Abstract
This study compared a noncontact infrared laser thermometer and 3 different brands of subcutaneous temperature transponding microchips with rectal thermometry in 50 rhesus macaques (Macaca mulatta). The data were analyzed by using intraclass correlation coefficients and limits of agreement. In addition, the technical capabilities and practicality of the thermometers in the clinical setting were reviewed. None of the alternative techniques investigated was equivalent to rectal thermometry in rhesus macaques. Temperatures obtained by using microchips had higher correlation and agreed more closely with rectal temperatures than did those obtained by the noncontact infrared method. However, transponding microchips did not yield consistent results. Due to difficulty in positioning nonsedated macaques in their homecage, subcutaneous microchips were not practical in the clinical setting. Furthermore, pair-housed macaques may be able to break or remove microchips from their cagemates.
Abbreviation: ICC, intraclass correlation coefficient
Body temperature is one of the fundamental parameters assessed in determining the health status of an animal in both the clinical and research settings. Core body temperature, which is considered the temperature of the hypothalamus or deep body sites, requires the use of invasive techniques such as pulmonary artery catheterization, urinary bladder catheterization, and esophageal or tympanic membrane probes.5,7,12-14,20 Therefore, a practical and minimally invasive method is required for routine temperature measurements. Veterinary medicine has relied almost exclusively on rectal thermometry, which is time- and labor-intensive.5,8 The stress of chemical or manual restraint may alter the validity of the temperature measurement obtained.12,20 Rectal temperatures can be influenced by the presence of feces in the rectum, and repeated sampling increases the risk of rectal bruising and tearing.3,6,20 In some species, such as rhesus macaques, the animal must be either sedated or trained to obtain rectal temperatures.
Alternative, less invasive thermometry techniques, such as noncontact infrared thermometry and subcutaneous microchips offer advantages in veterinary medicine that include speed, convenience, decreased stress to the animal (because chemical restrain is unnecessary), and decreased occupational risk from bites and scratches to the person performing the capture and restraint. Noncontact infrared thermometry is completely noninvasive. Subcutaneous microchip implantation is minimally invasive, requiring only injection of the microchip through a large-gauge needle; after initial implantation, measuring the temperature is completely noninvasive.
To decrease the possibility of exposure of personnel to Macacine herpesvirus 1 via bites or scratches, physical examinations and other procedures are performed on sedated macaques at our facility. Currently, unless an animal is very ill and able to be hand-caught, sedation is required to obtain a rectal temperature. Monkeys are restrained in a squeeze cage, and chemical sedation is administered intramuscularly. Much of the research using macaques at our facility involves inoculation with infectious diseases, such as malaria. The ability to measure body temperature frequently while an animal is nonsedated and in its cage would enhance our ability to detect illness prior to clinical signs.
Studies in other species have explored using subcutaneous microchips2-6,10,15,17,20 and noncontact infrared thermometry2,17,19-21 for temperature measurement. In the absence of any published studies regarding the accuracy or reliability of these alternative methods for temperature determination in rhesus macaques, the objective of the current study was to determine correlation and agreement of noncontact infrared thermometry and 3 brands of subcutaneous temperature transponders compared with rectal thermometry in rhesus macaques. In addition, the differences in cost, technical capabilities, and practical clinical aspects of each thermometer are discussed.
Materials and Methods
Animals.
This study used 50 (20 female, 30 male) rhesus macaques (Macaca mulatta; age, 3.5 to 11 y; weight, 3.4 to 11.7 kg). These macaques were free from overt clinical signs of illness, deemed to be in good health, and were negative by tuberculin skin test at least 1 mo prior to the study. Animals were obtained from and housed within the Walter Reed Army Institute of Research– Naval Medical Research Center, an AAALAC-accredited research facility. The protocol was reviewed and approved by the Walter Reed Army Institute of Research IACUC. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals.9
Macaques were housed singly or in same-sex pairs. The environment was maintained at 68 to 72 °F, with a relative humidity of 30% to 70% and on a 12:12-h light:dark cycle. The animals were fed a commercial diet (Lab Diet 5038, Purina Mills International, Brentwood, MO). Water was provided ad libitum. A variety of food and toy enrichments were used in their husbandry and care program.
Equipment.
Equipment used in this study included a rectal digital thermometer, a noncontact infrared thermometer, and 3 brands of subcutaneous temperature transponding microchips.
The rectal thermometer (2180C TurboTemp Electronic Thermometer, ALARIS Medical Systems, San Diego, CA) was battery-powered and operated within a temperature range of 80 to 106 ± 0.2 °F (26.7 to 41.1 ± 0.2 °C). The device was calibrated by the manufacturer, and no more than 1 wk before the device was used in these experiments, the institute's Medical Maintenance Division verified the accuracy of the rectal thermometer by inserting it into a circulated hot water bath.
The noncontact infrared thermometer (Transcat Fluke 62 Mini Infrared Thermometer, Raytek, Santa Cruz, CA) had a temperature range of 0 to 750 ± 3 °F (−18 to 400 ± 2 °C), with a response time of less than 0.5 s and the ability to take an instantaneous measurement at a maximal distance of 1.5 m (4 ft). The cost was approximately US$100 for the thermometer. The thermometer was calibrated by the manufacturer at an additional cost of US$230.00.
Three types of commercially available subcutaneous temperature transponding microchips were used in this study. For all 3, each microchip was preloaded in a sterile single-use syringe and coated with materials that bound to the animal's subcutaneous tissues to help prevent migration. Microchip A (HomeAgain TempScan, Intervet–Schering Plough Animal Health, Summit, NJ) operated at 125 kHz; each had a unique unalterable 10-digit alphanumeric identification code. Temperature measurements were obtained by using a compatible reader (HomeAgain Universal WorldScan Pocket Reader; Destron Fearing, South St Paul, MN). The reader was battery-powered, had an operating temperature of 32 to 122 °F (0 to 50 °C), and read and identified microchips operating at multiple radio frequencies, including 125, 128, and 134.2 kHz. When a microchip was detected, the scanner beeped, the identification code was displayed in an LCD window, and then the temperature was given. The reader had no memory capabilities. This microchip system had the fewest technologic capabilities of the 3 and was the least expensive: the scanner cost US$325, and each microchip cost US$12.50.
Microchip B (LifeChip with Bio-Thermo 985 microchips, Destron Fearing) operated at 134.2 kHz, and each had a unique unalterable 15-digit identification code. Temperature measurements were obtained by using a compatible reader (DTR-4 Handheld Reader, Destron Fearing). The reader was battery-powered and came with a charger and power cord. It had an operating temperature of −13 to 113 °F (−25 to 45 °C) and identified and read microchips operating at 125 or 134.2 kHz. When a microchip was detected, the scanner beeped once, vibrated, and displayed the code and temperature in an LCD window. The reader was used with software (provided with reader) that enabled, disabled, and erased the memory; downloaded data from the DTR-4 reader; and exported data to a spreadsheet program. The reader contained an internal memory capable of storing a maximum of 2047 identification codes. This system had more technologic capabilities than did microchip system A and was more expensive: the scanner cost US$950, and each microchip was US$26.
Microchip C (IPTT-300 Microchips, Bio Medic Data Systems, Seaford, DE) had a factory-calibrated range of 90 to 110 °F (32 to 43 °C). By using DASHost software (Bio Medic Data Systems), each microchip could be programmed with as many as 32 alphanumeric characters in any coding sequence (for example, the tattooed identification number of the animal). In addition, the coding sequence might represent information such as the study number, genotype, investigator name, project number, animal's sex, or animal's date of birth. This system was the only one tested that was programmable. Temperature measurements were obtained by using a compatible reader (DAS-7007S Straight Wireless Handheld Reader, Bio Medic Data Systems). The reader was battery-powered and came with a recharging stand and wireless communication module. Features of the reader included the abilities to enable, disable, and erase the memory and to turn on or off the date, time, temperature, vibration, and sound. The reader had an internal memory capable of storing as many as 8000 identification codes. The reader could be used in ‘hands free’ mode so, that microchips could be scanned without pressing of the scan button each time. Data could be imported wirelessly from the reader to a computer and into a spreadsheet program. This system had the most technologic capabilities of the 3 tested and a correspondingly increased price: the scanner cost US$3350, DASHost software was US$300, and each microchip was US$10.
Thermometry.
The macaques were chemically restrained by using ketamine (5 to 10 mg/kg) with acepromazine (0.05 to 0.10 mg/kg). Sedatives were administered intramuscularly in either the caudal thigh or quadriceps, during brief restraint with the squeeze cage. Prior to implantation, microchips C were programmed with each macaque's individual tattoo number. The hair between the shoulder blades was shaved by using electric clippers and the skin disinfected with alcohol. The 3 types of temperature-sensing microchips were implanted subcutaneously on the dorsum between the shoulder blades into each of 50 macaques, by using manufacturer-provided injectors, in a random order, approximately 1-in. apart. This procedure involved tenting of the skin over the shoulder-blade area, quick insertion of a large-bore needle delivery device containing the microchip, and depression of the plunger on the device that expelled the microchip from the delivery device. The implant sites were closed with tissue glue. The implantation location was chosen to prevent self-removal of the microchip by the macaque. Microchips were implanted a minimum of 7 d prior to temperature measurements to allow resolution of any inflammation that might result from device implantation or shaving. Each of the 3 types of microchip was scanned before and after placement to verify correct implantation and proper function. The number and implantation site (left, center, or right back) of each microchip were recorded for each macaque. Permanent markers (color-coded as to microchip type) were used to draw a circle around each implantation site, to facilitate microchip location at the time of temperature measurement.
Macaques were sedated a total of 3 times at intervals of 1 wk or greater. At each time point, 6 temperature readings (1 rectal, 2 noncontact infrared, 3 microchips) were obtained randomly over 5 min and recorded. To obtain body temperature by using the microchips, the reader was waved within a few centimeters of the implantation site until a beep was heard, indicating that the reader had detected the microchip. The rectal temperature was taken by using the electronic rectal thermometer, which was prepared by placing a small amount of lubricant on the tip of a covered probe. The probe was inserted rectally approximately 1 in. and held until the unit gave a digital reading. Body surface temperatures were obtained by using the noncontact infrared thermometer on the unshaven skin of the chest and abdomen. These areas were chosen because they have the least hair and are presented frequently by macaques in their cages. Measurements were taken by pointing the infrared laser at the target site from a distance of approximately 15 cm.
Statistical analysis.
Data were analyzed by using the Shrout and Fleiss Interclass Correlation Coefficient (ICC).18 The ICC is used to estimate the correlation of one variable between 2 members within a group. In the current study, the ICC was used to assess the similarity between the rectal temperature and each temperature obtained by using an alternate measurement method. A sample size of 50 subjects with 2 observations per subject (rectal and alternative temperature method [microchip or noncontact infrared thermometry]) achieved 83% power to detect an ICC of 0.9 under the alternative hypothesis when the ICC under the null hypothesis was 80% correlation by using an F-test with a significance level of 0.05. Statistical analysis was conducted by using SAS 9.3 (SAS Institute, Cary, NC).
Data also were analyzed by using Bland–Altman analysis to determine agreement between 2 measurement systems.1 This analysis can be used to determine whether a new technique agrees sufficiently to replace an established standard.1 Rectal temperature measurements were the standard for comparison with the results of the other thermometry techniques. Agreement to within 3 °F with a 95% confidence was the criterion established prior to the study for determining that a measurement system was equivalent to rectal thermometry. Therefore, 95% limits of agreement that extended beyond 3 °F indicated that the difference between alternative and rectal temperature measurements would exceed 3 °F for approximately 5% of the animals, a situation that would be clinically unacceptable. This analysis was conducted by using SAS 9.3 (SAS Institute).
The data were analyzed by using both correlation and agreement. Correlation measured the strength of the linear relationship of the alternative temperature method compared with rectal temperature but not the agreement between them. It was possible to have a linear relationship but not agreement. For example, if the temperature obtained by using an alternative measuring method was always 5° higher than the rectal temperature, then the values would show perfect correlation but zero agreement.1 The best temperature measuring method will have both correlation and agreement with rectal thermometry.
Results
The ICC between temperatures obtained by using each alternative thermometry method and rectal thermometry were calculated (Table 1). The temperatures measured by using microchips were inconsistent among the 3 sampling times, and those obtained by noncontact infrared thermometry were consistently poor.
Table 1.
Intraclass correlation coefficients between temperatures obtained by using each alternative method and rectal thermometry
| Time 1 | Time 2 | Time 3 | |
| Microchip A compared with rectal | 0.272 | 0.584 | 0.430 |
| Microchip B compared with rectal | 0.332 | 0.684 | 0.437 |
| Microchip C compared with rectal | 0.313 | 0.767 | 0.416 |
| Infrared at chest compared with rectal | −0.794 | 0.074 | −0.012 |
| Infrared at abdomen compared with rectal | −0.079 | 0.007 | 0.060 |
An intraclass correlation coefficient of <0.40 signifies poor correlation; 0.40 to 0.59 signifies fair correlation; 0.60 to 0.74 signifies good correlation; and >0.74 signifies excellent correlation.
Bland–Altman analysis of the temperatures obtained by using the various thermometry methods (Table 2) revealed considerable disagreement between rectal temperatures and those from all other modalities. At times 1 and 2, none of the methods were within the preestablished 3 °F to be considered equivalent to rectal thermometry. At time 3, microchips A and C had the lowest mean differences and were barely within the 3 °F limit of agreement.
Table 2.
Bland–Altman agreement between temperatures (°F) obtained by using each alternative method and rectal thermometry
| Time 1 |
Time 2 |
Time 3 |
||||||
| Mean difference | Limits of agreement | Mean difference | Limits of agreement | Mean difference | Limits of agreement | |||
| Rectal – microchip A | 2.52 | –0.26, 5.30 | 2.94 | 0.47, 5.42 | 0.67 | –1.53, 2.87 | ||
| Rectal – microchip B | 2.98 | 0.43, 5.54 | 3.29 | 1.34, 5.24 | 1.50 | –0.75, 3.75 | ||
| Rectal – microchip C | 2.31 | –0.42, 5.04 | 2.63 | 1.04, 4.22 | 0.77 | –1.33, 2.88 | ||
| Rectal – infrared at chest | 8.97 | 2.59, 15.35 | 8.70 | 3.35, 14.05 | 8.90 | 3.75, 14.06 | ||
| Rectal – infrared at abdomen | 9.76 | 1.15, 18.26 | 9.34 | 2.07, 16.61 | 12.12 | 5.39, 18.86 | ||
Both the noncontact infrared thermometer and microchip readers were easy and convenient to use on sedated monkeys. Both methods provided a temperature measurement within seconds, contrasted to the minutes required for the rectal temperature. However, reading the microchips from nonsedated macaques in their homecages was impractical because the reader had to be within a few centimeters to the microchip to register a reading. Therefore, the macaques had to have their dorsum facing out of the cage; most animals were difficult to position or did not remain in position for the reading. In contrast, the noncontact infrared thermometer was easy to use on nonsedated macaques in their homecages: the beam could be directed at the desired area, and distance was not a factor in obtaining a temperature reading.
Microchip failure.
Of the 50 macaques implanted, 16 had microchips that failed to provide a temperature reading during at least one of the 3 measurement times. These 16 animals were radiographed, and the findings are summarized in Table 3. Among microchips A, 14 malfunctioned: 4 failed to read but were present on radiographs, 2 were missing on radiographs, 6 returned the identification code but no temperature reading, and 2 read intermittently and were present on radiographs. In addition, 4 microchips C malfunctioned: 2 were missing from radiographs, and the remaining 2 were present on radiographs but appeared to be broken (Figure 1). Of the 2 faulty microchips B, one was missing from radiographs, and the other failed to read but was present on radiographs.
Table 3.
Summary of the 16 macaques with undetectable microchips
| Macaque | Microchip failure rate (maximum, 3) | Description of failure(s) | Radiograph results (3 microchips expected) | Pair-housed? |
| 1 | 1 | Microchip A: no reading | 3 microchips | yes |
| 2 | 1 | Microchip A: read code but no temperature | 3 microchips | yes |
| 3 | 1 | Microchip A: intermittent (no reading at time 2) | 3 microchips | yes |
| 4 | 2 | Microchips A and C: no reading | 3 microchips; microchip C appeared broken | yes |
| 5 | 1 | Microchip A: read code but not temperature | 3 microchips | no |
| 6 | 2 | Microchips A and C: no reading | 1 microchip; microchips A and C missing | yes |
| 7 | 1 | Microchip B: no reading | 2 microchips; microchip B missing | yes |
| 8 | 2 | Microchips B and C: no reading | 3 microchips; microchip C appeared broken | yes |
| 9 | 1 | Microchip A: no reading | 3 microchips | yes |
| 10 | 1 | Microchip A: no reading | 2 microchips; microchip A missing | yes |
| 11 | 2 | Microchip A: read code but not temperature; microchip C: no reading | 2 microchips; microchip C missing | yes |
| 12 | 1 | Microchip A: read code but no temperature | 3 microchips | no |
| 13 | 1 | Microchip A: read code but no temperature | 3 microchips | no |
| 14 | 1 | Microchip A: no reading | 3 microchips | no |
| 15 | 1 | Microchip A: read code but not temperature | 3 microchips | no |
| 16 | 1 | Microchip A: intermittent (no reading at time 3) | 3 microchips | yes |
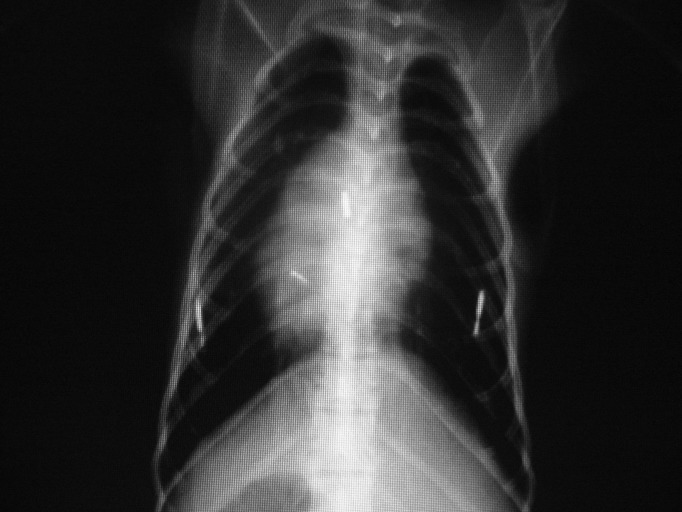
Figure 1.
Dorsal–ventral thoracic radiograph of a rhesus macaque. The left and right microchips are in the correct positions and appear to be intact. The center microchip appears to be broken, as indicated by the 2 smaller fragments.
Discussion
Temperature transponding microchips are commonly used to measure animal temperature in research. Some studies have found microchips to be a reliable alternative to rectal thermometry in tested species2-5,10,15,20 whereas others have not.5,6,17 In the current study, temperatures obtained by using microchips had higher correlation and agreed more closely with rectal temperatures than did those measured by using the noncontact infrared method. However, microchip thermometry was not a reliable method of determining body temperature in rhesus macaques: the microchip readings were not consistently repeatable. Subcutaneous temperatures may differ from rectal temperatures because the subcutaneous temperatures may be affected by ambient temperatures or wet fur. In addition, a lag time between changes in core and subcutaneous temperatures could account for some of the disagreement between temperatures. Furthermore, the location of microchip implantation and the amount of subcutaneous adipose tissue could influence temperature readings. Our results showed that microchips B and C had higher ICC than did other modalities, whereas microchips A and C provided the greatest agreement between subcutaneous and rectal temperatures. Overall, microchip C had the highest correlation and greatest agreement with rectal thermometry.
The current study showed that detecting microchips in nonsedated macaques in their homecages was impractical because most animals were difficult to position or did not remain in position for the reading. Large macaques were extremely strong and tended to be positioned face-forward in the squeeze cage, with their feet providing resistance, preventing reading of the microchips. Small macaques were easier to position appropriately in the squeeze cage but could turn around and did not stay still long enough in the appropriate position to yield readings. Overall, the macaques became stressed after repeated attempts to position them adequately in the squeeze cage for microchip reading. In addition, macaques that faced forward appeared to be frightened by the approach of the reader and struggled more. The readings that were obtained on nonsedated macaques occurred only when their positioning was perfect, which was rare, and sometimes even with good positioning, the microchips were still undetectable. Perhaps the metal bars of the cage interfered with reading the microchips. Another issue is that obtaining readings from animals that have been struggling could give falsely elevated temperatures.12,20
In this study, 26 of 50 macaques were pair-housed and 24 of 50 were singly housed. At least one microchip failed in 11 of the 26 pair-housed macaques, compared with 5 of the 24 singly housed animals. In addition, the 6 broken or missing microchips all occurred in pair-housed macaques. There were no missing or broken microchips in singly housed animals. Although missing microchips may have been due to incorrect implantation, this was unlikely because placement was verified after implantation. That all macaques with missing or broken microchips were pair-housed suggests that cagemates can break or remove microchips from each other, although these behaviors were not observed by the caretaking or research staff. As social animals, rhesus macaques should be housed in stable pairs or groups. Single housing should be the exception and justification provided based on experimental reasons, social incompatibility, or veterinary concerns for animal wellbeing.9 Therefore, before microchips are placed in macaques, researchers should consider that these devices may be removed or broken by cagemates.
Noncontact infrared thermometry has been studied for use in research2,11,16,17,19-21 Some studies have found noncontact infrared thermometry to be a useful alternative to rectal thermometry11,16,21 whereas others have not.2,17,19,20 In the current study, the measurements from the noncontact infrared thermometer had consistently poor correlation with and no agreement to rectal temperatures. Noncontact infrared thermometers measure surface temperatures and, as for subcutaneous transponders, the temperatures obtained with noncontact infrared thermometers may be influenced by ambient temperatures or wet fur. In addition, the presence of hair seemed to affect the ability of the beam to reach the skin surface sufficiently to measure temperatures. Readings from the abdomen were less effective than those from the chest, and this difference may reflect that rhesus macaques typically have less hair on their chests than their abdomens. The noncontact infrared thermometer was easy to use on nonsedated animals in their homecages, and distance was not a factor in obtaining a temperature reading. The infrared thermometer was relatively inexpensive and provided no memory or notable technologic capabilities.
In conclusion, this study compared methods of body temperature measurement in rhesus macaques that would ideally reduce the need for handling, sedation, and anesthesia. None of the alternative technologies investigated were equivalent to rectal thermometry in rhesus macaques. Temperatures obtained by using microchips showed the greatest agreement with rectal temperatures, although microchip thermometry did not consistently yield acceptable results. Due to difficulty in positioning nonsedated monkeys in their homecage, microchips were not practical in the clinical setting. Noncontact infrared thermometry was easy to use on nonsedated macaques in the clinical setting, but values were not correlated to those obtained by using rectal thermometry.
Acknowledgments
This work was performed at Walter Reed Army Institute of Research, Naval Medical Research Center, Silver Spring, MD, USA 20912-7500, and was funded by the Walter Reed Army Institute of Research. There is no objection to its presentation or publication. The opinions and assertions contained herein are the private ones of the author and are not to be construed as such or reflecting the views of the Department of Defense. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
I would like to thank Cara Olsen, Meng Shi, Robert Burks, Rodney Sturdivant, Joe Gruver, Dawn Wolf, Andrea Duarte, Deborah Rush, Jeffery Dressing, Walter Cruz, and Laura Brown for assistance.
References
- 1.Bland JM, Altman DG.1986. Statistical methods for assessing agreement between 2 methods of clinical measurement. The Lancet 1: 307–310.
- 2.Chen PH, White CE. 2006. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 45:57–63 [PubMed] [Google Scholar]
- 3.Cilia J, Piper DC, Upton N, Hagan JJ. 1998. A comparison of rectal and subcutaneous body temperature measurement in the common marmoset. J Pharmacol Toxicol Methods 40:21–26 [DOI] [PubMed] [Google Scholar]
- 4.Dunney S, Shuster D, Heaney K, Payton A. 1997. Use of an implantable device to monitor body temperature in swine. Contemp Top Lab Anim Sci 36:62 [Google Scholar]
- 5.Goodwin S. 1998. Comparison of body temperatures of goats, horses, and sheep measured with a tympanic infrared thermometer, an implantable microchip transponder, and a rectal thermometer. Contemp Top Lab Anim Sci 37:51–55 [PubMed] [Google Scholar]
- 6.Greer RJ, Cohn LA, Dodam JR, Wagner-Mann CC, Mann FA. 2007. Comparison of 3 methods of temperature measurement in hypothermic, euthermic, and hyperthermic dogs. J Am Vet Med Assoc 230:1841–1848 [DOI] [PubMed] [Google Scholar]
- 7.Hayes JK, Collette DJ, Peters JL, Smith KW. 1996. Monitoring body-core temperature from the trachea: comparison between pulmonary artery, tympanic, esophageal, and rectal temperatures. J Clin Monit 12:261–269 [DOI] [PubMed] [Google Scholar]
- 8.Huang HP, Shih H. 1998. Use of infrared thermometry and effect of otitis externa on external ear canal temperature in dogs. J Am Vet Med Assoc 213:76–79 [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 10.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim 32:260–269 [DOI] [PubMed] [Google Scholar]
- 11.Loughmiller JA, Spire MF, Dritz SS, Fenwick BW, Hosni MH, Hogge SB. 2001. Relationship between mean body surface temperature measured by use of infrared thermography and ambient temperature in clinically normal pigs and pigs inoculated with Actinobacillus pleuropneumoniae.. Am J Vet Res 62:676–681 [DOI] [PubMed] [Google Scholar]
- 12.Martin BJ. 1995. Tympanic infrared thermometry to determine cat body temperature. Contemp Top Lab Anim Sci 34:89–92 [PubMed] [Google Scholar]
- 13.Milewski A, Ferguson KL, Terndrup TE. 1991. Comparison of pulmonary artery, rectal, and tympanic membrane temperatures in adult intensive care unit patients. Clin Pediatr (Phila) 30 Suppl:13–16 [DOI] [PubMed] [Google Scholar]
- 14.Nierman DM. 1991. Core temperature measurement in the intensive care unit. Crit Care Med 19:818–823 [DOI] [PubMed] [Google Scholar]
- 15.Quimby JM, Olea-Popelka F, Lappin MR. 2009. Comparison of digital rectal and microchip transponder thermometry in cats. J Am Assoc Lab Anim Sci 48:402–404 [PMC free article] [PubMed] [Google Scholar]
- 16.Saegusa Y, Tabata H. 2003. Usefulness of infrared thermometry in determining body temperature in mice. J Vet Med Sci 65:1365–1367 [DOI] [PubMed] [Google Scholar]
- 17.Shelton LJ, Jr, White CE, Felt SA. 2006. A comparison of noncontact, subcutaneous, and rectal temperatures in captive owl monkeys (Aotus sp.). J Med Primatol 35:346–351 [DOI] [PubMed] [Google Scholar]
- 18.Shrout PE, Fleiss JL. 1979. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428 [DOI] [PubMed] [Google Scholar]
- 19.Sikoski P, Banks ML, Gould R, Young RW, Wallace JM, Nader MA. 2007. Comparison of rectal and infrared thermometry for obtaining body temperature in cynomolgus macaques (Macaca fascicularis). J Med Primatol 36:381–384 [DOI] [PubMed] [Google Scholar]
- 20.Stephens Devalle JM. 2005. Comparison of tympanic, transponder, and noncontact infrared laser thermometry with rectal thermometry in strain 13 guinea pigs (Cavia porcellus).. Contemp Top Lab Anim Sci 44:35–38 [PubMed] [Google Scholar]
- 21.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 37:126–131 [DOI] [PubMed] [Google Scholar]