Abstract
Collecting and analyzing available information on the building plans, concepts, and workflow from existing animal facilities is an essential prerequisite for most centers that are planning and designing the construction of a new animal experimental research unit. Here, we have collected and analyzed such information in the context of the European project Infrafrontier, which aims to develop a common European infrastructure for high-throughput systemic phenotyping, archiving, and dissemination of mouse models. A team of experts visited 9 research facilities and 3 commercial breeders in Europe, Canada, the United States, and Singapore. During the visits, detailed data of each facility were collected and subsequently represented in standardized floor plans and descriptive tables. These data showed that because the local needs of scientists and their projects, property issues, and national and regional laws require very specific solutions, a common strategy for the construction of such facilities does not exist. However, several basic concepts were apparent that can be described by standardized floor plans showing the principle functional units and their interconnection. Here, we provide detailed information of how individual facilities addressed their specific needs by using different concepts of connecting the principle units. Our analysis likely will be valuable to research centers that are planning to design new mouse phenotyping and archiving facilities.
Abbreviation: BRC, Biological Resource Centre; CR, Charles River; GMC, German Mouse Clinic; HMGU, Helmholtz Centre Munich Central Animal Facility; HZI, Helmholtz Centre for Infection Research; ICS, Institut Clinique de la Souris; IRC, Intragene Resource Center; IVC, individually ventilated cage; JAX, The Jackson Laboratory; MLC, Mary Lyon Centre; RSF, Wellcome Trust Sanger Institute Research Support Facility; TCP, Toronto Centre for Phenogenomics
The mouse is one of the leading experimental model systems to understand human biology and to develop new treatments for human disease. More than 35,000 mutant mouse lines and about 4000 lines from genetic reference populations will become available over the next 10 y, and all of these lines will have to be characterized, archived, and disseminated to the scientific community.1,4 Infrafrontier, the European infrastructure for phenotyping and archiving of the mouse mammalian genome, is a scientific program funded by the European Commission, which aims to develop a common European infrastructure for high-throughput systemic phenotyping, archiving, and dissemination of mouse models.16 Prerequisite for a high-quality, state-of-the-art scientific project is the availability of sophisticated and large-capacity infrastructures. Numerous documents and publications that provide design standards and technical criteria for the construction of animal research facilities already exist.7-9,13,17 However, they do not describe the relationships and interconnection between breeding, large-scale phenotyping, and archiving. To adapt existing and newly planned facilities for large-scale phenotyping and archiving, it is extremely helpful to have information on existing animal facilities and to analyze their organization and functioning. Therefore, a working group (WP5) of the Infrafrontier network assembled a comprehensive description of existing large phenotyping and archiving mouse facilities. A team of experts visited 9 research facilities and 3 commercial breeders in Europe, Canada, the United States, and Singapore. During the visits, information was collected about the basic organization of the facilities (hygiene, flow of material, mice, and personnel), their phenotyping (outline of laboratory spaces, procedures, assays, pipelines, and others) and archiving (outlines of rooms, special requirements, concepts) facilities, building plans (outline of rooms, floors, traffic), and technical specifications (construction materials and interior finishes).
We translated this information into standardized schematic floor plans and tables that outline the specific characteristics, basic structure, and interconnection of various functional units for each facility. From these data, we deduced several general concepts regarding the architecture of a state-of-the-art, large-scale phenotyping and archiving mouse facility. Our results summarize the structural organization of several modern large-scale animal phenotyping facilities, and this compilation likely will be useful for researchers, facility managers, and architects who are planning to construct new or upgrade existing facilities.
Materials and Methods
Data collection.
Several large mouse phenotyping and breeding facilities were selected for site visits, with the main emphasis on European facilities that are Infrafrontier partners. Additional large facilities in North America and in Singapore were included, and 3 commercial breeding facilities were visited to obtain insights into specific aspects of large-colony mouse breeding, for a total of 11 sites. The facilities from Infrafrontier partners were: the Mary Lyon Centre (MLC; Harwell, UK); Wellcome Trust Sanger Institute Research Support Facility (RSF; Hinxton, UK); Institut Clinique de la Souris (ICS; Strasbourg, France); Intragene Resource Center (IRC; Orleans, France); Helmholtz Zentrum München Central Animal Facility (HMGU; Munich, Germany); the German Mouse Clinic (GMC; Munich, Germany); Helmholtz Centre for Infection Research (HZI; Braunschweig, Germany); and Toronto Centre for Phenogenomics (TCP; Toronto, Canada). Additional facilities outside Europe included The Jackson Laboratory (JAX; Bar Harbor, ME) and the Biologic Resource Centre (BRC; Biopolis, Singapore). The commercial breeders were Charles River (CR; Lyon, France); Harlan (Horst, The Netherlands); and JAX.
Visits were conducted between September 2008 and November 2009. The visiting team was composed of members from Infrafrontier Working Group 5 and an architect. The 14 members of the visiting team represented specialists in archiving and phenotyping (scientists), facility operations (facility managers that were veterinarians and an economist), and facility planning (architect). The architect has extensive, specialized expertise in the design and construction of animal facilities. Each facility was visited by a subgroup of the visiting team such that at least one specialist from each field participated at each visit. The visits generally included presentations by the facility's scientific management, a comprehensive tour of the animal facility, and discussions with the heads of units and staff as well as facility managers. Standardized data sheets were used for reporting: (1) a building data sheet to record detailed information about the building itself, animal holding units, design of the holding units, support infrastructure, technical specifications, and construction materials; (2) individual data sheets for either phenotyping or archiving units to capture information about phenotyping assays, pipelines, types of archives, services, and so forth. The data sheets from each member of the visiting team were collected and collated to compendium data sheets.
Data analysis.
The results of each facility visit were summarized in a detailed report. Standardized floor plans describing the principle structure with respect to hygiene levels and functional units as well as the flow of material, animals, caretakers, and scientists were generated from building plans provided by the visited facilities. Summary tables were prepared for animal holding, building specifications, phenotyping, archiving, and other units (core breeding, quarantine, transgenic unit). An executive summary for each visited facility was generated to provide a short overview about the facility building itself, cage capacity, research context, concepts, special building features, phenotyping, archiving, other units, specialties, unexpected problems encountered, and lessons learned. The final documents were sent to the management of the visited facilities for approval and authorization. All detailed data that were collected during the visits were included in an internal Infrafrontier report. The full report and standardized data sheets are available on request from the Infrafrontier Working Group 5 coordinator (KS).
Results
Floor plans.
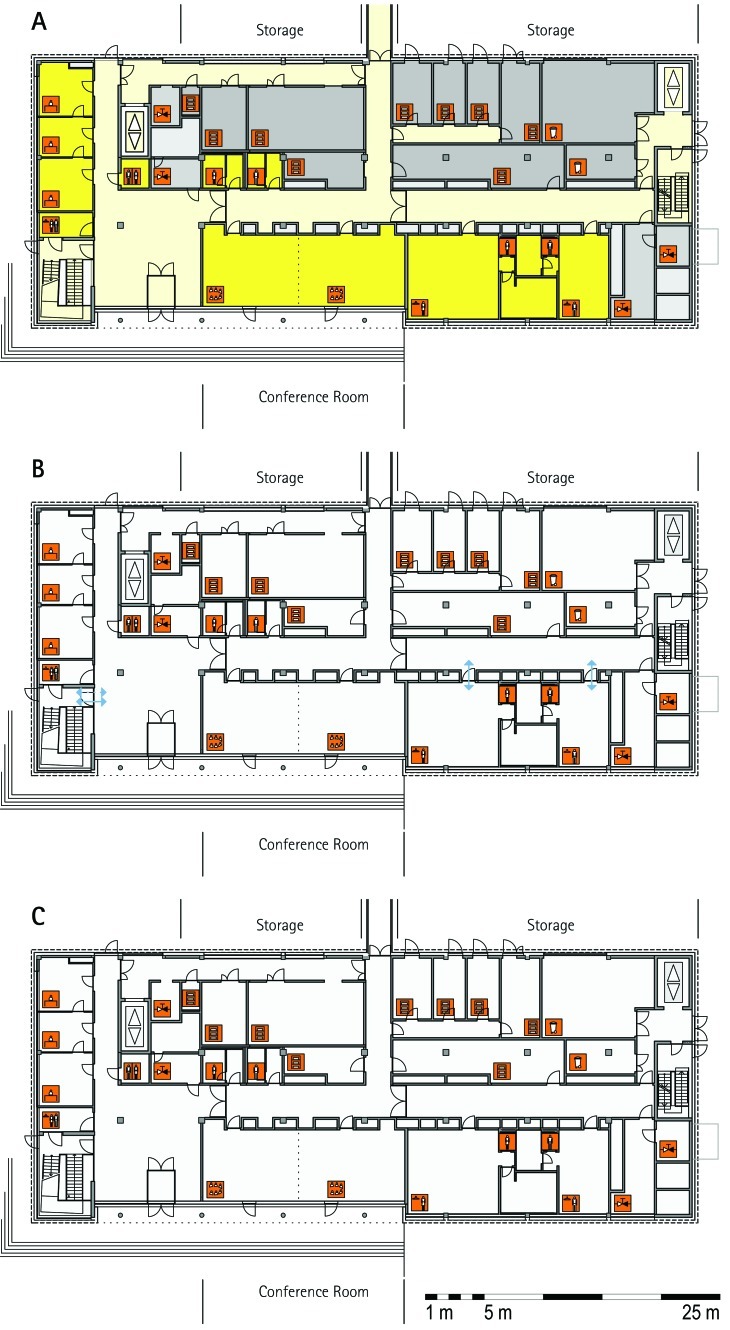
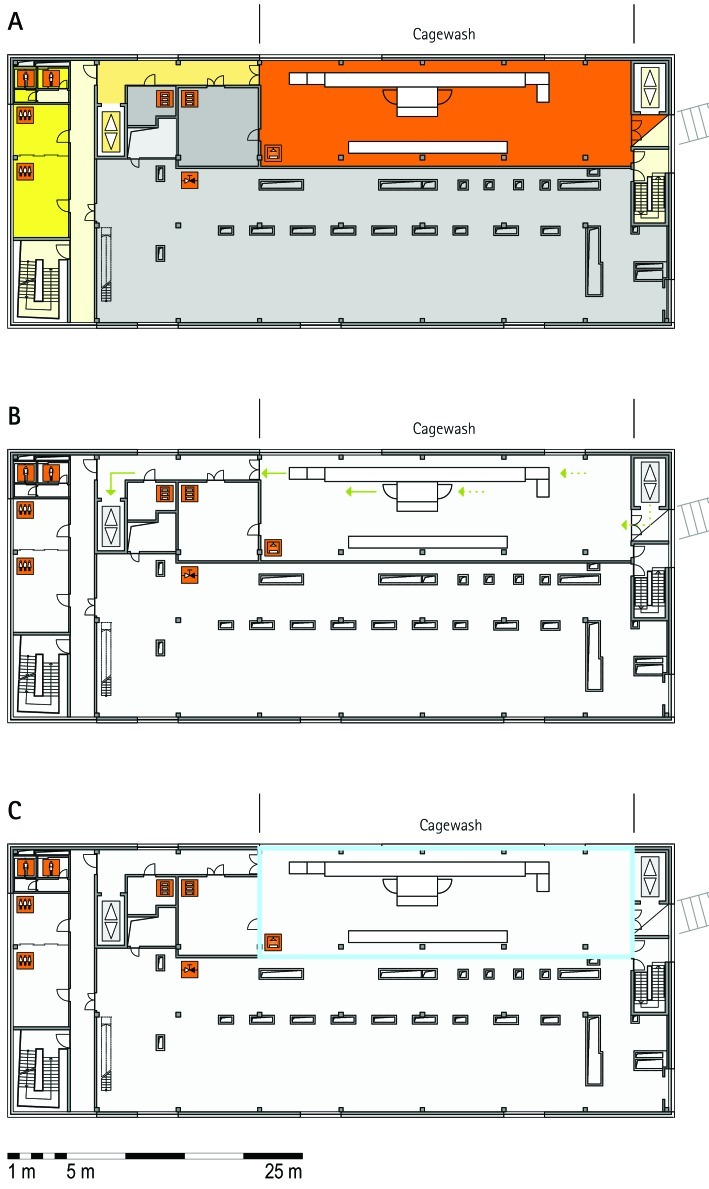
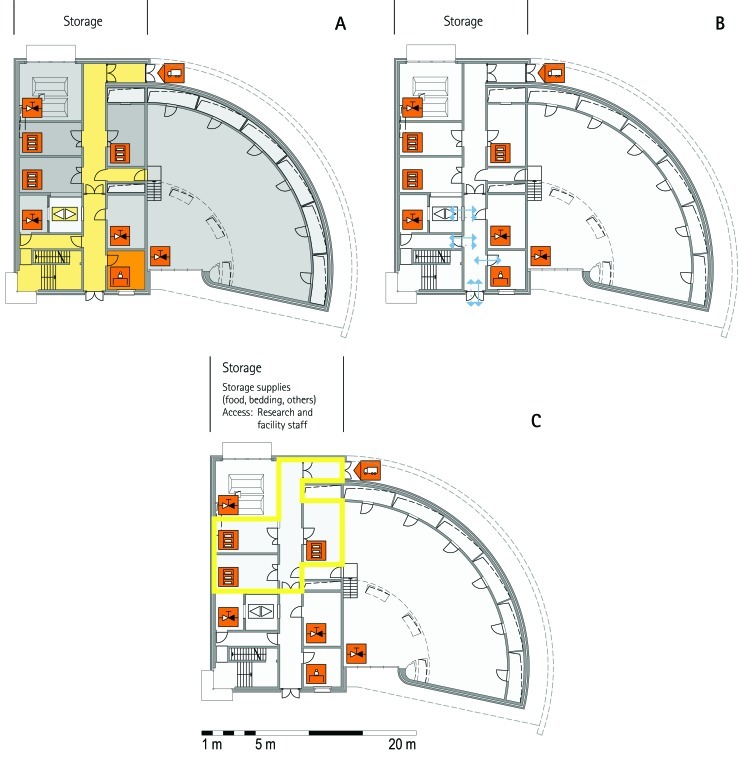
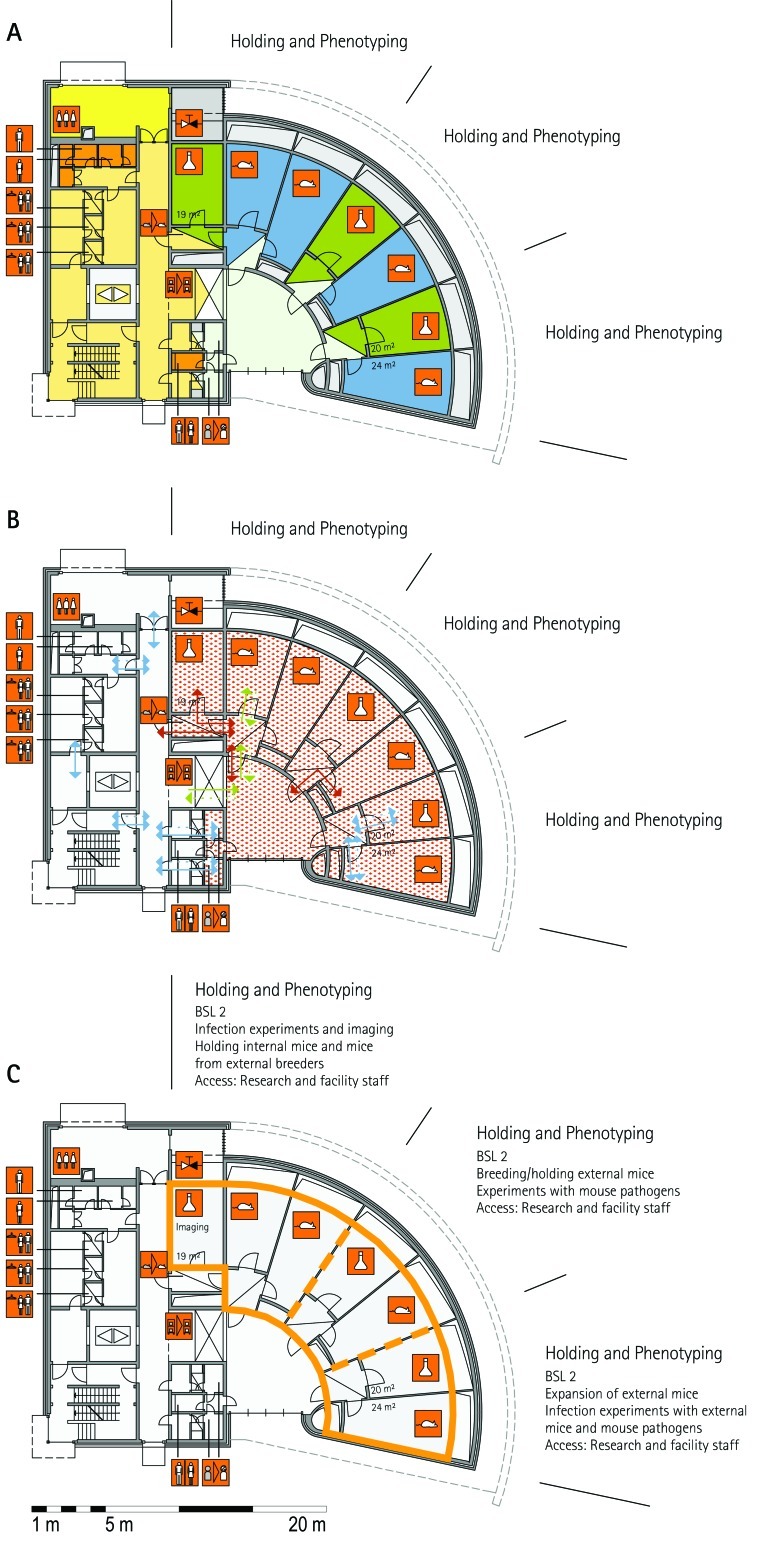
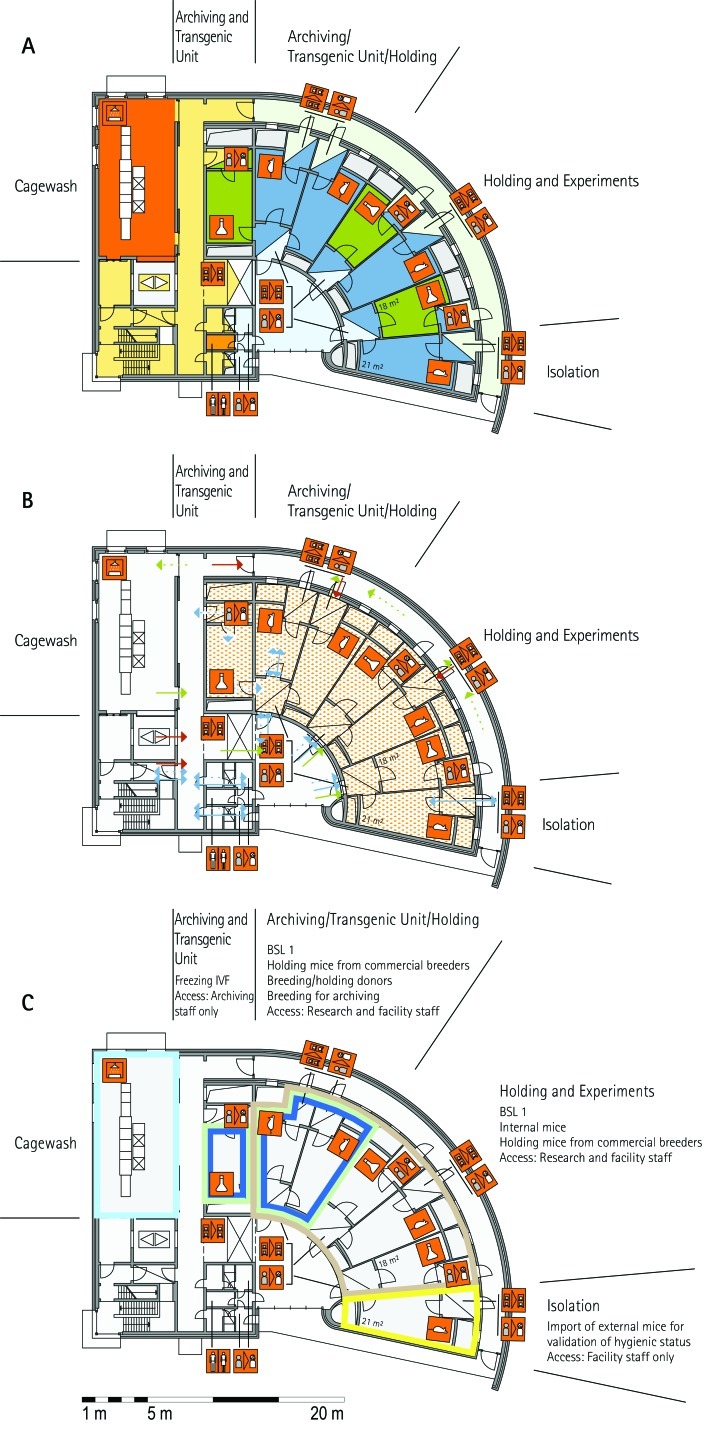
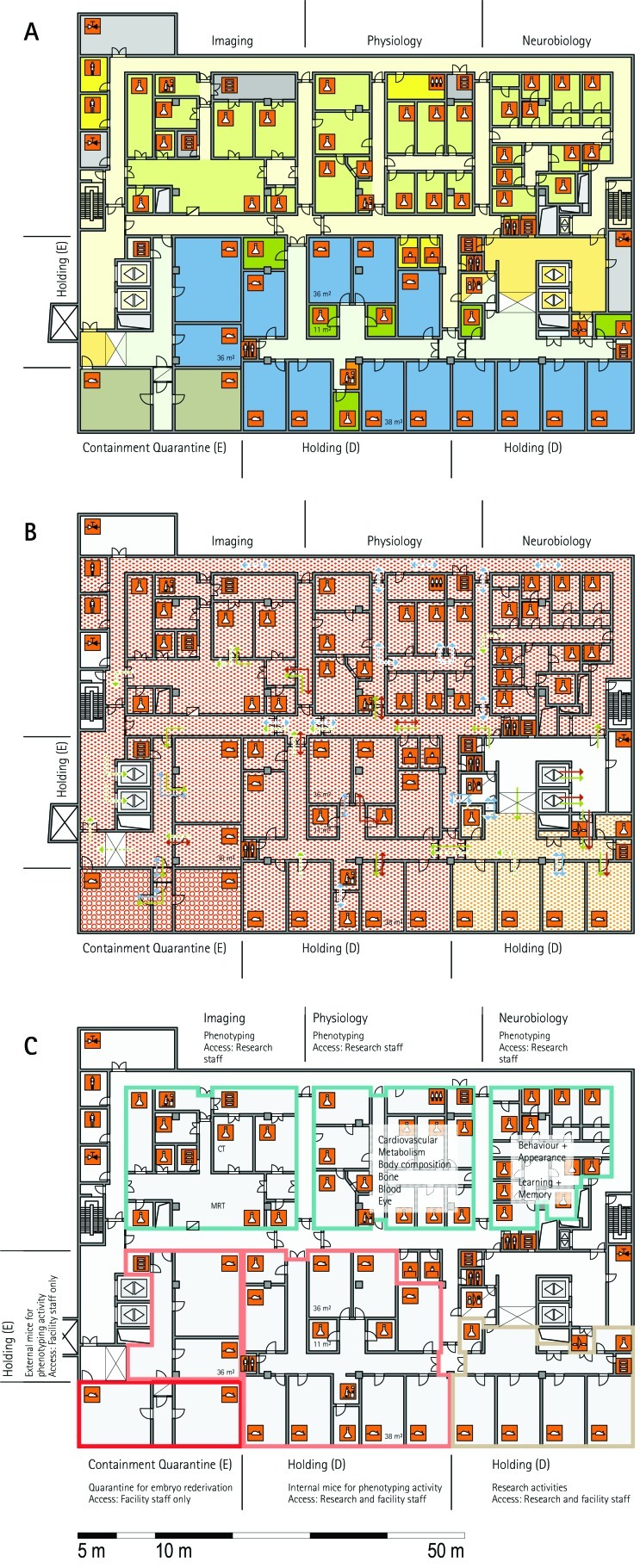
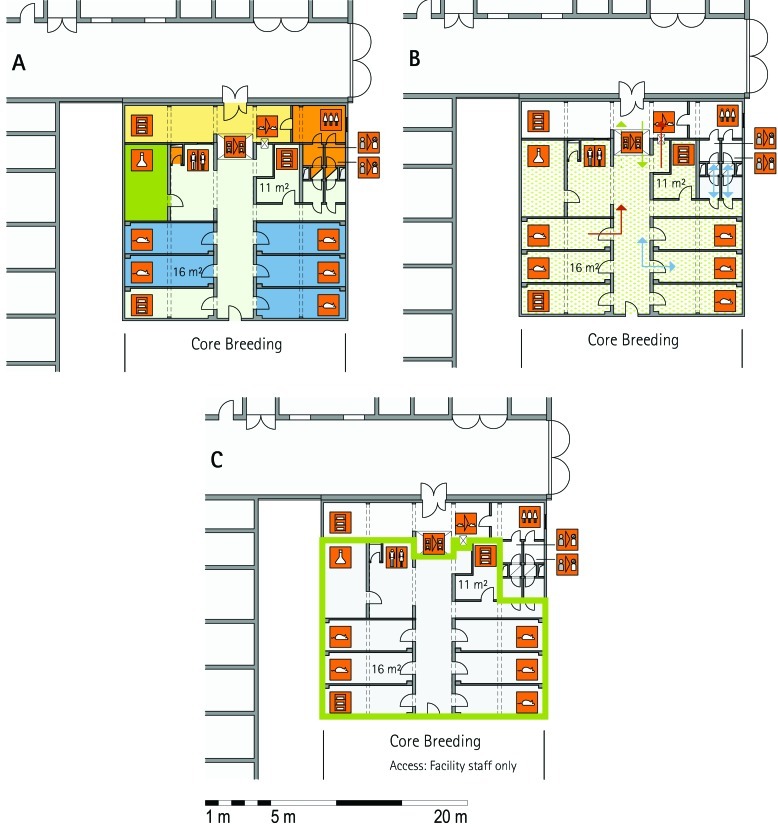
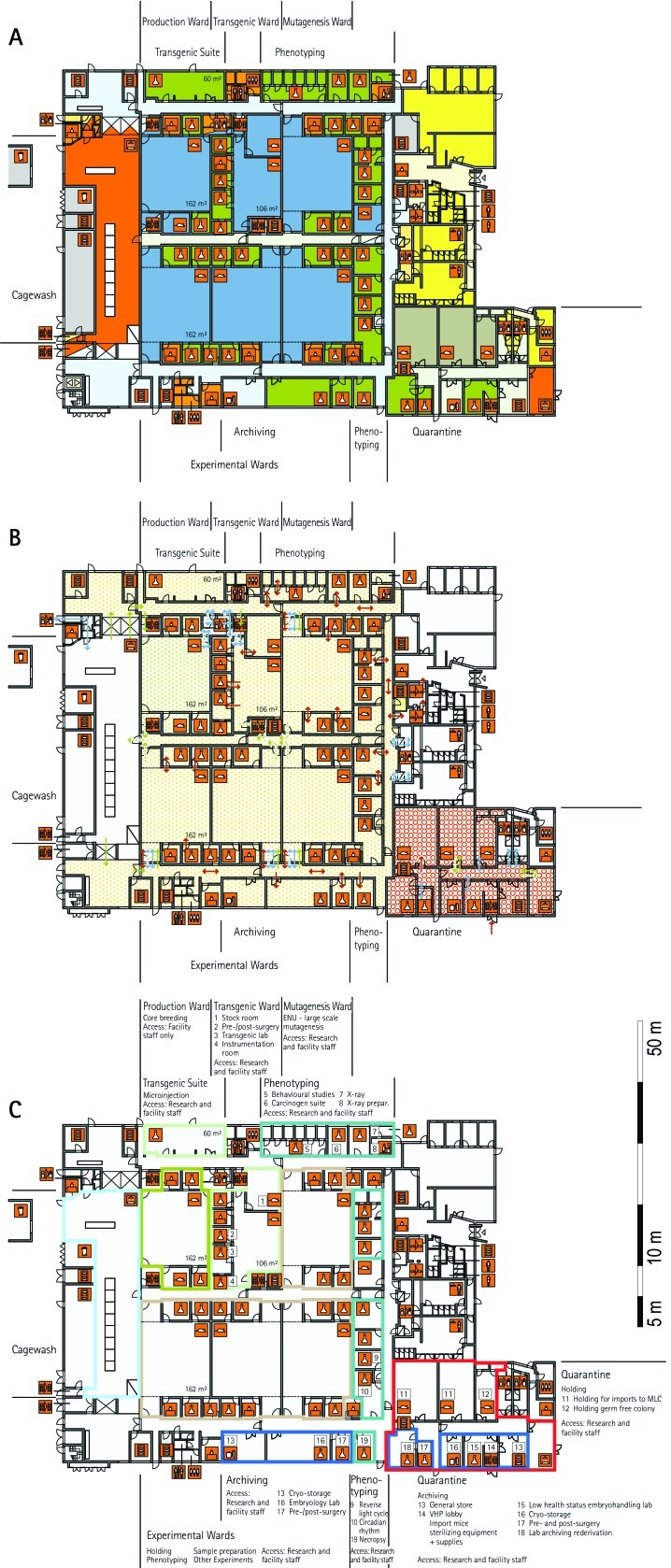
From all of the blueprints that we received during the visits, we generated standardized schematic floor plans for each facility (Figures 1 through 20). These floor plan schemes include all aspects that are important for the structural design; they also reflect the basic concepts for hygiene zones and trafficking of material and people. Three schemes were generated for each floor of a facility: zoning, flow and hygiene levels, and functional units.
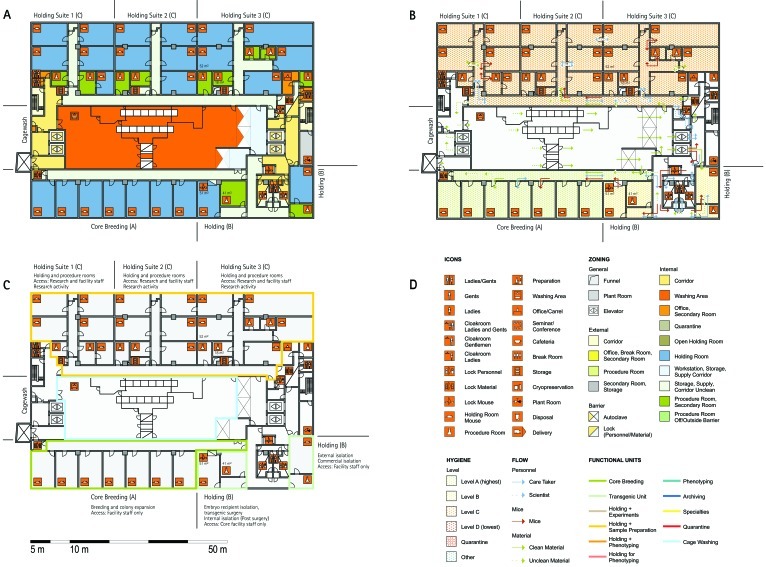
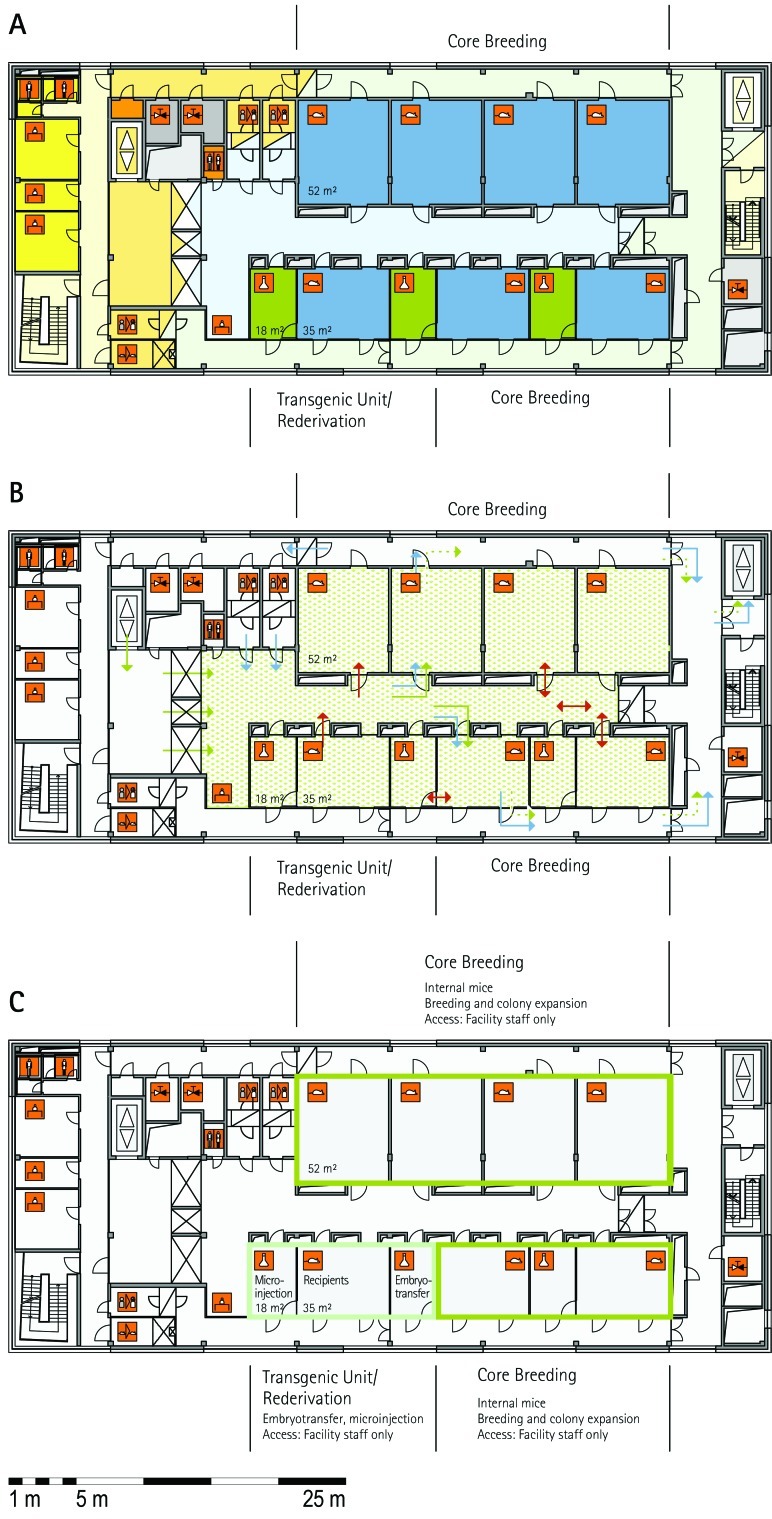
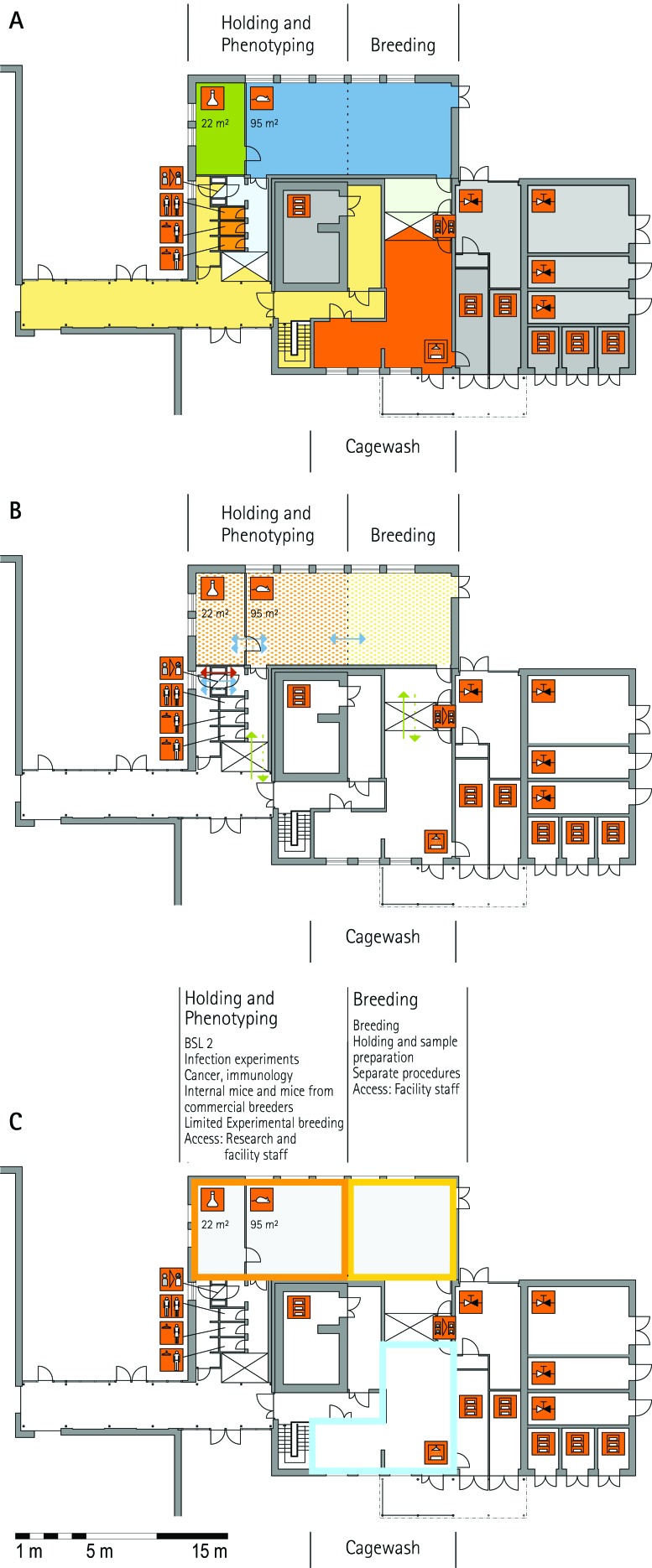
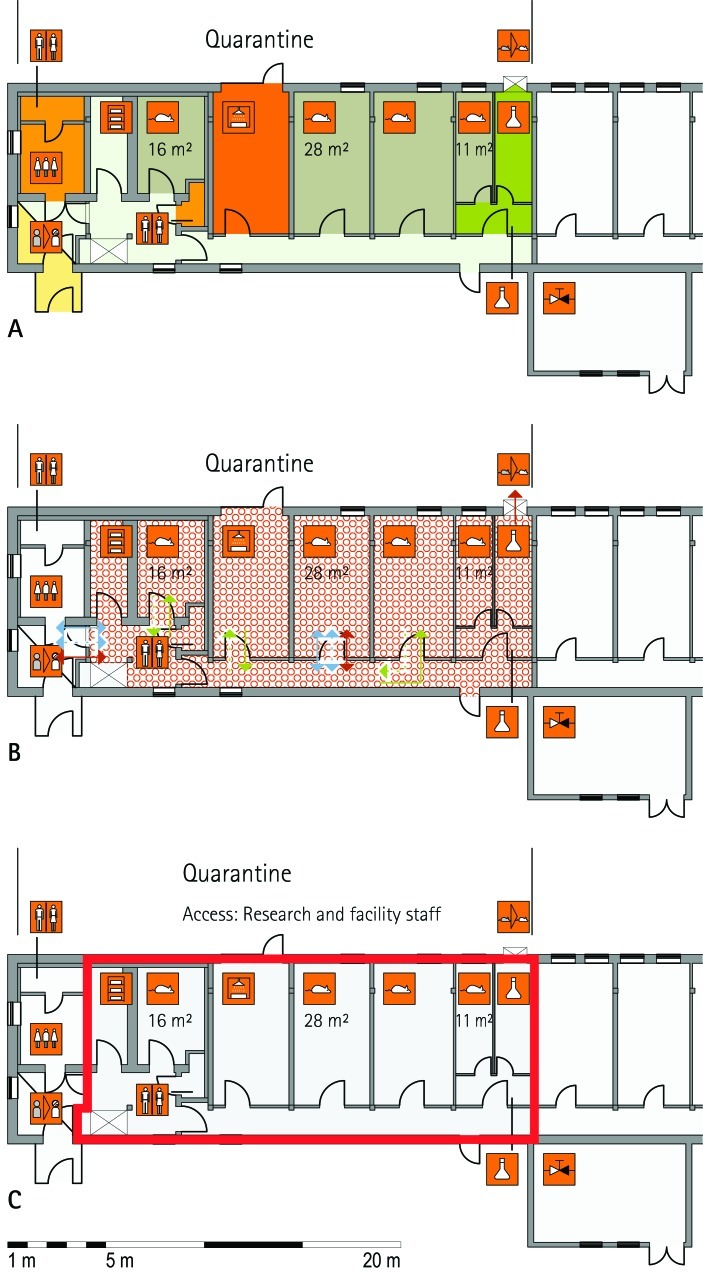
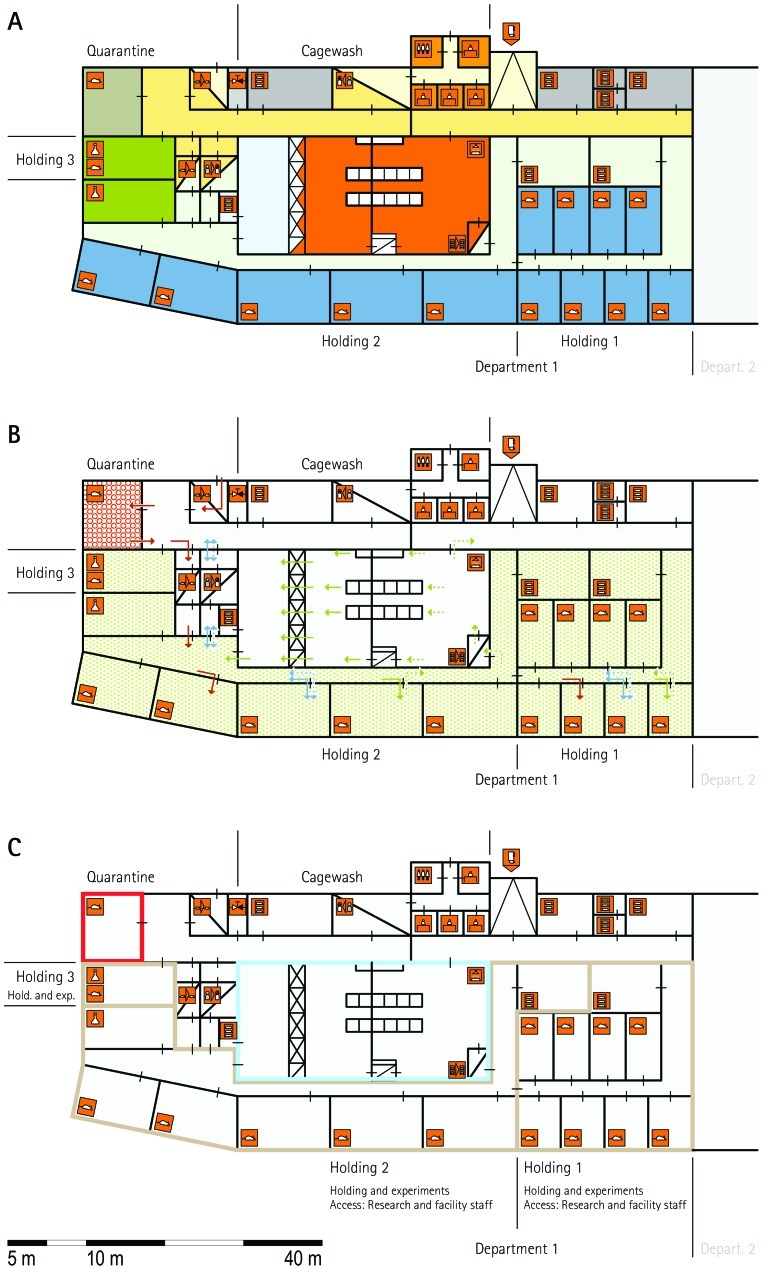
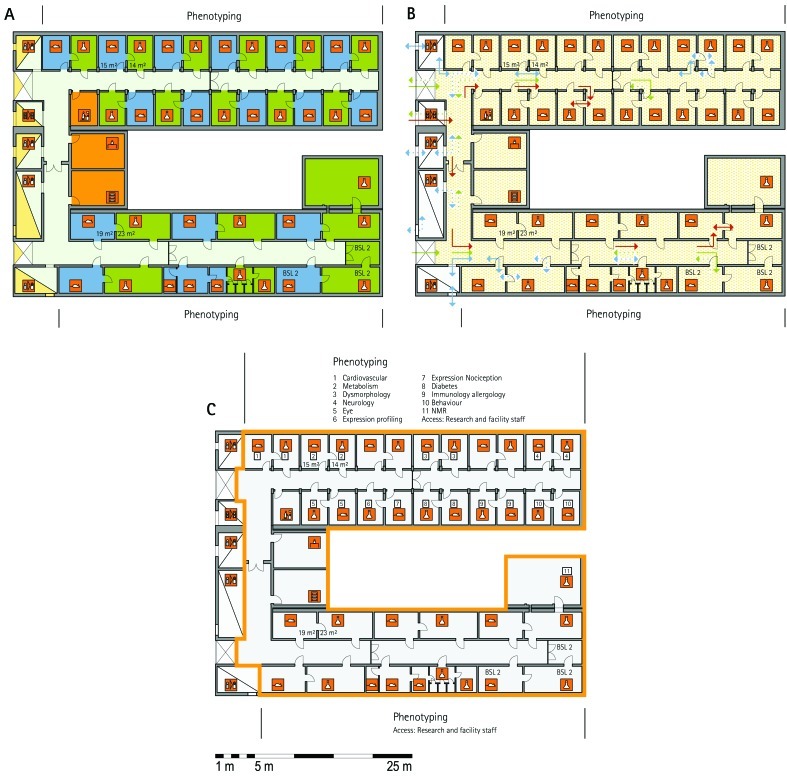
Figure 1.
Schematic floor plans—facility 1, plan 1. Three types of schematic floor plans that outline the layout of the rooms and floors, the hygiene zones, and traffic flow were generated from the information collected at the visited facilities. (A) Zoning scheme. The zoning scheme shows the various types of rooms, which are represented by icons. In addition, color coding indicates areas outside and within the barrier as well as the various functional units. (B) Flow and hygiene levels scheme. The flow and hygiene levels scheme shows the flow of personnel (caretaker, scientists), mice, and material (clean and soiled) and the various hygiene levels. (C) Functional unit scheme. Details of the functional use of the various units and access of the facility staff and scientists are presented. (D) Description of the icons and colors used in the various floor plans.
Figure 3.
Schematic floor plans—facility 1, plan 3. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 4.
Schematic floor plans—facility 1, plan 4. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 6.
Schematic floor plans—facility 2, plan 2. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 10.
Schematic floor plans—facility 4, plan 1. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 13.
Schematic floor plans—facility 4, plan 4. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 16.
Schematic floor plans—facility 4, plan 7. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 17.
Schematic floor plans—facility 4, plan 8. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 18.
Schematic floor plans—facility 4, plan 9. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
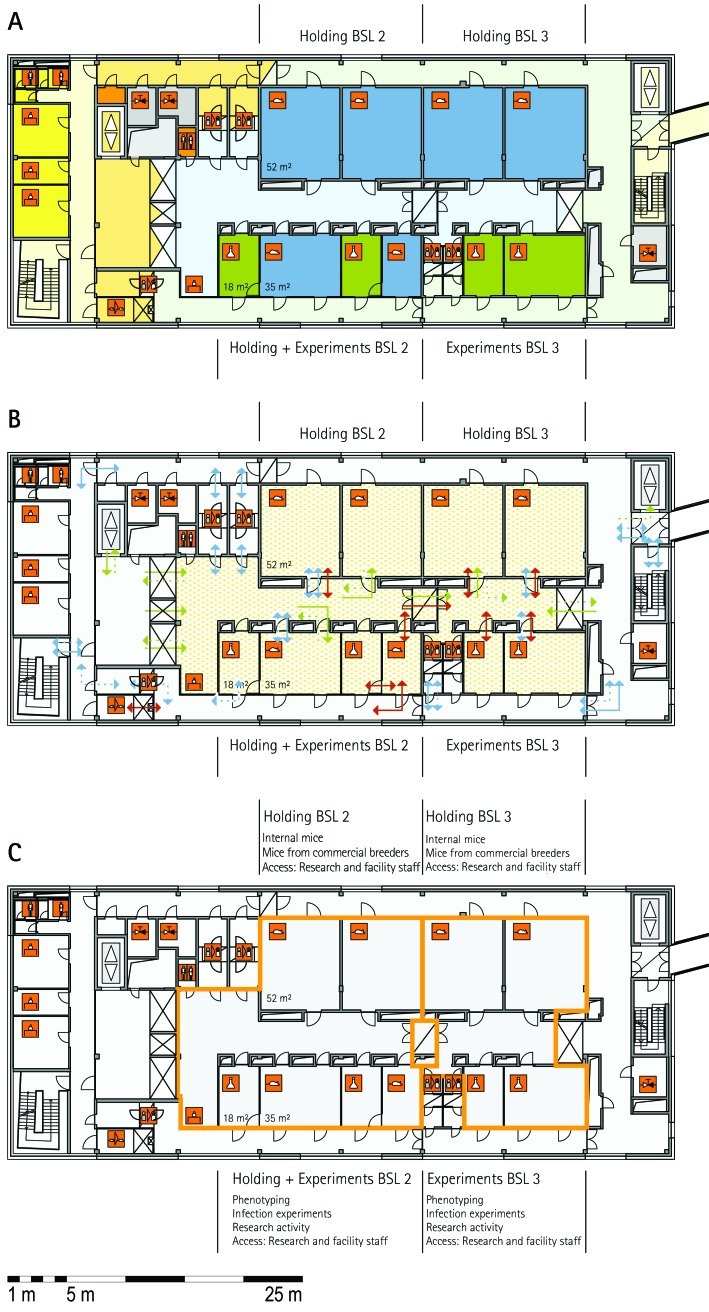
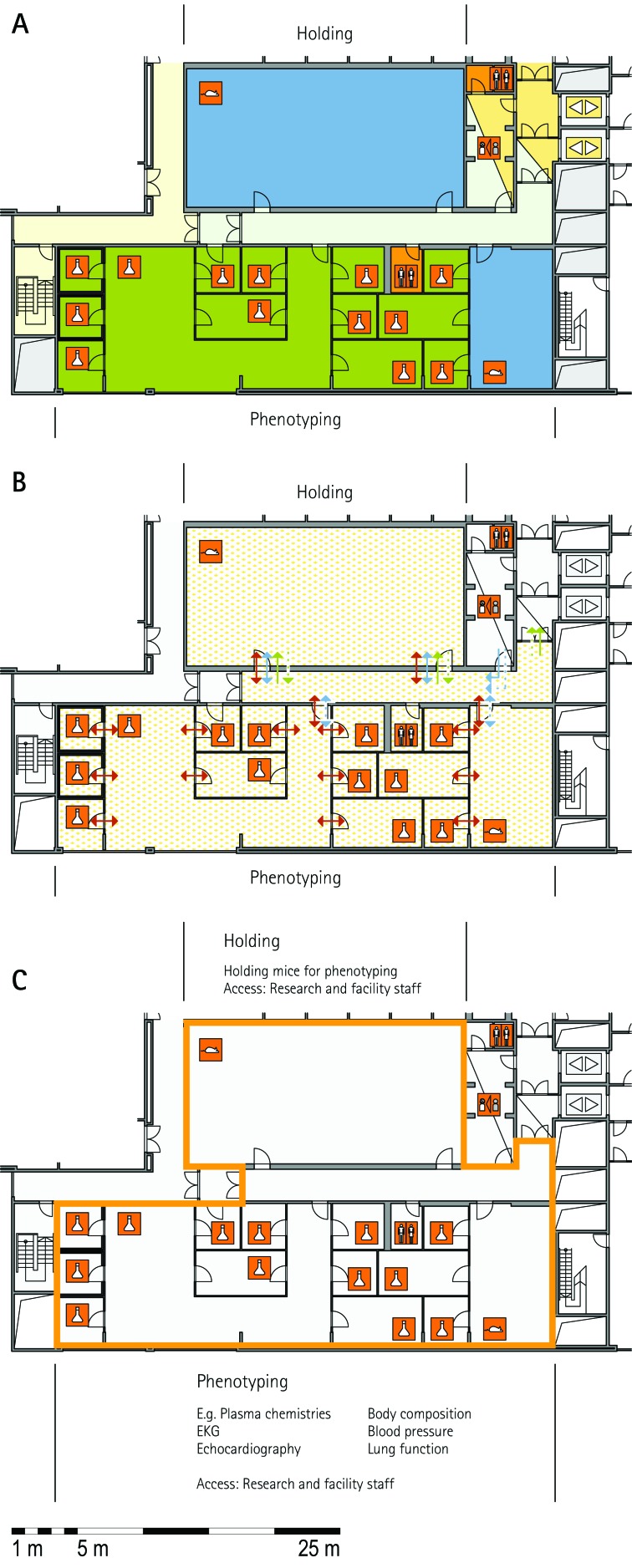
The zoning scheme (for example, Figure 1 A) shows different types of rooms represented by icons and color codes which indicate areas outside and behind the barrier as well as the different functional units. Different color codes depict the arrangement of housing and laboratory rooms, other functional rooms and the corridors.
The flow and hygiene levels scheme (for example, Figure 1 B) represents the flow of caretakers and scientists, mice, and clean and soiled material and the various hygiene levels. The different hygiene levels become readily obvious through the color-coding. The core breeding represents the highest hygiene level, whereas holding and phenotyping units have lower hygiene levels (Figures 1 B, 2 B, 7 B, 9 B, 11 B, and 14 B). The quarantine area (Figures 2 B, 8 B, 15 B, and 20 B) has a distinct color code, because this unit usually holds animals of undefined hygiene status. The flow of mice reflects the routes of animals between holding and phenotyping units or within the phenotyping unit (Figures 2 B, 5 B, 9 B, 12 B, 19 B, and 20 B). The flow pattern of clean and soiled material outlines the circular flow of material from the cleaning facility to the housing rooms and back (Figures 1 B, 9 B). The flow of personnel shows that caretakers have exclusive access to units at high hygiene levels, and scientists have access to experimental units where phenotyping archiving or other experimental procedures are performed (Figures 1 B and 9 B). The functional unit scheme (for example, Figure 1 C) presents details about the location and interconnection of various functional units and outlines access rules for facility staff and scientists. We also included details about the type of experiments or procedures performed in the different functional units. Our standardized representation of all floors and units of the visited facilities facilitates comparison of the various concepts and solutions applied.
Figure 2.
Schematic floor plans—facility 1, plan 2. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 7.
Schematic floor plans—facility 2, plan 3. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 9.
Schematic floor plans—facility 3. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 11.
Schematic floor plans—facility 4, plan 2. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 14.
Schematic floor plans—facility 4, plan 5. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 8.
Schematic floor plans—facility 2, plan 4. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 15.
Schematic floor plans—facility 4, plan 6. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 20.
Schematic floor plans—facility 6. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 5.
Schematic floor plans—facility 2, plan1. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 12.
Schematic floor plans—facility 4, plan 3. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Figure 19.
Schematic floor plans—facility 5. (A) Zoning scheme. (B) Flow and hygiene levels scheme. (C) Functional unit scheme. For a description of the icons and colors used in the floor plans, see the legend to Figure 1.
Building specifications.
The general characteristics of the building specifications from all visited facilities are summarized in Table 1, which lists the common features of most facilities and the specific features of individual facilities. The visited facilities represent the current state of the art in animal facility design, because most of the buildings were constructed between 2001 and 2008 (MLC, RSF, ICS, GMC, TCP, HZI, BRC) or were refurbished during this period (HMGU, IRC, JAX).
Table 1.
Building specifications
| Features common to most facilities | Facilities with unique features |
||
| Building site | Campus area | TCP | Hospital area downtown |
| Building type | Stand-alone | TCP | Separate; next to hospital building |
| HMGU | Integrated into a large facility building on a single level | ||
| BRC | Located on a single level in a large basement shared among 5 buildings | ||
| JAX | Facilities integrated into a large research building on different levels | ||
| Structure | Holding floors above ground | BRC, TCP | Holding floors underground |
| Technical plants located above holding floors | HZI | Technical plants located in roof space and basement; supply to the holding floors via ducts | |
| ICS | Technical plants located in roof space; supply of the holding floors via shafts | ||
| IRC | Technical plants located in basement below holding floors | ||
| Construction | Concrete skeleton | MLC, RSF | Steel skeleton |
| Harlan, IRC | Massive | ||
| Walls | Dry partition walls | HZI | Concrete and clean-room wall system (demountable) |
| BRC | Clean-room wall system | ||
| HMGU, IRC | Masonry | ||
| Floor | Concrete–screed–epoxy coating flooring system | ICS | Tiles (will be replaced by PVC flooring with welded-joint sheets) |
| MLC, RSF | PVC flooring | ||
| Ceiling | Clean-room ceiling panel system | HZI, GMC, ICS, IRC | Concrete, polyurethane, or acryl coating |
| Air supply | Holding rooms: HEPA filtration; 15 to 20 air changes hourly; 20 to 22 °C | HZI, JAX | 12 air changes hourly |
| MLC, RSF | F9 filtration | ||
| Disinfection | H2O2 by generator; decentralized | HZI | Centralized H2O2 gassing by ventilation plants |
| ICS | Chlorine dioxide; decentralized | ||
| Specialties | Design | RSF, TCP, BRC | Renovation or conversion of each room from technical floor is possible |
Ten of the 11 facilities are located on a campus in a rural area; only one facility is located at a downtown urban site in a hospital area. The number of floors, shape of the building, and other features are strongly dependent on the space available and the connections to other buildings. The designs of the buildings are highly dependent on constraints imposed by the property site. Examples of facilities that contain holding and procedure rooms and the entire support infrastructure on a single floor are RSF and HMGU. BRC represents a variant design, in which the entire facility is located in a large basement on a single floor that connects several buildings. In contrast, other facilities involve several floors (TCP, HZI, ICS). Elevators are used to connect the multiple floors and to transport clean and soiled material to and from the washing area. In facilities that include several floors, a clear separation into different functional units was achieved by physically separation. HZI is an example of an animal facility with various units located in separate buildings that were constructed at different times in response to a particular need or because of increased demand. The IRC animal facility is located in a building that was constructed more than 50 y ago. Several refurbishments and renovations have upgraded the infrastructure and the installation for animal holding. In almost all visited facilities, the holding rooms are located above-ground; exceptions are BRC and TCP, which are located underground.
Additional characteristics common between many facilities in regard to their construction characteristics were the wall systems, floor covering, and ceiling (Table 1). A HEPA-filtered air supply with 15 to 20 air changes hourly and a facility temperature of 20 to 22 °C were standard features in most facilities, with only small deviations at HZI and JAX (12 air changes hourly) and MLC and RSF (F9 filtration, equivalent to a filtering efficiency of greater than 95%).
At RSF, TCP, and BRC, future construction or renovation of each room will be possible without disturbing the barrier in neighboring sections, because the rooms can be accessed from a technical floor above. However, a technical floor above the holding room may cause hygiene problems, because joints in the ceilings have to be sealed properly, and appropriate noise protection, usually about 37 to 45 dB, for the holding rooms must be installed.
In conclusion, the design of facility buildings has been influenced by many factors, including connections to other buildings, the need to adapt to the physical environment (building site), the space available and its location, and national regulations. Furthermore, the designs of all of the visited facilities had to integrate specific local needs, implement all necessary functional units, and provide the required capacities. The facility design also had to meet the requirements and needs of the facility management, facility personnel, and scientists who are working in and using the facility. Unlike the varied designs, the building specifications of the 11 facilities visited were quite similar, even though they are located on different continents (Europe, North America, and Asia), in different climate zones, and under different national regulations.
Animal holding specifications.
All visited facilities house mice behind bioexclusion barriers to protect the animals from undesirable microbes. This barrier system includes several components: cages, cage racks, rooms, standard operating procedures, and training of staff.6,14 Many similarities are obvious between the various facilities in regard to hygiene status, caging system, washing area, corridor system, barrier access, and material supply (Table 2). The hygiene status of the animal holding units is denoted as SPF and, in one case, specific opportunistic pathogen-free. The exact criteria are defined by the individual facility, but most are based on those recommended by the Federation for Laboratory Animal Science Associations. At several facilities, the hygiene level ‘not defined’ describes an unknown health status of imported mice, which usually are housed in bioinclusion quarantine under negative-pressure ventilation.
Table 2.
Animal holding specifications
| Features common to most facilities | Facilities with unique features |
||
| Hygienic status | SPF (but not defined in quarantine) | GMC | Controlled hygiene status based on health reports |
| IRC | SPF and specific opportunistic pathogen free | ||
| BRC, HZI, MLC, RSF, IRC | SPF and experimental infections | ||
| MLC | SPF and gnotobiotic | ||
| Biosafety level | Biosafety Levels 1 and 2 | ICS, JAX, CR, Harlan | Only Biosafety Level 1 |
| BRC, HZI, RSF | Biosafety levels 1, 2 and 3 | ||
| Cage system | IVC | ICS, BRC, HMGU | IVC and open cages |
| IRC | IVC, open cages, and isolators | ||
| ICS, MLC | IVC and isolators | ||
| JAX | Disposable cages (quarantine) | ||
| Corridor system | Delivery and disposal for rooms through a single corridor | HZI, MLC, RSF | Separation of clean and unclean corridors |
| Barrier supply | Autoclaves located in washing area | HZI, HMGU, IRC | Autoclaves before unit barrier |
| Access—caretaker | 3-compartment lock with air shower | Harlan, IRC, HMGU | 3-compartment lock with wet shower |
| HMGU, BRC, TCP | 2-compartment lock with air or wet shower | ||
| ICS, JAX | 1-compartment lock with sit-over | ||
| Access—scientist | 2-compartment lock with air shower | MLC, RSF | 3-compartment lock with air shower |
| ICS | 1-compartment lock with sit-over | ||
| IRC | Interconnected doors | ||
| Washing area | Tunnel washer, rack washer, bottle washer | MLC, RSF, TCP | Robotics for handling cages for tunnel washer |
| IRC | Tunnel washer only | ||
| Automatic bedding dispensing and disposal system | HMGU, ICS, IRC | Either automatic bedding dispensing or automatic waste system | |
| Specialties | Design | MLC | Centralized large wards with holding room; satellite procedure rooms and office |
| TCP, ICS | Suites with a combination of similar-sized holding rooms and procedure rooms located on a single corridor | ||
| Media supply Lock device | TCP, HMGU (in part) BRC | Central water supply for cages Air shower for 4 persons | |
The preferred cage systems are individually ventilated cages (IVC). Some facilities use open cages and isolators in addition to IVC. Most facilities that are using open cages currently plan to switch to IVC. Isolators are used mainly in the quarantine units, to avoid contamination through the import of mice of unknown health status. Experimental infections are performed under the containment requirements of Biosafety Levels 2 and 3.
Most facilities use a single-corridor system for the delivery of clean and disposal of soiled material. IRC, a facility that was built in 1965, was planned to have a 2-corridor system but is now used with a one-corridor system. Only 3 facilities—MLC, RSF, and HZI—use a 2-corridor system, which separates the trafficking of clean and soiled materials. At HZI, a new mouse house (T2) was planned to incorporate a 2-corridor system but will be operated as single-corridor system. The use of static microisolation or IVC cages renders a 2-corridor system dispensable.
Caretakers often pass through the barrier via a 3-compartment lock with air or wet showers. Also in operation are two-compartment locks with air or wet showers or a one-compartment lock with a ‘sit-over,’ a bench on which a person sits and removes outdoor shoes, swings the legs to the interior, puts on new shoes or overshoes, and then continues entering the facility. In contrast, scientists and facility personnel at all visited facilities are not obliged to use a wet shower. However, scientists at all facilities visited do not have access to areas at high hygiene levels, such as the core breeding unit. At the various facilities, scientists typically enter the barrier via a 2-compartment lock with air shower, 3-compartment lock with air shower, one-compartment lock with sit-over, or interconnected doors.
Most holding rooms have a medium capacity (1000 to 2000 cages), but smaller holding rooms housing 100 to 700 cages also are present. Large holding rooms housing more 2500 cages were built only by commercial breeders. Ideally the size of the holding rooms should match the demands of the respective functional unit: for example, small holding rooms are more convenient for animals going into phenotyping assays. In contrast, large holding rooms with high stocking rates are more useful for breeding and colony management.
Some facilities used various specific concepts through the design of the holding rooms. MLC uses large ‘wards,’ which each comprise a large holding room, procedure rooms, and an office. At the TCP, ‘suites’ comprise units of holding rooms, with procedure rooms and suite-dedicated housekeeping facilities. A similar set-up is found at ICS, where a single procedure room is connected to 2 holding rooms.
The washing area is an essential element of the support infrastructure usually is equipped with tunnel washers, rack washers, and bottle washers. Some facilities have installed automated washing systems that use robots for cage handling in and out of the tunnel washer. Furthermore, most visited facilities use an automatic bedding dispensing and disposal system.
Principle functional units.
The preceding analyses enabled us to define several principal functional units that together define a state-of-the-art large-scale mouse facility: core breeding, breeding and holding, phenotyping, archiving, containment quarantine, transgenic unit, and support infrastructure. These units are tightly connected (Figure 21).
Figure 21.
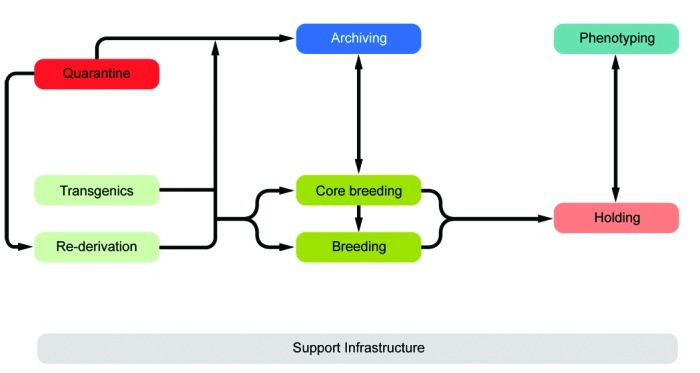
Principal phenotyping and archiving units and their interconnections.
Core breeding unit.
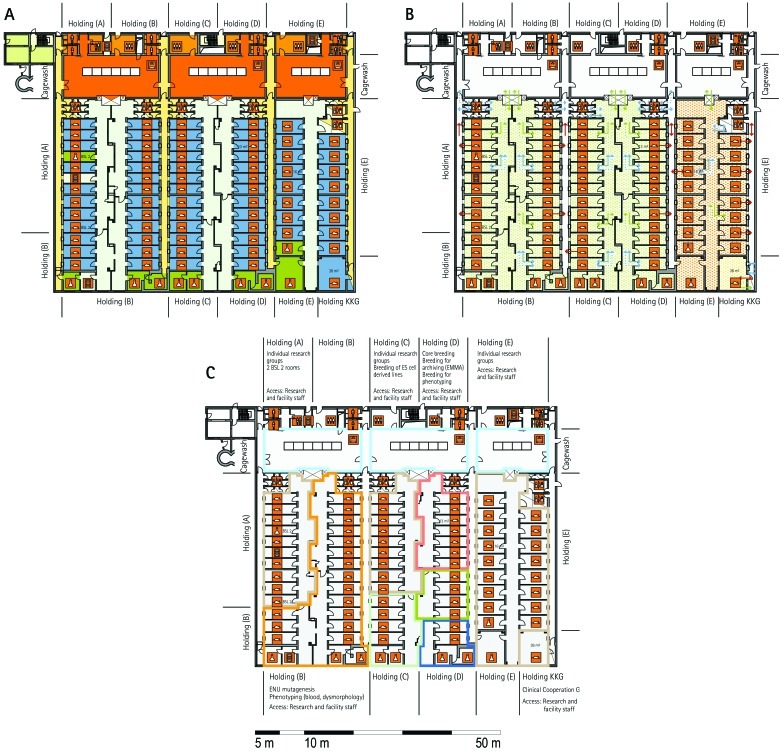
Most visited facilities have established so-called ‘core breeding units’, which have the highest hygiene level and access restrictions in the entire animal facility (MLC, ICS, HMGU, TCP, IRC, HZI, JAX). These units are in use for the central breeding and maintenance of the most valuable stocks and house the recipients for transgenic and rederived embryos. The core breeding unit is comparable to the production units at commercial breeders. In general, the core breeding unit and other breeding or holding units are strictly separated. To minimize the risk of contamination, only animal caretakers and veterinary staff but no scientists have access to this unit (ICS, HMGU, IRC, TCP, HZI). In most facilities, the caretakers that are dedicated to the core breeding unit do not have access to other units. The MLC production ward, which represents its core breeding unit, has a personnel and animal lock in addition to the central barrier. The core breeding units comprise holding rooms exclusively (TCP) or are combined with very few and small procedure rooms (RSF, HMGU, IRC, HZI).
Breeding–holding unit.
From the core breeding unit, mice are moved into holding units, which house mice prior to phenotyping or other technical procedures and expand colonies of mice for experimentation. According to the extent and type of experiments, different holding units—holding and sample preparation, holding and experiments, holding for phenotyping, and holding and phenotyping— are combined with other functional units. Some holding units are used exclusively for other functional units, such as the transgenic and archiving units (IRC, TCP, HZI, HMGU). Because cohorts have to be held and bred for phenotyping, holding units are of great importance especially for large phenotyping centers. Two principal variants were apparent in most visited facilities: separate units for holding and phenotyping of mice (HMGU, TCP, IRC), or a combined unit for holding and phenotyping (RSF, ICS, GMC, HMGU, IRC, HZI, HZI, JAX). In addition, holding units often were used for breeding and minor procedures such as blood sampling, tail biopsy, and so forth. Therefore, in some facilities, only a few small procedure rooms are associated with the holding rooms (HMGU, TCP, IRC). This set-up contrasts with that at other holding units, where extensive experimentation also takes place. These holding rooms often are combined with multiple procedure rooms (MLC, IRC, HZI). Other variations include the assignment of dedicated holding units to specific research groups (HMGU). At TCP, holding units are defined by their hygiene level or the origin of the mice housed in the holding unit.
Phenotyping unit.
Mouse mutants and genetic variants are analyzed in phenotyping units (Table 3). Some facilities represent so-called ‘mouse clinics,’ which are central European infrastructures specialized in comprehensive mouse phenotyping (GMC, MLC, RSF, ICS). The newly constructed Canadian mouse clinic TCP services several research groups in neighboring research-intensive hospitals. In addition to these specialized clinics, most visited facilities perform phenotyping assays (HZI, IRC, BRC, JAX).
Table 3.
Characteristics of phenotyping units
| Features common to most facilities | Facilities with unique features |
||
| Building specifics | Holding rooms in combination with procedure rooms for phenotyping | GMC | Doublet of holding and procedure rooms for phenotyping |
| ICS | Different holding rooms for different tests | ||
| HZI | BSL2 and BSL3 units for experimental infections | ||
| MLC, JAX–PHENO 1 | Large holding room in combination with several procedure rooms | ||
| Interface between holding and phenotyping | Mice return to holding rooms after phenotyping | IRC, JAX–PHENO 2 | Mice do not return after phenotyping |
| Import for mice for phenotyping | Rederivation | GMC | Import with accepted health status |
| ICS, IRC | Import with accepted health status; retesting | ||
| HZI | Rederivation and import of mice from commercial breeders | ||
| BRC | Import via quarantine | ||
| Hygiene | Barrier | IRC, JAX–PHENO 2 | No barrier |
| TCP | Barrier and units outside barrier | ||
| Phenotyping assays | Phenotyping ‘pipelines’ | HZI, IRC, BRC | No phenotyping pipelines |
| ICS, JAX | Standard and custom phenotyping pipelines | ||
| Service | Centrally offered | HZI, RSF, BRC | No services |
| IRC | Limited by capacity | ||
| GMC, MLC | Based on scientific collaboration | ||
The design of a phenotyping unit strongly depends on the individual local needs and strategies as well as the procedures for importing mice into the unit. Facilities using only rederived mice for phenotyping need large quarantine capacity for rederivation and an appropriate capacity to expand the colony in the breeding unit (HZI, MLC, RSF, TCP, JAX). The advantage of using only rederived mice for phenotyping is that all mice can be phenotyped by using the same shared equipment and then moved into the same holding room. In contrast, if mice are not rederived, the health status is different between individuals. In this case, when mice have been phenotyped by using common equipment, all mice have the same hygiene status as that of the group with the lowest status. At other facilities, cohorts are imported for phenotyping when they come from external facilities with an accepted health status (GMC) or after an acceptable health status has been confirmed (IRC, ICS). Facilities that accept imported cohorts have phenotyping units that are completely independent from other functional units, for example, quarantine and breeding units. Another variant is found at TCP, where the holding rooms for mice that are analyzed in the phenotyping unit are strictly separated from other holding units. The advantage of this concept is that a lower hygiene standard can be applied to such holding units.
The set-up of the procedure rooms is very much dependent on the constraints of the phenotyping assays themselves and in some cases requires specialized capabilities: for example, light:dark cycle, temperature, and MRI. The room size can vary considerably, depending on the equipment used or the assay performed; for example, at MLC, several small rooms are used for behavioral assays. An important feature of the phenotyping unit is the interface between the holding rooms and the procedure rooms. The ‘commuter concept’ makes use of large central holding rooms; mice are transported to and from the holding rooms to the procedure rooms (MLC, JAX, TCP). At TCP, small movable racks with stand-alone ventilation are used to accommodate brief holding near the procedure rooms to minimize transport or relocation stress in mice undergoing phenotyping. Another completely different concept is used at GMC, where one procedure room is combined with one holding room along a corridor. The cages were moved through this ‘phenotyping street’ for successive assays. Alternatively, dedicated holding rooms can be used for phenotyping assays (ICS). In places where the phenotyping unit was located outside the barrier, mice do not return to the holding rooms behind the barrier (IRC, JAX–PHENO 2).
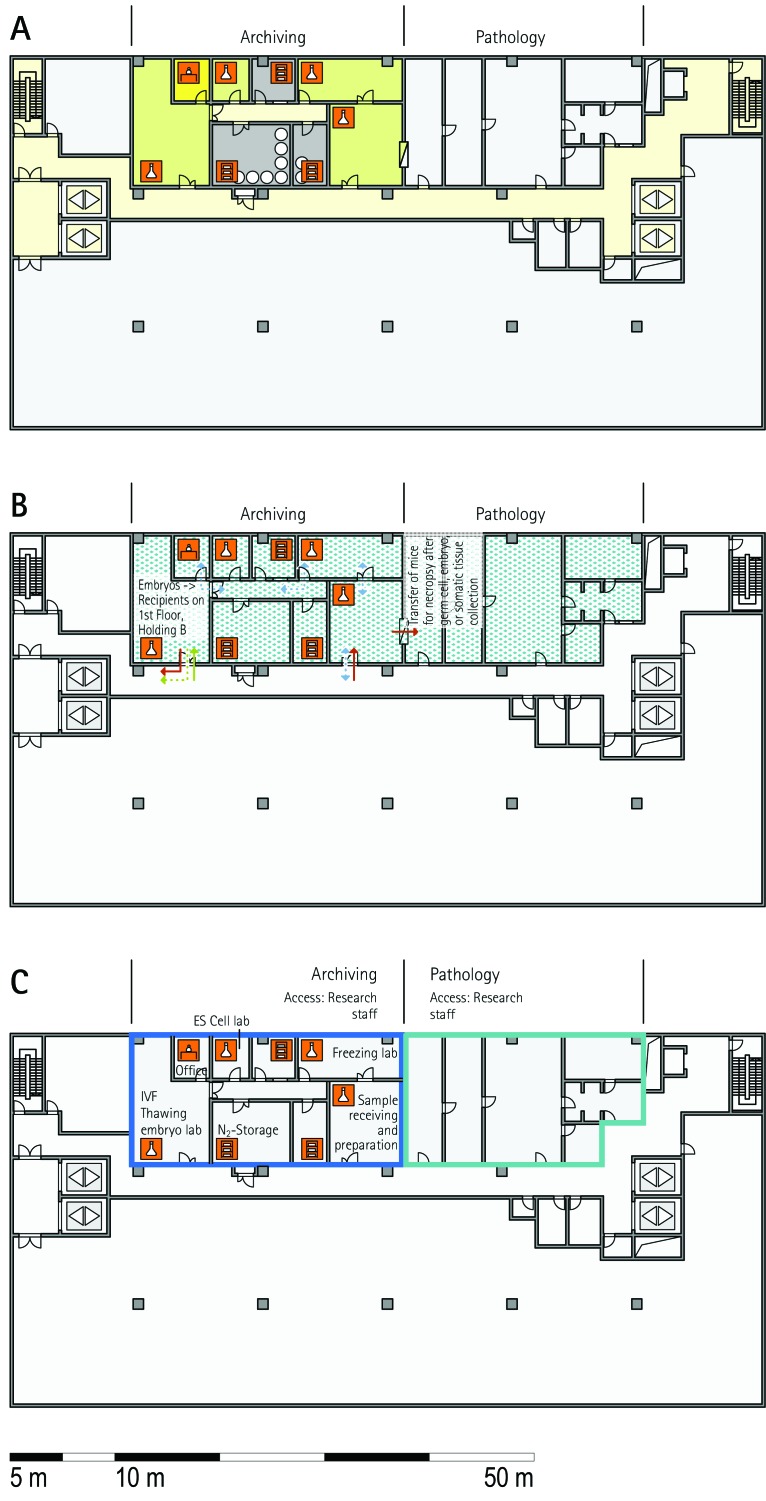
Archiving unit.
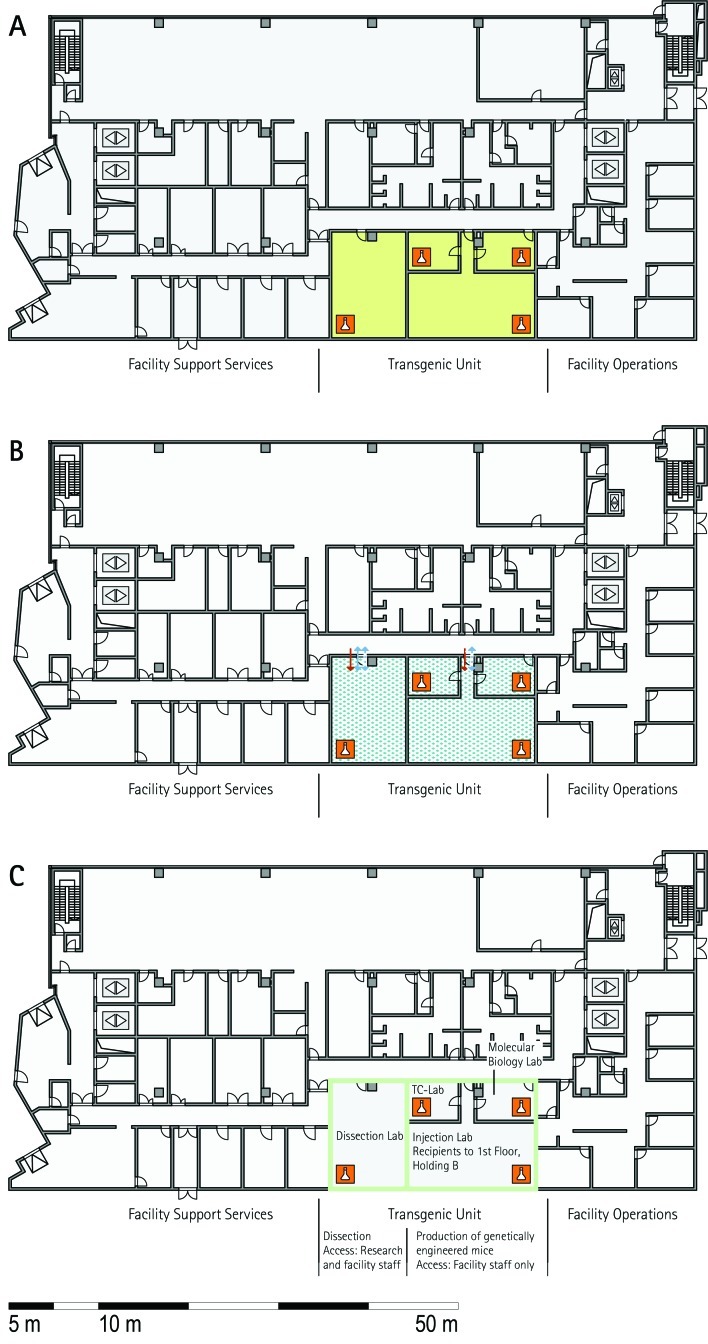
The archiving unit collects, preserves, and distributes mouse lines in a variety of formats, including embryonic stem cells, germplasm, embryos, and somatic tissue. All archiving units of the visited facilities offer services for internal users, but most of them also offer services to external customers (MLC, HMGU, ICS, TCP, IRC, BRC, JAX) or provide commercial services (JAX; Table 4). All visited facilities bank frozen embryos. Furthermore, sperm banking is widely used or planned to be implemented. In addition, biobanks for serum and blood, tissues, and ovaries have been established, and highly requested lines also are maintained as live strains. Several facilities provide mouse technology services (rederivation by IVF, speed expansion, strain rescue; Table 4). Archiving units consist of a set of procedure rooms for dissection, embryo handling, microinjection, a freezing laboratory, and archive. In addition, holding rooms for embryo transfer recipients, offspring, and oocyte donors have to be available. Storage in cryo-containers requires a second backup in another room at a remote site, which is often located in another building (MLC, HMGU, IRC, HZI, JAX). At TCP, the archiving unit includes a processing area for mice and is located completely outside the barrier; corresponding mouse holding occurs in a holding room behind the barrier. This arrangement provides the opportunity for external users to provide mice for archiving without importation into the holding facility. In contrast, other facilities place their archiving units entirely within the barrier. The archiving unit must have close connections to the quarantine unit for the preservation of mice of unknown health status; some facilities dedicate specific rooms of the quarantine area to archiving. These rooms were used for the import of mice from external facilities, embryo handling, and storage. Rooms often are shared between the archiving and transgenic units (RSF, ICS, HZI) or for rederivation (MLC, IRC). Shared usage of the archiving unit with other functional units is based on opportunities or needs for common equipment (for example, for microinjection) or mouse technologies.
Table 4.
Characteristics of archiving units
| Features common to most facilities | Facilities with unique features |
||
| Hygiene | Behind barrier | JAX | Behind and outside barrier |
| HMGU | Behind and outside barrier; quarantine | ||
| MLC, IRC | Behind barrier and quarantine | ||
| TCP | Outside barrier | ||
| Rooms | Shared with transgenic unit and rederivation | MLC, RSF, ICS, IRC, HZI | — |
| Located in quarantine | MLC, HMGU, IRC | — | |
| Archives | Frozen embryo banking; sperm banking (in place or at least planned) | ICS, HMGU, TCP | Also serum and blood banking |
| HMGU, MLC, RSF, TCP, JAX | Also tissue banking | ||
| TCP, JAX | Also ovary banking | ||
| HMGU, JAX | Also living mice | ||
| HMGU, MLC, TCP | Also N-ethyl-N-nitrosourea DNA and sperm | ||
| HZI, BRC | No sperm banking | ||
| BRC | No archives yet established | ||
| Services | Embryo and sperm cryopreservation and recovery | ICS, RSF, HZI | Only embryo cryopreservation and recovery |
| TCP, JAX | Also ovary transplantation; ovary cryopreservation and recovery | ||
| HZI | No external services | ||
| Strain services | Rederivation by IVF speed expansion; strain rescue | ICS, HZI | Only speed expansion |
| RSF, BRC, HZI | Only rederivation by IVF | ||
| TCP | Also speed cryopreservation (sperm and wildtype ovary) | ||
| JAX | Rederivation from induced pluripotent stem cells (in development) | ||
| HZI | No services | ||
Quarantine unit.
The main function of the quarantine unit is to receive mice of unknown or unacceptable health status (MLC, RSF, ICS, HMGU, TCP, IRC, HZI, BRC). Quarantine rooms often also are used to expand mice for archiving or rederivation of strains (TCP, IRC, JAX, HMGU). When mouse lines from external sources need to be phenotyped, they usually are imported via the quarantine unit. When integrated into a building, the quarantine area is strictly and physically separated from all other breeding and holding units (RSF, TCP, IRC, Harlan), or mice are kept in isolators (ICS). In other facilities (HMGU, HZI), the quarantine unit is located in stand-alone buildings.
Usually only dedicated caretakers have access to the quarantine unit (MLC, ICS, TCP, IRC, HZI, Harlan). In addition to holding and procedure rooms, the quarantine unit had a separate cage washing area at some facilities (MLC, HMGU, HZI), to strictly separate this unit from all others. An issue of great importance is the cage capacity of the quarantine area, which has to match the requirements of the facility's scientific activities. This aspect was thoroughly addressed in discussions with scientists during the planning phase of the animal facility. When the import of mice into a facility is only acceptable after rederivation (MLC, HMGU, RSF, HZI, JAX), the capacity of the quarantine unit needs to be sufficiently large to avoid becoming a bottleneck for subsequent activities (for example, phenotyping, archiving, and rederivation). A sufficiently large capacity similarly is required when the health status of mice must be confirmed before they enter the animal facility (IRC, ICS).
Transgenics unit.
The transgenic unit represents an essential functional unit for the production of genetically modified mouse lines. Most visited animal facilities have a transgenics unit (MLC, RSF, ICS, HMGU, TCP, IRC, HZI, BRC). For the reasons mentioned earlier, rooms often are shared between the transgenic and archiving units (RSF, ICS, IRC, HZI, HMGU). At RSF, there is a complete overlap of rooms. Similar to the archiving unit, some procedure rooms of the transgenic units may be located outside the barrier (for example, TCP). In contrast, transgenic units in other facilities are located completely within the barrier (MLC, RSF).
Lessons learned.
A systematic listing of common facility design errors and problems has been described elsewhere.12 However, the visiting team also collected feedback from people working or managing the visited centers to evaluate the most common changes that were necessary during or after building of the facility.
Size and capacity of holding rooms and quarantine.
A major issue at all facilities concerned the size and capacity of holding rooms. Large holding rooms are effective for large breeding capacities but are noisy and require considerable movement of facility personnel to service the room on a daily basis. Therefore, even when large rooms are chosen, a few small holding rooms should still be available. In addition, the cage capacity required in the holding units often was underestimated. That is, the cage capacities during the planning stage did not sufficiently account for future demands due to new technologies, changes in research projects, and new collaborations. Similarly, the cage capacities in the quarantine units frequently were too small to accommodate the import of large numbers of mouse lines from external facilities.
Size and number of procedure rooms.
The size and number of procedure rooms were often inadequate to fulfill the various requirements of the many diverse phenotyping assays. Whereas some specialized equipment requires large rooms, behavioral assays (for example) necessitate many small rooms. Therefore, a general solution is to build in flexibility by using light movable separating walls, which can be removed or rearranged to meet changing demands even after the facility has been built.
Support infrastructure and building specification.
Other suggested design improvements concerned the support infrastructure and building specifications. Frequently mentioned bottlenecks focused on autoclave capacity and storage space. Some facilities had to change the floor covering and install wireless networks. Several facilities built 2-corridor systems. However, because many new facilities (at least in Europe) are now using IVC, separation into dirty and clean corridors no longer seems mandatory.
Reduction of costs.
Building facilities with one-corridor systems reduces construction costs considerably. Furthermore, many facilities would now install more efficient energy-saving technologies (for example, covering pipes and ducts with mineral-fiber insulating material and introducing heat recovery technologies). These installations increase construction costs but considerably reduce operating costs over the lifetime of the facility.
Discussion
Several reviews, books, and publications by governmental organizations address animal facility planning and design.2,3,7-9,15,17 In addition, specialized information for designing mouse facilities5,6,13,14 and phenotyping facilities10,11 have been published. These documents provide recommendations for the planning and design with respect to codes, regulations, equipment, technical constraints, and cost issues. In addition, visiting other facilities is highly recommended9 before the planning stage to profit from existing solutions and concepts. However, none of the documents currently available has attempted to systematically describe existing facilities and outline their different designs and concepts. Furthermore, no standardized description of existing facilities has been published to facilitate the comparison of various facilities.
Therefore, we set out to provide a comprehensive description of existing large-scale mouse facilities. Here, we report the results of site visits to 9 large mouse production, phenotyping, and archiving centers by a team of experts including scientists, facility managers, and an architect. Our report provides detailed standardized information including schematic floor plans on several existing animal facilities dedicated to large-scale phenotyping of complex basic research projects and archiving. These visits made it very clear that an animal facility has to accommodate many different constraints and needs, including specific local needs and regulations and the number and types of phenotyping assays. Therefore, a unified description of how facilities throughout the world have solved these challenges is a valuable resource during the planning of a new facility. Our current report likely will help researchers, facility managers, architects, and governmental personnel to design and construct state-of-the-art facilities and thereby implement concepts that improve animal welfare and meet the needs of their local research communities.
Furthermore, our analysis of the designs revealed several principal functional units that together describe a state-of-the-art phenotyping and archiving mouse facility: core breeding, breeding and holding, phenotyping, archiving, transgenics and rederivation, quarantine, and supporting infrastructure units. The various solutions for the functional interconnection of these units are reflected in the design of each facility.
Acknowledgments
This study was funded by the European Commission within its 7th Framework Programme (SP4-Capacities-211404). We thank Susanne Löblein and Steve Thomas for preparing the figures. Furthermore we express our gratitude to all the people from the visited facilities for their time, effort, and generous sharing of information, especially Tom Weaver and Sarah Wells (MLC), James Bussell and Ramiro Ramirez-Solis (Wellcome Trust Sanger Institute RSF), Yann Herault, Tania Sorg, and Abdel Ayadi (ICS), Valérie Gailus-Durner (GMC), Susan Marschall (HGMU), Lise Phaneuf (TCP), Sébastien Paturance and Cécile Fremond (IRC), John Fitzpatrick and Molly Bogue (JAX), Sathivel Ponniah and Colin Lee Fook Kok (BRC), Jean-Luc Sardou and Jean-Loius Bequet (CR), and Robbert Otten and Roel Bruggink (Harlan).
References
- 1.Brown SD, Hancock JM, Gates H. 2006. Understanding mammalian genetic systems: the challenge of phenotyping in the mouse. PLoS Genet 2:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Council on Animal Care 2003. Guidelines on laboratory animal facilities—characteristics, design, and development. Ottawa, Ontario: Canadian Council on Animal Care. [Google Scholar]
- 3.Council of Europe. 2006. European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS No. 123). Guidelines for accommodation and care of animals (article 5 of the convention). Approved by the Multilateral Consultation. Cons 123 (2006 ) 3.
- 4.Cox RD, Brown SD. 2003. Rodent models of genetic disease. Curr Opin Genet Dev 13:278–283 [DOI] [PubMed] [Google Scholar]
- 5.Gonder JC, Laber K. 2007. A renewed look at laboratory rodent housing and management. ILAR J 48:29–36 [DOI] [PubMed] [Google Scholar]
- 6.Hessler JR.2009. Barrier housing for rodents, p 335–345. In: Hessler JR, Lehner NDM. Planning and designing research animal facilities. London (UK): Elsevier Academic Press.
- 7.Hessler JR, Leary SL.2002. Design and management of animal facilities, p 127–172. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press.
- 8.Hessler JR, Lehner NDM. 2009. Planning and designing research animal facilities. San Diego (CA): Academic Press [Google Scholar]
- 9.Holgate B.2010. Planning, design and construction of efficient animal facilities, p 124–135. In: Hubrecht R, Kirkwood J. The UFAW handbook on the care and management of laboratory and other research animals. Oxford (UK): Wiley–Blackwell.
- 10.Klaunberg BA, Davis JA. 2008. Considerations for laboratory animal imaging center design and setup. ILAR J 49:4–16 [DOI] [PubMed] [Google Scholar]
- 11.Klaunberg BA, Lizak MJ. 2004. Considerations for setting up a small-animal imaging facility. Lab Anim (NY) 33:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehner NDM, Hessler JR.2009. Common facility design errors and problems, p 179–183. In: Hessler JR, Lehner NDM. Planning and designing research animal facilities. London (UK): Academic Press.
- 13.Lipman NS.2007. Design and management of research facilities for mice, p 271–319. In: Fox JG Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. The mouse in biomedical research, 2nd ed, vol 3. Burlington (MA): Academic Press.
- 14.Lipman NS.2009. Rodent facilities and caging systems, p 265–288. In: Hessler JR, Lehner NDM. Planning and designing research animal facilities. London (UK): Academic Press.
- 15.National Institutes of Health 2008. Design requirements manual for biomedical laboratories and animal research facilities. Bethesda (MD): NIH [Google Scholar]
- 16.Raess M, Hrabe de Angelis M. 2007. Infrafrontier mouse models and phenotyping data for the European biomedical research community. EMBnet.journal 15:16–19 [Google Scholar]
- 17.Ruys T. 1991. Handbook of facility planning. New York (NY): Van Nostrand Reinhold [Google Scholar]