Abstract
Tooth eruption requires osteoclastogenesis and subsequent bone resorption. Secreted frizzled-related protein-1 (SFRP-1) negatively regulates osteoclastogenesis. Our previous studies indicated that SFRP-1 is expressed in the rat dental follicle, with reduced expression at days 3 and 9 close to the times for the major and minor bursts of osteoclastogenesis respectively, but it remains unclear as to what molecules contribute to its reduced expression at these critical times. Thus, it was the aim of this study to determine what molecules regulate the expression of SFRP-1 in the dental follicle. To that end, the dental follicle cells were treated with cytokines that are maximally expressed at days 3 or 9, and SFRP-1 expression was determined. Our study indicated that CSF-1, a molecule maximally expressed in the dental follicle at day 3, down-regulated SFRP-1 expression. As to EMAP-II, a highly expressed molecule in the dental follicle at day 3, it had no effect on the expression of SFRP-1. However, when EMAP-II was knocked downed by siRNA, the expression of SFRP-1 was elevated, and this elevated SFRP-1 expression could be reduced by adding recombinant EMAP-II protein. This suggests that EMAP-II maintained a lower level of SFRP-1 in the dental follicle. TNF-α is a molecule maximally expressed at day 9, and this study indicated that it also down-regulated the expression of SFRP-1 in the dental follicle cells. In conclusion, CSF-1 and EMAP-II may contribute to the reduced SFRP-1 expression seen on day 3, while TNF-α may contribute to the reduced SFRP-1 expression at day 9.
Keywords: Dental follicle, Tooth eruption, Gene Expression, SFRP-1, Osteoclastogenesis
INTRODUCTION
The dental follicle (DF) plays a crucial role in tooth eruption, because surgical removal of it prevents the tooth from erupting [1]. Structurally, the DF is a loose connective tissue sac that encases the unerupted tooth, and it is adjacent to alveolar bone at its periphery. For the tooth to erupt, the alveolar bone must be resorbed to create an eruption pathway [2, 3]. Research has revealed that osteoclast precursors (mononuclear cells) are recruited into the DF and then differentiate into osteoclasts, the multinucleate cells required for bone resorption and formation of the eruption pathway [4, 5]. For the eruption of the first mandibular molars of rats, osteoclast precursors are recruited into the DF by the chemoattractic molecules: colony-stimulating factor-1 (CSF-1) and monocyte chemoattractant protein-1 (MCP-1), both of which are maximally expressed in the DF at postnatal day 3 [6, 7, 8]. Our recent study found that endothelial monocyte-activating polypeptide II (EMAP-II) also contributes to this recruitment [9]. The fusion of the recruited osteoclast precursors is regulated by another crucial molecule, receptor activator of nuclear factor-kappa B ligand (RANKL), which is expressed in the DF [10]. Moreover, osteoprotegerin (OPG), a decoy receptor for RANKL and a negative osteoclastogenesis regulator, is down-regulated by CSF-1 in the DF at day 3 [11]. With RANKL presence in the DF, a decrease in OPG level at day 3 provides a favorable microenvironment to promote osteoclast formation.
Secreted frizzled related protein-1 (SFRP-1) was originally identified in a mouse eye cDNA library, with its distribution found in many other tissues such as brain, heart, kidney, lung, retina and spleen [12], as well as periodontal tissues infected with the bacteria P. gingivalis [13]. SFRP-1 is a secreted soluble protein with a frizzled-like cystein-rich domain (Fz) [12]. Because of its frizzled-like domain, SFRP-1 functions as an antagonist of Wnt signaling by competitively binding to Wnt proteins. As a negative regulator of Wnt signaling, SFRP-1 plays an important role through Wnt signaling in many aspects of cell proliferation, morphology, differentiation, motility, and bone homeostasis [14]. In addition, SFRP-1 has been identified as a tumor suppressor [15], and is involved in apoptosis of periodontal ligament fibroblasts [16].
Studies in recent years have revealed the role of SFRP-1 in bone formation. SFRP-1 expression has been identified in osteogenic cells such as osteoblasts and chondrocytes [17]. The expression of SFRP-1 is increased in human osteoblast cells during advancing osteoblast differentiation, with a peak level at the preosteocytic stage and a decline in mature osteocytes, followed by an accelerated rate of cell death [18]. Knockout of SFRP-1 in mice reduces the apoptosis of both osteoblasts and osteocytes, enhances the proliferation and differentiation of these cells, and increases trabecular bone formation [19]. By contrast, overexpression of SFRP-1 in mice inhibits osteoblast differentiation and its function, and reduces bone formation [20]. These results indicate that SFRP-1 inhibits bone formation through its effect on osteoblasts.
Other studies have shown that SFRP-1 inhibits osteoclastogenesis. Initial studies indicated that reduction of SFRP-1 by antiserum in the co-culture of murine osteoblasts and splenic cells stimulates osteoclastogenesis [17]. Similarly, knockdown of SFRP-1 by siRNA in KUSA osteoblast cells also enhances osteoclastogenesis in the co-culture of KUSA cells and bone marrow cells [17], which suggests that enhanced osteoclastogenesis is related to the reduced SFRP-1 in osteoblasts. Moreover, increased osteoclastogenesis is also observed in the bone marrow cell cultures from SFRP-1 knockout mice as compared to wild-type mice [19]. Direct inhibition of osteoclastogenesis by SFRP-1 has been shown by addition of recombinant SFRP-1 protein in culture. In cultures of murine splenic cells, SFRP-1 inhibited osteoclastogenesis in a dose-dependent manner [17]. This inhibition was also observed in cultures of mouse RAW 264.7 cells or purified rat bone marrow cells in which stromal cells/osteoblasts are not present [17, 21], which suggests that SFRP-1 may exert its inhibitory effect on osteoclastogenesis through osteoclast precursors directly. In addition, SFRP-1 also can bind to RANKL to block osteoclastogenesis [17].
By DNA microarray, we found that SFRP-1 was expressed in the rat DF, with its expression reduced at postnatal days 3 and 9 [21], the approximate times for the major and minor bursts of osteoclastogenesis respectively [5, 22]. We also showed that SFRP-1 inhibited osteoclastogenesis in cultures of rat bone marrow cells [21]. Because of the crucial role of the DF in tooth eruption, this study was conducted to determine what molecules regulate the reduced expression of SFRP-1 in the rat DF for osteoclastogenesis and tooth eruption.
MATERIALS AND METHODS
DF Cell Cultures
Harlan Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) were housed in the School of Veterinary Medicine at Louisiana State University in compliance with a protocol approved by the Institute of Animal Care and Use Committee (IACUC). The DF was isolated surgically from the first mandibular molars of 5- to 6-day-old rats and trypsinized to obtain the DF cells as previously described [23]. The DF cells were cultured for 5 to 6 passages to ensure uniform cell population in an incubator at 37 °C and 5% CO2 in minimum essential medium (MEM) (Sigma-Aldrich, St. Louis, MO, USA) plus 10% (V/V) newborn calf serum, 1 mM sodium pyruvate, 1% penicillin/streptomycin and 0.2% fungizone. The cells of passages 6 to 9 were used in this study.
DF Cell Treatments
The DF cells of passages 6 to 9 were grown in T-25 flasks until confluence. The cells were then cultured for an additional 8 h in serum-free MEM medium supplemented with 0.3% (v/v) bovine serum albumin (BSA) and placed in the same medium before the experimental treatments. The DF cells were treated with the following cytokines separately for time course studies: human EMAP-II (Pepro Tech, Rocky Hill, NJ, USA) at a concentration of 50 ng/ml for 3, 6, 9, 12 and 24 h; human CSF-1 (Pepro Tech) at a concentration of 50 ng/ml for 3, 6, 9, 12 and 24 h; and rat IL-1α (Pepro Tech) at concentrations of 5, 10, 15 and 20 ng/ml for 3, 6, 9 and 12 h. In addition, the DF cells were treated with rat TNF-α (Pepro Tech) at a concentration of 15ng/ml for 3, 6, 9 and 12 h in a time-course study, and at concentrations of 0, 5, 10, 15, or 20ng/ml for 6 h in a concentration-course study. The concentrations and times for the above cytokines were based on our previous studies [9, 10, 24]. The DF cells were collected for RNA isolation after designated treatments. Each of above treatments was repeated four times, each with a different set of DF cell cultures.
RNA Isolation
Total RNA was isolated from the DF cells using an automatic system QIAcube (Qiagen Inc., Valencia, CA, USA), with RNA mini column and DNase I digestion per manufacturer’s instructions. The RNA was quantified using a NanoDrop 8000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The RNA quality was ensured by an A260/A280 ratio of 2.0 or higher.
In Vitro Transfection of the DF Cells with siRNA
A Dicer substrate siRNA was designed using online software provided by Integrated DNA Technologies (Coralville, IA, USA), and synthesized by the same company as two single strands: sense strand 5′-rUrGrCrUrUrCrUrCrCrCrArCrArUrCrGrArCrUrUrCrCrUrCrArC-3 ′ a n d a n t i-sense strand 5′-rGrGrArGrGrArArGrUrCrGrArUrGrUrGrGrGrArGrArArGCA-3′ based on EMAP-II mRNA sequences (Genbank accession no. NM_053757). The siRNA duplex was annealed from these two single strands, targeting the rat EMAP-II mRNA at nucleotides 582–606. Scrambled siRNA not targeting any mRNA was used for the controls. For transfection, the DF cells were cultured in T-25 flasks one day before transfection in MEM medium plus 1 mM sodium pyruvate and 10% heat-inactivated fetal calf serum such that the cells were 30–40% confluent at the time of transfection. Transfection was carried out using Lipofectamine™ RNAiMAX per manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Briefly, siRNA and transfection reagent were diluted to 50 nM and 50-fold respectively in 500 μl Opti MEM I Reduced Serum Medium (Invitrogen). After 5 min of incubation at room temperature, the diluted siRNA and transfection reagent were combined, and incubated at room temperature for another 25 min to form siRNA-Lipofectamine™ RNAiMAX complex. The resultant 1 ml of complex was added to 4 ml of the DF cell culture to bring the final siRNA concentration to 5 nM. For controls, the DF cells were transfected with scrambled siRNA under the same conditions. The cultures were incubated at 37 °C with 5% CO2 for 24, 36, 48, 60 and 72 h. After designated time periods following transfection, the DF cells were collected for determination of gene expression using real-time RT-PCR.
To determine if EMAP-II can decrease SFRP-1 expression after EMAP-II expression was knocked down by siRNA, the DF cells were transfected first with control siRNA (Csi) or EMAP-II siRNA (Esi) as above. After 36 h at which SFRP-1 expression was significantly elevated in response to EMAP-II knockdown, the transfected DF cells were subjected to human EMAP-II (50 ng/ml) (E) treatment for an additional 6, 12 or 24 h.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real-time RT-PCR
To determine gene expression, 2 μg total RNA was reverse transcribed by M-MLV Reverse Transcriptase (Invitrogen) to synthesize the first strand cDNA per the manufacturer’s instructions. The reverse transcription was performed at 37 °C for 1 h, followed by 10 minute incubation at 70 °C to inactivate the reverse transcriptase.
Real-time RT-PCR was used to analyze the expression of SFRP-1 in the DF cells. The following primers were designed based on its mRNA sequences (GenBank accession no. AF167308): 5′-TAAAGAATGGCGCCGACTGTC-3′ and 5′-TGGCTGTGAGCAAGAACTGGC-3′. The real-time RT-PCR was performed as our previous study [10] with SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, USA). The relative gene expression (RGE) was calculated with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control using the formula 2−ΔΔCT. The primer sequences for GAPDH were 5′-GTGGACCTCATGGCCTACATG-3′ and 5′-TCAGCAACTGAGGGCCTCTC-3′.
Immunostaining
For detection of SFRP-1 protein expression in the DF cells, the DF cells were cultured on coverslips coated with poly-L-lysine in 6-well plates, and treated with or without human CSF-1 (50 ng/ml) (Pepro Tech) for 12 h. The cells then were fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.2% Triton X-100. After blocking with 2% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in Tris-buffered saline (TBS) buffer for 1 h at room temperature, the cells were incubated with rabbit IgG or rabbit anti-human SFRP-1 polyclonal primary antibody (Catolog # ab4193) (Abcam, Cambridage, MA, USA) at a concentration of 5 μg/ml overnight at 4 °C in TBS buffer containing 2% normal goat serum. Next, the cells were washed three times with TBS buffer and incubated for 1 h with biotinylated goat anti-rabbit IgG secondary antibody (1:100 dilutions in TBS buffer). Following three washes with TBS buffer, the cells were further incubated for 30 min with avidin-biotinylated horseradish peroxidase (HRP) (Vector Laboratories), and then incubated with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) (Vector Laboratories) for 5 min before counterstaining with hematoxylin.
Statistical Analysis
Data from the TNF-α concentration-course study and EMAP-II siRNA knockdown were analyzed using SAS program (version 9.1) (SAS Institute, Cary, NC, USA). Analysis of variance (ANOVA) was carried out to evaluate SFRP-1 gene expression, and then the means were separated with the least significant difference (LSD) test at a significance level of P ≤ 0.05. Data from other experiments were analyzed using paired t-tests at P ≤ 0.05.
RESULTS
CSF-1 Decreases SFRP-1 Expression
CSF-1 is maximally expressed in the rat DF of the first mandibular molar at postnatal day 3 [6], and it down-regulates OPG expression [11]. To determine if CSF-1 decreases SFRP-1 expression, the DF cells were treated with CSF-1 for 3 to 24 h, and the SFRP-1 expression was determined by real-time RT-PCR. As seen in Figure 1, SFRP-1 expression was reduced at 6 h after CSF-1 treatment. The maximal reduction of SFRP-1 expression was reached at 9 to 12 h, with a maximal reduction of 32% as compared to their time-wise controls. The expression level at 24 h was lower than the controls, but it was not statistically significant.
Figure 1.
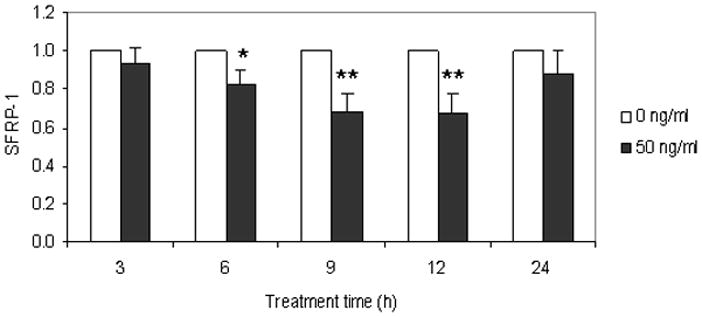
Inhibition of SFRP-1 expression in the dental follicle cells by CSF-1. The dental follicle cells were treated with CSF-1 at a concentration of 50ng/ml for 3 to 24 h, and the expression of SFRP-1 was determined by real-time RT-PCR. The results are presented as the means ± standard deviations of four independent experiments. An asterisk (*) and asterisks (**) indicate significant difference (P<0.05) and highly significant difference (P<0.01) between the treatments and its paired controls respectively.
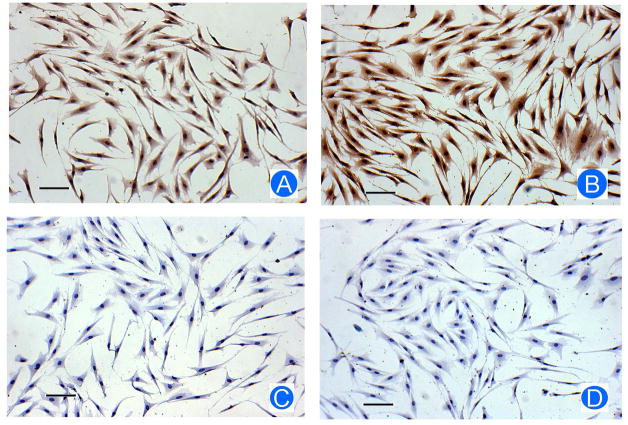
As CSF-1 decreased SFRP-1 gene expression, immunostaining was performed to determine if SFRP-1 protein was also decreased. As shown in Figure 2A, CSF-1 treatment resulted in a reduced staining for SFRP-1 as compared to the control without CSF-1 treatment (Figure 2B), indicating that CSF-1 decreased SFRP-1 protein expression. Thus, CSF-1 reduced both SFRP-1 gene expression and protein expression.
Figure 2.
Reduction of SFRP-1 protein in the dental follicle cells by CSF-1. After being treated with CSF-1, the dental follicle cells were immunostained for SFRP-1 protein level. The treated cells showed a reduced staining of SFRP-1 protein (A) as compared with control cells without treatment (B). In the staining controls in which primary antibody was replaced by IgG, no staining was observed in the cells treated with CSF-1 (C) or the cells without treatment (D). Scale bar: 50 μm.
IL-1α, which is maximally expressed in the stellate reticulum adjacent to the DF at postnatal days 1 to 3 [25], did not affect the expression of SFRP-1 in the DF cells (data not shown).
Up-regulation of SFRP-1 by EMAP-II knockdown
EMAP-II is maximally expressed in the dental follicle at postnatal day 3 [9]. To determine if EMAP-II decreases SFRP-1 expression, the DF cells were treated with human EMAP-II but there was no effect on SFRP-1 expression (data not shown). This may be attributed to a high level of constitutive EMAP-II expression (about 60% of GAPDH expression at postnatal day 3) that might mask the treatment effect. Therefore, EMAP-II siRNA, which knocks down EMAP-II expression by more than 95% in our previous study [9], was used to knock down EMAP-II expression and then SFRP-1 expression was determined. As shown in Figure 3, knockdown of EMAP-II expression resulted in an increase of SFRP-1 expression from 24 to 72 h. SFRP-1 expression was significantly higher from 36 to 60 h, with a maximal increase of 1.7-fold at 48 h. This suggests that high level of EMAP-II in the DF cells suppresses SFRP-1 expression and maintains its low level.
Figure 3.

Up-regulation of SFRP-1 in the dental follicle cells by knockdown of EMAP-II with siRNA. The DF cells were transfected with a siRNA at a concentration of 5 nM that targets EMAP-II mRNA or a control siRNA that does not target any sequence. The expression of SFRP-1 was determined by real-time RT-PCR from 24 to 72 h after transfection. The results are presented as mean ± standard deviation of four independent experiments. An asterisk (*) and asterisks (**) indicate significant difference (P<0.05) and highly significant difference (P<0.01) between the treatments and paired controls respectively.
To determine if EMAP-II decreases SFRP-I expression after EMAP-II was knocked down, the cells were treated with EMAP-II after 36 h of transfection with siRNA. As shown in Figure 4, knockdown of EMAP-II expression by siRNA (Esi) resulted in an increase of SFRP-1 expression as compared to control siRNA knockdown (Csi). In contrast, siRNA knockdown plus EMAP-II treatment (Esi+E) significantly decreased SFRP-1 expression at 12 and 24 h as compared to EMAP-II knockdown only (Esi). At 12 h, SFRP-1 expression was reduced by EMAP-II to a level similar to that without EMAP-II knockdown (Csi). This demonstrates the inhibitory effect of EMAP-II on SFRP-1 expression in the DF cells.
Figure 4.
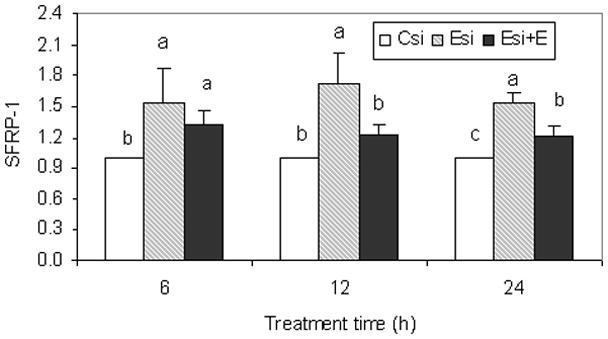
The dental follicle cells were transfected with a siRNA that targets EMAP-II mRNA (Esi) (slashed bars) or a control siRNA that does not target any sequences (Csi) (open bars) for 36 h, and then the cells were further treated with human recombinant EMAP-II (Esi+E) (close bars) at a concentration of 50ng/ml for an additional 6, 12 and 24 h. The SFRP-1 expression in the dental follicle cells were determined by real-time RT-PCR. The results are presented as the means ± standard deviations of four replications, and significant difference (P<0.05) within each time point between the means was indicated by different letters.
TNF-α Decreases SFRP-1 Expression
Because TNF-α is maximally expressed at postnatal day 9 [26], the experiments were conducted to determine if it decreases SFRP-1 expression. As shown in Figure 5A in a concentration-course study, TNF-α significantly decreased SFRP-1 expression at a concentration of 10 ng/ml, with a maximal reduction of 25%. Reduction in SFRP-1 expression was similar from concentrations of 10 to 20ng/ml. Similarly, SFRP-1 expression was decreased significantly at 6 to 9 h by TNF-α as seen in time-course study (Figure 5B).
Figure 5.

Inhibition of SFRP-1 expression in the dental follicle cells by TNF-α. The dental follicle cells were treated with TNF-α at concentrations of 0 to 20 ng/ml for 6 h (A), and at a concentration of 15 ng/ml for 3 to 12 h (B). The expression of SFRP-1 in the dental follicle cells was determined by real-time RT-PCR. The results are presented as the means ± standard deviations of four independent experiments. A different letter between the means (A) or an asterisk (*) (B) indicated significant difference (P<0.05).
DISCUSSION
Osteoclastogenesis is required for resorption of alveolar bone to provide a pathway for tooth eruption [2]. It is well recognized that osteoclasts differentiate from precursors in the presence of CSF-1 and RANKL through a complicated pathway [27], in which OPG negatively regulates osteoclastogenesis. As a soluble receptor, OPG inhibits osteoclastogenesis by acting as a decoy receptor to bind to RANKL so as to block the cell-to-cell interaction [28]. In vivo and in vitro studies indicated that OPG inhibits osteoclastogenesis and prevents bone loss caused by excessive bone resorption [28], and it is regarded as a protective molecule for bone loss. Consequently, a decline in OPG expression could favor osteoclastogenesis. Such a reduced OPG expression occurs at day 3 in the DF of the first mandibular molar of the rat, and thus provides a favorable microenvironment for the major burst of osteoclastogenesis at this time [29]. This reduction of OPG expression is due to an up-regulation of CSF-1 at day 3 which, in turn, down-regulates OPG [11]. Given that another osteoclastogenesis inhibitor, SFRP-1, is expressed in the DF, what are the molecule(s) that may down-regulate it in order for osteoclastogenesis to occur?
In our previous studies using a DNA microarray, we found that SFRP-1 had a chronologically reduced expression in the DF of the first mandibular molar of the rat at days 3 and 9 [21] and that these times approximately correlated with the major and minor bursts of osteoclastogenesis in the DF. Thus, we hypothesized that the molecules with maximal expression in the DF or adjacent stellate reticulum at day 3 and day 9 might act to down-regulate SFRP-1 expression to promote osteoclastogenesis. To test this hypothesis, this study was carried out to determine if EMAP-II, CSF-1, IL-1α, and TNF-α could down-regulate SFRP-1 expression in the DF cells. Our study indicated that CSF-1, EMAP-II and TNF-α indeed did down-regulate SFRP-1 expression.
CSF-1 plays important roles in the regulation of tooth eruption because CSF-1 mutant rats are toothless [30]. Our previous studies showed that CSF-1 regulates osteoclastogenesis through its recruitment of osteoclast precursors and its down-regulation of OPG [7, 11]. In this study, we showed that CSF-1 also down-regulates the expression of SFRP-1 in the DF cells. Because CSF-1 is maximally expressed at day 3 in the first mandibular molar, it may be responsible for the down-regulation of SFRP-1 in the DF at day 3 in vivo. Thus, the reduced expression of both OPG and SFRP-1 might provide a favorable microenvironment to allow for the major burst of osteoclastogenesis at day 3.
EMAP-II is highly and maximally expressed in the dental follicle at day 3, and it acts as a chemoattractant to recruit osteoclast precursors, as shown in our previous study [9]. In this study, direct treatment of EMAP-II on the DF cells had no effect on the expression of SFRP-1. This may be due to the high level of EMAP-II secreted by the DF cells that masks the treatment effect. Therefore, siRNA was used to knock down EMAP-II expression. After EMAP-II was knocked down, the SFRP-1 expression was significantly up-regulated, indicating that the constitutive high levels of EMAP-II suppress SFRP-1 expression. Moreover, after EMAP-II was knocked down, the up-regulated SFRP-1 expression could be reduced by recombinant EMAP-II protein, suggesting that EMAP-II could inhibit SFRP-1 expression if there were no high levels of EMAP-II. This also suggests that the high level of EMAP-II helps to maintain the lower levels of SFRP-1 seen at day 3. However, it remains unclear as to how EMAP-II suppresses SFRP-1 expression.
As mentioned above, both EMAP-II and CSF-1 contributed to the down-regulation of SFRP-1 expression, but they might act differently. Regarding EMAP-II, its high levels both in vitro and in vivo may help to maintain the lower levels of SFRP-1, while CSF-1 may directly down-regulate the expression of SFRP-1. In addition, EMAP-II may be partially responsible for the high levels of CSF-1 at day 3, as knockdown of EMAP-II by siRNA resulted in reduction of CSF-1 [9]. Thus, it is possible that EMAP-II acts alone or partially through its up-regulation of CSF-1 to suppress SFRP-1 expression. Given that RANKL level is low at day 3, the down-regulation of SFRP-1 by EMAP-II and CSF-1 is quite significant.
Unlike CSF-1 and EMAP-II, IL-1α had no effect on SFRP-1 expression in the DF cells, a finding similar to in KUSA osteoblast cells [17]. It is not expected, however, given that IL-1α enhances CSF-1 expression [31], and in this study we showed that CSF-1 down-regulated SFRP-1 expression. This could be explained by the dual functions of IL-1α. On the one hand, IL-1α enhances CSF-1 expression [31], which then down-regulated SFRP-1 expression as shown in this study. On the other hand, IL-1α enhances the expression of cyclooxygenase-2 (COX-2), resulting in production of prostaglandin E2 (PGE2) in intestinal myofibroblast cells and adrenal cells [32, 33], and PGE2 up-regulates SFRP-1 expression in KUSA cells [17]. Therefore, it is possible that absence of effect of IL-1α on SFRP-1 expression is the outcome of its down-regulation of SFRP-1 through CSF-1 and its up-regulation of SFRP-1 through production of PGE2, leading to no change in SFRP-1 expression.
Besides the major burst of osteoclastogenesis at day 3, there is a minor one at day 10 [22]. However, the OPG level at this time is not down-regulated as opposed to day 3. This raises a question regarding what microenvironment favors osteoclastogenesis at this time. This could be explained by a reduction in SFRP-1 as shown in this study and an increase in RANKL as our previous studies indicated [10, 21]. An increase in RANKL expression may be regulated by TNF-α, a molecule that is maximally expressed at day 9 [26]. In this study, we found that TNF-α also down-regulates SFRP-1 expression in the DF cells, suggesting that down-regulation of SFRP-1 at day 9 may be regulated by TNF-α. The down-regulated SFRP-1, coupled with increased RANKL expression, could create a favorable condition for the minor burst of osteoclastogenesis. In addition to up-regulating RANKL expression and down-regulating SFRP-1 expression, TNF-α may also promote osteoclastogenesis directly [34].
CONCLUSIONS
Our studies have shown that CSF-1, a molecule maximally expressed in the DF at day 3, down-regulates SFRP-1 expression in the DF cells. This down-regulation could result in a reduced SFRP-1 level in the DF at day 3, thereby promoting the major burst of osteoclastogenesis seen at this time. Similarly, TNF-α, a molecule maximally expressed in the DF at day 9, also down-regulates SFRP-1 expression in the DF cells, and could do the same in the DF to create a favorable microenvironment for the minor burst of osteoclastogenesis seen at day 10. Moreover, our studies have shown that high EMAP-II suppresses SFRP-1 expression in the DF cells, and an in vivo high level of EMAP-II at day 3 could maintain a low SFRP-1 level, thereby promoting the major burst of osteoclastogenesis. In addition, CSF-1 and EMAP-II may function together to maintain a low SFRP-1 level in the DF seen at day 3 to promote the major burst of osteoclastogenesis.
Acknowledgments
This research was supported by NIH grant DE008911-20 to G.E.W and S.Y.
Footnotes
Declaration of Interest
The authors declare no conflicts of interest in this study.
References
- 1.Cahill DR, Marks SC., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol Med. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 2.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise GE. Cellular and molecular basis of tooth eruption. Orthod Craniofac Res. 2009;12:67–73. doi: 10.1111/j.1601-6343.2009.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks SC, Jr, Cahill DR, Wise GE. The cytology of the dental follicle and adjacent alveolar bone during tooth eruption in the dog. Am J Anat. 1983;168:277–289. doi: 10.1002/aja.1001680303. [DOI] [PubMed] [Google Scholar]
- 5.Wise GE, Fan W. Changes in the tartrate-resistant acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J Dent Res. 1989;68:150–156. doi: 10.1177/00220345890680021001. [DOI] [PubMed] [Google Scholar]
- 6.Wise GE, Lin F, Zhao L. Transcription and translation of CSF-1 in the dental follicle. J Dent Res. 1995;74:1551–1557. doi: 10.1177/00220345950740090801. [DOI] [PubMed] [Google Scholar]
- 7.Que BG, Wise GE. Colony-stimulating factor-1 and monocyte chemotactic protein-1 chemotaxis for monocytes in the rat dental follicle. Arch Oral Biol. 1997;42:855–860. doi: 10.1016/s0003-9969(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 8.Wise GE, Que BG, Huang H. Synthesis and secretion of MCP-1 by dental follicle cells – implications for tooth eruption. J Dent Res. 1999;78:1677–1681. doi: 10.1177/00220345990780110301. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Wise GE. Expression of endothelial monocyte-activating polypeptide II in the rat dental follicle and its potential role in tooth eruption. Eur J Oral Sci. 2008;116:334–340. doi: 10.1111/j.1600-0722.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113:404–409. doi: 10.1111/j.1600-0722.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 11.Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84:837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattner A, Hsieh J, Smallwood PM, Gilber DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cystein-rich ligand-binding domain of frizzled receptors. PNAS. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CH, Amar S. Inhibition of SFRP-1 reduces severity of periodontitis. J Dent Res. 2007;86:873–877. doi: 10.1177/154405910708600913. [DOI] [PubMed] [Google Scholar]
- 14.Hoang BH, Thomas JT, Abdul-Karim FW, Correia KM, Conlon RA, Luyten FP, Ballock RT. Expression pattern of two frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev Dyn. 1998;212:364–372. doi: 10.1002/(SICI)1097-0177(199807)212:3<364::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Fukui K, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Seiko Y. Transcriptional silencing of secreted related protein 1 (SFRP1) by promoter hypermethylation in non-small-cell lung cancer. Oncogen. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Amar S. Secreted frizzled-related protein 1 (SFRP-1) protects fibroblasts from ceramide-induced apoptosis. J Biol Chem. 2004;279:2832–2840. doi: 10.1074/jbc.M308102200. [DOI] [PubMed] [Google Scholar]
- 17.Hausler KD, Horwood NJ, Chuman Y, Fisher J, Ellis J, Martin TJ, Rubin JS, Gillespie MT. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19:1873–1881. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- 18.Bodien PVN, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 19.Bodine PVN, Zhao W, Kharode YP, Bex FJ, Lambert A, Goad ME, Guar T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocr. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 20.Yao W, Cheng Z, Shahnazar M, Dai W, Johnson ML, Lane NE. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010;25:190–199. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Wise GE. A DNA microarray analysis of chemokine and receptor genes in the rat dental follicle – Role of secreted frizzled-related protein-1 in osteoclastogenesis. Bone. 2007;41:266–272. doi: 10.1016/j.bone.2007.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cielinski MJ, Jolie M, Wise GE, Ando DG, Marks SC., Jr . Colony-stimulating factor-1 (CSF-1) is a potent stimulator of tooth eruption in the rat. In: Davidovitch Z, editor. The biological mechanisms of tooth eruption, resorption and replacement by implants. Birmingham, AL: EBSCO Media; 1994. pp. 429–436. [Google Scholar]
- 23.Wise GE, Lin F, Fan W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992;267:483–492. doi: 10.1007/BF00319370. [DOI] [PubMed] [Google Scholar]
- 24.Wise GE, Ren Y, Yao S. Regulation of osteoclastogenesis gene expression in dental follicle cells. J Dent Res. 2003;82:298–302. doi: 10.1177/154405910308200411. [DOI] [PubMed] [Google Scholar]
- 25.Wise GE, Lin F, Zhao L. Immunolocalization of interleukin-1 α in rat mandibular molars and its enhancement after in vivo injection of epidermal growth factor. Cell Tissue Res. 1995;280:21–26. doi: 10.1007/BF00304507. [DOI] [PubMed] [Google Scholar]
- 26.Wise GE, Yao S. Expression of tumor necrosis factor-alpha in the rat dental follicle. Arch Oral Biol. 2003;48:47–54. doi: 10.1016/s0003-9969(02)00153-x. [DOI] [PubMed] [Google Scholar]
- 27.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda H, Shima N, Nakagawa N, Mochizuki S, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higasshio K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 29.Wise GE, Lumpkin SJ, Huang H, Zhang Q. Osteoprotegerin and osteoclast differentiation factor in tooth eruption. J Dent Res. 2000;79:1937–1942. doi: 10.1177/00220345000790120301. [DOI] [PubMed] [Google Scholar]
- 30.Van Wesenbeeck L, Odgren PR, Mackay CA, D’Angelo M, Safadi FF, Popoff SN, Hul WW, Marks SC., Jr The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. PNAS. 2002;99:14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wise GE, Lin F. Regulation and localization of colony-stimulating factor-1 mRNA in cultured rat dental follicle cells. Arch Oral Biol. 1994;39:621–627. doi: 10.1016/0003-9969(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 32.Hinterleitner TA, Saada JI, Berschneider HM, Powell DW, Valentich JD. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of CI-secretion in T84 cells. Am J Physiol Cell Physiol. 1996;271:C1262–1268. doi: 10.1152/ajpcell.1996.271.4.C1262. [DOI] [PubMed] [Google Scholar]
- 33.OC’onnel NA, Kumar A, Chatzipanteli K, Mohan A, Agarwal RK, Head C, Bornstein SR, Abou-Samra AB, Gwosdow AR. Interleukin-1 regulates corticosterone secretion from the rat adrenal gland through a catecholamine-dependent and prostaglandin E2-independent mechanism. Endocrinology. 1994;135:460–467. doi: 10.1210/endo.135.1.8013385. [DOI] [PubMed] [Google Scholar]
- 34.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]