Bacteroidetes [51], [52]. A similar result was observed in the biofilm from the Irish Sea [53]. On the other hand, major storage products in phytoplankton, such as cyanophycin in cyanobacteria and starch, were also found to be potential substrates for TBDTs in some bacteria. This observation explains in part the ability of Gammaproteobacteria and CFB to consume a wide range of phytoplankton-derived DOM.
DOM also originates in part from the degradation of terrestrial plant materials, such as phytic acid, which is transported to the open sea through rivers [54]. Phytic acid can be an abundant source of phosphorus and carbon for bacteria in coastal waters and is it also used for chelating Fe [54]. Phytic acid-specific TBDT sequences were abundant in the metagenomic data.
Aside from the prevalence of TBDT sequence from (meta-) genomic data, the other ‘omic’ datasets including (meta-) proteomics and (meta-) transcriptomics suggested that TBDTs are physiologically and ecologically significant. The metaproteome sampled from South Atlantic surface waters showed that TBDTs were predominant in membrane proteins, wherein the Gammaproteobacteria and CFB group bacteria were the most abundant from the open ocean to the coastal seawater [6]. These TBDT proteins were closely related to Gammaproteobacteria and members of the CFB, Alphaproteobacteria, and Deltaproteobacteria. TBDTs were detected in abundance in the metaproteomic samples from the upwelling in the South China Sea off Vietnam, where the Alteromonadales represented the abundant taxa in this research (unpublished data). Transcripts associated with TBDT were significantly overrepresented in the high-molecular weight DOM treatment from the new dominant Alteromondales bacteria, whereas Pelagibacter and Prochlorococcus decreased in relative abundance [7]. TBDTs also comprise the most abundant group of transport-related transcripts within the CFB group bacteria sequences in natural environments [8].
However, ABC transporter transcripts dominated the acquisition of DOM monomers, with minor contributions from the TRAP in the southeastern U.S. coastal seawater wherein the pelagic microorganism Roseobacter and SAR11 are dominant [4]. Carbohydrate-related ABC transporter transcripts from Roseobacter bacteria accounted for more than 30% of all the DOM-related transporter sequences in the coastal ecosystem [4], suggesting that they outcompeted other marine bacteria for carbohydrates. SAR11 bacteria typically contributed to a significant fraction of amino acid assimilation in surface waters [55]. In the Sargasso Sea, the periplasmic compounds of ABC transporter systems for amino acids were found to be the most abundant peptides from SAR11 [3], suggesting that they were able to take up amino acid. Not surprisingly, based on their known abundance in the wild and the high proportion of ABC transporter in Roseobacter and SAR11 bacteria genomic data, the ABC transport proteins were the most frequently detected proteins at such high abundance in marine environments. Hence, the difference in the expressed transporter profiles in marine environments is primarily caused by the geographic distribution of the dominant bacteria population. However, the sampling or analysis method influenced the results of membrane proteomics [5]. The peptide search in the metaproteomic study in the Sargasso Sea revealed only the environmental protein-coding sequences from SAR11 and cyanobacteria [3].
A previous study indicated that relatively abundant bacteria cannot dominate the consumption of all DOMs and that a diverse assemblage of bacteria is essential for the complete degradation of complex DOM in the oceans [50]. The abundant operational taxonomic unit belongs to the members of SAR11, Roseobacter, cyanobacteria, CFB group, and Gammaproteobacteria (such as OMG and Alteromonadales) across the GOS sites [56]. The major types of high-affinity carbohydrate and amino acid transporters known in the Roseobacter and SAR11 bacteria include ABC systems [3], [4]. In contrast, CFB group bacteria, such as Polaribacter sp. MED152, possess relatively few transporters for free amino acids and lack carbohydrate-specific ABC transporters [57]. The Shewanella species (order Alteromonadales) carried TBDTs for GlcNA or chito-oligosaccharides transport across the outer membrane and a specific permease for GlcNA transport, but lacked GlcNA-specific PTS or ABC systems in the cytoplasmic membrane [58]. Thus, a broad array of different substrate utilization patterns for Gammaproteobacteria and CFB bacteria in the marine environment could be assumed to occur ubiquitously because of the expressions of TBDTs, although these bacteria possibly represented a lower percentage of overall microbial community nutrient acquisition compared with the high abundance of ABC transporters in pelagic microorganisms, such as SAR11, Roseobacter, and cyanobacteria.
In conclusion, transporter sequence information, such as diversity and substrate specificity, provide useful and relevant clues for the DOM utilization of a bacterium, bacterial group, or even a microbial community. In this study, TBDTs for DOM transportation were found to be abundant across marine environments. Based on the prevalence of TBDT and ABC transporter sequence data, the major bacterial groups were found to have distinct DOM uptake patterns. Such a metagenomic analysis can potentially contribute to the understanding of the molecular bases of bacterial activities in the ocean, particularly by highlighting the contributions of Gammaproteobacteria and CFB group bacteria with TBDTs to the overall ecosystem function.
Materials and Methods
Data Preparation
The NR database (3.8 GB, 12,061,831 sequences; 4,118,133,053 total letters) of protein sequences was downloaded from the National Center for Biotechnology Information (NCBI), and the predicted proteomes of Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363 from our laboratory were compared with them. The amino-acid sequences and the site metadata (sample location and environment conditions were listed in Table S3) derived from 57 samples in the GOS [30] expedition were retrieved from MG-RAST (version 2) [59]. The GSa in the GOS data sets was excluded from analysis because it was a suspected contaminant in samples from that site [35]. Comparison of the functional annotation of the metagenomics from the GOS was performed using the tools provided in MG-RAST with a maximal e-value of 1e-5 and a minimal alignment length >100. DNA sequences matching “Ton and Tol transport systems” and “ABC transporter”were exported. The ‘Ton and Tol transport systems’ sequences were translated to create a ‘GOS Ton and Tol protein’, using BLASTX [36] against the GOS AA sequence database (E-value threshold 1e-10). Sequence searches for transporters in the bacterial genome were carried out using BLASTP [36] (E-value threshold 1e-3) and a custom-made database comprising the members of various transporter families based on the Transporter Classification system [60]–[62]. NCBI annotations of the resulting proteins were scanned manually to remove non-transporter proteins.
Identification of TBDTs
NCBI-NR protein sequence searches for TBDTs were carried out using HMMER hmmsearch [63] with Pfam hidden Markov models (PF00715 for the N-terminal plug domain and PF00593 for the C-terminal membrane-spanning B-barrel domain) and the NR database. Both matched HMM profile sequences were additionally verified by manual inspection and kept for further analysis. Furthermore, NCBI annotations of the resulting proteins were scanned manually to remove sequences that were labeled as spurious. A BLASTP search of the “GOS Ton and Tol protein database” homolog was conducted against a database containing all TBDT proteins at NCBI with a conservative E-value cutoff of 1e-5. All recruited sequences were subjected to verification using reciprocal BLASTP. The 15,905 and 44,073 homologs of TBDT were identified separately in NCBI-NR protein and GOS metagenomics. The genomic organization of the bacterial TBDT genes was visualized on a linear genome map using the Genome2D program [64].
TBDT Clustering
TBDTs with known substrates were combined with the predicted TBDT homologs. All vs all BLASTP searches were performed for the TBDTs and a Lek clustering algorithm [37], [38] was applied to cluster proteins. An E-value cutoff of 1e-40 for the BLASTP results and a Lek similarity cutoff of 0.6 were used to build gene family clusters. The retrieved predicted TBDT sequences from GOS and from NR were clustered into 783 clusters containing more than two members.
Hit Normalization
To reconcile the effects of gene size on hit retrieval, the number of hits (Ng) at all GOS sampling sites were size-normalized to the relative average length of the gene (Lx) compared to the length of recA from E. coli K12 (LrecA: 1,062 bp) using the equation Nn = LrecA/Lx × Ng, where Nn represents normalized hits and Lx is the average length of TBDT genes each cluster or the average length of all genes in a ABC transporter system (data is available at http://www.tcdb.org/ [62]). To remove the bias of average genome size on the sampling of gene from a given metagenomic community, the number of recA-normalized hits for six single-copy genes (recA, atpD, gyrB, dnaK, rpoB and tufA) was averaged per site as described previously [65]. The frequency of TBDT andABC transporter genes relative to the number of single-copy gene hits for each site was then calculated as: number of size-normalized gene hits/average number of size-normalized six single-copy gene hits.
Bacterial Culture
Citromicrobium bathyomarinum JL354 and Citromicrobium sp. JLT1363 were maintained and precultured aerobically at 28°C with RO medium as previously [66], and stirred at a rate of 160 rpm in the dark. To establish the starvation-induced stationary phase, the feed of medium to the chemostat was switched off at the end of half-maxima optical density (1/2 ODmax) and subsequently changed (inoculum size: 2% v/v) to a nutrient mix with trace elements (1 mL per L medium) and glucose (final concentration 0.5%). The trace element stock solution contained per L: 3.15 g FeCl3.6H2O, 4.36 g Na2-EDTA.2H2O, 0.02 mg vitamin B12, 0.08 mg biotin, 0.4 mg vitamin B1, 0.2 mg vitamin B5, 10.0 mg CoCl2.6H2O, 9.8 mg CuSO4.5H2O, 22.0 mg ZnSO4.7H2O, 180.0 mg MnCL2.4H2O and 6.3 mg Na2MoO4.2H2O. The stationary phase samples were taken after the cell density had reached a constant value. Cells were collected and centrifuged at 10, 000× g for 20 min at 4°C. The samples for further proteomic analysis were frozen in liquid nitrogen and stored at −80°C.
Protein Extraction and OFFGEL Digestion
Whole cell lysates were prepared as described previously [67]. Cells were washed with 10 mM Tris-HCl, pH 8.0 and lysed in SDT-lysis buffer using a 1∶10 sample to buffer ratio for 5 min at 95°C. Brief sonication was performed to reduce the viscosity of the lysate, which was centrifuged for 5 min at 16,000×g to remove debris. OFFGEL digest was processed with Endoproteinase Lys-C (Roche, Indianapolis, IN, USA) and Trypsin (Promega, Madson, USA). Filter Aided Sample Preparation was used when in-solution digest was carried out as described previously [67] to desalt large amounts of peptide mixtures for OFFGEL separation.
Protein Characterized by LC-MS/MS
A Finnigan™ LTQ™ linear ion trap MS (Thermo Electron) equipped with an electrospray interface was connected to the LC setup for eluted peptide detection. Data-dependent MS/MS spectra were obtained simultaneously. Each scan cycle consisted of one full MS scan in profile mode followed by five MS/MS scans in centroid mode with the following Dynamic Exclusion™ settings: repeat count 2, repeat duration 30 s, exclusion duration 90 s. Each sample was analyzed in triplicate.
MS/MS spectra were automatically searched against the NR protein database using the BioworksBrowser rev. 3.1(Thermo Electron, San Jose, CA). Protein identification results were extracted from SEQUEST out files with BuildSummary [68]. The peptides were constrained to be tryptic and up to two missed cleavages were allowed.
Carbamidomethylation of cysteines was treated as a fixed modification, whereas oxidation of methionine residues was considered as a variable modification. The mass tolerance allowed for the precursor ions was 2.0 Da and for fragment ions was 0.8 Da. The protein identification criteria were based on Delta CN (≥0.1) and cross-correlation scores (Xcorr, one charge ≥1.9, two charges ≥2.2, three charges ≥3.75. Gene ontology information was assigned for the identified proteins using InterProScan searches implemented in Blast2GO [69].
Supporting Information
In Citromicrobium bathyomarinum JL354 all operons contain the TBDT gene (marked in red).
(TIF)
In Citromicrobium sp. JLT1363 all operons contain the TBDT gene (marked in red).
(TIF)
Functional category distribution for the identified proteins in Citromicrobium bathyomarinum JL354 based on their annotations in the Gene Ontology (GO) cell component (A), molecular function (B) and biological processes (C) vocabularies.
(TIF)
Functional category distribution for the identified proteins in Citromicrobium sp. JLT1363 based on their annotations in the Gene Ontology (GO) cell component (A), molecular function (B) and biological processes (C) vocabularies.
(TIF)
Functional classification of TBDTs among sequenced genomes.
(XLS)
Functional classification of TBDTs among marine bacterial genomes.
(XLS)
The number and frequency of TBDT and ABC transporter sequences each GOS site.
(XLS)
A list of expressed proteins identified in proteomic analysis of Citromicrobium bathyomarinum JL354.
(XLS)
A list of expressed proteins identified in proteomic analysis of Citromicrobium sp. JLT1363.
(XLS)
Acknowledgments
Professor John Hodgkiss is thanked for his help with the English in this manuscript. We gratefully acknowledge the thoughtful comments, valuable suggestions, and helpful criticisms of the reviewers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NSFC (Natural Science Foundation of China) 40906079, National Basic Research Program of China 2011CB808800, key NSFC 91028001, Public Science and Technology Research Funds Projects of Ccean 201105021 and the Scientific Research Foundation for Returned Scholars, Ministry of Education of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kujawinski EB. The impact of microbial metabolism on marine dissolved organic matter. Annu Rev Mar Sci. 2011;3:567–599. doi: 10.1146/annurev-marine-120308-081003. [DOI] [PubMed] [Google Scholar]
- 2.Jiao NZ, Herndl GJ, Hansell DA, Benner R, Kattner G, et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Microbiol. 2010;8:593–599. doi: 10.1038/nrmicro2386. [DOI] [PubMed] [Google Scholar]
- 3.Sowell SM, Wilhelm LJ, Norbeck AD, Lipton MS, Nicora CD, et al. Transport functions dominate the SAR11 metaproteome at low-nutrient extremes in the Sargasso Sea. ISME J. 2009;3:93–105. doi: 10.1038/ismej.2008.83. [DOI] [PubMed] [Google Scholar]
- 4.Poretsky RS, Sun S, Mou X, Moran MA. Transporter genes expressed by coastal bacterioplankton in response to dissolved organic carbon. Environ Microbiol. 2010;12:616–627. doi: 10.1111/j.1462-2920.2009.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowell SM, Abraham PE, Shah M, Verberkmoes NC, Smith DP, et al. Environmental proteomics of microbial plankton in a highly productive coastal upwelling system. ISME J. 2011;5:856–865. doi: 10.1038/ismej.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris RM, Nunn BL, Frazar C, Goodlett DR, Ting YS, et al. Comparative metaproteomics reveals ocean-scale shifts in microbial nutrient utilization and energy transduction. ISME J. 2010;4:673–685. doi: 10.1038/ismej.2010.4. [DOI] [PubMed] [Google Scholar]
- 7.McCarren J, Becker JW, Repeta DJ, Shi Y, Young CR, et al. Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea. Proc Natl Acad Sci U S A. 2010;107:16420–16427. doi: 10.1073/pnas.1010732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottesen EA, Marin III R, Preston CM, Young CR, Ryan JP. Metatranscriptomic analysis of autonomously collected and preserved marine bacterioplankton. ISME J. 2011;5:1881–1895. doi: 10.1038/ismej.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boos W, Eppler T. Winkelmann G, editor. Prokaryotic binding protein-dependent ABC transporters. 2001. pp. 77–114. editor. Microbial Transport Systems. New York: Wiley-VCH.
- 10.Jiao NZ, Zheng Q. The microbial carbon pump: from genes to ecosystems. Appl Environ Microbiol. 2011;77:7439–7444. doi: 10.1128/AEM.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erni B. Winkelmann G, editor. Glucose transport by the bacterial phosphotransferase system (PTS): an interface between energy- and signal transduction. 2001. pp. 115–138. editor. Microbial Transport Systems. New York: Wiley-VCH.
- 12.Mulligan C, Fischer M, Thomas GH. Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea. FEMS Microbiol Rev. 2011;35:68–86. doi: 10.1111/j.1574-6976.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 13.Moeck GS, Coulton JW. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 14.Hopkinson BM, Barbeau K. Iron transporters in marine prokaryotic genomes and metagenomes. Environ Microbiol. 2012;1:114–128. doi: 10.1111/j.1462-2920.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 15.Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: structure of the BtuB:TonB complex. Science. 2006;312:1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
- 16.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of thiamin biosynthesis in prokaryotes: new genes and regulatory mechanisms. J Biol Chem. 2002;277:48949–48959. doi: 10.1074/jbc.M208965200. [DOI] [PubMed] [Google Scholar]
- 17.Schauer K, Gouget B, Carrière M, Labigne A, de Reuse H. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol Microbiol. 2007;63:1054–1068. doi: 10.1111/j.1365-2958.2006.05578.x. [DOI] [PubMed] [Google Scholar]
- 18.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-Dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schauer K, Rodionov DA, de Reuse HD. New substrates for TonB-dependent transport: do we only see the “tip of the iceberg”? Trends Biochem Sci. 2008;33:330–338. doi: 10.1016/j.tibs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, et al. Plant carbohydrate scavenging through TonB-Dependent receptors: a feature shared by phytopathogenic and aquatic bacteria. PLoS ONE. 2007;2:e224. doi: 10.1371/journal.pone.0000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulanger A, Déjean G, Lautier M, Glories M, Zischek C, et al. Identification and regulation of the N-acetylglucosamine utilization pathway of the plant pathogenic bacterium Xanthomonas campestris pv. campestris. J Bacteriol. 2010;192:1487–1497. doi: 10.1128/JB.01418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Ochiai A, Fukuda Y, Hashimoto W, Murata K. A putative lipoprotein of Sphingomonas sp. strain A1 binds alginate rather than a lipid moiety. FEMS Microbiol Lett. 2008;28:221–226. doi: 10.1111/j.1574-6968.2008.01354.x. [DOI] [PubMed] [Google Scholar]
- 23.Eisenbeis S, Lohmiller S, Valdebenito M, Leicht S, Braun V. NagA-dependent uptake of N-acetyl-glucosamine and N-acetyl-chitin oligosaccharides across the outer membrane of Caulobacter crescentus. J Bacteriol. 2008;190:5230–5238. doi: 10.1128/JB.00194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neugebauer H, Herrmann C, Kammer W, Schwarz G, Nordheim A, et al. ExbBD-dependent transport of maltodextrins through the novel MalA protein across the outer membrane of Caulobacter crescentus. Appl Environ Microbiol. 2005;187:8300–8311. doi: 10.1128/JB.187.24.8300-8311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller TR, Delcher AL, Salzberg SL, Saunders E, Detter JC, et al. Genome sequence of the dioxin-mineralizing bacterium Sphingomonas wittichii RW1. J Bacteriol. 2010;192:6101–6102. doi: 10.1128/JB.01030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim BL. The distribution of TonB-dependent receptors in nitrogen-fixing nodulating bacteria. Microbes Environ. 2010;25:67–74. doi: 10.1264/jsme2.me10102. [DOI] [PubMed] [Google Scholar]
- 27.Mirus O, Strauss S, Nicolaisen K, Haeseler AV, Schleiff E. TonB-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 2009;25:1–25. doi: 10.1186/1741-7007-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frickey T, Lupas A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics. 2004;20:3702–3704. doi: 10.1093/bioinformatics/bth444. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Stoeckert CJ, Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, et al. The Sorcerer II Global Ocean Sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 2007;5:398–431. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao NZ, Zhang R, Zheng Q. Coexistence of two different photosynthetic operons in Citromicrobium bathyomarinum JL354 as revealed by whole-genome sequencing. J Bacteriol. 2010;192:1169–1170. doi: 10.1128/JB.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Q, Zhang R, Jiao NZ. Genome sequence of Citromicrobium strain JLT1363, isolated from the South China Sea. J Bacteriol. 2011;193:2074–2075. doi: 10.1128/JB.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biers EJ, Sun S, Howard EC. Prokaryotic genomes and diversity in surface ocean waters: interrogating the global ocean sampling metagenome. Appl Environ Microbiol. 2007;75:2221–2229. doi: 10.1128/AEM.02118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauro FM, McDougald D, Thomas T, Williams TJ, Egan S, et al. The genomic basis of trophic strategy in marine bacteria. Proc Natl Acad Sci U S A. 2009;106:15527–15533. doi: 10.1073/pnas.0903507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLong EF, Karl DM. Genomic perspectives in microbial oceanography. Nature. 2005;437:336–342. doi: 10.1038/nature04157. [DOI] [PubMed] [Google Scholar]
- 36.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 38.Wu D, Wu M, Halpern A, Rusch DB, Yooseph S, et al. Stalking the fourth domain in metagenomic data: searching for, discovering, and interpreting novel, deep branches in marker gene phylogenetic trees. PLoS ONE. 2011;6:e18011. doi: 10.1371/journal.pone.0018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamarca BBD, Zhu W, Arceneaux JEL, Byers R, Lundrigan MD. Participation of fad and mbt genes in synthesis of Mycobactin in Mycobacterium smegmatis. Appl Environ Microbiol. 2004;186:374–382. doi: 10.1128/JB.186.2.374-382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vraspir JM, Butler A. Chemistry of marine ligands and siderophores. Annu Rev Mar Sci. 2009;1:43–63. doi: 10.1146/annurev.marine.010908.163712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JL, Kunz DA. Cyanide utilization in Pseudomonas fluorescens NCIMB 11764 involves a putative siderophore. FEMS Microbiol Lett. 1997;156:61–67. [Google Scholar]
- 42.Tang K, Huang HZ, Jiao NZ, Wu CH. Phylogenomic analysis of marine Roseobacters. PLoS ONE. 2010;5:e11604. doi: 10.1371/journal.pone.0011604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A. 2009;106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilmes B, Kock H, Glagla S, Albrecht D, Voigt B, et al. Cytoplasmic and periplasmic proteomic signatures of exponentially growing cells of the psychrophilic bacterium Pseudoalteromonas haloplanktis TAC125. Appl Environ Microbiol. 2011;77:1276–1283. doi: 10.1128/AEM.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christie-oleza JA, Fernandez B, Nogales B, Bosch R, Armengaud J. Proteomic insights into the lifestyle of an environmentally relevant marine bacterium. ISME J. 2011;6:124–135. doi: 10.1038/ismej.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowell SM, Norbeck AD, Lipton MS, Nicora CD, Callister SJ. Proteomic analysis of stationary phase in the marine bacterium “Candidatus Pelagibacter ubique”. Appl Environ Microbiol. 2008;74:4091–4100. doi: 10.1128/AEM.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aluwihare LI, Repeta DJ. A comparison of the chemical characteristics of oceanic DOM and extracellular DOM produced by marine algae. Mar Ecol Prog Ser. 1999;186:105–117. [Google Scholar]
- 48.Dafner EV, Wangersky PJ. A brief overview of modern directions in marine DOC studies Part II–Recent progress in marine DOC studies. J Environ Monit. 2002;4:55–69. doi: 10.1039/b107279j. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy M, Hedges J, Benner R. Major biochemical composition of dissolved high molecular weight organic matter in seawater. Mar Chem. 1996;55:281–297. [Google Scholar]
- 50.Cottrell MT, Kirchman DL. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol. 2000;66:1692–1697. doi: 10.1128/aem.66.4.1692-1697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunliffe M, Murrell JC. The sea-surface microlayer is a gelatinous biofilm. ISME J. 2009;3:1001–1003. doi: 10.1038/ismej.2009.69. [DOI] [PubMed] [Google Scholar]
- 52.Obernosterer I, Catala P, Lami R, Caparros J, Ras J, et al. Biochemical characteristics and bacterial community structure of the sea surface microlayer in the South Pacific Ocean. Biogeosci Discuss. 2007;4:2809–2844. [Google Scholar]
- 53.Cox MJ, Joint I, Edwards C, McCarthy AJ. Identification of carbohydrate metabolism genes in the metagenome of a marine biofilm community shown to be dominated by Gammaproteobacteria and Bacteroidetes. Genes. 2010;1:371–384. doi: 10.3390/genes1030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzumura M, Kamatani A. Origin and distribution of inositol hexaphosphate in estuarine and coastal sediments. Limnol Oceanogr. 1995;40:1254–1261. [Google Scholar]
- 55.Malmstrom RR, Kiene RP, Cottrell MT, Kirchman DL. Contribution of SAR11 bacteria to dissolved dimethylsulfoniopropionate and amino acid uptake in the North Atlantic Ocean. Appl Environ Microbiol. 2004;70:4129–4135. doi: 10.1128/AEM.70.7.4129-4135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yooseph S, Nealson KH, Rusch DB, McCrow JP, Dupont CL, et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature. 2010;468:60–66. doi: 10.1038/nature09530. [DOI] [PubMed] [Google Scholar]
- 57.González JM, Fernández-Gómez B, Fernàndez-Guerra A, Gómez-Consarnau L, Sánchez O, et al. Genome analysis of the proteorhodopsin-containing marine bacterium Polaribacter sp. MED152 (Flavobacteria). Proc Natl Acad Sci U S A. 2008;105:8724–8729. doi: 10.1073/pnas.0712027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang C, Rodionov DA, Li X, Laikova ON, Gelfand MS, et al. Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J Biol Chem. 2006;281:29872–29885. doi: 10.1074/jbc.M605052200. [DOI] [PubMed] [Google Scholar]
- 59.Meyer F, Paarmann D, D’Souza M, Olson R, Glass EM, et al. The Metagenomics RAST server-A public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinf. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milton H, Saier Winkelmann G, editor. Families of transporters: a phylogenetic overview. 2001. pp. 1–22. editor. Microbial Transport Systems. New York: Wiley-VCH.
- 61.Ren Q, Chen K, Paulsen IT. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res. 2007;35:D274–D279. doi: 10.1093/nar/gkl925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saier MH, Jr, Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information, Nucl Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eddy SR. A new generation of homology search tools based on probabilistic inference. Genome Inform. 2009;23:205–211. [PubMed] [Google Scholar]
- 64.Baerends R, Smits W, de Jong A, Hamoen L, Kok J, et al. Genome2D: a visualization tool for the rapid analysis of bacterial transcriptome data. Genome Biol. 2004;5:R37. doi: 10.1186/gb-2004-5-5-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howard EC, Sun S, Biers EJ, Moran MA. Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environ Microbiol. 2008;10:2397–2410. doi: 10.1111/j.1462-2920.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 66.Tang K, Zong R, Zhang F, Xiao N, Jiao NZ. Characterization of the photosynthetic apparatus and proteome of Roseobacter denitrificans. Curr Microbiol. 2009;60:124–133. doi: 10.1007/s00284-009-9515-7. [DOI] [PubMed] [Google Scholar]
- 67.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:3–7. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 68.Dai J, Shieh CH, Sheng QH, Zhou H, Zeng R. Proteomic analysis with integrated multiple dimensional liquid chromatography/mass spectrometry based on elution of ion exchange column using pH steps. Anal Chem. 2005;77:5793–5799. doi: 10.1021/ac050251w. [DOI] [PubMed] [Google Scholar]
- 69.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In Citromicrobium bathyomarinum JL354 all operons contain the TBDT gene (marked in red).
(TIF)
In Citromicrobium sp. JLT1363 all operons contain the TBDT gene (marked in red).
(TIF)
Functional category distribution for the identified proteins in Citromicrobium bathyomarinum JL354 based on their annotations in the Gene Ontology (GO) cell component (A), molecular function (B) and biological processes (C) vocabularies.
(TIF)
Functional category distribution for the identified proteins in Citromicrobium sp. JLT1363 based on their annotations in the Gene Ontology (GO) cell component (A), molecular function (B) and biological processes (C) vocabularies.
(TIF)
Functional classification of TBDTs among sequenced genomes.
(XLS)
Functional classification of TBDTs among marine bacterial genomes.
(XLS)
The number and frequency of TBDT and ABC transporter sequences each GOS site.
(XLS)
A list of expressed proteins identified in proteomic analysis of Citromicrobium bathyomarinum JL354.
(XLS)
A list of expressed proteins identified in proteomic analysis of Citromicrobium sp. JLT1363.
(XLS)