Abstract
Chagas disease, an important cause of heart disease in Latin America, is caused by the parasite Trypanosoma cruzi, which typically is transmitted to humans by triatomine insects. Although autochthonous transmission of the Chagas parasite to humans is rare in the United States, triatomines are common, and more than 20 species of mammals are infected with the Chagas parasite in the southern United States. Chagas disease has also been detected in colonies of nonhuman primates (NHP) in Georgia and Texas, and heart abnormalities consistent with Chagas disease have occurred at our NHP center in Louisiana. To determine the level of T. cruzi infection, we serologically tested 2157 of the approximately 4200 NHP at the center; 34 of 2157 primates (1.6%) tested positive. Presence of the T. cruzi parasite was confirmed by hemoculture in 4 NHP and PCR of the cultured parasites. These results strongly suggest local transmission of T. cruzi, because most of the infected NHP were born and raised at this site. All 3 species of NHP tested yielded infected animals, with significantly higher infection prevalence in pig-tailed macaques, suggesting possible exploration of this species as a model organism. The local T. cruzi strain isolated during this study would enhance such investigations. The NHP at this center are bred for use in scientific research, and the effects of the Chagas parasite on infected primates could confuse the interpretation of other studies.
Abbreviation: NHP, nonhuman primate; TNPRC, Tulane National Primate Research Center
Most nonhuman primates (NHP) used in research in the United States are now raised at 1 of 8 National Primate Research Centers or other institutions in the United States and are shipped as needed for studies, thereby avoiding the importation of pathogens from their native countries. However, knowledge about the infection status of these research animals within colonies in the United States is important with regard to colony health, the outcome of the scientific studies that use these NHP, and the safety of caregivers and laboratory workers.
Infection with the hemoflagellate parasitic pathogen Trypanosoma cruzi, the causative agent of Chagas disease, has been reported sporadically in NHP colonies in the United States (Table 1). Chagas is the most serious parasitic disease in Latin America, and despite control efforts, its negative socioeconomic impact is rising.33 Of the approximately 8 million people infected,26 20% to 30% will develop chronic disease; most of these persons will die of heart disease (70% to 85%), digestive disorders (15% to 30%), or neurologic disease (less than 5%). Most (80%) transmission occurs through insect vectors, specifically through contact of parasite-containing feces (which are deposited while the insect is taking a blood meal) with mammalian mucous membranes or through a break in the skin. In addition, approximately 1% to 12% of offspring of infected mothers will acquire the parasite by congenital transmission.7 Insect-vector-mediated autochthonous transmission to humans is rare in the United States, with only 7 documented cases.11,18 However, a robust sylvan cycle exists in the United States, including T. cruzi-infected triatomine insects and a variety of mammals.31,36
Table 1.
Reports of T. cruziinfection in nonhuman primates in the United States
| Species | No. of NHP infected/ no. tested | State where infection identified (and likely acquired) | Evidence (method of detection) | Reference |
| Pileated gibbon (Hylobates pileatus) | 1/1 | Louisiana | Amastigotes in myocardium at necropsy | 30 |
| Rhesus macaque (Macaca mulatta) | 1/1 | Maryland (Texas or Georgia) | Blood culture after inadvertently transferred to immunosuppressed NHP; recipient confirmed by blood smears, serology, and xenodiagnosis | 9 |
| Rhesus macaque | 20/236 (8.5%) | Texas | Index case: amastigotes observed at necropsy; 19 additional by serology | 17 |
| Squirrel monkey (Saimiri sciureus) | 2/2 | Louisiana | Microscopy, hemoculture, and xenodiagnosis | 12 |
| Yellow baboon (Papio cynocephalus) | 1/1 | Texas | Amastigotes noted at necropsy | 13 |
| Crested black macaque (Macaca nigra) | 1/1 | Oregon (Texas) | Flagellates observed in spinal fluid; amastigotes in brain at necropsy; serology | 25 |
| Lion-tailed macaque (Macaca silenus) | 7/11 (64%) | Georgia | Hemoculture and PCR | 27 |
| Ring-tailed lemur (Lemur catta) | 1/19 (5%) | Georgia | Hemoculture and PCR | 27 |
| Pig-tailed macaque (Macaca nemestrina) | 1/1 | Washington (Louisiana) | Hemoculture, PCR, and serology | 29 |
| Ring-tailed lemur (Lemur catta) | 21/41 (51%) | Georgia | Hemoculture, PCR, and serology | 14 |
| Black-eyed lemur (Eulemur macaco flavifrons) | 1/5 (20%) | Georgia | Serology | 14 |
| Black and white ruffed lemur (Varecia variegata variegata) | 3/4 (75%) | Georgia | Serology | 14 |
| Chimpanzee (Pan troglodytes) | 1 | Texas | Amastigotes on necropsy, PCR, and immunohistochemistry | 5 |
T. cruzi was first identified in the United States in 1916 in the triatomine insect vector Triatoma protracta.19 Eleven species of triatomine insects live in the southern two-thirds of the United States, where they are commonly known as ‘kissing bugs’ (because as night feeders, they often feed on the face) or ‘cone-nosed bugs.’ T. sanguisuga is the species reported most commonly in Louisiana.10 On average, the prevalence of infection in the insect vectors is 25%,36 although much higher prevalence is reported in some areas, including Louisiana (56%).10 T. cruzi has been identified in more than 20 mammalian species across the southern United States; the most important of these mammals are rodents, raccoons, opossums, and armadillos.6,16 Recent studies showed that the highest prevalence of antibodies against T. cruzi occurred in raccoons (0% to 68%, range depends on state) and opossums (17% to 52%).6
The first case of T. cruzi in a NHP in the United States occurred at the Delta Regional Primate Research Center (Covington, LA; now called the Tulane National Primate Research Center [TNPRC]), where a gibbon (Hylobates pileatus) from Malaysia died of symptoms of Chagas disease30. This case suggested local transmission because Chagas is endemic only to the Americas (Table 1). Additional cases of T. cruzi infection in NHP have been reported in Louisiana, Texas, and Georgia. Additional infected NHP were identified in Washington, Oregon, and Maryland; the suspicion was that they previously were infected in Texas, Louisiana, or Georgia (Table 1). Because these NHP were either imported from outside the United States, where T. cruzi has not been reported to occur naturally, or were born and raised in the United States, these cases point to T. cruzi infection that was acquired in the United States. To understand the prevalence of locally acquired T. cruzi infection at the TNRPC, we conducted a serologic survey of 2157 NHP residing at the center.
Materials and Methods
Study design.
To determine the prevalence of T. cruzi infection in NHP at the TNPRC, we first validated an immunochromatographic rapid, dipstick assay for the detection of T. cruzi antibodies in NHP. We then used this test to assay plasma samples from 2157 NHP from the center. T. cruzi infection in 4 seropositive NHP was confirmed by hemoculture and PCR of the cultured parasites.
Study site.
TNPRC is an AAALAC-accredited facility and 1 of 8 national NHP centers funded by the NIH. The center houses approximately 4200 NHP including 13 different species; the majority are rhesus macaques (Macaca mulatta). The NHP that compose the breeding colonies are housed in single-species groups in 70 outdoor corrals and field cages, where the animals have the potential to come into contact with triatomine vectors living in the area. The colonies are managed in accordance with all IACUC regulations that prescribe the humane care and use of laboratory animals (Animal Welfare Act1 and the Guide for the Care and Use of Laboratory Animals).15 The NHP were fed a standard primate diet supplemented with fresh fruits, vegetables, and forage (seed and nut) mix with water available ad libitum.
Validation of immunochromatographic dipstick assay.
The immunochromatographic dipstick assay (Trypanosoma Detect Rapid Test, InBios International, Seattle, WA) has been shown to be a sensitive and specific assay for detecting T. cruzi antigens in human sera.21 This assay was tested on 7 archived NHP serum samples that had previously tested positive for T. cruzi antibodies by using 1 of 3 serologic tests (enzyme immunosorbance assay, indirect immunofluoresence, and complement fixation) at the Centers for Disease Control and Prevention. In addition, the dipstick assay was tested on 16 NHP samples positive for the nonpathogenic, but antigenically similar, T. rangeli parasite and 10 NHP serum samples negative for both parasites (all provided by the Centers for Disease Control and Prevention, Atlanta, GA). Each dipstick assay on these control sera was performed (in triplicate) according to the manufacturer's instructions. We tested for agreement between the dipstick assay and the archived NHP controls with the κ agreement statistic (κ = 1 is complete agreement; 0 is random) by using JMP version 9 (SAS, Cary, NC).
Study animals.
Whole blood was collected from 2172 NHP at the TNPRC by venipuncture into tubes containing EDTA during routine health exams between 2003 to 2004 under a protocol approved by the animal care and use committees of both the TNPRC and Loyola University New Orleans. For sample collection, NHP were anesthetized with an intramuscular injection of ketamine hydrochloride (10 mg/kg); 15 plasma samples were hemolyzed and therefore not analyzed further. Three primate species were tested: 1311 rhesus macaques (M. mulatta), 388 pig-tailed macaques (M. nemestrina), and 458 baboons (Papio spp. [primarily P. anubis and P. cynocephalus hybrids]). These NHP were residing in 33 of the primate center's 70 outdoor housing areas. The average daily census of the species studied during 2004 (only 31 samples were collected in 2003) was 2776 rhesus macaques, 388 pig-tailed macaques, and 548 baboons. The colony is maintained with more female than male animals as breeding populations; the male-to-female ratio was 1:1.64 for rhesus macaques, 1:3.34 for pig-tailed macaques, and 1:2.81 for baboons.
Assessment of infection.
Serology.
The NHP plasma samples were transferred to Loyola University New Orleans, stored at −20 °C, and brought to room temperature before serologic testing. Each of the plasma samples was tested for the presence of antibodies against T. cruzi by using the dipstick assay (Trypanosoma Detect Rapid Test, InBios International) according to the manufacturer's instructions. Because of the large number of samples, each experimental sample was tested once, and positive plasma samples were retested in duplicate. All specimens showed identical results on all 3 replicates.
Hemoculture and PCR.
To further confirm the presence of T. cruzi in serologically positive samples, an additional 5 mL of blood from 7 of the serologically positive NHP underwent hemoculture of the buffy coat according to standard procedures.27 In addition, PCR using T. cruzi-specific primers (the minicircle primers TC3 and TC4) was performed on DNA isolated from parasites cultured from the blood.11
Microscopy.
The buffy coat from the first approximately 100 blood samples was examined by using wet mounts (magnification, 40×) and Giemsa-stained blood smears (magnification, 100×) for 2 min each by trained observers.
Statistical analysis.
We tested for differences in infection prevalence among species by logistic regression. The Fisher exact test was used to test for sex-biased infection prevalence for each species. All statistical analyses were done by using JMP version 9 (SAS). A P value less than 0.05 was considered significant.
Results
Validation of the rapid test—control samples.
The dipstick assay showed a positive result on 6 of 7 T. cruzi-positive control NHP serum samples. Furthermore, all 16 T. rangeli-positive control NHP serum samples tested negative for antibodies against T. cruzi by the dipstick assay. All 10 negative-control NHP serum samples also were negative by the dipstick assay. The κ coefficient (κ = 0.90, SEM = 0.09, P < 0.0001) indicated strong agreement between the dipstick assay and the archived NHP controls. Assays performed in triplicate showed identical results, demonstrating excellent reproducibility.
Serologic testing of the NHP plasma samples.
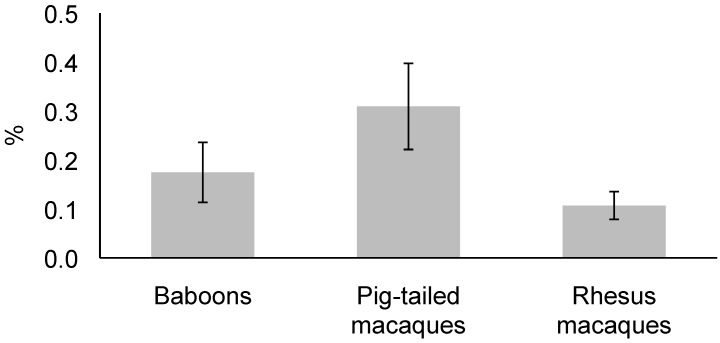
Of the 2157 NHP plasma samples, 34 (1.6%) tested positive for T. cruzi antibodies by the dipstick assay. These data included all 3 species: pig-tailed macaques (M. nemestrina, 12 [35%] of the 34 positive samples), rhesus macaques (M. mulatta, 14 [41%] of the positive samples), and baboons (Papio spp., 8 [24%] of the positive samples). The species showing the highest infection prevalence was pig-tailed macaques (3.1% of those tested), followed by baboons (1.7% of those tested) and rhesus macaques (1.1% of those tested). Statistical analysis using logistic regression indicated that infection prevalence was higher in pig-tailed macaques than in rhesus macaques (likelihood ratio: χ2 = 5.13, P < 0.05, Figure 1). The infected primates were dispersed throughout the TNPRC, with positive NHP found in 17 of the 33 corrals and field cages tested.
Figure 1.
Trypanosoma cruzi infection prevalence for 3 species of nonhuman primates at TNPRC. Infection prevalence in pig-tailed macaques is significantly (P < 0.05) higher than that in rhesus macaques. Error bar, SEM.
Microscopy, hemoculture, and PCR.
None of the wet mounts or Giemsa-stained blood smears showed trypanosomes, but these methods are known to lack sensitivity in chronically infected mammals.22
To confirm that the positive serology accurately reflected T. cruzi infection, hemoculture was performed by using blood from 7 of the 34 seropositive NHP. Within 6 wk, trypanosomes appeared in 4 of these cultures (Figure 2). DNA isolated from the cultured trypanosomes was confirmed as T. cruzi by PCR using T. cruzi-specific primers11 and obtaining the characteristic 276-bp fragment (data not shown).
Figure 2.
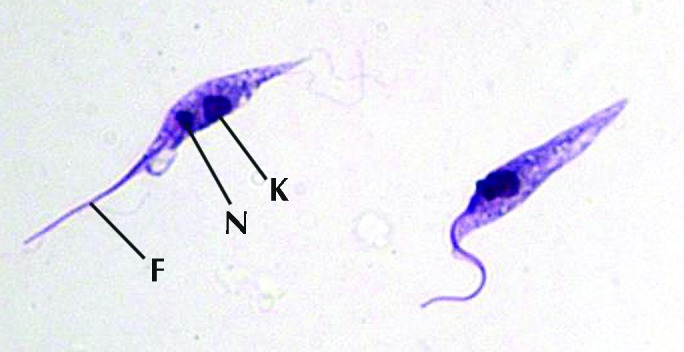
Giemsa-stained T. cruzi epimastigotes cultured from buffy coat of female M. nemestrina. k, kinetoplast; n, nucleus; f, flagellum.
Of the 34 NHP that tested positive, most (25 of 34, 73.5%) were born and raised at the TNPRC. Another 7 (3 baboons and 4 pig-tailed macaques) of these NHP were born at the University of Washington Primate Center to mothers that had been born in captivity. One baboon was born in Kenya (where T. cruzi has never been reported) and lived for some time in Ohio, and the remaining NHP (a baboon) was born at the Yerkes Primate Center (Atlanta, GA) to a mother that had been born in captivity before both dam and offspring were transferred to the TNPRC.
More female (26 of 34, 76%) than male (8 of 34, 24%) NHP were infected, reflective of the sex bias in the population. The infected male-to-female ratio was 1:1 for rhesus macaques, 0:12 for pig-tailed macaques, and 1:7 for baboons. The Fisher exact test revealed no significant differences (P > 0.05) in infection prevalence between sexes for any of the 3 species examined. In addition, one mother–daughter pair was infected, suggesting the possibility of congenital transmission.
Discussion
In the current study, we report the low prevalence of T. cruzi infection in a NHP colony in Louisiana. Only 34 (1.6%) of 2157 NHP tested showed positive serology for T. cruzi by a dipstick assay. Further confirmation of the serologic test was provided by hemoculture of buffy coat from 4 NHP and by PCR amplification of a T. cruzi-specific band from the cultured parasites. Because the vast majority of the seropositive NHP (25 of 34, 73.5%) were captive-bred and raised in Louisiana; these findings strongly suggest local transmission of T. cruzi. Perhaps 2 of the 9 NHP born outside of Louisiana acquired the parasite in those other states, given that insect vectors or vectors and infected animals were reported in Ohio32 and Georgia.6,20 However, the 7 NHP born in Washington likely acquired the infection after their transfer to Louisiana, because T. cruzi has not been reported in that state. Infection was not evident by wet mount or Giemsa-stained blood smears in any of the samples, consistent with the low level of blood parasitemia in chronic infections.28
Local acquisition of T. cruzi by these NHP is not surprising, given that this colony is near (approximately 50 miles) the location of the sixth reported autochthonous human case in the United States11 and is in an area known to have a high number of infected vectors and wild animals. Previous reports in Louisiana have shown that more than 50% of the local insect vector, T. sanguisuga, is infected.8,11 In addition, in Louisiana, T. cruzi infection has been documented in 1.1% to 28.8% of armadillos,3,34 37.5% of opossums,3 and 4.7% to 22.1% of dogs.4,24 In some of these cases, the T. cruzi infection prevalence was higher than those found in countries with endemic infection in humans.23 The difference in prevalence in armadillos and dogs is likely a real difference in infection prevalence among geographic localities and a reflection of the different sensitivities of the different detection methods used. Because the method used to measure T. cruzi prevalence in armadillos (hemoculture) is much less sensitive than is the dipstick assay used in the current study, our results suggest that infection levels in other mammals are greater than those seen in our NHP.
NHP are thought to contract the parasite by eating the insect vectors, in addition to known routes of transmission of the parasite for humans, such as contamination of the bite wound or mucous membranes. In fact, NHP in Georgia have been observed to handle and partially consume a triatomine bug.27
Unexpectedly, 35% of our infected NHP were pig-tailed macaques. This result is surprising, given that pig-tailed macaques comprised only 18% of the primates tested. In fact, pig-tailed macaques were infected significantly (P < 0.05) more often than were rhesus macaques but not baboons. Perhaps pig-tailed macaques are especially susceptible and therefore might be explored as an animal model for Chagas disease; limited studies to date have used NHP as model systems for Chagas disease.2,35 In addition, the availability of local T. cruzi strains may be useful for Chagas studies using local NHP. Unfortunately, all but one cultured strain was lost in the aftermath of Hurricane Katrina; this strain could be useful for further studies on NHP, and additional strains could be cultured from the positive NHP. Of particular interest was the identification of a mother–offspring pair among the seropositive NHP. This finding suggests possibility of congenital transmission, an area of considerable interest in the Chagas community, and one that could be pursued in this colony.
The strong suggestion of local, ongoing transmission at this NHP colony has important implications for colony health. The low prevalence suggests that continuing modifications and control efforts (for example, removal of vegetation near cages, rodent control) are mostly effective; although it is unlikely all transmission can be avoided, given the high prevalence of infected insect vectors. In some previous reports of T. cruzi infection in NHP in the United States, the NHP were immunosuppressed and represent serendipitous findings that likely underestimate true prevalence.9,17,25 When possible, monitoring T. cruzi infection in colonies in the Southern United States is recommended to understand the actual prevalence. The infection status of the NHP can affect the results of other studies, and infected animals could transmit the parasite to caregivers and lab workers, such as through contamination with infected blood.
In summary, the current study reports a low level of T. cruzi prevalence in a NHP colony in Louisiana. Chagas should be considered as a differential diagnosis for unexplained heart disease and it is important to continue the insect and pest control programs.
Acknowledgments
We appreciate the assistance of Dr Liz Didier (TNPRC) in facilitating these studies, Dr Frank Cogswell for instigating this research, and Mr Frank Steurer (Centers for Disease Control and Prevention, Atlanta, GA) for supplying the test sera. This research was informed by work done on NIH grant R15 AI079672-01A1 and supported in part by the Loyola University SJ Mullahy Fund for Undergraduate Research, the Washington NPRC Base Grant P51 RR000166, and the TNPRC Base Grant P51 RR000164 awarded by the National Center for Research Resources (NCRR), NIH.
References
- 1. Animal Welfare Act as Amended. 2007. 7 USC §2131–2159.
- 2.Andrade MC, Dick EJ, Jr, Guardado-Mendoza R, Hohmann ML, Mejido DC, VandeBerg JL, DiCarlo CD, Hubbard GB. 2009. Nonspecific lymphocytic myocarditis in baboons is associated with Trypanosoma cruzi infection. Am J Trop Med Hyg 81:235–239 [PMC free article] [PubMed] [Google Scholar]
- 3.Barr SC, Brown CC, Dennis VA, Klei TR. 1991. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from Southern Louisiana. J Parasitol 77:624–627 [PubMed] [Google Scholar]
- 4.Barr SC, Dennis VA, Klei TR. 1991. Serologic and blood culture survey of Trypanosoma cruzi infection in four canine populations of Southern Louisiana. Am J Vet Res 52:570–573 [PubMed] [Google Scholar]
- 5.Bommineni YR, Dick EJ, Jr, Estep JS, Van de Berg JL, Hubbard GB. 2009. Fatal acute Chagas disease in a chimpanzee. J Med Primatol 38:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ. 2010. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from 6 states across the Southern United States. Vector Borne Zoonotic Dis 10:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlier Y, Truyens C. 2010. Maternal-fetal transmission of Trypanosoma cruzi. In: Telleria J, Tibayrenc M. American trypanosomiasis, Chagas disease: 100 years of research. London (UK): Elsevier. [Google Scholar]
- 8.Cesa K, Caillouet KA, Dorn PL, Wesson DM. 2011. High Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) prevalence in Triatoma sanguisuga (Hemiptera: Redviidae) in Southeastern Louisiana. J Med Entomol 48:1091–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicmanec JL, Neva FA, McClure HM, Loeb WF. 1974. Accidental infection of laboratory-reared Macaca mulatta with Trypanosoma cruzi. Lab Anim Sci 24:783–787 [PubMed] [Google Scholar]
- 10.de la Rua N, Stevens L, Dorn PL. 2011. High genetic diversity in a single population of Triatoma sanguisuga (LeConte, 1855) inferred from two mitochondrial markers: cytochrome b and 16S ribosomal DNA. Infect Genet Evol 11:671–677 [DOI] [PubMed] [Google Scholar]
- 11.Dorn PL, Perniciaro L, Yabsley M, Roellig D, Balsamo G, Diaz J, Wesson DM. 2007. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 13:605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhard M, D'Alessandro A. 1982. Congenital Trypanosoma cruzi infection in a laboratory-born squirrel monkey, Saimiri sciureus. Am J Trop Med Hyg 31:931–933 [DOI] [PubMed] [Google Scholar]
- 13.Gleiser CA, Yaeger RG, Ghidoni JJ. 1986. Trypanosoma cruzi infection in a colony-born baboon. J Am Vet Med Assoc 189:1225–1226 [PubMed] [Google Scholar]
- 14.Hall CA, Polizzi C, Yabsley MJ, Norton TM. 2007. Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherines Island, Georgia. J Parasitol 93:93–96 [DOI] [PubMed] [Google Scholar]
- 15.2011. Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 16.John DT, Hoppe KL. 1986. Trypanosoma cruzi from wild raccoons in Oklahoma. Am J Vet Res 47:1056–1059 [PubMed] [Google Scholar]
- 17.Kasa TJ, Lathrop GD, Dupuy HJ, Bonney CH, Toft JD. 1977. An endemic focus of Trypanosoma cruzi infection in a subhuman primate research colony. J Am Vet Med Assoc 171:850–854 [PubMed] [Google Scholar]
- 18.Kjos SA, Snowden KF, Olson JK. 2009. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis 9:41–50. [Google Scholar]
- 19.Kofoid A, McCulloch I. 1916. On Trypanosoma triatomae, a new flagellate from hemipteran bugs from the nests of the wood rat Neotoma fuscipes. Univ Calif Publ Zool 16:113–126 [Google Scholar]
- 20.Le Conte JL. 1855. Remarks on 2 species of American Cimex. Proc Acad Nat Sci Philadelphia 7:404 [Google Scholar]
- 21.Lorca M, Contreras MDC, Salinas P, Guerra A, Raychaudhuri S. 2008. Evaluación de una prueba rápida para el diagnóstico de la infección por Trypanosoma cruzi en suero. Parasitol Latinoam 63: 29–33 [Google Scholar]
- 22.Luquetti AO, Schmunis GA. 2010. Diagnosis of Trypanosoma cruzi infection. In: Telleria J, Tibayrenc M. American trypanosomiasis, Chagas disease: 100 years of research. London (UK): Elsevier. [Google Scholar]
- 23.Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. 2009. Prognostic impact of Chagas disease in the United States. Am Heart J 157:22–29 [DOI] [PubMed] [Google Scholar]
- 24.Nieto PD, Boughton R, Dorn PL, Steurer F, Raychaudhuri S, Esfandiari J, Goncalves E, Diaz J, Malone JB. 2009. Comparison of 2 immunochromatographic assays and the indirect immunofluorescence antibody test for diagnosis of Trypanosoma cruzi infection in dogs in South Central Louisiana. Vet Parasitol 165:241–247 [DOI] [PubMed] [Google Scholar]
- 25.Olson LC, Skinner SF, Palotay JL, McGhee GE. 1986. Encephalitis associated with Trypanosoma cruzi in a Celebes black macaque. Lab Anim Sci 36:667–670 [PubMed] [Google Scholar]
- 26.Pan American Health Organization. 2006. [Quantitative estimates of Chagas disease in the Americas]. Washington (DC): Pan American Health Organization. [Spanish] [Google Scholar]
- 27.Pung OJ, Spratt J, Clark CG, Norton TM, Carter J. 1998. Trypanosoma cruzi infection of free-ranging lion-tailed macaques (Macaca silenus) and ring-tailed lemurs (Lemur catta) on St Catherine's Island, Georgia, USA. J Zoo Wildl Med 29:25–30 [PubMed] [Google Scholar]
- 28.Rassi A, Marcondes de Rezende J, Luquetti A, Rassi Junior A. 2010. Clinical phases and forms of Chagas disease. In: Telleria J, Tibayrenc M. American trypanosomiasis, Chagas disease: 100 years of research. London (UK): Elsevier. [Google Scholar]
- 29.Schielke JE, Selvarangan R, Kyes KB, Fritsche TR. 2002. Laboratory diagnosis of Trypanosoma cruzi infection in a colony-raised pigtailed macaque. Contemp Top Lab Anim Sci 41:42–45 [PubMed] [Google Scholar]
- 30.Seibold HR, Wolf RH. 1970. American trypanosomiasis (Chagas’ disease) in Hylobates pileatus. Lab Anim Care 20:514–517 [PubMed] [Google Scholar]
- 31.Stevens L, Dorn PL, Schmidt JO, Klotz JH, Lucero D, Klotz SA. 2011. Kissing bugs. The vectors of chagas. Adv Parasitol 75:169–192 [DOI] [PubMed] [Google Scholar]
- 32.Uhler PR. 1876. List of the hemiptera of the region west of the Mississippi River, including those collected during the Hayden explorations of 1873. Washington (DC): Department of the Interior. [Google Scholar]
- 33. World Health Organization. The World Health Report 2002: reducing risks, promoting health life. Geneva (Switzerland): World Health Organization. [DOI] [PubMed]
- 34.Yaeger RG. 1988. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana. Am J Trop Med Hyg 38:323–326 [DOI] [PubMed] [Google Scholar]
- 35.Zabalgoitia M, Ventura J, Anderson L, Carey KD, Williams JT, Vandeberg JL. 2003. Morphologic and functional characterization of Chagasic heart disease in nonhuman primates. Am J Trop Med Hyg 68:248–252 [PubMed] [Google Scholar]
- 36.Zeledón R, Beard CB, Pinto Dias JC, Leiby DA, Dorn PL, Coura JR. 2012. An appraisal of the status of Chagas disease in the United States. London (UK): Elsevier. [Google Scholar]