Abstract
Stereotypic behaviors are repetitive invariant behaviors that are common in many captive species and potentially indicate compromised welfare and suitability as research subjects. Adult laboratory mice commonly perform stereotypic bar-gnawing, route-tracing, and back-flipping, although great individual variation in frequency occurs. Early life factors (for example, level of maternal care received) have lasting effects on CNS functioning and abilities to cope with stress and therefore may also affect stereotypic behavior in offspring. Access to maternal resources and care are influenced by the number of pups in a litter; therefore, we examined both litter size and its potential correlate, weight at weaning, as early environmental predictors of adult stereotypic behavior in laboratory mice. Further, we assessed the effects on offspring stereotypic behavior of delaying the separation of mother and pups (weaning) beyond the standard 21 d of age. Analyzing stereotypic behavior in 3 different mouse colonies composed of 2 inbred strains (C57BL/6N and C57BL/6J) and an outbred stock (CD1[ICR]) revealed significant positive correlation between litter size and stereotypic behavior in female, but not male, mice. Weight and age at weaning did not significantly affect levels of stereotypy in either sex. Litter size therefore may be a useful indicator of individual predisposition to stereotypic behavior in female laboratory mice.
Abbreviation: B6, C57BL/6N; CD, CD1(ICR)
Stereotypic behavior, an easily measured behavior that shows great individual variation, may confound some mouse-based research,7 and is potentially affected by the early familial environment. Stereotypic behaviors have traditionally been defined as invariant, repetitive, and apparently functionless26,29 and are common in many captive species, including laboratory mice. In laboratory mice, activities meeting this description include bar-gnawing, back-flipping, and route-tracing.28 More recently stereotypic behaviors have been defined as being induced by frustration, attempts to cope, or CNS dysfunction27—a definition based on biological cause rather than phenotype. The specific term ‘stereotypy’ has been suggested to apply to the subset of these behaviors that is caused specifically by dorsal striatal dysfunction.27 Bar-mouthing, route-tracing, and similar repetitive behaviors in laboratory mice are less severe and prevalent in enriched environments21,34 and are more severe in previously enriched mice that have enrichments removed, suggesting that these behaviors are induced by frustration.21 Preliminary evidence that they may also be true stereotypies comes from their link with a form of generalized behavioral inflexibility termed ‘recurrent perseveration,8 where responses in a learned task are more repetitive than the response behavior observed in control animals. Such findings speak to both the welfare significance of laboratory mouse stereotypies and their potential implications for many aspects of mouse behavior during testing.
In many species, early maternal deprivation (such as premature weaning) increases later risks of stereotypic behaviors.20 In laboratory mice, lower offspring weights or ages at weaning increase the likelihood of developing bar-mouthing and similar activities in adulthood. In addition, lighter and younger animals show an increased number of escape attempts immediately after weaning, suggesting that lower weight and age at weaning may be associated with a greater need for milk and stronger motivation to return to the mother.35,36 Differences in resource access during development and resulting effects on developmental stage at the time of weaning therefore may be one reason why individual mice vary in their adult stereotypic behavior.35,36 Litter size can be one factor that affects the amount of maternal care received per offspring: sharing maternal resources among multiple siblings can result in slower growth, delayed onset of independent feeding, lower dominance status, and even lower probabilities of survival for particular progeny.12,18,31 Allocation of maternal resources may explain why offspring from larger litters were slower to develop coordinated movements in a swim-task32 and why some studies19,30 (but not all5,9) find long-term increases in emotionality in adult rodents from larger litters. Together, these findings suggest that individual mice from large litters may be at increased risk of later stereotypic behavior because they receive less maternal care in infancy and because they weigh less at weaning.
We examined both litter size and its potential correlate, weight at weaning, as predictors of stereotypic behavior in adult laboratory mice. Stereotypic behavior was assessed in 3 different mouse colonies that were composed of 2 inbred strains (C57BL/6N [B6] and C57BL/6J) and an outbred stock (CD1[ICR]). In 2 of the colonies, we also tested the effects of weaning at standard or delayed ages. The third colony, in which mice had previously been used to study pup dispersal behavior2, was maintained in enriched or nonenriched housing as adults,3 and litter-size effects on adult stereotypic behavior were investigated post hoc. We predicted that due to the expected negative influence of litter size on development, mice from larger litters would be smaller at weaning and adult stereotypic behavior would be greater in mice from larger litters or mice that weighed less at weaning. We also predicted that delayed weaning would reduce stereotypic behavior and that pups from larger litters might particularly benefit from this practice.
Materials and Methods
Animals.
Procedures for all mice were approved by the University of Guelph's Animal Care Committee and conducted in accordance with CCAC guidelines.4 All colonies were free of pathogens. Stereotypic behavior in adulthood was assessed similarly across colonies, as described later (see Stereotypic Behavior).
Colony 1.
Adult virgin male and female mice of the inbred C57BL/6N (B6) and outbred CD1(ICR) strains were purchased from Charles River Laboratories (Senneville, Quebec, Canada) and, on arrival to the Mount Sinai Hospital Animal Facility (Toronto, Canada), were housed in standard polycarbonate mouse cages (model N10HT, Ancare, Bellmore, NY; 13 cm high × 28 cm deep × 19 cm wide). Cages were maintained inside a single temperature (20 ± 1 °C)- and humidity (50% to 60%)-controlled room, under a 12:12-h photocycle. Mice had ample corncob bedding (Bed O'Cobs, Andersons, Maumee, OH) and ad libitum access to nesting material, dry food pellets (LabDiet 5053, Aberfoyle, Ontario, Canada), and sterilized water; cage cleaning was performed weekly. A week after their arrival, 2 female and one male (from the same strain) mouse were housed together. At the first sign of pregnancy, female mice were housed individually. Of the 14 litters born, 2 were excluded from the study due to extreme sex biases; the 12 remaining litters, which comprised a total of 104 pups (n = 24 same-sex sibling groups) from 7 B6 and 5 CD1 dams, were studied. Litters were weaned and weighed at either 21 d (7 litters) or 35 d (5 litters), earmarked for identification, and housed in same-sex sibling groups (n per cage = 2 to 5).
Colony 2.
C57BL/6J mice were bred and housed under standard conditions (22 ± 2 °C; relative humidity, 45% ± 5%) at a large commercial mouse facility, in typical joined duplex-style cages (model 3, Thoren Caging Systems, Hazelton, PA; 14 cm high × 30 cm deep × 14 cm wide). All mice had ample bedding of white pine shavings (Crobb Box, Ellsworth, ME) and ad libitum access to bottled acidified water (pH 2.8 to 3.1) and dry food pellets (Purina Mills, Richmond, IN); cage cleaning was performed weekly. We weaned and weighed 50 litters (182 pups in total) at 21 ± 1 d (n = 16 litters), 28 ± 1 d (n = 15 litters), or 35 ± 1 d (n = 19 litters) from dams of standard C57BL/6J breeding stock between their second and fifth parity, allowing natural litter size variation. Litter size was randomized among treatment groups rather than counter-balanced, because of the larger sample sizes in colony 2 compared with colony 1. At weaning, 2 male and 2 female mice from each litter were chosen at random and rehoused by sex (n per cage = 2).
Colony 3.
Adult virgin C57BL6/J mice were bred and housed at the University of Guelph Central Animal Facility, inside a single temperature (20 ± 1 °C) and humidity (50% to 60%)-controlled room under a 12:12-h photocycle. Mice had ample corncob bedding (Teklad 7097, Harlan Laboratories, Mississauga, Canada) and nesting material (Ancare, Bellmore, NY) and unlimited access to dry food pellets (Teklad 2014, Harlan Laboratories) and water (nonsterilized); cage cleaning was performed weekly. A total of 82 pups from 17 dams were weaned and weighed at 35 d and housed in same-sex sibling groups (n = 2 to 3). From birth to day 14, all mice were housed in standard polycarbonate mouse cages (as described for colony 1 mice), furnished with nesting material and a ‘mouse house’ (nontoxic polycarbonate; catalog no. K3327, Bio-Serv, Frenchtown, NJ). On day 14, mice were moved into new housing units for a dispersal experiment;2 each unit consisted of 2 identical cages connected by a tube, which the mother was unable to access. Therefore, one cage was a natal cage containing the dam, and the other a ‘dispersal cage’ that only the pups could reach. In addition, we used 2 cage styles so that there were 2 types of dispersal units, which varied slightly in dimension.2 At weaning, mice were housed in either enriched (9 litters) or standard (8 litters) conditions, counterbalanced by this early cage style. Environmental enrichment consisted of larger cages, additional nesting materials (tissue or shredded paper towel), and toys (sterilized PVC tubes, nylon dog bones, yogurt containers) rotated biweekly.3
Stereotypic behavior.
Mice (age, 6 to 7 mo) were observed in their home cages for stereotypies including bar-gnawing, back-flipping, and route-tracing; these actions were recorded as stereotypic if repeated 3 times without interruption.33 Beginning 1 h after lights off, individual mice were observed for stereotypic behavior and inactivity (mouse remained at rest, hunched, and motionless, for more than 8 s) by using a 1-0 sampling method. Every mouse was observed continuously for 20 s once every 45 min across a 3-h session, such that each mouse was scored 4 times daily. A stereotypy score of 1 was assigned when a mouse performed a stereotypic behavior at any time during the 20-s observation time. When a mouse was inactive at any point during the 20-s period, an inactivity score of 1 was assigned. Daily mean levels of stereotypy and overall activity were calculated as proportions of total observations and averaged by sex and family. Stereotypy also was calculated as a proportion of total active time. These data were collected for 4 d, which revealed the behavior as being consistent across days, and analyses used the final mean score of behavior across all 4 d.
Statistical analyses.
General linear models were run separately for male and female mice by using Minitab 13 (Minitab, State College, PA). Litter size was a covariate; weaning age (in Colonies 1 and 2) was an independent variable; and all interactions were included. Colony 1 data also were blocked by strain. Colony 3 mice were all weaned at the same age (35 d), but pre- and postweaning housing conditions varied; therefore, early- and late-housing types were included as blocking factors in colony 3 analyses. In addition, the interaction of litter size with housing type was included.
Whenever significant effects of litter size were found, litter size was tested as a predictor of offspring weight at weaning by using litter size as a covariate and blocking by weaning age or housing and its interactions. To investigate whether weight at weaning predicted adult stereotypic behavior as previously reported,35,36 we replaced litter size with weight at weaning in the models. Models were checked for orthogonality by comparing the sequential and adjusted sums of squares in model outputs.10 If this analysis indicated nonorthogonality, models were analyzed with the main effects in all possible orders, reporting only the smallest F ratio and largest P value obtained. In addition, data were checked to ensure they met model assumptions of homogeneity of variance, and log transformations were applied when necessary. Differences were considered significant at a P value of less than 0.05, and significant interactions were reanalyzed according to the interaction term to investigate their causes further. We report stereotypic behaviors as a proportion of total active time; reporting the findings as a proportion of total observations yielded qualitatively similar results.
Results
Litter size and stereotypic behavior.
In colony 1, CD1 female mice showed more stereotypic behavior as adults than did B6 female mice (P = 0.04, F[1,6] = 6.8), as did female mice from larger litters (Figure 1). In contrast, the behavior of male mice in colony 1 was not significantly influenced by strain (P > 0.05; F[1,4] = 1.0) or litter size (P > 0.05; F[1,4] = 2.1). Delayed weaning age did not significantly affect stereotypic behavior (female mice: P > 0.05, F[1,6] = 2.7; male mice: P > 0.05, F[1,4] = 2.1), even in mice from larger litters (P > 0.05).
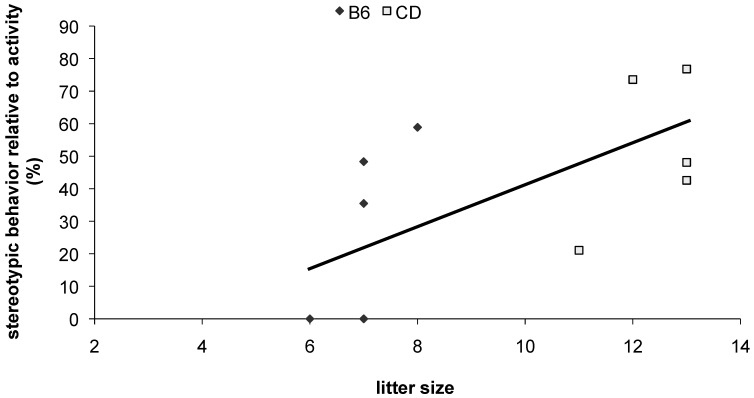
Figure 1.
Stereotypic behavior in colony 1 female mice. Those from larger litters were more stereotypic as adults (general linear model: P = 0.006, F[1,6] = 17.2; analyses were blocked for strain effects). B6, C57BL/6N; CD, CD1(ICR).
Similar results were found for colony 2: litter size positively predicted stereotypic behavior in adult female mice (Figure 2), whereas male stereotypic behavior was not significantly affected (P > 0.05, F[1,40] = 0.27). In addition, weaning age did not significantly affect stereotypic behavior (female mice: P > 0.05, F[2,39] = 1.3; male mice: P > 0.05, F[2,40] = 1.4), but again even in mice from larger litters (P > 0.05).
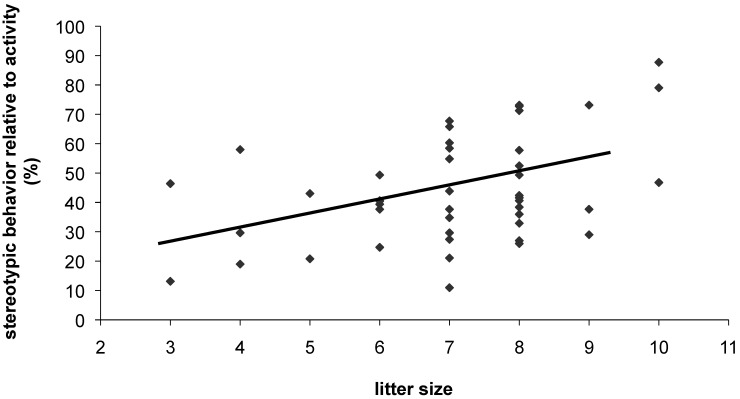
Figure 2.
Stereotypic behavior in colony 2 female mice. Litter size positively predicted stereotypic behavior in adults (P = 0.004, F[1,39] = 9.4).
In colony 3, female stereotypic behavior was affected by an interaction between postweaning enrichment and litter size (Figure 3). Litter size significantly predicted stereotypic behavior in female mice that were weaned into enriched (P = 0.005, F[1,4] = 30.6) but not into standard (P > 0.05, F[1,4] = 0.14) cages. In male mice, stereotypies were not significantly influenced by litter size (P > 0.05, F[1,10] = 1.0), enrichment (P > 0.05, F[1,10] = 0.48), or the interaction between enrichment and litter size (P > 0.05, F[1,4] = 0.9).
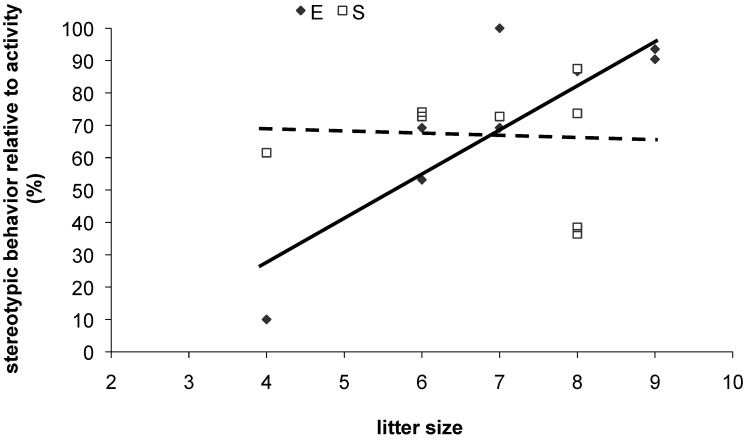
Figure 3.
Stereotypic behavior in colony 3 female mice. Postweaning enrichment significantly interacted with litter size (general linear model: P = 0.042, F[1,10] = 5.4). Litter size significantly predicted stereotypic behavior in female mice weaned into enriched (E; P = 0.005, F[1,4] = 30.6) but not standard, nonenriched (S; P > 0.05, F[1,4] = 0.14) cages.
Litter size, weight at weaning, and stereotypic behavior.
Litter size was not a significant predictor of weight at weaning in colony 1 (female mice: P > 0.05, F[1,6] = 0.34; male mice: P > 0.05, F[1,4] = 0.41) or colony 2 (female mice: P > 0.05, F[1,33] = 1.7; male mice: P > 0.05, F[1,36] = 0.10). Litter size was a significant negative predictor of weight at weaning in colony 3 female mice (P = 0.002, F[1,10] = 17.4) but not male mice (P > 0.05, F[1,10] = 3.9). Furthermore, weight at weaning was not a significant predictor of adult stereotypic behavior (colony 1 female mice: P > 0.05, F[1,6] = 0.19; colony 1 male mice: P > 0.05, F[1,4] = 1.9; colony 2 female mice: P > 0.05, F[1,35] = 0.90; colony 2 male mice: P > 0.05, F[1,36] = 0.49; colony 3 female mice: P > 0.05, F[1,9] = 4.2; colony 3 male mice: P > 0.05, F[1,9] = 0.95). Late-weaned litters were heavier at weaning than were early-weaned litters (colony 1 female mice: P = 0.001, F[1,6] = 188.0; colony 1 male mice: P = 0.001, F[1,4] = 236.2); colony 2 female mice: P = 0.001, F[2,33] = 112.0; colony 2 male mice: P = 0.001, F[2,36] = 107.7).
Discussion
Stereotypic behavior in adult female mice covaried positively with litter size in Colonies 1 and 2, and in the subset of females weaned into enriched conditions in colony 3. Therefore, female mice from larger litters showed more stereotypy as adults as compared with female mice from smaller litters. In contrast, male stereotypic behavior in adulthood was not significantly related to litter size.
Although this result was partially consistent with our hypothesis that large litters are most likely to develop motor stereotypies, the data were inconsistent with our proposed mechanism. We had hypothesized that offspring that share early resources with many siblings and thus receive less maternal investment would develop more slowly,12,18 with this effect and their lighter weights at weaning acting to elevate their later stereotypic behavior. Except in colony 3’s female mice, however, litter size did not significantly predict offspring weight at weaning. In addition, weight at weaning did not predict later stereotypic behavior, such that the results of the current study failed to replicate previous findings.35,36 Furthermore, extending the duration of pup access to maternal resources by delaying weaning did not reduce stereotypic behavior, even in mice from larger litters and despite a subsequent increase in the pups’ weights at weaning. This finding was contrary to previous findings in laboratory mice35,36 and to studies of weaning age in other species including American mink (Neovison vison) and African striped mice (Rhabdomys pumilio).14,20 Therefore, although litter size seems a useful predictor of adult female stereotypy in laboratory mice, the effect did not seem mediated by reduced milk intake leading to smaller body weights.
An alternative cause of these effects could be the early social environment: aspects of early experience that do not affect milk transfer and thus weight gain. Such early social effects have been implicated in other studies. In one study of motor coordination,32 litter size effects were evident during early lactation, even before the emergence of weight differences due to litter size effects on nutrition. Likewise in African striped mice, pups that receive both paternal and maternal care are less stereotypic in adulthood than are conspecifics reared by the dam alone.16 These effects due to biparental care are unlikely to be mediated by milk transfer and did not affect pup weight, and so demonstrate that other aspects of parental care are key14—a hypothesis further suggested by the fact that in African striped mice, weight at weaning does not predict later stereotypic behavior, even though age at weaning does.14 One potentially important nonnutritive aspect of parental care is the amount of licking and grooming directed to pups early in life. In neonatal rats, this behavior is a powerful determinant of adult offspring response to stressors and other aspects of adult behavior. 6,24 In larger litters, each individual pup receives less maternal grooming because there are many pups for her to lick.6 This hypothesis potentially explains 2 aspects of our data: the lack of weaning age effect— because in mice, maternal licking of pups is negligible beyond postnatal day 182,17—and why litter size affected female mice more than male mice. Because maternal licking reportedly is biased toward male mice,1 female mice may experience a greater loss in maternal licking, or this activity falls below a critical level, in female mice with numerous siblings. An alternative aspect of the early social environment is interactions with siblings, which has been proposed as one means by which litter size can affect offspring behavioral development, for example, social play in juveniles22 and emotional and maternal behavior in adulthood.23,31 The cause-and-effect relationship between litter size and stereotypic behavior inherent in our original hypothesis, if correct, would then be consistent with the corpus of work showing lasting behavioral effects of the early social and physical environment (for example, social isolation25 and enrichment3,21,34).
The hypotheses we offer assume that developing in a larger litter causes later effects on adult phenotypes. However, our data were merely correlational, and perhaps increased stereotypic behavior and large litter sizes covary for completely different reasons. In particular, maybe highly stereotypic dams have bigger litters and their offspring then inherit this behavior. For example, in both African striped mice and farmed American mink, offspring with stereotypic parents are more stereotypic as adults than are offspring of nonstereotypic parents.11,16,26 Furthermore, stereotypic mothers of these species have higher reproductive outputs, including larger litter sizes.13,15 Whether the same pattern holds for laboratory mice is as yet unknown.
One last unexpected result warrants mention. In Colonies 1 and 2, mice were housed in standard cages, and adult female mice displayed the expected link between large litter size and stereotypy. In colony 3, mice were housed in both standard and enriched cages, but only those housed in enriched cages showed the expected association between litter size and stereotypy. Adult mice housed in standard cages, including mice from small litters, had considerable stereotypic behavior (for example, higher than that in colony 2). This finding might be explained by the nonstandard infant housing systems used in this colony. Infants here were used in studies on preweaning dispersal behavior, during which they lived in 2 cages connected by a long tunnel. Therefore at weaning, colony 3 mice moved to standard cages experienced a decrease in cage size and complexity. Such decreases in environmental quality can elevate stereotypic behavior,21 and such ‘negative contrast’ effects might have masked the litter-size effects that were evident in their colony-mates that were weaned to large enriched cages instead.
We also assessed another abnormal repetitive behavior: barbering (incessant hair-plucking).3 However, due to small sample sizes and the qualitative nature of the data, the results (all nonsignificant) were inconclusive and so are not presented here. Whether litter size similarly affects barbering now needs further research, as do the mechanisms underlying the links we found between bar-mouthing and similar stereotypies in adult female mice and their litter size in infancy. Such research should include the assessment of dam stereotypy and the cross-fostering of litters, to investigate the roles and heritability of maternal stereotypy; the experimental manipulation of litter size, to investigate whether its effects are actually causal; the study of maternal licking and grooming, and of pup–pup interactions, to assess the importance of these aspects of early social experience; and the measurement of adult levels of stress, anxiety, and perseveration to investigate whether these measures are affected concurrently.
Whatever the mechanisms, however, our current findings have immediate implications. Given the links between mouse stereotypies and other aspects of functioning, such as recurrent perseveration, our work combines with other studies of litter size effects to suggest that controlling for litter size could reduce variability in behavioral data and reduce the number of mice required in some research. Currently, many millions of mice are subjects in neurobehavioral research globally each year, and millions of mice intended for such research are shipped annually from commercial suppliers, but no knowledge about a mouse's litter size is transferred from vendor to researcher. This lack makes it impossible to control for natural litter size variation in purchased stock. However, statistically controlling for litter size could reduce variance in experimental data and support the use of fewer animals.
In conclusion, we here report that litter size positively predicts adult stereotypic behavior in female laboratory mice. Results generated in 3 separate mouse colonies, using 2 inbred strains and an outbred stock, demonstrate high external validity. Future work should investigate the driving force behind this relationship. The current findings encourage further investigation into early social environments as predictors of individual predisposition to stereotypic behavior and as means for improving the validity of behavioral research.
Acknowledgments
We thank the Roder laboratory at Mount Sinai Hospital (Toronto, Ontario, Canada) for hosting the colony 1 study, the Animal Welfare Institute for funding the colony 1 study, the ACLAM Foundation for funding the colony 2 study, and NSERC for funding the colony 3 study.
References
- 1.Alleva E, Caprioli A, Laviola G. 1989. Litter gender composition affects maternal behavior in primiparous mouse dams (Mus musculus). J Comp Psychol 103:83–87 [DOI] [PubMed] [Google Scholar]
- 2.Bechard A, Mason G. 2010. Leaving home: a study of laboratory mouse pup independence. Appl Anim Behav Sci 125:181–188 [Google Scholar]
- 3.Bechard A, Meagher R, Mason G. 2011. Environmental enrichment reduces the likelihood of alopecia in adult C57BL6/J mice. J Am Assoc Lab Anim Sci 50:171–174 [PMC free article] [PubMed] [Google Scholar]
- 4. Canadian Council on Animal Care. 1995. Guide to the care and use of experimental animals, vol 2. Ottawa (Canada): Canadian Council on Animal Care.
- 5.Dimitsantos E, Escorihuela RM, Fuentes S, Armario A, Nadal R. 2007. Litter size affects emotionality in adult male rats. Physiol Behav 92:708–716 [DOI] [PubMed] [Google Scholar]
- 6.Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. 2002. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav 73:61–75 [DOI] [PubMed] [Google Scholar]
- 7.Garner JP. 2005. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J 46:106–117 [DOI] [PubMed] [Google Scholar]
- 8.Garner JP, Thogerson CM, Dufour BD, Würbel H, Murray JD, Mench JA. 2011. Reverse-translational biomarker validation of abnormal repetitive behaviors in mice: an illustration of the 4Ps modeling approach. Behav Brain Res 219:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gervais MC, Defries JC, Kuse AR. 1977. Open-field behavior in mice: effect of litter size. Behav Biol 20:519–522 [DOI] [PubMed] [Google Scholar]
- 10.Grafen A, Hails R. 2002. Modern statistics for the life science. New York (NY): Oxford University Press [Google Scholar]
- 11.Hansen CPB. 1993. Stereotypies in ranch mink—the effect of genes, litter size, and neighbors. Behav Processes 29:165–178 [DOI] [PubMed] [Google Scholar]
- 12.Hudson R, Trillmich F. 2008. Sibling competition and cooperation in mammals: challenges, developments, and prospects. Behav Ecol Sociobiol 62:299–307 [Google Scholar]
- 13.Jeppesen LL, Heller KE, Bildsoe A. 2004. Stereotypies in female farm mink (Mustela vison) may be genetically transmitted and associated with higher fertility due to effects on body weight. Appl Anim Behav Sci 86:137–143 [Google Scholar]
- 14.Jones M, Mason G, Pillay N. 2010. Early social experience influences the development of stereotypic behaviour in captive-born striped mice Rhabdomys. Appl Anim Behav Sci 123:70–75 [Google Scholar]
- 15.Jones M, van Lierop M, Mason G, Pillay N. 2010. Increased reproductive output in stereotypic captive Rhabdomys females: potential implications for captive breeding (Rhabdomys). Appl Anim Behav Sci 123:63–69 [Google Scholar]
- 16.Jones M, van Lierop M, Pillay N. 2008. All a mother's fault? Transmission of stereotypy in striped mice (Rhabdomys). Appl Anim Behav Sci 115:82–89 [Google Scholar]
- 17.König B, Markl H. 1987. Maternal care in house mice. Behav Ecol Sociobiol 20:1–9 [Google Scholar]
- 18.König B, Riester J, Markl H. 1988. Maternal care in house mice (Mus musculus): II. J Zool (Lond) 216:195–210 [Google Scholar]
- 19.LaBarba RC, White JL. 1971. Litter size variations and emotional reactivity in BALB/c mice. J Comp Physiol Psychol 75:254–257 [DOI] [PubMed] [Google Scholar]
- 20.Latham N, Mason G. 2008. Maternal deprivation and the development of stereotypic behaviour. Appl Anim Behav Sci 110:84–108 [Google Scholar]
- 21.Latham N, Mason G. 2010. Frustration and perseveration in stereotypic captive animals: is a taste of enrichment worse than none at all? Behav Brain Res 211:96–104 [DOI] [PubMed] [Google Scholar]
- 22.Laviola G, Alleva E. 1995. Sibling effects on the behavior of infant mouse litters (Mus domesticus). J Comp Psychol 109:68–75 [DOI] [PubMed] [Google Scholar]
- 23.Laviola G, Terranova ML. 1998. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev 23:197–213 [DOI] [PubMed] [Google Scholar]
- 24.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney M. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277:1659–1662 [DOI] [PubMed] [Google Scholar]
- 25.Lukkes JL, Watt MJ, Lowry CA, Forster GL. 2009. Consequences of postweaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason GJ. 1991. Stereotypies: a critical review. Anim Behav 41:1015–1037 [Google Scholar]
- 27.Mason GJ.Mason G, Rushen J. 2006. Stereotypic behaviour: fundamentals and applications to animal welfare and beyond, p 325–357. In: Stereotypies in captive animals, 2nd ed. Wallingford (CT): CAB International.
- 28.Nevison CM, Hurst JL, Barnard CJ. 1999. Why do male ICR(CD1) mice perform bar-related (stereotypic) behaviour? Behav Processes 47:95–111 [DOI] [PubMed] [Google Scholar]
- 29.Ödberg FO. 1978. Introduction to abnormal behaviours: stereotypies, p. 475–480. In: Proceedings of the First World Congress on Ethology Applied to Zootechnics. Madrid (Spain): Industrias Grafices Espana.
- 30.Priestnall R. 1973. The effects of litter size and postweaning isolation or grouping on adult emotionality in C3H mice. Dev Psychobiol 6:217–224 [DOI] [PubMed] [Google Scholar]
- 31.Ricceri L, Moles A, Crawley J. 2007. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res 176:40–52 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T. 1998. Effects of litter size on behavioral development in mice. Reprod Toxicol 12:613–617 [DOI] [PubMed] [Google Scholar]
- 33.Vickery S, Mason G. 2003. Behavioural persistence in captive black bears: implications for reintroduction. Ursus 14:35–43 [Google Scholar]
- 34.Würbel H, Chapman R, Rutland C. 1998. Effect of feed and environmental enrichment on development of stereotypic wire-gnawing in laboratory rodents. Appl Anim Behav Sci 60:69–81 [Google Scholar]
- 35.Würbel H, Stauffacher M. 1997. Age and weight at weaning affect corticosterone level and development of stereotypies in ICR mice. Anim Behav 53:891–900 [Google Scholar]
- 36.Würbel H, Stauffacher M. 1998. Physical condition at weaning affects exploratory behaviour and stereotypy development in laboratory mice. Behav Processes 43:61–69 [DOI] [PubMed] [Google Scholar]