Abstract
The term barrier function as applied to human skin often connotes the physical properties of this organ that provide protection from its surrounding environment. This term does not generally include skin pigmentation. However, skin pigmentation, which is the result of melanin produced in melanocytes residing the basal layer of the skin and exported to the keratinocytes in the upper layers, serves equally important protective function. Indeed, changes in skin pigmentation are often the most readily recognized indicators of exposure of skin to damaging agents, especially to natural and artificial radiation in the environment. Several recent studies have shed new light on a) the mechanisms of involved in selective effects of subcomponents of UV radiation on human skin pigmentation and b) the interactive influences between keratinocytes and melanocytes, acting as ‘epidermal melanin unit’, that manifest as changes in skin pigmentation in response to exposure to various forms of radiation. This article provides a concise review of our current understanding of the effects of the non-ionizing solar radiation, at cellular and molecular levels, on human skin pigmentation.
INTRODUCTION
Human skin interfaces between the body and external environment, acts as a barrier against physical, chemical and biological attacks from the environment. Exposure to solar radiation and sun sensitivity are associated with increased risk for skin cancers (1, 2). The incidence of skin cancer (including melanoma and non-melanoma skin cancers) has been increasing substantially in the United States (3, 4). Solar UV radiation induces genotoxic effects by damaging wide variety of bio-organic molecules including DNA, proteins and other small molecules such as folate. Biological macromolecules or small compounds present in the skin act as chromophores and absorb radiation of specific wavelength to bring about a cascade of reactions within the cell. Nucleic acid, urocainic acids, co-factors NADPH and NADH, aromatic amino acids- tryptophan and tyrosine, riboflavins, porphyrins and melanins and their precursors are all chromophores present in the skin. They absorb protons and undergo a series of structural and chemical changes (5). Melanin synthesized in the melanocytes plays an important role in protecting the skin from radiation-induced damage. Melanocytes transfer melanosomes to keratinocytes, where melanin is localized above the nucleus in the form of a cap like structure to protect the cellular DNA (6). UV radiation induces immediate pigment darkening (IPD) by chemical modification of melanin, and possibly spatial redistribution of melanosomes in keratinocytes and melanocytes (7). UV exposure also leads to delayed tanning (DT) by new synthesis of melanin over several days after UV exposure and persists for weeks (8). UV-induced pigmentation is thought to play a protective role by preventing DNA damage and accumulation of mutations. Given the importance of melanin and skin pigmentation in providing protection from solar radiation, the evolutionary aspects of human skin pigmentation have received much attention. Skin pigmentation is thought to have evolved as protective adaptation from the damaging effects of direct exposure of the skin to solar radiation (9). However, since melanin pigment absorbs solar radiation, which is required for synthesis of vitamin D in the skin, the primary site of production of this molecule essential for a wide range of physiological processes, loss of dark pigmentation and evolution of fair skin, in order to maximize the absorption of solar radiation for vitamin D synthesis (10) but while simultaneously increasing the susceptibility to its damaging effects, is thought to be the evolutionary price of migration of early humans to higher latitudes. This review provides an overview of the current knowledge on the biological mechanisms of the effects of solar radiation on melanocytes, melanin and human skin pigmentation.
BIOLOGY OF SKIN PIGMENTATION
Human skin color phenotype is determined by the amount and distribution of melanin and ranges from white to black with various shades in between. Fitzpatrick scale of skin phototype classification is based on the color of the skin and eye and ability of the skin to burn and tan when exposed to UV radiation. Type I-III are white skin, type I- always burns, never tans; type II- always burns, minimal tan; type III- burns minimally, tans moderately and gradually; whereas type IV- light brown skin, burns minimally and tans well; type V- brown skin, rarely burns, tans deeply and type VI- dark brown/black skin, never burns, tans deeply (11). Skin pigmentation is determined by over a 100 genes including those that encode transcription factors, enzymes, hormones, autocrine and paracrine factors and their receptors. There are several excellent and comprehensive reviews on biochemistry, cell biology and genetics of skin pigmentation (6, 12–14). Here, only a brief outline of the biology of pigmentation as it relates to its response to environmental radiation will be presented. The photoprotective function of skin resides primarily in the keratinocytes present in the outer layers of the epidermis with the melanin pigment producing melanocytes embedded within the basal layer of keratinocytes (6). The pigment melanin is synthesized in the melanocytes and helps to protect the skin from the deleterious effects of solar radiation by several mechanisms (15). Accordingly, patients with impaired production of melanin, for example as in vitiligo, or lack pigment altogether as in albinos, have higher rate of skin cancers (16, 17).
Two chemically distinct types of melanins are found in the skin; an insoluble black-brown eumelanin and an alkali soluble red-yellow pheomelanin. Eumelanin is a polymeric compound consisting of 5, 6-dihydroxyindole (DHI) and 5, 6- dihydroxyindole carboxylic acid (DHICA) monomers and is the primary melanin involved in photoprotection, with the precursors DHI and DHICA having considerable antioxidant properties (18, 19). Pheomelanin is also a polymeric compound; however it is made up of sulfur and nitrogen containing benzothiazine monomers. The biochemical mechanisms of the initial reactions by which these two types of melanins are synthesized are well understood. However, the terminal reactions that make the insoluble polymers, especially pheomelanin, are not understood and thought to occur through spontaneous reactions. This makes the microenvironment within the melanosome, where these pigments are formed critically important (20). The pathway begins with the production of DOPA and DOPA quinone from tyrosine, catalyzed by the enzyme tyrosinase (TYR), the most important and rate-limiting enzyme in pigmentation. Tyrosinase catalyzes the only step common to both eumelanin and pheomelanin synthesis. Mutations in tyrosinase cause albinism, a deficiency of production of both types of melanin, externally characterized by the complete or partial lack of skin, hair and eye pigmentation (21). Pheomelanin synthesis pathway is thought to proceed spontaneously, controlled by the concentrations of metabolites and inherent pH controlled by the other pigmentary gene products. Synthesis of eumelanin on the other hand, is catalyzed by additional tyrosinase related enzymes TYRP1 and TYRP2 (also known as dopachrome tautomerase, DCT) (22, 23). Tyrosinase and TYRPs are membrane bound enzymes. They are synthesized and transported through the ER-Golgi secretory pathway and finally incorporated into the melanosomes (20). The relationship between total melanin content, ratio of eumelanin:pheomelanin ratio and the activity levels of the key melanogenic enzyme tyrosinase has been investigated in an exhaustive study by Ito and his coworkers (24). Classifying melanocytes isolated from individuals based on visual skin pigmentation as very light, light, fairly dark and dark, it was noted that total melanin content correlated with tyrosinase activity levels across all skin types, and while eumelanin levels correlated with visual skin pigmentation, pheomelanin levels were consistently higher in lighter skin (24). The melanocyte master controller known as micropthalmia transcription factor, MITF, controls many aspects of skin pigmentation- from development of melanocytes to their response to environmental stimuli including expression of melanogenic enzymes (25).
The mature melanosomes when fully deposited with melanin pigment are transported from the melanocyte cell body into the dendrites and transferred to the keratinocytes, where melanosomes localize to the perinuclear area (26). Mutations in cytoskeletal components that affect melanosome movement within the melanocyte result in overall pigment lightening due to the inability of the melanosomes to be transported along the dendrites and thus transferred to the keratinocytes (27). The transfer of melanosomes to keratinocytes is known to be regulated by protease-activated receptor 2 (PAR2) and its peptide activator SLIGRL (26). Although the molecular details of this process and how it is regulated are poorly understood, it generally accepted that it provides an additional site of regulation for both constitutive and facultative skin pigmentation.
ELECTROMAGNETIC RADIATION AND PIGMENTATION
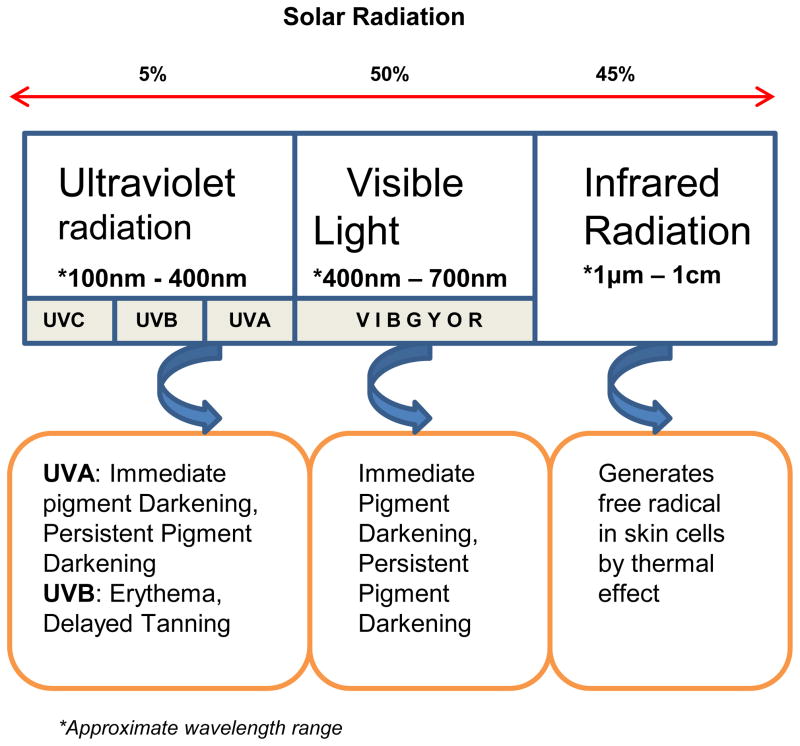
Electromagnetic radiation consists of electrical, radio, microwave, infrared, visible, UV, X-rays and gamma-rays. Solar radiation that reaches the earth’s surface is primarily nonionizing radiation (UVR, visible light, and infrared radiation) (Fig.1). Radio waves have the largest wavelength but lowest frequency and energy, whereas gamma rays have small wavelength but highest energy. Radio frequency energy is used for several therapeutic and cosmetic purposes (28). There are several reports of its efficacy in non-invasive skin tightening (29). Radio waves have can penetrate into deeper layers of the skin and generate heat. This thermal effect of radiofrequency radiation causes tightening of the skin in the sub-dermal layers (30). Karinen et al. found differences in protein expression in volunteers forearm skin exposed to radiofrequency modulated electromagnetic field (RF-EMF, mobile phone radiation) and suggested protein expression in human skin might be affected by the exposure to RF-EMF (31). The exponential increase in the usage of mobile phones, which emit radiofrequency, has prompted some studies on the effects prolonged use of mobile phones on skin and risk of melanoma (32). Infrared radiation with intermediate energy causes an increase in the vibrational energy of biomolecules and causes the production of free radicals within the skin (33). Microwave radiation is also a form of non-ionizing radiation that does not possess enough energy to cause significant harm to the skin. However, intense exposure to this type radiation can cause change in dielectric field within cells that increases friction between polar water molecules, and thus increases the ambient temperature and can lead to burns. However, only limited information is available on the effects of radio or microwave radiation on human skin pigmentation.
Figure 1.
Solar radiation spectrum and effects of radiation on skin pigmentation.
CHROMOPHORES IN THE SKIN
Solar radiation is absorbed by endogenous chromophores like nucleic acids, aromatic amino acids and melanin precursors present in the epidermis (5). DNA absorbs 4-fold less of UVA than UVB radiation resulting in production of DNA photo-damage and mutations. Tyrosine and tryptophan absorb UV radiation and bind to DNA and produce photodamage, and also cause damage to adjacent amino acids within proteins. Melanin absorbs UV radiation and reduces the UV-induced damage in the melanocytes and keratinocytes. Moan et al. proposed that the evolutionary and biological role of facultative skin pigmentation is to protect folate and its derivatives against photodegradation (34). Recently, rhodopsin, a photopigment that is present in the eye and involved in visual transduction has been reported to be present in melanocytes and contribute to UV phototransduction in these cells (35). However, the full complement of chromophores in the skin and specifically in the melanocytes and the mechanisms of cutaneous phototransduction remain to be fully understood.
UV RADIATION
Effects of UV radiation on the skin have been studied extensively due to the physiological and the pathological implications of natural and recreational exposure to solar and non-solar UV radiation sources. Solar UV spectrum spans from 100 nm to 400 nm wavelength and it is divided into UVA (320 nm-400 nm), UVB (280 nm-320nm) and UVC (100 nm-280 nm). UV radiation of wavelengths shorter than ~310 nm are absorbed by earth’s ozone layer and therefore natural exposure to UVC component of the solar radiation is thought to be minimal. Approximately 90–95% of UVA and 5–10% of UVB in the solar radiation reaches human skin (36, 37). UVA can penetrate deep into dermis and 20–50% of solar UVA can reach the depth of the melanocytes, whereas only 9–15% solar UVB reaches melanocytes in the skin (6). The causative role of UV radiation in melanoma has been investigated extensively. This topic has been covered by several comprehensive reviews (37–40).
Increased production and distribution of melanin within melanocytes and the conversion of keratinocytes into a hornlike substance or stratified squamous epithelium are the morphological and physiological response to UV radiation in skin (41). Higher dose of UV radiation initiate apoptosis in keratinocytes and melanocytes. De Leeuw et al. irradiated melanocytes (skin type II) and keratinocytes with UV and found melanocytes less sensitive to UV radiation than keratinocytes (42). UV-induced oxidative stress increases the production of ROS and in response to the accumulating ROS, keratinocytes release factors that act on melanocytes and induce hyperpigmentation. A potential target for cosmetic skin-lightening systems would be to increase the anti-oxidants (43).
Geographical differences in bioactivity, strength, seasonal distribution of UVA and UVB have been recorded, but influence of these differences on human skin pigmentation is not well understood. For example, latitudinal bands of UVA distribution are wider than UVB and higher levels of UVA exist towards the poles. Western Europe receives highest UVA compared to UVB and whereas equatorial regions receive slightly less UVA than tropical and subtropical areas (9). In an elegant review, Jablonski and Chaplin summarized the effects of UVA and UVB on human body and selective mechanisms involved in the evolution of pigmentation. They proposed that evolutionarily two clines of human skin pigmentation were produced by natural selection based on UV radiation- one at the equator with darker skin pigmentation and the second with depigmented skin in response to lower UVB availability and its requirement for vitamin D photosynthesis. In a detailed discussion of the effects of UV on folate, which is essential for cell division, DNA repair and melanogenesis, these authors propose that photolysis of folate directly by UVA and depletion of folate by ROS may have played a role in evolution of pigmentation. Additionally, sensitivity of tyrosinase to high levels of ROS generated in response to UVA exposure is thought to disrupt melanin production (9). In addition to evolution of skin types in response to UV, solar UV continues remain a significant factor in human skin pigmentation.
Exposure to UVA induces immediate pigment darkening (IPD) within a short time, as little as few hours, by acting on preexisting melanin. UVA can also cause persistent pigment darkening (PPD) in the skin. Both types of skin darkening by UVA are more readily observed in dark-skinned individuals than in fair-skinned persons. Recent extensive in vivo studies on human subjects by Hearing’s laboratory, using state of the art immunohistochemical and molecular methodologies, have contributed significantly to our understanding of the mechanisms of UV induced pigment changes (44–46). Based on their study on subjects repeatedly irradiated with UV, five times a week for two weeks, Choi et al. reported that UVA, unlike UVB, induces minimal changes in expression of pigmentation related genes (45). In another study, where subjects were given a challenge dose of UVA following the 2-week UVA exposure, Miyamura et al reported that although UVA exposure resulted in DNA and cellular damage, the UVA-induced pigmentation/tanning offered no photoprotective effect upon UVA challenge (44). Thus, the UVA exposure can cause formation of singlet oxygen radicals which induce DNA-strand break, nuclear base damage and mutations (47). Garland et al. hypothesized that exposure to UVA could induce melanoma and demonstrated potential carcinogenic effect of UVA in cultured human melanocytes. Endogenous pigment and/or melanin-related molecules are thought to enhance UVA-induced DNA damage via photo-sensitization reactions that generate reactive oxygen species (ROS) (48). UVA generated ROS can damage mitochondria and induce apoptosis in cell culture (49). Pheomelanin and/or melanin intermediates are thought to act as photosensitizers in DNA single-strand breaks caused by UVA (50). Marrot et al. also demonstrated that endogenous pigments and/or melanin-related molecules enhance DNA damage, evidenced by higher DNA breakage in melanocytes than in fibroblasts and higher in cells with high melanin content (51).
Although UVA reaches the dermal layers of the skin and its effects on photoaging and keratinocyte invasiveness are well studied (52), melanocytes seem to be more susceptible to the damaging effects of UVA. For example, exposure of cultured melanocytes and keratinocytes from the same donor to UVA and UVB showed that UVA-induced oxidative lesions contributed to a larger extent to DNA damage in melanocytes than in keratinocytes (53). Cui et al. demonstrated that keratinocytes absorb UV radiation and activate p53 mediated propiomelanocortin (POMC) promoter, which leads to an increased expression of POMC, and POMC derived beta-endorphins and alpha-melanocyte stimulating (alpha-MSH) hormone (54). Keratinocyte- secreted alpha-MSH stimulates melanocytes and increases pigment production by binding to melanocortin 1 receptor (MC1R) receptor and activation of cAMP pathway (55). Increased alpha-MSH secretion in response to UV exposure can cause both melanocyte proliferation and increased melanogenesis (56). Scott et al. showed that while the alpha-MSH, the ligand for MC1R increased MC1R mRNA expression, treatment of melanocytes with the MC1R antagonist agouti protein or UV downregulated MC1R expression. These authors also investigated regulation of MC1R by paracrine and endocrine factors and suggested differential regulation of MC1R may contribute to the variation in constitutive and UV induced pigmentation in humans (57). MC1R signaling, presumably via paracrine mechanism, appears to participate in both constitutive and facultative skin pigmentation. Thus, the critical role of MC1R as key regulator of skin pigmentation has been well documented. Accordingly, allelic variants of MC1R that result in receptor molecules with varying response to binding to alpha-MSH have been shown to be associated with red hair phenotype, tanning response to solar radiation and susceptibility to melanoma and non-melanoma skin cancers (13, 58, 59).
Exposure to UVB radiation, on the other hand, causes erythema and delayed tanning (DT) and UVB is more efficient than UVA in causing these effects (60). UVB-induced DT is a result of increased melanin synthesis and skin darkening that develops days after UV exposure (34). Repetitive 2-week exposure of human skin to UVB radiation significantly increased the levels of eumelanin and pheomelanin in the skin (61). Miyamura et al. exposed human skin and three-dimensional reconstructed human skin equivalents to 2-week repetitive UVB radiation and then challenged with a 2 MED of UV dose and observed a significant increase in melanin content and melanocyte density in human skin and human skin equivalents (44). In another detailed study using immunohistochemistry and whole human genome microarray techniques, Choi et al. showed exposure of human skin to UVB radiation increased melanin content and increased expression of melanocyte-specific genes and proteins (45). Thus, UVA or UVB effects on skin pigmentation are distinct, where UVB induce skin pigmentation by increasing melanin synthesis, whereas UVA elicit visible pigmentation by oxidation or distribution of existing melanin (44, 61, 62).
UVB exposure also causes DNA damage by formation of covalent bonds between adjacent thymidine molecules (thymidine dimers), pyrimidine dimers (CPD) and other protoproducts such as cytidine and cytidine-thymidine dimers (63). Folate and its derivatives such as 5-methyltetrahydrofolate can also absorb UVB radiation; however, due to its lower penetration, unlike UVA, UVB does not cause significant photodegradation of folate and its metabolites (64). Repeated and prolonged UVB exposure is known to be potential inducer of skin cancer. Carcinogenic effects of UVB have been extensively investigated in relation to melanomagenesis and other skin cancers and reviewed elsewhere (38).
VISIBLE LIGHT
Solar radiation also consists of radiation of 400–700 nm wavelength that is visible to human eye. Visible light causes skin erythema at a much higher dose. Kollias and Baqer studied the change in pigmentation brought about by visible and near infrared region (polychromatic radiation 390–1700 nm). These authors used the lower inner arm skin of volunteers to determine the changes in skin color that occur following irradiation with this broad band light. Using remittance spectroscopic technique, they found increase in skin pigmentation without evidence of erythema- associated color change (65). Porges et al, on the other hand, observed pigment changes accompanied by erythema. They exposed individuals of skin types II, III and IV to visible light and observed induction of IPD, immediate erythema and persistent delayed tanning reaction. IPD and immediate erythema faded over a period of 24 hours, whereas tanning response remained for 10 days (66). Mahmoud et al. in their review speculated that this discrepancy could be due to use and lack of non-standardized light sources for studies on the effects visible light on human skin pigmentation (67). A recent study on induction of pigmentation in Fitzpatrick skin types IV-V by UV radiation and a better defined light source for visible light showed that IPD induction by visible region of sunlight is not significantly different from that of UV fraction. However, increase in persistent pigment darkening (PPD) is significantly lower in visible region than compared to UV fraction. These authors suggest that UV and visible light interact with the same precursors, however UV can induce 25 times more pigmentation per J cm−2 compared to visible light (68). These studies may be relevant in view of the increasing ‘light pollution’, the excessive or obtrusive artificial light that surrounds us both day and night, that is being recognized as significant human health hazard.
GROWTH FACTOR RESPONSES TO RADITAION
In addition to MSH, production and secretion of other paracrine factors in the skin that influence melanocyte biology are known to be affected by exposure to radiation (69). Among these factors, basic FGF (bFGF) may be considered one of the most important factors. UVA and UVB stimulate bFGF secretion by both kertainocytes and melanocytes (70). Endothelin-1 (ET-1) is of considerable interest in the context of melanogenic response to UV. ET-1 binds to its cognate receptor ET B on melanocytes and appears to have a biphasic effect on melanin production-stimulatory effect at low concentration and inhibitory effect at higher concentrations. More importantly, ET-1 also modulates the survival response of melanocytes to UVB exposure. Interestingly, UVA appears to significantly downregulate ET-1 production by keratinocytes (71). Another keratinocyte-derived factor, macrophage migration inhibitory factor (MIF), which is secreted in response to UVB was recently shown to increase skin pigmentation indirectly by increasing expression of PAR-2 that enhances phagocytosis of melanosomes by keratinocytes. The indirect effect of MIF on pigmentation is supported by the observation that there was no effect on melanin production when cultured human melanocytes were treated with this factor in the absence of keratinocytes (72). MIF also stimulated secretion of stem cell factor (SCF), an important keratinocyte-derived factor involved in melanocyte survival and UVB induced pigmentation (72, 73). Hepatocyte growth factor (HGF) is known to promote cell motility and proliferation of keratinocytes and melanocytes. Dermal fibroblast is the main source of HGF in the skin and UV irradiated keratinocytes strongly induce the production of HGF. Fibroblasts secrete HGF when neighboring keratinocytes are exposed to UVR, but not when they themselves are exposed to UV, suggesting a paracrine mechanism. Mildner et al., data suggest that upregulation of HGF plays an important role in skin homeostasis after UV irradiation (74). Keratinocyte growth factor (KGF) increases the melanosome transfer from melanocytes to keratinocytes and thus increases the amount of melanin transferred when exposed to UV radiation. KGF-induced melanosome transfer was more significant in keratinocytes isolated from fair-skinned individuals compared to keratinocytes isolated from dark skin, due to an increased expression of KGF receptor in fair-skinned keratinocytes (75). The Nerve growth factor (NGF) released by keratinocytes prevents UV induced apoptosis in melanocytes by upregulating Bcl2 expression and this UV induced protection is thought to be especially significant in the lower layers of the skin (76, 77). UVB irradiated keratinocytes secrete the antioxidant adult T cell leukemia-derived factor/thioredoxin (ADF/TRX) and increase the binding capacity/binding of the melanocyte stimulating hormone (MSH) and an increase in melanogenesis, a protective mechanism for keratinocytes and melanocytes (78). Keratinocytes releases nitric oxide (NO) in response to UV radiation which decreases cell growth and increases the dendricity and melanin synthesis in melanocytes by upregulating the production of tyrosinase and TYRP1 (79). ACTH and TGF-beta1increases the melanin production in melanocytes as well as the cornification in keratinocytes (41). These studies illustrate the cooperative influences within the epidermal melanin unit that consists of one melanocyte and several keratinocytes acting as a functional unit.
CONCLUSION
Natural variation in skin pigmentation, the most readily visible human trait with significant social and psychological implications, is a complex and dynamic biological feature that appears to have evolved in response to geographical migration of early humans and the varying exposure to environmental solar radiation. This intrinsic response of skin to radiation continues to be exploited by humans for therapeutic and cosmetic purposes. However, while our knowledge on the genetics, cell biology and biochemistry of skin pigmentation has expanded exponentially, our understanding of the effects of radiation on skin pigmentation remains incomplete. Availability of more well defined experimental radiation sources and state of the art non-invasive methods of assessment of pigmentary changes in vivo could further refine our classification of subcategories of radiation, specifically UVA and UVB. Such refinements will have implications for many therapeutic as well a cosmetic uses of radiation for affecting skin pigmentation.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 AR-056087 to V. Setaluri and was partially supported by Career Development Award from Dermatology Foundation to N. Maddodi.
Biographies
Nitya Maddodi graduated with PhD degree from India and completed his post-doctoral training in Department of Dermatology, University of Wisconsin school of Medicine and Public Health. He is currently working as an Assistant Scientist and focusing his research on role of oncogenic BRAF and MAPK signaling in melanoma tumorigenesis.
Ashika Jayanthy graduated from Illinois Institute of Technology with a B.S in Biochemistry. She is currently working as an Associate Research Specialist in Dr. Vijaysaradhi Setaluri’s laboratory at Department of Dermatology, University of Wisconsin School of Medicine and Public Health.
Vijaysaradhi Setaluri is the Cripps-Ratcliff Professor in the Department of Dermatology at University of Wisconsin School of Medicine and Public Health. He received his PhD in Biochemistry from the Center for Cellular & Molecular Biology at Osmania University, India and his post doctoral training at the Memorial Sloan-Kettering Cancer Center in New York. Dr. Setaluri’s laboratory studies the biology of melanocytes and melanoma. His contributions to pigment cell biology include identification of melanosomal protein sorting signals, characterization of immune response to TYRP1 and regulation of TYRP1 gene expression in melanoma, role of ion-channel TRPM1 and calcium homeostasis in pigmentation. Ongoing research in his laboratory is focused on understanding the molecular mechanism of trans-differentiation of malignant melanocytes and the role of autophagy as melanocyte homeostasis.
Footnotes
This paper is part of the Special Issue in Commemoration of the 70th birthday of Dr. David R. Bickers
References
- 1.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton-Bishop JA, Chang YM, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, Fitzgibbon E, Kukalizch K, Randerson-Moor J, Elder DE, Bishop DT, Barrett JH. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. 2011;47:732–741. doi: 10.1016/j.ejca.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer facts and figures 2011. Vol. 2011 Atlanta: American Cancer Society; 2011. [Google Scholar]
- 5.Young A. Chromophores in human skin. Phys Med Biol. 1997;42:789–802. doi: 10.1088/0031-9155/42/5/004. [DOI] [PubMed] [Google Scholar]
- 6.Costin GE, V, Hearing J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 7.Routaboul C, Denis A, VA Immediate pigment darkening: description, kinetic and biological function. Eur J Dermatol. 1999;9:95–99. [PubMed] [Google Scholar]
- 8.Hönigsmann H. Erythema and pigmentation. Photodermatology, Photoimmunology & Photomedicine. 2002;18:75–81. doi: 10.1034/j.1600-0781.2002.180204.x. [DOI] [PubMed] [Google Scholar]
- 9.Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proceedings of the National Academy of Sciences. 2010;107:8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen AWC, Jablonski NG. Vitamin D: In the evolution of human skin colour. Medical Hypotheses. 2010;74:39–44. doi: 10.1016/j.mehy.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Roberts W. Skin type classification systems old and new. Dermatol Clin. 2009;27:529– 33. doi: 10.1016/j.det.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Sturm RA, Box NF, Ramsay M. Human pigmentation genetics: the difference is only skin deep. BioEssays. 1998;20:712–721. doi: 10.1002/(SICI)1521-1878(199809)20:9<712::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. The Melanocortin-1 Receptor is a Key Regulator of Human Cutaneous Pigmentation. Pigment Cell Res. 2000;13:156–162. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 14.BuscÅ R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 15.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar B. The role of melanocytes in the repair of UV related DNA damage in keratinocytes. Pigment Cell Res. 1998;11:110–3. doi: 10.1111/j.1600-0749.1998.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 17.Asuquo ME, Ngim O, Ebughe G, Bassey EE. Skin cancers amongst four Nigerian albinos. Int J Dermatol. 2009;48:636–638. doi: 10.1111/j.1365-4632.2009.04017.x. [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J Eur Acad Dermatol Venereol. 2011;25:1369–1380. doi: 10.1111/j.1468-3083.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- 19.Blarzino C, Mosca L, Foppoli C, Coccia R, De Marco C, RMA Lipoxygenase/H2O2-catalyzed oxidation of dihdroxyindoles: synthesis of melanin pigments and study of their antioxidant properties. Free Radic Biol Med. 1999;26:446–53. doi: 10.1016/s0891-5849(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 20.Prota G, editor. Melanins and Melanogenesis. Academic Press, Inc; San Diego CA: 1992. [Google Scholar]
- 21.Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol. 2009;18:741–749. doi: 10.1111/j.1600-0625.2009.00896.x. [DOI] [PubMed] [Google Scholar]
- 22.Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 23.Simon JD. Seeing red: pheomelanin synthesis uncovered. Pigment Cell Melanoma Res. 2009;22:382–383. doi: 10.1111/j.1755-148X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 24.Wakamatsu K, Kavanagh R, Kadekaro AL, Terzieva S, Sturm RA, Leachman S, Abdel-Malek Z, Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 25.Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007;127:751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]
- 26.Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, Shapiro SS. Inhibition of melanosome transfer results in skin lightening1. J Invest Dermatol. 2000;115:162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 27.Wei QIN, Wu X, Hammer J. The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport. J Muscle Res Cell Motil. 1997;18:517–527. doi: 10.1023/a:1018659117569. [DOI] [PubMed] [Google Scholar]
- 28.Ion L, Raveendran SS, Fu B. Body-contouring with radiofrequency-assisted liposuction. J Plast Surg Hand Surg. 2011;45:286–93. doi: 10.3109/2000656X.2011.613263. [DOI] [PubMed] [Google Scholar]
- 29.Woolery-Lloyd H, KJN Skin tightening. Curr Probl Dermatol. 2011;42:147–52. doi: 10.1159/000328284. [DOI] [PubMed] [Google Scholar]
- 30.El-Domyati M, El-Ammawi TS, Medhat W, Moawad O, Brennan D, Mahoney MG, Uitto J. Radiofrequency facial rejuvenation: Evidence-based effect. J Am Acad Dermatol. 2011;64:524–535. doi: 10.1016/j.jaad.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karinen A, Heinavaara S, Nylund R, Leszczynski D. Mobile phone radiation might alter protein expression in human skin. BMC Genomics. 2008;9:77. doi: 10.1186/1471-2164-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardell L, Carlberg M, Hansson Mild K, Eriksson M. Case-control study on the use of mobile and cordless phones and the risk for malignant melanoma in the head and neck region. Pathophysiology. 2011;18:325–333. doi: 10.1016/j.pathophys.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Darvin ME, Haag SF, Lademann J, Zastrow L, Sterry W, Meinke MC. Formation of free radicals in human skin during irradiation with infrared light. J Invest Dermatol. 2010;130:629–631. doi: 10.1038/jid.2009.283. [DOI] [PubMed] [Google Scholar]
- 34.Moan J, Nielsen KP, Juzeniene A. Immediate pigment darkening: its evolutionary roles may include protection against folate photosensitization. FASEB J. 2011 doi: 10.1096/fj.11-195859. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Wicks Nadine L, Chan Jason W, Najera Julia A, Ciriello Jonathan M, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Current Biology. 2011;21:1906–1911. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Semin Cutan Med Surg. 2011;30:222–228. doi: 10.1016/j.sder.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Narayanan DL, Saladi RN, Fox JL. Review: Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 38.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma†. Photochem Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- 40.Von Thaler AK, Kamenisch Y, Berneburg M. The role of ultraviolet radiation in melanomagenesis. Exp Dermatol. 2010;19:81–88. doi: 10.1111/j.1600-0625.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 41.Dissanayake N, Mason R. Modulation of skin cell functions by transforming growth factor-beta1 and ACTH after ultraviolet irradiation. J Endocrinol. 1998;159:153–163. doi: 10.1677/joe.0.1590153. [DOI] [PubMed] [Google Scholar]
- 42.De Leeuw SM, Janssen S, Simons JWIM, Lohman PHM, Vermeer BJ, Schothorst AA. The UV action spectra for the clone-forming ability of cultured human melanocytes and keratinocytes. Photochem Photobiol. 1994;59:430–436. doi: 10.1111/j.1751-1097.1994.tb05060.x. [DOI] [PubMed] [Google Scholar]
- 43.Ochiai Y, Kaburagi S, Okano Y, Masaki H, Ichihashi M, Funasaka Y, Sakurai H. A Zn(II) – glycine complex suppresses UVB-induced melanin production by stimulating metallothionein expression. Int J Cosmet Sci. 2008;30:105–112. doi: 10.1111/j.1468-2494.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 44.Miyamura Y, Coelho SG, Schlenz K, Batzer J, Smuda C, Choi W, Brenner M, Passeron T, Zhang G, Kolbe L, Wolber R, Hearing VJ. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011;24:136–147. doi: 10.1111/j.1755-148X.2010.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi W, Miyamura Y, Wolber R, Smuda C, Reinhold W, Liu H, Kolbe L, Hearing VJ. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. J Invest Dermatol. 2010;130:1685–1696. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, Hearing VJ. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci. 2010;123:3102–3111. doi: 10.1242/jcs.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. Ultraviolet A and melanoma: A review. J Am Acad Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 48.Garland CF, Garland FC, Gorham ED. Rising trends in melanoma an hypothesis concerning sunscreen effectiveness. Ann Epidemiol. 1993;3:103–110. doi: 10.1016/1047-2797(93)90017-x. [DOI] [PubMed] [Google Scholar]
- 49.Godar DE. UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol. 1999;112:3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 50.Wenczl E, Van der Schans GP, Roza L, Kolb RM, Timmerman AJ, Smit NPM, Pavel S, Schothorst AA. (Pheo)Melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol. 1998;111:678–682. doi: 10.1046/j.1523-1747.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 51.Marrot L, Belaidi JP, Meunier JR, Perez P, Agapakis-Causse C. The Human melanocyte as a particular target for UVA radiation and an endpoint for photoprotection assessment. Photochem Photobiol. 1999;69:686–693. [PubMed] [Google Scholar]
- 52.Jean C, Blanc A, Prade-Houdellier N, Ysebaert L, Hernandez-Pigeon H, Al Saati T, Haure M-J, Coluccia A-M-L, Charveron M, Delabesse E, Laurent G. Epidermal growth factor receptor/β-catenin/T-cell factor 4/matrix metalloproteinase 1: a new pathway for regulating keratinocyte invasiveness after UVA irradiation. Cancer Res. 2009;69:3291–3299. doi: 10.1158/0008-5472.CAN-08-1909. [DOI] [PubMed] [Google Scholar]
- 53.Mouret S, Forestier A, Douki T. The specificity of UVA-induced DNA damage in human melanocytes. Photochem Photobiol Sci. 2011;11:155–62. doi: 10.1039/c1pp05185g. [DOI] [PubMed] [Google Scholar]
- 54.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Box NF, Terzian T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008;21:525–533. doi: 10.1111/j.1755-148X.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 56.Im S, Moro O, Peng F, Medrano EE, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Activation of the cyclic AMP pathway by α-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Res. 1998;58:47–54. [PubMed] [Google Scholar]
- 57.Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Res. 2002;15:433–439. doi: 10.1034/j.1600-0749.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- 58.Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VJ, Abdel-Malek ZA. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci. 2002;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 59.Nan H, Kraft P, Qureshi AA, Guo Q, Chen C, Hankinson SE, Hu FB, Thomas G, Hoover RN, Chanock S, Hunter DJ, Han J. Genome-Wide Association Study of Tanning Phenotype in a Population of European Ancestry. J Invest Dermatol. 2009;129:2250–2257. doi: 10.1038/jid.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gange RW, Blackett AD, Matzinger EA, Sutherland BM, Kochevar IE. Comparative protection efficiency of UVA- and UVB-induced tans against erythema and formation of endonuclease-sensitive sites in DNA by UVB in human skin. J Invest Dermatol. 1985;85:362–364. doi: 10.1111/1523-1747.ep12276983. [DOI] [PubMed] [Google Scholar]
- 61.Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J Invest Dermatol. 2004;122:503–509. doi: 10.1046/j.0022-202X.2004.22223.x. [DOI] [PubMed] [Google Scholar]
- 63.González E, González S. Drug photosensitivity, idiopathic photodermatoses, and sunscreens. J Am Acad Dermatol. 1996;35:871–885. doi: 10.1016/s0190-9622(96)90108-5. [DOI] [PubMed] [Google Scholar]
- 64.Steindal AH, Juzeniene A, Johnsson A, Moan J. Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem Photobiol. 2006;82:1651–1655. doi: 10.1562/2006-06-09-RA-915. [DOI] [PubMed] [Google Scholar]
- 65.Kollias N, Baqer A. An experimental study of the changes in pigmentation in human skin in vivo with visible and near infrared light. Photochem Photobiol. 1984;39:651–659. doi: 10.1111/j.1751-1097.1984.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 66.Porges SB, Kaidbey KH, GGL Quantification of visible light-induced melanogenesis in human skin. Photodermatol. 1988;5:197–200. [PubMed] [Google Scholar]
- 67.Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW. Effects of visible light on the skin†. Photochem Photobiol. 2008;84:450–462. doi: 10.1111/j.1751-1097.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 68.Ramasubramaniam R, Roy A, Sharma B, Nagalakshmi S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem Photobiol Sci. 2011;10:1887–1893. doi: 10.1039/c1pp05202k. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi Y, V, Hearing J. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- 71.Tada A, Suzuki I, Im S, Davis M, Cornelius J, Babcock G, Nordlund J, Abdel-Malek Z. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575–584. [PubMed] [Google Scholar]
- 72.Enomoto A, Yoshihisa Y, Yamakoshi T, Ur Rehman M, Norisugi O, Hara H, Matsunaga K, Makino T, Nishihira J, Shimizu T. UV-B Radiation Induces Macrophage Migration Inhibitory Factor – Mediated Melanogenesis through Activation of Protease-Activated Receptor-2 and Stem Cell Factor in Keratinocytes. Am J Pathol. 2011;178:679–687. doi: 10.1016/j.ajpath.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic Expression of Two Paracrine Melanogenic Cytokines, Stem Cell Factor and Endothelin-1, in Ultraviolet B-Induced Human Melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mildner M, Mlitz V, Gruber F, Wojta J, Tschachler E. Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. J Invest Dermatol. 2007;127:2637–2644. doi: 10.1038/sj.jid.5700938. [DOI] [PubMed] [Google Scholar]
- 75.Cardinali G, Bolasco G, Aspite N, Lucania G, Lotti LV, Torrisi MR, Picardo M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J Invest Dermatol. 2007;128:558–567. doi: 10.1038/sj.jid.5701063. [DOI] [PubMed] [Google Scholar]
- 76.Zhai S, Yaar M, Doyle SM, Gilchrest BA. Nerve growth factor rescues pigment cells from ultraviolet-induced apoptosis by upregulating BCL-2 levels. Exp Cell Res. 1996;224:335–343. doi: 10.1006/excr.1996.0143. [DOI] [PubMed] [Google Scholar]
- 77.Stefanato CM, Yaar M, Bhawan J, Phillips TJ, Kosmadaki MG, Botchkarev V, Gilchrest BA. Modulations of nerve growth factor and Bcl-2 in ultraviolet-irradiated human epidermis. J Cutan Pathol. 2003;30:351–357. doi: 10.1034/j.1600-0560.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 78.Funasaka Y, Ichihashi M. The effect of ultraviolet B induced adult T cell leukemia-derived factor/thioredoxin (ADF/TRX) on survival and growth of human melanocytes. Pigment Cell Res. 1997;10:68–73. doi: 10.1111/j.1600-0749.1997.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 79.Roméro-Graillet C, Aberdam E, Clément M, Ortonne JP, Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99:635–642. doi: 10.1172/JCI119206. [DOI] [PMC free article] [PubMed] [Google Scholar]