Abstract
Different types of oscillations in the olfactory bulb (OB), including θ (1 to 4 and 5 to 12 Hz), β (13 to 30 Hz), and γ oscillations (31 to 64 and 65 to 90 Hz), are important in olfactory information processing and olfactory-related functions and have been investigated extensively in recent decades. The awake and anesthetized states, 2 different brain conditions, are used widely in electrophysiologic studies of OB. Chloral hydrate, pentobarbital, and urethane are commonly used anesthetics in these studies. However, the influence of these anesthetics on the oscillations has not been reported. In the present study, we recorded the local field potential (LFP) in the OB of rats that were freely moving or anesthetized with these agents. Chloral hydrate and pentobarbital had similar effects: they slightly affected the power of θ oscillations; significantly increased the power of β oscillations; significantly decreased the power of γ oscillations, and showed similar recovery of γ oscillations. Urethane had very different effects: it significantly increased oscillations at 1 to 4 Hz but decreased those at 5 to 12 Hz, decreased β and γ oscillations, and showed no overt recovery in γ oscillations. These results provide experimental evidence of different effects of various anesthetics on OB oscillations and suggest that the choice of anesthetic should consider the experimental application.
Abbreviation: LFP, local field potential; OB, olfactory bulb
Oscillations of the electrical activity in the brain, which are reflected by the local field potentials (LFP) recorded by extracellular electrodes, have been studied extensively to explore brain functions since the early days of neurophysiology.12 In the brain, neural oscillations often reflect the behavior of neurocircuits and offer a unique opportunity to explore the temporal evolution of interconnected neuronal groups accomplishing various tasks.11,12
Electrical oscillations in the olfactory bulb (OB), an important information processing center of the central olfactory system, have been studied since they were first observed in 1950.1 The oscillations are classified into different types according to frequency. In OB, the 3 typical types of oscillations are: θ band (about 1 to 4 Hz and 5 to 12 Hz), β band (about 13 to 30 Hz), and γ band (about 31 to 64 Hz and 65 to 90 Hz).17,19,28 All of these oscillations play important roles in basic olfactory information processing and olfactory cognition and may have additional functions such as olfactory associated-rewarding behavior.10,19
Oscillations in OB are largely brain-state–dependent. The awake and anesthetized states, 2 different brain conditions, are used often in electrophysiologic studies of OB.8,11,12 To obtain reliable responses to external stimulation, some experiments must be performed in anesthetized animals.8,11,12 Furthermore, brain activity levels can be manipulated readily by depth of anesthesia and choice of anesthetic.8,21
General anesthetics exert their effects by increasing the inhibitory or decreasing the excitatory neural activities across the brain, including OB.7,13 Although different anesthetics can have distinct mechanisms and effects,9,15,26 no systematic comparison of the effects of various anesthetics on the oscillations in OB had been performed previously. Chloral hydrate, pentobarbital, and urethane are used often in electrophysiologic studies in rats.2,5,21-23,31 Chloral hydrate reportedly targets both glycine and GABAA receptors; pentobarbital targets only the GABAA receptor; and urethane targets GABAA, glycine, and glutamate receptors.2,5,21-23,31 Here we explored the effects of chloral hydrate, pentobarbital, and urethane on different types of oscillations in rat OB. These results are relevant to the selection of appropriate anesthetics for studies of oscillations in rat OB.
Materials and Methods
Experiments were performed on adult male Sprague–Dawley rats (200 to 300 g) that were housed in individual cages under a constant temperature of 23 ± 1 °C, stable relative humidity, and a 12:12-h light:dark cycle. They had free access to food and water. All experimental and animal care procedures were approved in advance by the Chinese Academy of Science.
Surgery.
Rats were anesthetized with intraperitoneal pentobarbital (80 mg/kg body weight, dissolved in 0.9% sodium chloride) and mounted in a stereotaxic frame (Stoelting, Kiel, WI). After a midline scalp incision, one small hole was drilled into the cranium to accommodate a surgical screw to serve as a ground, and 2 other holes were drilled over both OB (1.5 mm lateral and 7.5 mm anterior to bregma) for recording. After opening the dura, a tungsten wire coated with polytetrafluoroethylene (diameter, 0.2 mm) was inserted into the granule cell layer of OB, to a depth of about 3 mm relative to the surface. Further confirmation of recording sites was performed by histologic staining. The 2 tungsten-wire electrodes and ground screw were attached to male pins that were secured in a rectangular 3 × 1 socket array and secured with dental acrylic. Subjects were allowed at least 1 wk to recover from surgery.
LFP recording.
LFP recording was performed in a rectangular chamber (40 cm × 30 cm) in a Faraday cage. LFP signals were sent to an amplifier (Dagan, Minneapolis, MN), and amplified 2000 times, and filtered (band-pass, 0.1 to 300 Hz). The amplified LFP signals were digitalized by using an analog-to-digital converter (Micro-1401, Cambridge Electronic Design, Cambridge, UK) at the sample rate of 2000 Hz and then saved onto the hard drive of a computer.
After habituating the rats to the recording conditions for about 30 min, baseline recording (40 min) was acquired, followed by injection of pentobarbital (80 mg/kg body weight), chloral hydrate (350 mg/kg body weight), or urethane (1.4 g/kg body weight). Data during the anesthetized state were acquired for 140 min. The durations of these recordings were long enough to include the time period in which each of the 3 anesthetics treatments was most effective.9 At these dosages, the rats showed signs of slight movement about 80 to 90 min after the administration of pentobarbital or chloral hydrate, whereas rats given urethane did not show any signs of emerging from anesthesia. To minimize the number of rats used, some animals underwent anesthesia and recording with a second anesthetic if chloral hydrate or pentobarbital was used during the first session. To avoid the interactions of the drugs, the intervals between 2 drug applications were at least 1 wk. Rats were euthanized after urethane was used, because of its detrimental effect on the health of the animals.6
Statistical analysis.
LFP signals (Figure 1) were analyzed by using the software program Spike2 (Cambridge Electronic Design), similar to our previous studies.21,22 After excluding artifacts (less than 1% of the total data), the total LFP data were divided into 18 segments, each containing 10 min of data. Time–frequency transformation was performed based on the segment (Hanning window; FFT size, 2048; frequency resolution, 0.977 Hz), and the spectral power was calculated for each frequency resolution. As in our pervious and other studies,17,21,22 LFP signals were divided into 3 main frequency bands of oscillations: θ oscillations (1 to 4 Hz and 5 to 12 Hz), β oscillation (13 to 30 Hz), and γ oscillations (31 to 64 Hz and 65 to 90Hz). For each frequency band, the power from all frequencies included was averaged. To observe the effect of the anesthetic on the oscillations, the baseline power (40 min of data before application of anesthetics) for each frequency band was normalized as 1, as in other studies.9
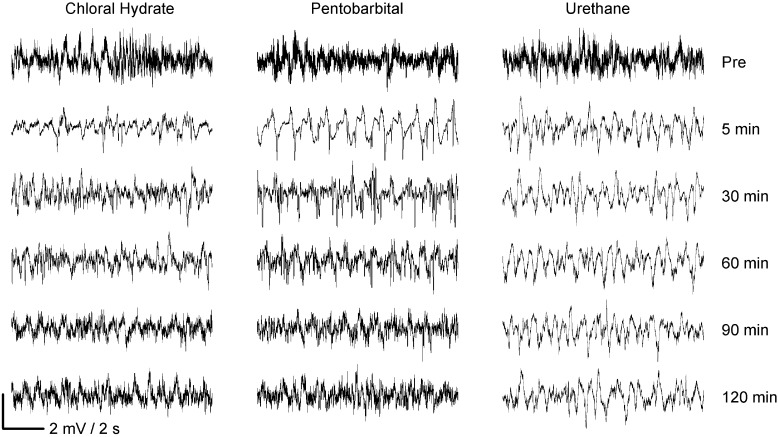
Figure 1.
General effects of chloral hydrate, pentobarbital, and urethane on the raw data of the LFP signal from rat OB. Pre, LFP data obtained before the application of anesthetics; 5 to 120 min, time after the application of anesthetics.
First, the one-sample Kolmogorov–Smirnov test was performed by using SPSS 13.0 (IBM, New York, NY) to determine whether the data were normally distributed. All data followed a normal distribution (P > 0.05). Then ANOVA with least significant differences for paired comparisons (Figures 2 through 4) or paired t tests (Figure 5) were used to assess differences between groups. Results were expressed as mean ± SEM. A P value of less than 0.05 was considered significant.
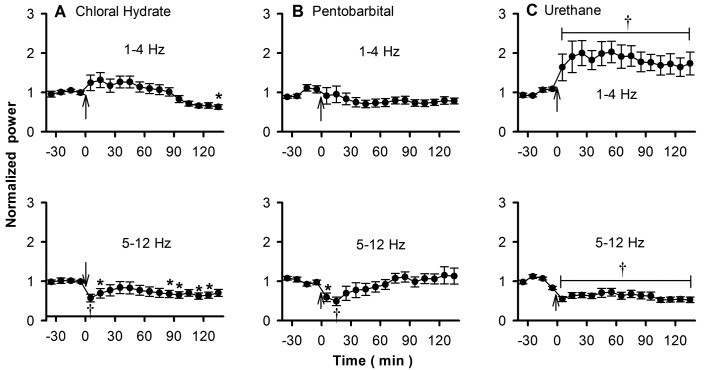
Figure 2.
The effects of (A) chloral hydrate, (B) pentobarbital, and (C) urethane on θ oscillations of rat OB. Top row, 1 to 4 Hz; bottom row, 5 to 12 Hz; *, P < 0.05; †, P < 0.01.
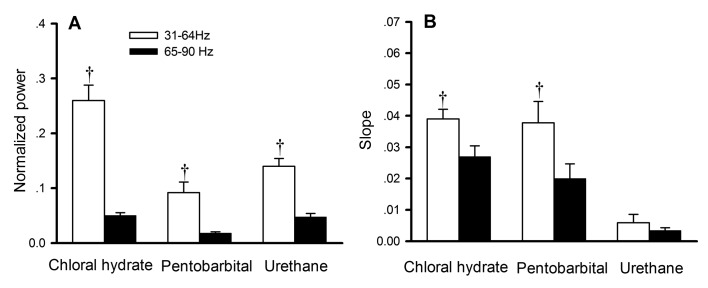
Figure 5.
Comparison of (A) suppression and (B) recovery between 31 to 64 Hz and 65 to 90 Hz. The normalized power data in panel A were obtained from the largest suppression in Figure 4. †, Significant (P < 0.01) difference between values for different frequency bands (31 to 64 Hz compared with 65 to 90 Hz) from the same anesthetic.
Results
General effects of the 3 anesthetics.
Before anesthesia, the freely moving rats either explored the surrounding environment or stayed still in the experimental cage. In both behavioral states, LFP signals recorded from the granule cell layer of OB showed high-frequency oscillations, which were decreased dramatically during anesthesia. At 5 min after the application of anesthetic, only low-frequency oscillations were apparent (Figure 1). With chloral hydrate and pentobarbital, high-frequency oscillations gradually began to return after about 30 min. There were no obvious recovery of the oscillations even 90 to 100 min after the injection of urethane (Figure 1).
Effects of anesthetics on θ oscillations.
To quantitatively compare the different effects of the 3 anesthetics on the oscillations of OB, we used the relative energy power of the oscillations as an index for further analysis. We first focused on θ oscillations (1 to 12 Hz), which we further divided into 2 subbands: 1 to 4 Hz, corresponding to the respiratory rhythm in the anesthetized or awake stationary state, and 5 to 12 Hz, corresponding to the exploring state.3,18 The power spectrum of 1 to 4 Hz changed slightly after application of pentobarbital or chloral hydrate. Power seemed to increase and then to decrease at about 90 min later, although nearly all of these fluxuations were not significant (ANOVA, F[14, 209] = 3.911; paired comparison, P > 0.05 except for the last segment; n = 15; Figure 2 A). Pentobarbital only slightly decreased the power spectrum (ANOVA, F[17, 269] = 0.579; P = 0.88; n = 18; Figure 2 B).
For the 5- to 12-Hz oscillations, both chloral hydrate and pentobarbital significantly suppressed the power spectrum within 20 to 30 min (chloral hydrate; ANOVA, F[14, 209] = 0.924; paired comparison, P < 0.01 for 20 min and P < 0.05 for 30 min; n = 15; Figure 2 A; pentobarbital: F[17, 269] = 2.003; paired comparison, P < 0.05 for 20 min and P < 0.01 for 30 min; n = 18; Figure 2B). About 30 min later, the difference was no longer significant, except at a few isolated time points (Figure 2 A).
Compared with chloral hydrate and pentobarbital, the effect of urethane on the θ oscillations of OB was simple and clear: the 1- to 4-Hz oscillations increased significantly (ANOVA, F[16, 254] = 0.825; paired comparison, all P < 0.05; n = 17; Figure 2 C) and the 5- to 12-Hz oscillations decreased significantly (ANOVA, F[16, 254] = 2.311; paired comparison, all P < 0.01; n = 17; Figure 2 C), and the power of the oscillations remained stable over the period we tested.
Effects of anesthetics on β oscillations.
About 40 min after the application of chloral hydrate or pentobarbital, the power of β oscillations increased significantly to about 1.5-fold of the baseline activities (chloral hydrate: ANOVA, F[14, 209] = 0.924; paired comparison, P < 0.01 for 40 to 110 min and P < 0.05 for 140 min; n = 15; Figure 3 A; pentobarbital: ANOVA, F[17, 269] = 2.003; paired comparison, P < 0.05 for all periods after 40 min; n =18; Figure 3 B). The effects of urethane were different in 2 respects: it significantly reduced the power of β oscillations (ANOVA, F[16, 254] = 5.090; paired comparison, all P < 0.001; n = 17; Figure 3 C) and exerted its effects rapidly (within 10 min).
Figure 3.
The effects of (A) chloral hydrate, (B) pentobarbital, and (C) urethane on β oscillations of rat OB. *, P < 0.05; †, P < 0.01.
Effects of anesthetics on γ oscillations.
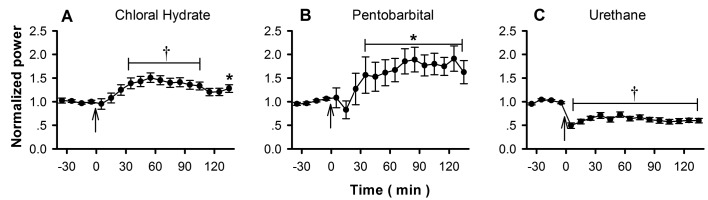
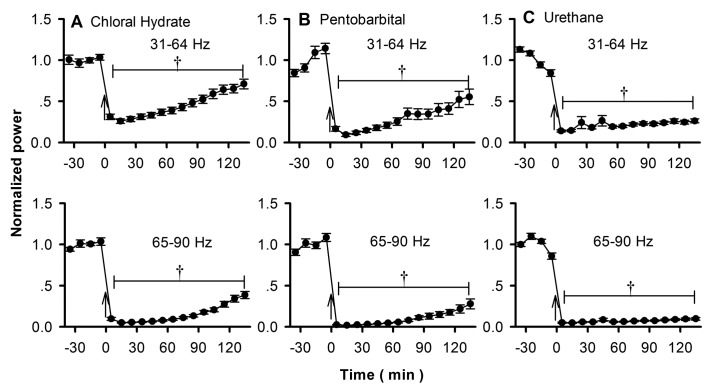
The γ oscillations of rodent OB range from about 30 to 90 Hz.17,19,28 As in other previous studies,21 we divided γ oscillations into 2 subbands—31 to 64 Hz and 65 to 90 Hz—which have been suggested to play different roles in olfactory functions.17,28 The power of both subbands decreased immediately, and the largest suppression occurred about 10 to 20 min after the application of anesthetic (Figure 4). Then the power of the γ oscillations recovered slowly after chloral hydrate or pentobarbital (Figure 4 A and B), but no recovery was noted for urethane (Figure 4 C). Linear regression analysis showed that the recovery in power was correlated with time for chloral hydrate (P < 0.0001 for all of the 15 OB evaluated) and pentobarbital (P < 0.0001 for 16 of the OB evaluated and P < 0.001 for the remaining 2 OB) but not for urethane (P < 0.01 for 7 OB and P > 0.05 for 10 OB).
Figure 4.
The effects of (A) chloral hydrate, (B) pentobarbital, and (C) urethane on γ oscillations of rat OB. Top row, 31 to 64 Hz; bottom row, 65 to 90 Hz.
The differentiated effects of anesthetics on subbands of γ oscillations were analyzed quantitatively based on the largest suppression, that is, 10 or 20 min after application of the anesthetic (Figure 4). Compared with the 31- to 64-Hz subband, the 65- to 90-Hz subband always had a larger suppression for all anesthetics (paired t test, P < 0.01 for all pairs; Figure 5 A). The recovery rate, which was reflected by the slope of the regression lines, was always slower for the 65- to 90-Hz subband with chloral hydrate or pentobarbital (paired t test, P < 0.01 for all pairs; Figure 5 B). This analysis was not done for urethane because it showed no significant recovery of high-frequency oscillations during the periods analyzed.
Discussion
Our data showed that chloral hydrate, pentobarbital, and urethane can affect the oscillations of OB profoundly. The effects of chloral hydrate and pentobarbital were similar: they had minimal effects on θ oscillations, increased β oscillations, decreased the 2 subbands of γ oscillations, and showed similar recovery of high-frequency oscillations. However, urethane behaved differently. It increased 1- to 4-Hz but decreased 5- to 12-Hz θ oscillations, decreased all β and γ oscillations, and demonstrated no recovery of high-frequency oscillations. These results clearly indicate the various effects of these anesthetics on oscillations in OB, providing helpful information for selecting anesthetics for specific research of OB oscillations.
Since the discovery of OB oscillations in hedgehogs,1 their functions and origins have been studied extensively.12,19,25,28 From these studies, it is clear that different types of oscillations are important for different OB functions and are modulated by internal and external brain states.10-12 Anesthetics exert their effects by increasing inhibitory or decreasing excitatory neural activities across almost the entire brain, thereby affecting brain states prominently. It is therefore unsurprising that anesthetics modulate OB oscillations, as we have shown in the present study: chloral hydrate, pentobarbital, and urethane all decreased γ oscillations, in agreement with results obtained from other brain areas.9 Single- or multiple-unit recording in same mitral cell has shown that the ketamine–xylazine, another commonly used anesthetic in olfactory studies, effectively decreases the spontaneous firing rate.27 Because the spike firing rate greatly influences γ oscillations,16 ketamine–xylazine and the 3 anesthetics we used in present study all may have similar effects on γ oscillation. The decreased γ oscillation that we observed might reflect a decreased firing rate and weak synaptic transmission, as reported in many other studies.24,29,30
Previously reported general effects of pentobarbital and chloral hydrate on different bands of oscillations in OB are similar in those reported in the present study,9,21,22 although some minor differences have been noted. Different anesthetics exert differential effects via distinct mechanisms.21,26 In OB, a large body of evidence suggests that the dendrodendritic synapses between mitral and granule cells are the main anatomic basis for these oscillations.28 The inhibitory transmitter released from these synapses is GABA, whose receptor is the common target of both chloral hydrate and pentobarbital, thereby providing the molecular basis for their similar effects.20,26 Furthermore, the effects of chloral hydrate and pentobarbital on γ oscillations were relatively stable and showed significant recovery over about 2 h, suggesting their potential use in studies in which baseline activities need to be manipulated.21,22
Urethane has been widely used as an anesthetic in animal experiments because it can be administrated readily, produces a long-lasting steady level of anesthesia with minimal effects on autonomic and cardiovascular systems.14,25 Urethane-anesthetized animals are thought to have similar physiologic and pharmacologic behaviors as unanesthetized animals.4,31 The mechanisms of urethane have been proved to be more complicated than those of chloral hydrate and pentobarbital: urethane shows modest potentiation of GABAergic and glycynergic transmission, modest depression of glutamatergic transmission, and moderate depression of cholinergic transmission.14,15 These mechanisms provide the molecular basis for the long-lasting and strong anesthetic effects of urethane and the rationale for the many differences from chloral hydrate and pentobarbital that we noted in our present study. These properties indicate that urethane is suitable for studies that require prolonged maintenance of a stable brain state.16,25 Previous studies have indicated that the urethane-anesthetized animals show steady periodic alternation between slow- and fast-wave states.24,29 In the current study, mainly slow waves of LFP were present. This discrepancy probably reflected the high dosage of urethane that we used (1.4 g/kg compared with1.2 g/kg in previous studies).24,29
The primary goal of our present study was to provide evidence to support the appropriate selection of anesthetics to explore OB oscillations effectively. Additional useful information would be obtained by combining OB recording with electroencephalography. Many studies have shown that oscillations in OB are very specific;12,18,21 therefore, it is more likely that the effects of anesthetics on OB are different from those on other brain areas. Detailed comparison among different brain regions is an interesting topic for future studies, particularly if coupled with multiple approaches to monitoring the animal's physiologic state (such as temperature, heart rate, and hindlimb pinch reflex).
Anesthetics are necessary in many electrophysiologic studies. The present study described the detailed effects of 3 common anesthetics on the oscillations of rat OB. The results show that chloral hydrate and pentobarbital had similar effects, which were quite different from those of urethane, thereby providing evidence for the need to select an appropriate anesthetic to explore OB oscillations effectively.
Acknowledgments
This work was supported by the National Natural Science Foundation of PR China (31100799 to A Li; 30788002 and 31171061 to F Xu), the Chinese Academy of Science (08B1021001 to F Xu), Wuhan National Laboratory for Optoelectronics (Z08004 to F Xu), and Wuhan Institute of Physics and Mathematics (Y1S1021001 to A Li; 08K1011001 to F Xu).
References
- 1.Adrian ED. 1950. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2:377–388 [DOI] [PubMed] [Google Scholar]
- 2.Buonviso N, Amat C, Litaudon P, Roux S, Royet JP, Farget V, Sicard G. 2003. Rhythm sequence through the olfactory bulb layers during the time window of a respiratory cycle. Eur J Neurosci 17:1811–1819 [DOI] [PubMed] [Google Scholar]
- 3.Cenier T, David F, Litaudon P, Garcia S, Amat C, Buonviso N. 2009. Respiration-gated formation of γ and β neural assemblies in the mammalian olfactory bulb. Eur J Neurosci 29:921–930 [DOI] [PubMed] [Google Scholar]
- 4.Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT.2008. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One 3: e2004. [DOI] [PMC free article] [PubMed]
- 5.David FO, Hugues E, Cenier T, Fourcaud-Trocme N, Buonviso N. 2009. Specific entrainment of mitral cells during γ oscillation in the rat olfactory bulb. PLOS Comput Biol 5:e1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field KJ, Lang CM. 1988. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim 22:255–262 [DOI] [PubMed] [Google Scholar]
- 7.Fontanini A, Bower JM. 2005. Variable coupling between olfactory system activity and respiration in ketamine–xylazine anesthetized rats. J Neurophysiol 93:3573–3581 [DOI] [PubMed] [Google Scholar]
- 8.Fontanini A, Katz DB. 2008. Behavioral states, network states, and sensory response variability. J Neurophysiol 100:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Guo L, Zhang J, Chen Y, Wang X, Zeng T, Tian S, Ma Y. 2008. Differential effects of ageing on the EEG during pentobarbital and ketamine anaesthesia. Eur J Anaesthesiol 25:826–833 [DOI] [PubMed] [Google Scholar]
- 10.Fuentes RA, Aguilar MI, Aylwin ML, Maldonado PE. 2008. Neuronal activity of mitral-tufted cells in awake rats during passive and active odorant stimulation. J Neurophysiol 100:422–430 [DOI] [PubMed] [Google Scholar]
- 11.Gelperin A, Ghatpande A. 2009. Neural basis of olfactory perception. Ann N Y Acad Sci 1170:277–285 [DOI] [PubMed] [Google Scholar]
- 12.Gervais R, Buonviso N, Martin C, Ravel N. 2007. What do electrophysiological studies tell us about processing at the olfactory bulb level? J Physiol Paris 101:40–45 [DOI] [PubMed] [Google Scholar]
- 13.Gyulai FE. 2004. Anesthetics and cerebral metabolism. Curr Opin Anaesthesiol 17:397–402 [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Harris RA. 2002. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94:313–318 [DOI] [PubMed] [Google Scholar]
- 15.Itoh K, Sakata M, Watanabe M, Aikawa Y, Fujii H. 2008. The entry of manganese ions into the brain is accelerated by the activation of N-methyl-D-aspartate receptors. Neuroscience 154:732–740 [DOI] [PubMed] [Google Scholar]
- 16.Kashiwadani H, Sasaki YF, Uchida N, Mori K. 1999. Synchronized oscillatory discharges of mitral–tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol 82:1786–1792 [DOI] [PubMed] [Google Scholar]
- 17.Kay LM. 2003. Two species of γ oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci 2:31–44 [DOI] [PubMed] [Google Scholar]
- 18.Kay LM. 2005. θ oscillations and sensorimotor performance. Proc Natl Acad Sci USA 102:3863–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. 2009. Olfactory oscillations: the what, how, and what for. Trends Neurosci 32:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LacKamp A, Zhang GC, Mao LM, Fibuch EE, Wang JQ. 2009. Loss of surface N-methyl-D-aspartate receptor proteins in mouse cortical neurones during anaesthesia induced by chloral hydrate in vivo. Br J Anaesth 102:515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li A, Gong L, Xu F. 2011. Brain-state–independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci USA 108:5087–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li AA, Gong L, Liu Q, Li X, Xu F. 2010. State-dependent coherences between the olfactory bulbs for δ and θ oscillations. Neurosci Lett 480:44–48 [DOI] [PubMed] [Google Scholar]
- 23.Mast TG, Griff ER. 2005. In vivo preparation and identification of mitral cells in the main olfactory bulb of the mouse. Brain Res Brain Res Protoc 15:105–113 [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Kashiwadani H, Kirino Y, Mori K. 2005. State-dependent sensory gating in olfactory cortex. Neuron 46:285–296 [DOI] [PubMed] [Google Scholar]
- 25.Neville KR, Haberly LB. 2003. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol 90:3921–3930 [DOI] [PubMed] [Google Scholar]
- 26.Pistis M, Belelli D, Peters JA, Lambert JJ. 1997. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br J Pharmacol 122:1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinberg D, Koulakov A, Gelperin A. 2006. Sparse odor coding in awake behaving mice. J Neurosci 26:8857–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas-Libano D, Kay LM. 2008. Olfactory system γ oscillations: the physiological dissection of a cognitive neural system. Cogn Neurodyn 2:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuno Y, Kashiwadani H, Mori K. 2008. Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J Neurosci 28:9227–9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuno Y, Mori K. 2009. Behavioral state-dependent changes in the information processing mode in the olfactory system. Commun Integr Biol 2:362–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson DA. 2010. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci 30:1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]