Abstract
Background
Little information exists regarding pediatric contrast enhanced ultrasonography.
Objective
To assess the safety and feasibility of contrast enhanced ultrasonography of pediatric abdominal and pelvic tumors.
Materials and Methods
This prospective study included eight boys and five girls (mean age, 10.8 years) with abdominal or pelvic tumors. Cohorts of three subjects underwent ultrasound with perflutren contrast agent at escalating dose levels.Neurological and funduscopic examination, electrocardiography, and continuous pulse oximetry were performed before and after contrast administration. Threeradiologists independently scored six imaging parameters on pre- and post-contrast sonography. Inter-reviewer agreement was measured by the Kappa statistic.
Results
No neurological, retinal, electrocardiographic, or pulse oximetry changes were attributable to the contrast agent. Two subjects reported minor, transient symptoms. Post-contrast ultrasound parameter scores improved slightly in 8 of 12 subjects. Post-contrast ultrasound inter-reviewer agreement improved slightly for detection of tumor margins (pre-contrast = 0.20, post-contrast = 0.26), local tumor invasion (pre-contrast = −0.01, post-contrast = 0.10) and adenopathy (pre-contrast = 0.35, post-contrast = 0.44).
Conclusions
Although our sample size is small, perflutren contrast agents appear to be safe and well tolerated in children. Contrast enhanced sonography of pediatric abdominal and pelvic tumors is feasible but larger studies are needed to define their safety and efficacy in this patient population.
Keywords: safety, feasibility, contrast enhanced ultrasound, pediatric solid malignancies
Introduction
Children being treated for malignant solid abdominal and pelvic tumors often undergo repeated computed tomography (CT) to monitor response. Because children are in a phase of rapid body development and organ growth, their bodies may be more sensitive to the damaging effects of ionizing radiation than those of adults [1,2]. The carcinogenic effects resulting from diagnostic imaging procedures are stochastic and the probability of secondary malignancy increases with increasing radiation exposure and is cumulative over time. Therefore, radiation-free modalities such as magnetic resonance imaging (MRI) and ultrasonography (US) should be used whenever possible. Children are excellent candidates for US of abdominal and pelvic structures because the transducer can be positioned near the structure of interest thereby reducing signal attenuation and artifact. Ultrasound is also portable, less expensive than CT or MRI, and requires no sedation. However, US images can lack the resolution of CT and MRI in large pediatric patients with deep-seated structures of interest. Unlike CT and MRI, contrast agents are not routinely used for US to improve visualization of normal and abnormal structures.
Two US contrast agents have been approved by the U.S. Food and Drug Administration (FDA) for use in adult cardiology patients because they substantially improve endocardial border delineation [3–9]. These “microbubble” agents comprise perfluorocarbon gas (perflutren) encased within an outer shell of protein or phospholipid. The microbubbles approximate the size of an erythrocyte and remain within the vascular space [10], where they are highly reflective on ultrasonography. Contrast-enhanced US (CEUS) is contraindicated in patients with right-to-left cardiac shunting, which can allow the microbubbles to enter the arterial circulation and potentially cause microvascular occlusion [11].
The use of US contrast agents has been reported predominantly for adult cardiac and hepatic imaging [3–5,12–19]. More recently, quantitative CEUS is emerging as a valuable method of monitoring tumor blood flow in preclinical and adult clinical trials [20–24]. Ultrasound contrast agents are known to be well-tolerated and safe in adults, [25–32] but there have been only a few cardiology studies investigating the safety of the intravenous administration of these agents in children [29,30]. One concern is that contrast-induced microemboli may occur in children due to their potentially different vascular anatomy. Organs most at risk would be the central nervous system, heart, and lungs. It is also unknown whether US contrast agents improve sonographic visualization of pediatric abdominal or pelvic structures. Therefore, we sought to assess the safety and feasibility of using a perflutren US contrast agent in pediatric patients for imaging of malignant solid abdominal and pelvic tumors.
Materials and Methods
Study design and patients
We investigated an injectable suspension of human serum albumin microspheres encapsulating octafluoropropane gas (Optison™, provided by General Electric Heath Care, Princeton, NJ) with a mean particle size of 2 – 4.5 μm and a mean pulmonary elimination half-life of 1.3 ± 0.69 (SD) min. This prospective, pediatric dose escalation study was approved by our Institutional Review Board and performed with HIPAA compliance under FDA IND 62,852. Patients aged ≥ 2 and ≤ 21 years with a known or suspected solid abdominal or pelvic tumor were eligible. Signed informed consent and assent were provided by patients, parents, or guardians, as appropriate. Patients’ demographics, primary diagnoses, and phase of therapy were extracted from medical records.
The study had two primary objectives: 1) to assess the toxicity profile of the US contrast agent in children and young adults and 2) to determine its optimal dose for visualization of abdominal and pelvic pediatric solid malignancies. The study was closed before the optimal dose could be determined because the contrast agent was voluntarily, temporarily withdrawn from the market due to manufacturing irregularities. During the time that the contrast agent was unavailable, newer contrast specific software was installed on our US machine that made it impractical to return to the original study design. Therefore, we instead assessed the feasibility and value of CEUS for visualization of abdominal and pelvic tumors at several contrast agent dose levels using grayscale imaging. A secondary objective, to assess the feasibility of quantifying contrast agent flow into pediatric solid tumors, is not addressed here. Each patient received two injections of the same dose of contrast agent: the first to assess tumor visualization (primary objective) and the second to address the secondary objective. Both injections were used to assess toxicity. Using a traditional Phase 1 toxicity schema, cohorts of three children were enrolled at escalating dose levels; three subjects were imaged at a single dose level before escalating to the next higher dose (Table 1). For this contrast agent the manufacturer’s recommended adult dose is 0.5 mL which may be increased with repeated injections to improve endocardial border delineation [11]. The most recent pediatric cardiology study based dosing of this agent on weight such that patients < 20 kg received 0.3 mL and ≥ 20 kg received 0.5 mL [29]. Because there is little data regarding the safety of these agents in children, and at the recommendation of the FDA, for our safety study the dose was based on body surface area, beginning at a very low dose of 0.125 mL/m2 and escalating in 0.075 mL/m2 increments to 0.350 mL/m2. This approach resulted in doses that were lower than or comparable to those recommended in the pediatric cardiology literature. We also conservatively set the maximal allowable single dose at 2.15 mL/m2; approximately half that recommended by the manufacturer for adults [11]. The Cancer Therapy Evaluation Program, Common Toxicity Criteria, Version 2.0, was used to define the stopping rule as 20% of patients with a Grade 3 or 4 adverse event attributable to the contrast agent [33].
Table 1.
Demographics, contrast agent dose level and volume, primary diagnosis, and tumor site of children with solid abdominal or pelvic tumors imaged with contrast-enhanced ultrasound (CEUS)
| Subject | Sex | Race | Age at Time of Imaging | a Dose Level | Contrast Agent Volume(mL) | Primary Diagnosis | Imaged Tumor |
|---|---|---|---|---|---|---|---|
| b1 | M | W | 20 yr 8 mo/20yr 10 mo | 1 and 2 | 0.32 and 0.71 | Hepatocellular carcinoma | Liver primary with nodal metastasis |
| 2 | M | W | 17 yr 6 mo | 1 | 0.24 | Extrarenal rhabdoid | Pelvic metastasis |
| 3 | F | B | 2 yr 9 mo | 1 | 0.07 | Neuroblastoma | Right adrenal primary and retroperitoneal adenopathy |
| 4 | M | W | 6 yr 3 mo | 2 | 0.25 | Wilms tumor | Kidney primary |
| c5 | M | W | 16 yr 2 mo | 2 | 0.35 | Ewing sarcoma | Pelvic primary |
| c6 | F | W | 9 yr 9 mo | 2 | 0.33 | Hepatoblastoma | Liver recurrence |
| 7 | F | South American Indian | 10 yr 5 mo | 2 | 0.26 | Rhabdomyosarcoma | Pancreas metastasis |
| 8 | M | W | 12 yr 9 mo | 3 | 0.56 | Hepatocellular carcinoma | Liver primary with nodal metastasis |
| 9 | M | W | 19 yr 9 mo | 3 | 0.73 | Rhabdomyosarcoma | Pelvic primary |
| 10 | M | W | 2 yr 9 mo | 3 | 0.28 | Neuroblastoma | Retroperitoneal primary |
| 11 | M | W | 2 yr 6 mo | 4 | 0.33 | Hepatoblastoma | Liver primary |
| 12 | F | W | 2 yr 6 mo | 4 | 0.33 | Neuroblastoma | Right adrenal primary |
| 13 | F | W | 5 yr 8 mo | 4 | 0.35 | Neuroblastoma | Right adrenal primary |
M, male; F, female; W, white; B, black
Dose level 1 = 0.175 mL/m2; dose level 2 =0.20 mL/m2;dose level 3 = 0.275 mL/m2;dose level 4 = 0.35 mL/m2
This subject was enrolled twice at two different dose levels
Imaging deemed inevaluable due to US machine mechanical index > 0.9
Because CEUS was compared to both pre-contrast US and contrast-enhanced CT, only patients scheduled for US and CT were eligible. Candidates underwent extensive eligibility pre-screening and baseline evaluations. To minimize the risk of microemboli, particular attention was paid to the central nervous system, heart, and lungs. A complete history and physical was required within two weeks before CEUS. Subjects with known sensitivity to human albumin or blood products; with past or current evidence of retinopathy, pulmonary hypertension, open heart surgery or cyanotic heart disease; who required sedation for US or were pregnant, lactating, undergoing intensive care, or unable to comply with study requirements were ineligible. We assessed subjects indirectly for cerebral microemboli by funduscopic retinal examination before and after CEUS. Baseline funduscopy was performed within one week before CEUSin the first 11 subjects. Because all post-CEUS retinal findings were normal, we then discontinued funduscopic examinations to avoid causing unnecessary discomfort to subjects. We also required a normal 12-lead electrocardiogram (ECG; General Electric, 5500 or 5000 Marquette machine, Milwaukee, WI) and baseline limited neurological examination tailored to assess the cranial nerves, gross motor skills, and mentation within one week before CEUS. Additional requirements were normal blood pressure, heart rate, and respiratory rate, and pulse oximetry showing oxygen saturation ≥ 96% on room air, within five minutes before contrast agent injection. Subjects were interviewed five minutes before injection to record pre-existing symptoms of illness or discomfort.
Thirteen patients were enrolled between June 2003 and August 2004, when the agent was temporarily, voluntarily, withdrawn from the market. One 20-year-old male was enrolled twice at two dose levels. Two subjects were excluded from imaging analysis because the US machine’s mechanical index was > 0.9 during CEUS (see scanning procedure below). These two subjects were included in the toxicity analysis.
Ultrasound and CT scanning procedures
In clinical practice a hand injection of this contrast agent is acceptable, however, in order to standardize the injection rate for research purposes we administered the agent (diluted in 2 mL of normal saline for the first eight subjects and in 1 mL for the remaining five) as a bolus through an indwelling central venous line, using a power injector. The first subject’s bolus was given at 0.2 mL/sec, which was too slow to allow adequate visualization. The second subject received the bolus at 0.8 mL/sec and the remaining 12, at 1 mL/sec. Second injections were administered identically after 10 minutes. A 50 mL/hr continuous infusion of sterile normal saline was administered for 30 minutes after the second injection. An Acuson Sequoia US machine (Mountain View, CA) using a 4V2, 8C2 or 6C2 MHz transducer (selected for maximum image resolution by the sonographer and principal investigator) was used for pre-contrast imaging and a 6C2 MHz transducer and the Cadence Contrast Agent Imaging CPS software (Acuson, Mountain view, CA), for contrast-enhanced imaging. This software obtained imaging in gray scale at a preset resonant frequency compatible with this contrast agent. For CEUS the ultrasound machine’s mechanical index was maintained at ≤ 0.9 to minimize microbubble destruction. Longitudinal and transverse sweeps through the tumor and abdominal or pelvic structures were obtained before and after the first injection of contrast agent and recorded as 10-second digital cine-clips that were stored on magneto-optical discs for later review. Post-contrast imaging was begun immediately after the injection and continued for approximately 5 to 7 minutes or until the contrast agent was no longer visible.
Because of the broad age range of the intended study population contrast-enhanced CT, rather than MRI, was chosen as the reference standard since it was felt there was less potential for variable image quality with CT. All CT examinations were performed at our institution on a LightSpeed Ultra helical eight-row detector CT scanner (GE Medical Systems, Milwaukee, WI) with oral and IV contrast material, 5 mm slice thickness and a 1.35:1 pitch. Patients were scanned using 120 kVp with the milliampere-second setting adjusted for body weight and a preset noise level of 5. Those able to follow breath-hold instructions (generally aged 6 years and older) were scanned during suspended inspiration. The area scanned included the abdomen and pelvis in nine examinations, the abdomen only in three and the pelvis only in two. CT examinations were performed within a mean of 10 days (range 2 – 25 days) of the CEUS examinations.
Toxicity monitoring
During CEUS, patients underwent continuous ECG monitoring (Welch-Allen Propaq monitor, Beaverton, OR). Within 5 minutes after the second injection, an ECG rhythm strip was printed and later interpreted by a cardiologist along with a twelve-lead ECG performed within 4 hours after CEUS. Oxygen saturation was continuously monitored by pulse oximetry during both injections and for 30 minutes after the second injection. Blood pressure and heart and respiratory rates were recorded one and five minutes after each injection and 30 minutes after the second injection. Subjects/guardians were interviewed after each injection and again 24 to 48 hours after CEUS to identify any immediate or delayed adverse effects attributable to the contrast agent. Focused neurological examination was performed within 30 minutes after the second injection in the first 12 subjects and within 2 hours in the remaining two. The heart and lungs were auscultated 30 minutes after the second injection. Follow-up funduscopy was performed within 24 hours after CEUS in the first 8 subjects and within 4 days in an additional two; one subject was lost to ophthalmologic follow-up.
Feasibility assessment
We assessed feasibility by using contrast-enhanced CT as the reference standard. After each cohort of three patients was imaged, their pre and post-CEUS and contrast-enhanced CT imaging was de-identified and reviewed by three pediatric radiologists with 18, 16 and 10 years of experience, who were blinded to subjects’ history and primary diagnosis. Each radiologist reviewed, in one sitting, all imaging for each cohort; CT and pre- and post-CEUS images were shuffled to approximate random order. Reviewers graded the conspicuity of lesions, tumor invasion of local structures, and associated adenopathy as: definitely present = 1, probably present = 2, equivocal = 3, probably not present = 4, or definitely not present = 5. Ability to visualize tumor margins was graded as: excellent (> 90% identified) = 1, well-defined (75%–90% identified) = 2, moderately defined (50%–74% identified) = 3, poorly defined (<50% identified) = 4, and not defined = 5. For each of these four parameters (conspicuity, local invasion, adenopathy, tumor margins) an US-CT agreement score was calculated for pre and post CEUS examinations for each reviewer. The agreement score was defined as 5 minus the absolute difference of scores between US and CT. The higher the score, the better the agreement, with 5 being the highest score indicating identical scores between US and CT (e.g., US = 4 and CT = 4, then US-CT agreement score = 5−|4−4| = 5). One was the lowest possible score indicating polar opposite US and CT scores (e.g., US = 5 and CT = 1, then the US-CT agreement score = 5−|5−1| = 1). If the CT score differed among the three reviewers, the median was used for purposes of calculating the reviewer’s US-CT agreement scores. Each reviewer also determined the number of solid organ metastases and whether the primary tumor was solid, mixed (approximately half and half) or cystic. A score of 1 was given if the tumor was solid, 2 if mixed and 3 if cystic. A score of 1 was given if there were no metastases, 2 if there was only one metastasis and 3 if more than one. For these two parameters (tumor appearance, number of metastases), the US-CT agreement score was defined as 3 minus the absolute difference of scores between US and CT. The total US-CT agreement score for each study subject was the sum of the individual parameter scores (conspicuity, local invasion, adenopathy, tumor margins, number of solid organ metastases and solid/mixed/cystic) from all three reviewers. The pre and post contrast US-CT agreement scores for each subject were then compared.
Statistical analysis
Descriptive statistics were used for all comparisons described above. We also investigated whether contrast enhancement improved inter-reviewer agreement for individual US parameters by comparing pre- and post-CEUS inter-reviewer agreement using weighted Kappa statistics with a linear weight for categories scaled from 1 to 5. Inter-reviewer agreement was defined as: poor ≤ 0; slight, 0–0.2; fair, 0.2–0.4; moderate, 0.4–0.6; substantial, 0.6–0.8; almost perfect, 0.8–1.0. All analyses were performed with SAS version 9.2 software (SAS Institute, Cary, NC).
Results
The 13 subjects underwent a total of 28 injections of ultrasound contrast material. Demographics, contrast agent dose and volume, primary diagnosis, and tumor site are shown in Table 1. Of note, six subjects received clinically relevant doses, i.e. doses comparable to current recommendations for this contrast agent (subject 1’s second enrollment dose and subjects 8 – 12, Table 1). Two subjects had not begun therapy, four were off therapy and had recurrent disease, and eight were undergoing treatment. All subjects’ cardiac rate and rhythm, oxygen saturation, blood pressure, and respiratory rate remained normal during and 30 minutes after each contrast injection. Post-CEUS auscultation of the heart and lungs was normal in all subjects, and all post-CEUS cardiac rhythm strips and 12-lead ECGs were interpreted as normal. One 2-year-old boy who received 0.275 mL/m2 of contrast agent demonstrated possible decreased deep tendon reflex on the post-CEUS neurological examination, but it was not attributed to the contrast agent. The patient was undergoing chemotherapy for retroperitoneal neuroblastoma, and it was unclear whether this potential change reflected technical difficulty in assessing deep tendon reflexes in a 2-year-old or the patient’s underlying disease or treatment. The patient’s other post-CEUS findings were normal. The remaining subjects had no change in neurological findings. None of the 10 subjects who underwent post-CEUS funduscopy showed evidence of retinal microemboli. Two subjects reported mild, transient symptoms during contrast agent injection. A 20-year-old young man (who received 0.125 mL/m2)experienced mild tinnitus and lightheadedness, and a 12-year-old boy (who received 0.275 mL/m2) experienced brief taste alteration. One 6-year-old boy (who received 0.20 mL/ m2) became hyperactive about one hour after contrast injection and remained irritable throughout the day and night; he was undergoing chemotherapy for Wilms tumor, and the cause of this reaction was unclear. No other delayed adverse reaction was attributed to the contrast agent.
There was a slight improvement in the average scores for 3 of the 4 individual parameters after administration of contrast material (Table 2). Eight of the 12 CEUS examinations showed slight improvement in total US-CT agreement scores compared to pre-contrast US-CT agreement scores (Table 3). Inter-reviewer agreement weighted Kappa values improved on CEUS for detection of tumor margins (pre-CEUS = 0.20, post-CEUS = 0.26) invasiveness (pre-CEUS = −0.01, post-CEUS = 0.10) and adenopathy (pre-CEUS = 0.35, post-CEUS = 0.44). On CEUS, there was moderate agreement on adenopathy, fair agreement on tumor margins and conspicuity, but only slight agreement on local invasiveness. Figures 1, 2, and 3 demonstrate representative changes in tumor appearance on pre- and post-contrast US as compared to contrast-enhanced CT images.
Table 2.
aIndividual parameter US-CT agreement scores pre and post contrast administration
| Trial Cohort | US-CT Agreement Score Pre Contrast | US-CT Agreement Score Post Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conspicuity | Tumor margin | Invasion | Adenopathy | Contrast Dose | Conspicuity | Tumor Margin | Invasion | Adenopathy | |
| 1 | 5.00 | 3.89 | 4.44 | 3.89 | 0.175 mL/m2 | 5.00 | 4.33 | 4.44 | 4.00 |
| 2 | 4.22 | 3.00 | 4.44 | 3.89 | 0.200 mL/m2 | 4.55 | 3.44 | 4.44 | 4.11 |
| 3 | 4.78 | 4.44 | 4.11 | 3.56 | 0.275 mL/m2 | 4.45 | 3.44 | 4.67 | 3.78 |
| 4 | 5.00 | 3.89 | 3.11 | 4.00 | 0.350 mL/m2 | 4.78 | 4.56 | 4.00 | 4.00 |
| Average | 4.75 | 3.81 | 4.03 | 3.83 | Average | 4.69 | 3.95 | 4.39 | 3.97 |
Each parameter was scored on a scale of 1 to 5. The aindividual parameter US-CT agreement score was defined as 5 minus the absolute difference of scores between US and CT. The higher the score, the better the agreement. 5 is the highest score, indicating indentical scores for US and CT (e.g., US = 4 and CT = 4, then US-CT agreement score = 5−|4−4| = 5; 1 is the lowest score, indicating polar opposite scores by US and CT (e.g., US = 5 and CT = 1, then US-CT agreement score = 5−|5−1| = 1).
Table 3.
a Total US-CT agreement scores before and after use of US contrast agent at four escalating doses
| Subject | Pre-contrast Score | Post-contrast Score | % Change |
|---|---|---|---|
| Cohort 1: Dose Level = 0.175 mL/m2 | |||
| 1 | 62 | 67 | 8.1% |
| 2 | 74 | 77 | 4.1% |
| 3 | 68 | 67 | −1.5% |
| Cohort 2: Dose Level = 0.200 mL/m2 | |||
| b4 | 47 | 52 | 10.6% |
| 5 | 67 | 70 | 4.5% |
| b6 | 59 | 62 | 5.1% |
| Cohort 3: Dose Level = 0.275 mL/m2 | |||
| 7 | 68 | 66 | −2.9% |
| 8 | 68 | 64 | −5.9% |
| b9 | 60 | 61 | 1.7% |
| Cohort 4: Dose Level = 0.350 mL/m2 | |||
| 10 | 70 | 75 | 7.1% |
| 11 | 68 | 68 | 0% |
| 12 | 60 | 67 | 11.7% |
Sum of individual parameter US-CT agreement scores for three reviewers for each subject.
The score does not include data for tumor appearance which could not be evaluated due to technical factors.
Fig. 1.

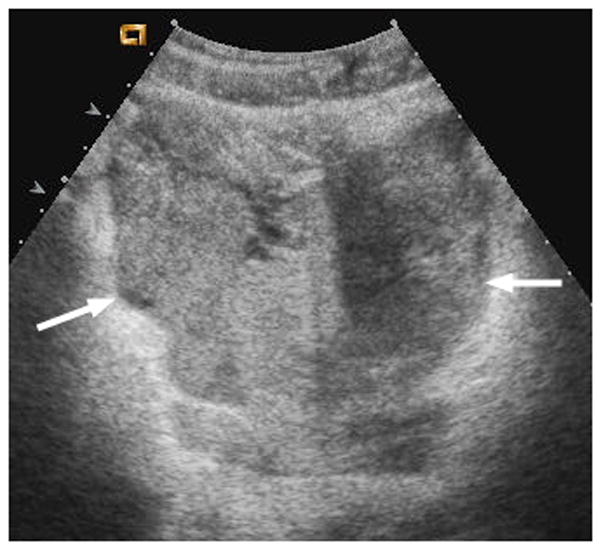
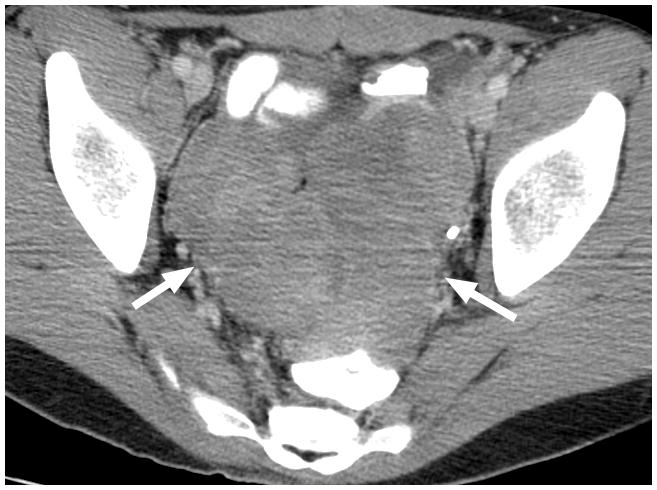
17-year-old boy with pelvic metastasis of renal rhabdoid tumor. (a) Non–contrast-enhanced transverse ultrasound (US) image showing the pelvic tumor (arrows). (b) After administration of 0.24 mL of perflutren contrast agent, the mass (arrows) is more echogenic, indicating enhancement of viable tumor. (c) Contrast-enhanced computed tomography (CT) image of the pelvic tumor (arrows) shows tumor configuration and definition of margins similar to that seen on US
Fig. 2.
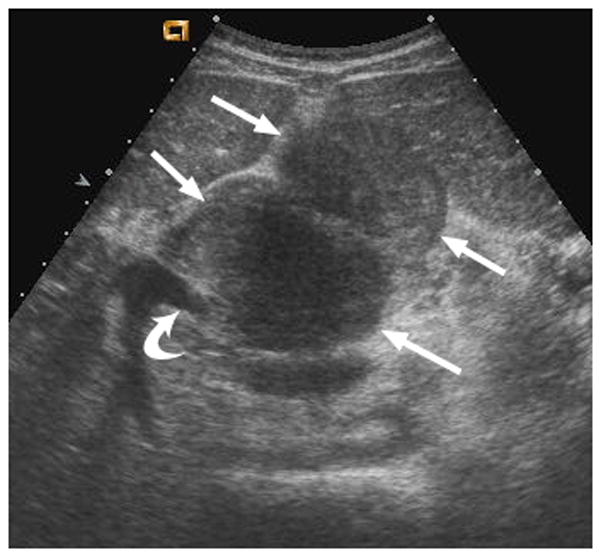

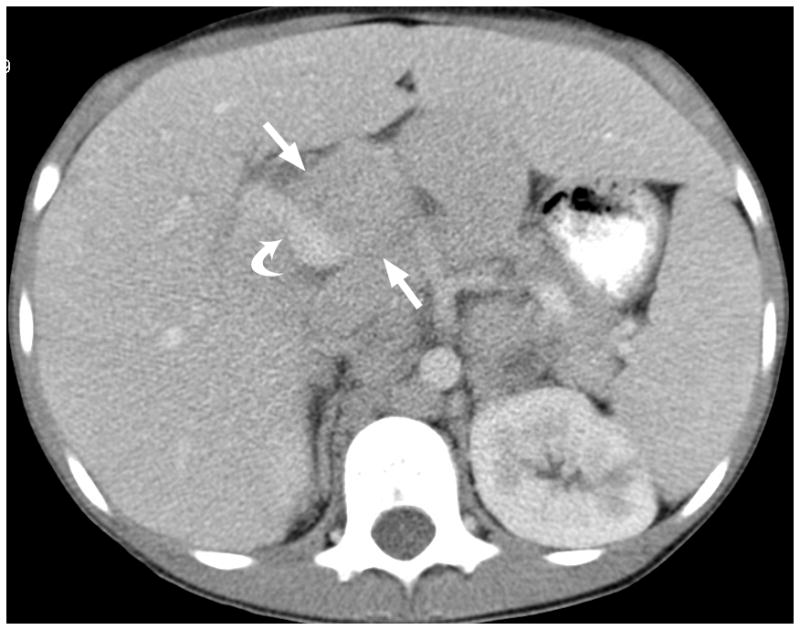
12-year-old boy with hepatocellular carcinoma and local nodal metastasis. (a) Non–contrast-enhanced transverse US image near the porta hepatis shows enlarged metastatic nodes (arrows) and main portal vein (curved arrow). (b) After administration of 0.56 mL of perflutren contrast agent, the main portal vein is opacified (curved arrow) and the relationship between the portal vein and adjacent node (straight arrows) is well defined. (c) Contrast-enhanced CT image at the level of the porta hepatis shows the enlarged node (arrows) adjacent to the main portal vein (curved arrow) as demonstrated by CEUS
Fig. 3.
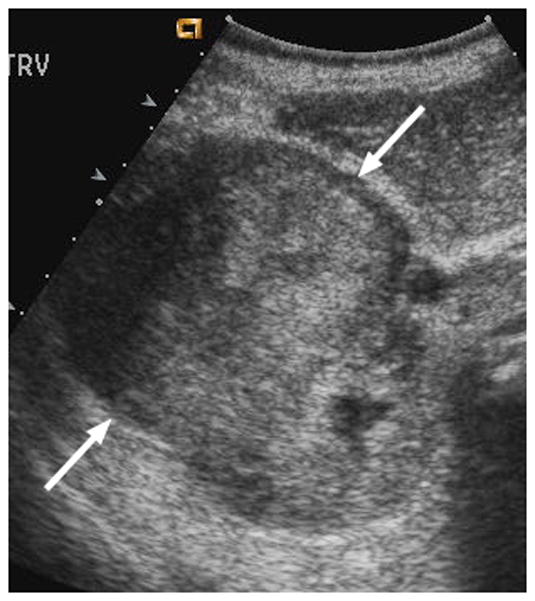
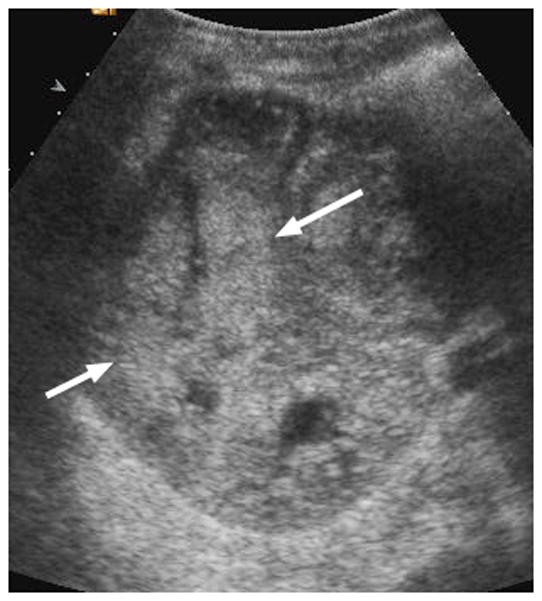
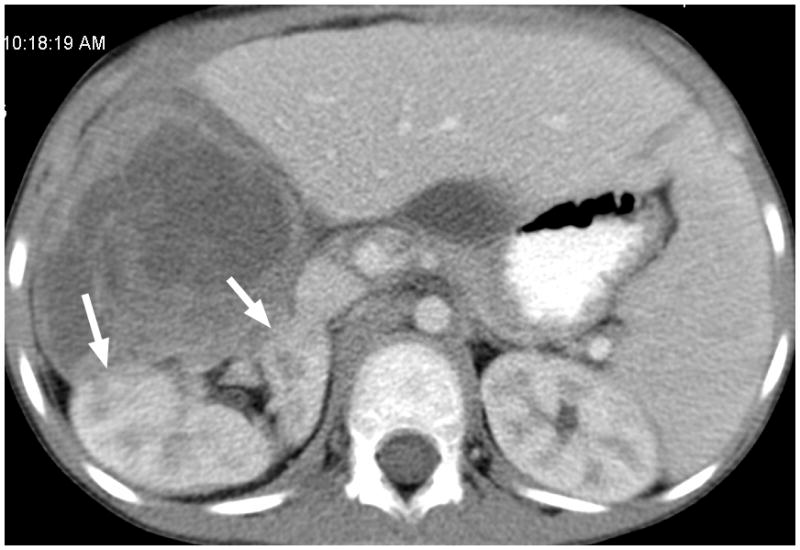
6-year-old boy with Wilms tumor. (a) Non–contrast-enhanced transverse US image of the primary right renal tumor (arrows). (b) After administration of 0.25 mL of perflutren contrast agent, there is slight enhancement in the lateral aspect of the tumor (arrows). Note that tumor margin is not well defined on pre- or post-contrast US images. (c) Contrast-enhanced CT image of the tumor better defines the interface between tumor and normal renal parenchyma (arrows)
Discussion
We have rigorously evaluated 13 subjects with pediatric solid abdominal or pelvic malignancies who received a total of 28 intravenous injections of an US contrast agent (perflutren in human serum albumin microspheres). Although this was a dose escalation study, based on current dosing recommendations for this contrast agent, six of our subjects received clinically relevant doses [11, 29]. Our toxicity assessments were tailored to detect microembolic events in several organ systems of major concern; the central nervous system, the heart and the lungs. We found that this contrast agent was safe and generally well tolerated in subjects as young as two years of age, children with newly diagnosed or recurrent cancer, and those undergoing chemotherapy or radiation therapy.
To assess for cerebral microemboli we performed retinal funduscopic examinations within 1 day (n = 8) to 4 days (n = 2) after CEUS and found no retinal changes or evidence of retinal microemboli. No neurological changes were attributed to the contrast agent. One subject became hyperactive after the contrast injection and experienced irritability until the following day. While irritability has been reported as a rare (< 0.5%) adverse reaction to this contrast agent in adults, the underlying cause in this case remains unclear and may have been related to the subject’s underlying disease status or treatment [11].
None of our subjects showed evidence of altered cardiac function, conduction, rate, or rhythm. Blood pressure, respiratory rate, cardiac and pulmonary auscultation, and continuous pulse oximetry remained normal in all subjects. Two subjects (15%; 2/13) reported mild symptoms during the administration of contrast agent that resolved within minutes: tinnitus and lightheadedness in one and taste alteration in the other. Altered taste was previously reported in 2% (4/203) of adults and 5% (1/20) of pediatric patients receiving this agent [3,29]. Tinnitus and lightheadedness have also been reported as rare in adults [11].
Although we were not able to identify the optimal dose of this contrast agent for imaging pediatric abdominal and pelvic tumors we found that visualization of imaging parameters improved slightly in 2/3s of our subjects after contrast administration. Our study was limited by the heterogeneity of tumor types, tumor sites, and body habitus. Because of this inherent variability, a larger study will be necessary to identify a dose that provides optimal tumor and organ enhancement in children. Also, newer contrast specific software that is now available, with color overlay and subtraction capabilities, should improve lesion conspicuity. Despite these limitations, we have shown that enhancement of various pediatric solid abdominal and pelvic tumors can be subjectively appreciated on CEUS even at low contrast agent doses.
Our preliminary data suggest that this US contrast agent is safe and well tolerated over a range of dosages in children with newly diagnosed, currently treated, or recurrent solid abdominal or pelvic malignancies. Further studies using current contrast-specific imaging software are needed to identify the optimal dose and to elucidate which pediatric abdominal and pelvic tumors are best visualized by CEUS. In the future, if CEUS is shown to increase diagnostic confidence, it may be possible to reduce the number and cost of CT scans and MRIs requiring sedation that are needed to monitor patients during therapy. Perhaps more importantly, because microbubble contrast agents remain in the vascular space, they can be used as surrogate markers of tumor blood flow. Because the newest class of anti-cancer agents, anti-angiogenic agents, alter tumor blood flow but may not substantially alter tumor size, CEUS is emerging as a promising modality for assessing response to these agents in adult cancers [20,21,34,35]. Our preclinical studies have shown that quantitation of US contrast agent flow into pediatric murine models of neuroblastoma and rhabdomyosarcoma allows accurate monitoring of changes in tumor blood flow induced by a variety of anti-angiogenic agents [22–24].
Conclusion
In this current small study we demonstrated the sonographic visualization of contrast agent flow into a variety of solid pediatric tumors in the clinical setting. We will continue to investigate the feasibility of quantitating US contrast agent flow into pediatric solid tumors in clinical trials. Our safety and imaging findings, coupled with the many advantages of sonography in children, uniquely position CEUS to become a valuable method of assessing solid tumors in pediatric cancer therapy trials. Larger studies are needed to confirm the safety and define the efficacy of these agents.
Acknowledgments
The authors wish to thank Stacey Glass, RDMS, RVT for excellent technical assistance in performing CEUS, Tommy Kettler for vigilant nursing assistance and patient monitoring, Dr. Robert Kaufman for thoughtful review of the manuscript, Erika Thompson for manuscript preparation, and the patients who participated in this study.
Supported in part by the American Lebanese Syrian Associated Charities (ALSAC), the Research and Education Foundation of the Society for Pediatric Radiology, and General Electric Healthcare
Reference List
- 1.Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JL, Cheirif J, Segar DS, et al. Improved left ventricular endocardial border delineation and opacification with Optison (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J Am Coll Cardiol. 1998;32:746–752. doi: 10.1016/s0735-1097(98)00311-8. [DOI] [PubMed] [Google Scholar]
- 4.Sakakura K, Tone K, Kakimoto H, Koyama M, Sekioka K. Improvement of endocardial border delineation during dobutamine stress echocardiography with Levovist. J Cardiol. 2003;41:277–283. [PubMed] [Google Scholar]
- 5.Mulvagh SL, Rakowski H, Vannan MA, et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Colonna P, Corda M, et al. Contrast-enhanced harmonic color Doppler for left ventricular opacification: improved endocardial border definition compared to tissue harmonic imaging and optimization of methodology in patients with suboptimal echocardiograms. Echocardiography. 2001;18:639–649. doi: 10.1046/j.1540-8175.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.Nanda NC, Wistran DC, Karlsberg RP, et al. Multicenter evaluation of SonoVue for improved endocardial border delineation. Echocardiography. 2002;19:27–36. doi: 10.1046/j.1540-8175.2002.00027.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshitani H, Takeuchi M, Hirose M, et al. Head-to-head comparison of fundamental, tissue harmonic and contrast harmonic imaging with or without an air-filled contrast agent, levovist, for endocardial border delineation in patients with poor quality images. Circ J. 2002;66:494–498. doi: 10.1253/circj.66.494. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, Goldman ME, Gillam LD, Cohen JL, Aurigemma GP, Gottdiener JS. Efficacy and safety of the novel ultrasound contrast agent perflutren (definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am J Cardiol. 2000;86:669–674. doi: 10.1016/s0002-9149(00)01050-x. [DOI] [PubMed] [Google Scholar]
- 10.Ophir J, Parker KJ. Contrast agents in diagnostic ultrasound. Ultrasound Med Biol. 1990;16:209. doi: 10.1016/0301-5629(90)90151-2. [DOI] [PubMed] [Google Scholar]
- 11.Optison [package insert] Oslo, Norway: GE Healthcare; 2005. Oct, [Google Scholar]
- 12.von HA, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound. 2010;38:1–9. doi: 10.1002/jcu.20626. [DOI] [PubMed] [Google Scholar]
- 13.Piscaglia F, Lencioni R, Sagrini E, et al. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med Biol. 2010;36:531–550. doi: 10.1016/j.ultrasmedbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Burns PN, Wilson SR. Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q. 2006;22:5–13. [PubMed] [Google Scholar]
- 15.Bhayana D, Kim TK, Jang HJ, Burns PN, Wilson SR. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977–983. doi: 10.2214/AJR.09.3375. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, Burns PN. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691–695. doi: 10.2214/AJR.07.3116. [DOI] [PubMed] [Google Scholar]
- 17.Kim TK, Jang HJ, Burns PN, Murphy-Lavallee J, Wilson SR. Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:58–66. doi: 10.2214/AJR.07.2493. [DOI] [PubMed] [Google Scholar]
- 18.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SR, Jang HJ, Kim TK, Burns PN. Diagnosis of focal liver masses on ultrasonography: comparison of unenhanced and contrast-enhanced scans. J Ultrasound Med. 2007;26:775–787. doi: 10.7863/jum.2007.26.6.775. [DOI] [PubMed] [Google Scholar]
- 20.Lassau N, Lamuraglia M, Vanel D, et al. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: prospective study of 49 cases. Ann Oncol. 2005;16:1054–1060. doi: 10.1093/annonc/mdi214. [DOI] [PubMed] [Google Scholar]
- 21.Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary results. Radiology. 2011;258:291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 22.Sims TL, McGee M, Williams RF, et al. IFN-beta restricts tumor growth and sensitizes alveolar rhabdomyosarcoma to ionizing radiation. Mol Cancer Ther. 2010;9:761–771. doi: 10.1158/1535-7163.MCT-09-0800. [DOI] [PubMed] [Google Scholar]
- 23.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 24.McCarville MB, Streck CJ, Dickson PV, Li CS, Nathwani AC, Davidoff AM. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240:73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- 25.Wei K. Contrast echocardiography: what have we learned from the new guidelines? Curr Cardiol Rep. 2010;12:237–242. doi: 10.1007/s11886-010-0105-x. [DOI] [PubMed] [Google Scholar]
- 26.Abdelmoneim SS, Bernier M, Scott CG, et al. Safety of contrast agent use during stress echocardiography: a 4-year experience from a single-center cohort study of 26,774 patients. JACC Cardiovasc Imaging. 2009;2:1048–1056. doi: 10.1016/j.jcmg.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Main ML, Goldman JH, Grayburn PA. Ultrasound contrast agents: balancing safety versus efficacy. Expert Opin Drug Saf. 2009;8:49–56. doi: 10.1517/14740330802658581. [DOI] [PubMed] [Google Scholar]
- 28.Aggeli C, Giannopoulos G, Lampropoulos K, Pitsavos C, Stefanadis C. Adverse bioeffects of ultrasound contrast agents used in echocardiography: true safety issue or “much ado about nothing”? Curr Vasc Pharmacol. 2009;7:338–346. doi: 10.2174/157016109788340695. [DOI] [PubMed] [Google Scholar]
- 29.McMahon CJ, Ayres NA, Bezold LI, et al. Safety and efficacy of intravenous contrast imaging in pediatric echocardiography. Pediatr Cardiol. 2005;26:413–417. doi: 10.1007/s00246-004-0795-1. [DOI] [PubMed] [Google Scholar]
- 30.Zilberman MV, Witt SA, Kimball TR. Is there a role for intravenous transpulmonary contrast imaging in pediatric stress echocardiography? J Am Soc Echocardiogr. 2003;16:9–14. doi: 10.1067/mje.2003.41. [DOI] [PubMed] [Google Scholar]
- 31.Incesu L, Yazicioglu AK, Selcuk MB, Ozen N. Contrast-enhanced power Doppler US in the diagnosis of acute appendicitis. Eur J Radiol. 2004;50:201–209. doi: 10.1016/S0720-048X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 32.Oldenburg A, Hohmann J, Skrok J, Albrecht T. Imaging of paediatric splenic injury with contrast-enhanced ultrasonography. Pediatr Radiol. 2004;34:351–354. doi: 10.1007/s00247-003-1092-5. [DOI] [PubMed] [Google Scholar]
- 33.Common Terminology Criteria (CTC) Cancer Therapy Evaluation Program. 1999 Apr 30; version 2.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf#search=“commontoxicity.
- 34.Lamuraglia M, Bridal SL, Santin M, et al. Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol. 2010;73:202–212. doi: 10.1016/j.critrevonc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]


