Abstract
Objectives
Dual inhibition of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) demonstrated initial promise in clinical trials. This phase II study tested the efficacy and safety of capecitabine, oxaliplatin, and cetuximab with or without bevacizumab as first-line treatment for metastatic colorectal cancer patients.
Methods
Patients were randomized to receive capecitabine 850 mg/m2 PO twice daily for 14 days, oxaliplatin 130 mg/m2 IV day 1, and cetuximab 400 mg/m2 IV loading dose followed by 250 mg/m2 IV days 1, 8, and 15 with (Arm A) or without (Arm B) bevacizumab 7.5 mg/kg IV day 1 every 21 days. Tumor samples were collected and retrospectively analyzed for KRAS mutation status. The primary endpoint was response rate, with time to progression (TTP) and overall survival (OS) as secondary objectives.
Results
Twenty-three patients (12 in Arm A, 11 in Arm B) were enrolled onto the study. Median follow-up was 25.9 months. Both treatments were well tolerated, with expected higher rates of grade 1/2 hypertension and bleeding in Arm A. The overall response rate was 54% (36.4% in Arm A and 72.7% in Arm B). Median time to progression was 8.7 months in Arm A and 14.4 months in Arm B. The median survival was 18.0 months in Arm A and 42.5 months in Arm B. The study was prematurely terminated after other studies reported inferior outcomes with dual antibody therapy.
Conclusions
Although terminated early, the study supports the detrimental effect of combining VEGF and EGFR inhibition in metastatic colorectal cancer.
Keywords: Metastatic colon cancer, Vascular endothelial growth factor (VEGF), Epidermal growth factor receptor (EGFR)
Introduction
The combination of a fluoropyrimidine and oxaliplatin is a common therapeutic strategy for the initial treatment of metastatic colon cancer [1]. Although the use of FOLFOX (infusional 5-fluorouracil plus oxaliplatin) is a common strategy in this setting, substitution of the oral fluoropyrimidine capecitabine for intravenous 5-fluorouracil has similar efficacy in combination with oxaliplatin [2–5]. Further improvement in outcome is achieved with the addition of the anti-VEGF antibody bevacizumab [6–8]. When the current study was conceived, initial reports suggested high response rates with the addition of cetuximab, an anti-EGFR antibody, to frontline chemotherapy [9–11]. More recently, the predictive value of KRAS mutation on response to EGFR targeted therapy has been validated, with responses noted only in patients with KRAS wild-type tumors [12–15].
The combination of cetuximab and bevacizumab demonstrated synergy in preclinical studies [16]. These studies demonstrated almost complete inhibition of VEGF expression and angiogenesis in vitro following treatment with anti-EGFR and anti-VEGF agents. A randomized phase II trial in fluoropyrimidine and oxaliplatin-refractory metastatic disease demonstrated a promising objective response rate of 20% when cetuximab and bevacizumab were given in combination [17]. Given encouraging preclinical and clinical rationale for frontline use of dual VEGF-EGFR blockage, we initiated this randomized phase II study to evaluate the combination of capecitabine, oxaliplatin, and cetuximab with or without bevacizumab as first-line therapy for metastatic colorectal cancer.
Patients and Methods
Patients
Patients age ≥18 with histologically confirmed metastatic adenocarcinoma of the colon or rectum who had not received prior chemotherapy for their disease were eligible for the study (prior adjuvant therapy was permitted if completed 12 months or more prior to study enrollment). Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–1 and adequate hematologic, clotting, hepatic, and renal function (creatinine clearance ≥ 50 ml/min). Exclusion criteria included clinically significant cardiovascular disease (e.g., uncontrolled hypertension >160/100 mmHg, myocardial infarction within the last 6 months, unstable angina), pregnant or breastfeeding females, patients with history of central nervous system disease including history of cerebrovascular accident within 6 months of enrollment, evidence of urine protein/creatinine ratio (UPC) ≥1.0, history of surgical procedure within 28 days of enrollment, history of allergic reaction to any of the study drugs, and ≥ grade 2 existing neuropathy. As this study was initiated and conducted prior to the emergence of data on cetuximab resistance associated with KRAS mutations, patients were treated with cetuximab regardless of KRAS mutation status (which was determined retrospectively) [12–14]. The protocol was open for accrual and received institutional review board approval at Fox Chase Cancer Center (FCCC) and regional community cancer programs participating in the Fox Chase Office of Extramural Research Program (OER), a clinical trial consortium in the Delaware Valley coordinated through Fox Chase Cancer Center.
Study Design and Treatment Plan
This was a randomized phase II trial for patients with previously untreated metastatic colorectal cancer who were candidates for frontline systemic therapy. Patients were randomized in a 1:1 fashion into two cohorts using a table generated by permuted block randomization. Patients randomized to arm A received the following in 21-day cycles: capecitabine 850 mg/m2 orally twice daily on days 1–14, oxaliplatin 130 mg/m2 infused over 2 h on day 1, cetuximab 250 mg/m2 weekly infused over 60 min after a loading dose on day 1 of cycle 1 of 400 mg/m2 infused over 120 min (patients were premeditated with an anti-histamine intravenously), and bevacizumab 7.5 mg/kg on day 1 infused over 90 min (administered following oxaliplatin injection). Patients randomized to arm B received the same regimen without bevacizumab.
Assessments
The following were obtained within 14 days of study initiation: medical history; physical examination; CT scan of the chest, abdomen, and pelvis; and laboratory studies, including a complete blood count (CBC), comprehensive metabolic profile (CMP), coagulation studies, carcinoembryonic antigen, and urine for protein/creatinine ratio. On day 1 of each treatment cycle (every 21 days), patients underwent physical examination, CBC,CMP, and urine for protein/creatinine ratio. During study treatment, patients were evaluated weekly with vital signs and routine blood tests (CBC and CMP).
Tumor measurements were obtained every two cycles (6 weeks) for the first four cycles and then every three cycles (9 weeks) thereafter. Response was evaluated by Response Evaluation Criteria in Solid Tumor (RECIST) criteria version 1.0 [18]. Patients continued treatment on study until evidence of progression of disease or unacceptable toxicity. Upon removal from study, patients were monitored at 3-month intervals until disease progression or death.
Dose Adjustments
Adverse events were graded according to National Cancer Institute Common Terminology Criteria Version 3.0. Oxaliplatin dose was reduced by one dose level in the presence of ≥grade 3 nausea/vomiting, neuropathy, thrombocytopenia, or neutropenia according to the following scale: 100, 85, and 65 mg/m2. Capecitabine was held for patients with ≥grade 2 diarrhea that persisted for≥2 days, ≥ grade 2 hand-foot syndrome, ≥ grade 3 nausea/vomiting, and ≥grade 3 hematologic toxicities. Upon resolution of toxicity, capecitabine was restarted with a reduction of one 500-mg tablet per day for subsequent cycles. Grade 2 or 3 hypertension that was controlled with anti-hypertensive medications resulted in reduction of the bevacizumab dose to 5 mg/kg. Grade 4 hypertension prompted discontinuation of bevacizumab. Development of grade 3 hemorrhage or any arterial thromboembolic event resulted in permanent discontinuation of bevacizumab. The dose of cetuximab therapy was adjusted in the presence of ≥grade 3 dermatologic toxicity, with delay of the infusion for 1–2 weeks and decreased dosing to 200 or 150 mg/m2.
Correlative Studies
KRAS Mutation Status Evaluation
Tumor samples were tested for the presence of a KRAS mutation on codons 12 and 13. DNA analyses were performed by the Fox Chase Clinical Molecular Genetics Laboratory. Extraction, isolation, and purification of DNA from formalin-fixed paraffin embedded tissue (FFPE) suitable for molecular analysis were conducted using WaxFree DNA with a DNA extraction kit (TrimGen WF-100). Ten to fifteen fresh cut unstained slides were usually suitable for analysis. DNA (~100 ng) was amplified by polymerase chain reaction (PCR) using primer sequences located on either side of the region of the coding exon of interest. PCR products were detected by agarose gel electrophoresis. Mutations were detected by sequencing of the purified PCR amplified product (BigDye Terminator v.1.1 Cycle Sequencing Kit, Applied Biosystems) and evaluated by capillary electrophoresis (ABI 3100, Applied Biosystems).
Statistical Analysis
The primary objective for this randomized phase II study was objective response rate (RR) for each regimen. Secondary objectives included determining the time to progression (TTP), overall survival (OS), and toxicity profiles of patients treated with each of these regimens. No direct comparison between regimens was planned. The projected sample size was powered by clinical RR endpoint considerations. A proportion of patients with responses less than 40% in each arm would be of no interest. The combination of cetuximab, capecitabine, and oxaliplatin with or without bevacizumab would be of interest if the proportion of patients with favorable responses in each arm was at least 60%. Forty patients per arm were needed to test the null hypothesis: p≤0.4 against the alternative hypothesis: p≥0.6 at the 7.44% level of significance and with 87% power. The null hypothesis would be rejected if at least 21 of 40 patients in each arm responded. An interim stopping rule for toxicity was planned as well. However, an early stopping rule for efficacy was not incorporated as both regimens contained agents with demonstrated activity in advanced colorectal cancer.
Study accrual was terminated after enrollment of 23 patients, based on emerging data from other studies that dual antibody therapy resulted in inferior outcomes compared to single antibody therapy [19, 20]. The results presented are for the primary and secondary objectives for the study as planned, using this smaller sample size. Time to progression and overall survival were defined from enrollment date to event, and RR was calculated. Descriptive statistics were applied to toxicity reporting. The median time to progression and overall survival was calculated by the Kaplan–Meier method. Patients were censored at the last time point known to be free of progression (for time to progression analysis) and last time point known to be alive (for overall survival).
Results
Patient Characteristics
Twenty-three patients from four institutions were enrolled on this study between June 2006 and May 2008. Patient characteristics are listed by treatment arm in Table 1. Despite the smaller than planned sample size, the groups were generally well balanced for patient and clinical characteristics, with the exception of a significantly greater number of patients with PS 0 and liver metastases in Arm B compared with Arm A. A total of 210 cycles were administered (99 cycles in Arm A and 111 cycles in Arm B). The median number of treatments was 8 overall (range <1–19), with 7.5 (<1–19) for arm A and 10 (3–16) for Arm B. Median follow-up for all patients was 25.9 months (18.14 months for Arm A and 33.53 months for Arm B).
Table 1.
Patient and tumor characteristics by treatment arm
| Characteristics | All enrolled | Arm A (n=12) | Arm B (n=11) | P-value | |||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | ||
| Gender | 0.32 | ||||||
| Male | 18 | 78.3 | 8 | 66.7 | 10 | 90.9 | |
| Female | 5 | 21.7 | 4 | 33.3 | 1 | 9.1 | |
| Median age (range) | 59 (42–78) | 59 (45–78) | 58 (42–74) | 0.6 | |||
| Race/ethnicity | 0.093 | ||||||
| Caucasian | 19 | 82.6 | 8 | 66.7 | 11 | 100 | |
| African American | 4 | 17.4 | 4 | 33.3 | 0 | 0 | |
| Performance status | 0.0028 | ||||||
| 0 | 13 | 56.6 | 3 | 25 | 10 | 90.9 | |
| 1 | 10 | 43.4 | 9 | 75.0 | 1 | 9.1 | |
| Adjuvant therapy | |||||||
| Yes | 2 | 8.6 | 0 | 0 | 2 | 18 | 0.22 |
| No | 21 | 91.3 | 12 | 100 | 9 | 82 | |
| Sites of metastases | |||||||
| Liver | 17 | 74 | 6 | 50 | 11 | 100 | 0.014 |
| Lung | 11 | 48 | 7 | 58 | 4 | 36 | 0.41 |
| Abdominal lymph nodes | 9 | 39 | 5 | 42 | 4 | 36 | 1.00 |
| 1–2 metastatic sites | 18 | 78 | 9 | 75 | 9 | 82 | 1.00 |
| >3 Metastatic sites | 5 | 22 | 3 | 25 | 2 | 18 | |
| Correlative studiesa | |||||||
| KRAS wild type | 15 | 75 | 7 | 70 | 8 | 80 | 0.5 |
| KRAS mutant | 5 | 25 | 3 | 30 | 2 | 20 | |
The correlative studies were assessed retrospectively following study completion on 20 patients for whom tissue samples were available (ten patients from each study arm).
The numbers in bold represent statistical significance P<0.05
Toxicity
All patients were evaluable for toxicity. A summary of toxicities which were at least possibly related to study treatment is presented in Table 2 by grade and treatment arm. Most of the observed toxicities were grades 1–2, with no grade 5 toxicities documented. Grade 1–2 fatigue was the most common toxicity observed in both treatment arms. Skin toxicities (most notably grade 1–2 rash), gastrointestinal, and neurologic toxicities were equally observed in both treatment arms. Interestingly, hand-foot syndrome was seen more often among patients enrolled on arm B (36% versus 8.3%). As expected, patients treated with bevacizumab demonstrated higher rates of any grade hypertension (50% versus 18.2%) and bleed (50% versus 36.4%). However, grade 3 and 4 deep vein thromboses were observed only among patients randomized to treatment arm B (3 patients; 27.2%). A higher rate of metabolic abnormalities such as hypomagnesemia was seen among patients treated with both cetuximab and bevacizumab (Arm A).
Table 2.
Toxicity by grade and treatment arm
| Toxicity | All patients N (%) (n=32) | Arm A N (%) (n=12) | Arm B N (%) (n=11) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 | |
| Hypersensitivity reaction | 4 (17.3) | 1 (4.3) | – | 3 (25) | 1 (8.3) | – | 1 (9.1) | – | – |
| Hematologic | |||||||||
| Neutropenia | 8 (34.7) | 1 (4.3) | – | 2 (16.7) | 1 (8.3) | – | 6 (54.5) | – | – |
| Infection with neutropenia | 1 (4.3) | – | – | – | – | – | 1 (9.1) | – | – |
| Infection without neutropenia | 7 (30.4) | 2 (8.7) | – | 3 (25) | 1 (8.3) | – | 4 (36.3) | 1 (9.1) | – |
| Thrombocytopenia | 8 (34.7) | 1 (4.3) | – | 2 (16.7) | – | – | 6 (54.5) | 1(9.1) | – |
| Vascular | |||||||||
| Hemorrhage | 10 (43.5) | – | – | 6 (50.0) | – | – | 4 (36.4) | – | – |
| DVT | – | 2 (8.7) | 1 (4.3) | – | – | – | – | 2 (18.1) | 1 (9.1) |
| Hypertension | 8 (34.8) | – | – | 6 (50.0) | – | – | 2 (18.2) | – | – |
| Constitutional symptoms | |||||||||
| Edema | 3 (13.0) | – | – | 2 (16.7) | – | – | 1 (9.1) | – | – |
| Fatigue | 22 (95.6) | – | – | 11 (91.6) | – | – | 11 (100) | – | – |
| Pain | 16 (69.5) | 2 (8.7) | – | 8 (66.6) | 1 (8.3) | – | 8 (72.7) | 1 (9.1) | – |
| Fever | 6 (26.0) | – | – | 2 (16.7) | – | – | 4 (36.3) | – | – |
| Skin changes | |||||||||
| Alopecia | 1 (4.3) | – | – | – | – | – | 1 (9.1) | – | – |
| Dry/cracked | 7 (30.4) | – | – | 3 (25) | – | – | 4 (36.3) | – | – |
| Fissures | 9 (39.1) | 1 (4.3) | – | 7 (58.3) | – | – | 2 (18.1) | 1 (9.1) | – |
| Nail changes | 4 (17.3) | 1 (4.3) | – | 2 (16.7) | – | – | 2 (18.1) | 1 (9.1) | – |
| Paronychia | 11 (47.8) | 1 (4.3) | – | 5 (41.6) | – | – | 6 (54.5) | 1 (9.1) | – |
| Rash | 17 (73.9) | 5 (21.7) | – | 9 (75) | 2 (16.7) | – | 8 (72.7) | 3 (27.2) | – |
| Hand-foot syndrome | 5 (21.7) | – | – | 1 (8.3) | – | – | 4 (36.4) | – | – |
| Gastrointestinal | |||||||||
| Constipation | 11 (47.8) | 2 (8.7) | – | 5 (41.7) | 2 (16.7) | – | 6 (54.5) | – | – |
| Diarrhea | 12 (52.1) | 8 (34.8) | – | 5 (41.6) | 4 (33.3) | – | 7 (63.6) | 4 (36.3) | – |
| Nausea | 16 (69.5) | – | – | 9 (75) | – | – | 7 (63.6) | – | – |
| Vomiting | 7 (30.4) | – | – | 5 (41.6) | – | – | 2 (18.1) | – | – |
| Metabolic | |||||||||
| Weight loss | 5 (21.7) | 1 (4.3) | – | 3 (25) | – | – | 2 (18.1) | 1 (9.1) | – |
| Hypomagnesemia | 8 (34.7) | 3 (13.0) | 1 (4.3) | 4 (33.3) | 1 (8.3) | 1 (8.3) | 4 (36.3) | 2 (18.1) | – |
| Hyperglycemia | 14 (60.8) | – | – | 6 (50.0) | – | – | 8 (72.2) | – | – |
| Hypoalbuminemia | 7 (30.4) | – | – | 2 (16.7) | – | – | 5 (45.5) | – | – |
| Hypokalemia | 3 (13.0) | 2 (8.7) | – | 1 (8.3) | – | – | 2 (18.1) | 1 (9.1) | – |
| Neurologic | |||||||||
| Parasthesias | 17 (73.9) | – | – | 8 (66.6) | – | – | 8 (72.2) | – | – |
| Parasthesias/pain | 2 (8.7) | – | – | 1 (8.3) | – | – | 1 (9.1) | – | – |
| Sensory neuropathy | 11 (47.8) | – | – | 5 (41.6) | – | – | 6 (54.5) | – | – |
| Insomnia | 6 (26.0) | – | – | 3 (25) | – | – | 3 (27.2) | – | – |
Clinical Outcome
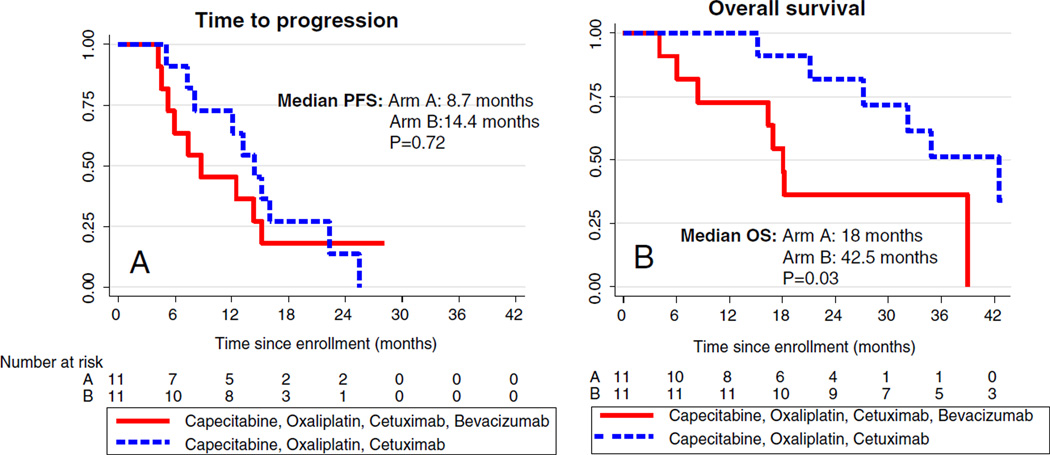
Twenty-two of 23 patients were evaluable for response (one patient discontinued after experiencing a hypersensitivity reaction to cetuximab during the first treatment) (Table 3). The overall RECIST confirmed response rate was 54% (36.4% in Arm A and 72.7% in Arm B). The median time to progression was 8.7 months for Arm A and 14.4 months for Arm B (p=0.72) (Fig. 1a). The median survival was 18.0 months in Arm A and 42.5 months in Arm B (p=0.03) (Fig. 1b). Survival at 12 and 24 months was 72.7% and 36.36% for patients treated on Arm A and 100% and 81.82% for patients treated on Arm B.
Table 3.
RECIST confirmed responses by treatment arm
| All enrolled | Arm A | Arm B | ||||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |
| Complete response | 2 | 9 | 1 | 9 | 1 | 9 |
| Partial response | 10 | 45 | 3 | 27 | 7 | 64 |
| Stable disease | 10 | 45 | 7 | 64 | 3 | 27 |
| Overall responsea | 12 | 55 | 4 | 36 | 8 | 73 |
Overall response = complete and partial response
Fig. 1.
Kaplan–Meyer curves of time to progression (a) and overall survival (b) by treatment arm. Patients randomized to arm A received treatment with capecitabine, oxaliplatin, cetuximab, and bevacizumab. Patients randomized to arm B were treated with the same regimen without bevacizumab
Correlative Studies
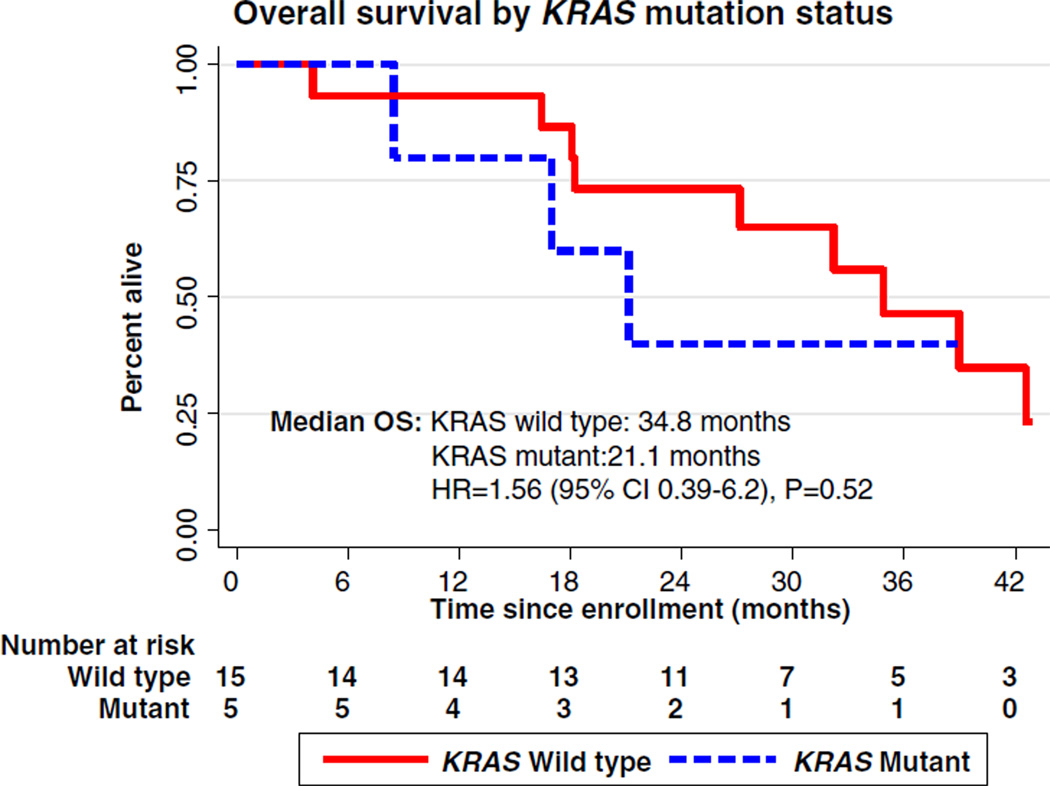
Tumor samples for correlative studies were available for 20 of the patients enrolled in this trial. KRAS mutations were found in 5 (25%) patients, evenly distributed between the two arms (Table 1). Median overall survival for KRAS wild-type patients was 34.8 months compared with 21.1 months among KRAS mutant patients (HR = 1.56; CI: 0.39–6.20) (p=0.52) (Fig. 2). Survival at 12 and 24 months was 93% and 73%, respectively, for KRAS wild-type patients versus 80% and 40%, respectively, for KRAS mutant patients. Additional analysis of the affect of KRAS mutation status by treatment arm was deferred due to the small sample size.
Fig. 2.
Overall survival by KRAS mutation status. Kaplan–Meier curve of overall survival of KRAS mutant and KRAS wild-type patients. HR=1.567; 95% CI: 0.396–6.20
Discussion
Despite the early termination of our study due to emerging data [19, 20], several conclusions can be drawn from this randomized phase II study. Although not designed to serve as a direct comparison between arms, the dual antibody containing arm (A) appeared to be inferior to the non-bevacizumab containing arm (B). The PACCE and CAIRO-2 studies similarly demonstrated decreased survival among patients treated with the combination of VEGF and EGFR inhibition. The multicenter PACCE study evaluated the combination of chemotherapy (FOLFOX or FOLFIRI) plus bevacizumab with or without panitumimab and demonstrated inferior OS in the dual antibody arm (19.4 vs 24.5 months for oxaliplatin-based therapy; HR, 1.43;95% CI, 1.11 to 1.83) [20]. The CAIRO2 trial studied the combination of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab and also noted inferior OS for the dual antibody arm (19.4 versus 20.3 months) [19]. Our study differed from the aforementioned studies in that the single antibody arm contained the anti-EGFR antibody cetuximab rather than a VEGF inhibitor. However, the results are quite similar. In addition, a recently reported single institution phase II study of the combination of capecitabine, oxaliplatin, cetuximab, and bevacizumab reported an overall response rate of 43% and a median overall survival of 18.8 months. These results are worse than would be expected with chemotherapy and single antibody treatment, with increased toxicity [21].
Despite the small sample size, patient characteristics were well balanced between the two treatment arms, with the exception of an increased number of patients with ECOG performance status 0 in arm B. We think that this is unlikely to explain the large difference in outcome between the arms. In an updated analysis of Intergroup trial N9741 which compared several frontline chemotherapy regimens, patients with a PS 0 had only a trend toward improved outcome compared to PS 1 patients [22]. Toxicity is also unlikely to explain the difference in clinical outcome. The most notable difference in toxicity between the two arms in our study was an increased incidence of hand-foot syndrome among patients enrolled on the single antibody treatment arm. This group was treated for a longer period of time (111 cycles versus 99), and thus, longer exposure to capecitabine may explain this finding. The addition of bevacizumab to cetuximab resulted in the expected increased rates of bleeding and hypertension. These observations are in line with the PACCE and CAIRO-2 studies which demonstrated increased rates of grade 3 and 4 toxicities in the dual antibody arms [19, 20]. The higher rate of adverse events was not thought to be the sole explanation for the decreased efficacy of the dual antibody arms in the large studies reported, as all events were manageable and were not a cause for patient withdrawal from the study [23]. This was similarly noted in our trial, with the most frequent adverse events being of grade 1 or 2 in severity.
Differences in KRAS status are also unlikely to explain clinical outcome differences. In both PACCE and CAIRO-2, differences in KRAS mutation status did not provide an explanation for the decreased response to the dual antibody approach. Approximately 40% of patients were found to have KRAS mutations in both studies. Inferior TTP and OS were documented with the addition of cetuximab even among the sub-population of patients with wild-type KRAS tumors, who would be expected to be responsive to EGFR targeted therapy [19, 20]. Although our study was designed prior to the discovery of the predictive nature of KRAS mutational status on response to EGFR targeted therapy, no difference in the incidence of KRAS mutations was noted between the two arms, and thus, it cannot explain the difference in patient outcomes.
The exact mechanism behind the inferior outcome with dual antibody therapy remains elusive despite extensive analysis of the two large randomized clinical trials [23, 24]. Downstream target alteration by one antibody which reduces the activity of the other antibody may account for this negative interaction. Alternatively, pharmacodynamic interactions between the two drugs may contribute to this effect [24]. The relationship between hypoxia and efficacy of cetuximab therapy has also been suggested as a possible explanation. Preclinical studies have validated bevacizumab as an inducer of a hypoxic tumor environment and upregulation of hypoxia induced factor-1 alpha (HIF-1α) [25]. Studies have also shown that down regulation of HIF-1α is required for maximal anti-tumor activity of cetuximab [26]. Recently, the group led by Zeng demonstrated activation of KRAS in hypoxic colorectal cancer cells which resulted in inhibition of apoptosis and stimulation of angiogenesis [27]. Based on these observations, treatment with bevacizumab may potentially result in hypoxia with KRAS activation and thus counteract the intended therapeutic effect of cetuximab. Further study to elucidate the mechanism of this negative interaction is clearly warranted.
Conclusion
In summary, although prematurely terminated due to emerging data, the current randomized phase II study supports the inferior clinical outcome of metastatic colorectal cancer patients receiving dual EGFR and VEGF inhibition. Dual antibody therapy should thus not be routinely undertaken in the treatment of metastatic colorectal cancer.
Acknowledgments
Supported by grant number P30 CA006927 from the National Cancer Institute.
Additional financial support for the study was provided by Bristol-Myers Squibb and Roche.
National clinical trial number: NCT00321100
Footnotes
Conflict of Interest Dr. Barbara Burtness receives research funding from Genentech.
Dr. Steven Cohen receives research funding from Genentech and BMS.
All other authors state that they have no relevant conflicts of interest.
Contributor Information
Efrat Dotan, Email: efrat.dotan@fccc.edu, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Neal J. Meropol, University Hospitals Seidman Cancer Center, Case Western Reserve University, Cleveland, OH, USA
Barbara Burtness, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Crystal S. Denlinger, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA
James Lee, Hematology Oncology Associates, SJ, Mt. Holly, NJ, USA.
David Mintzer, Pennsylvania Oncology Hematology Associates, Philadelphia, PA, USA.
Fang Zhu, Department of Biostatistics and Bioinformatics, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Karen Ruth, Department of Biostatistics and Bioinformatics, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Holly Tuttle, Extramural Research Program, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Judi Sylvester, Extramural Research Program, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Steven J. Cohen, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA
References
- 1.Goldberg RM, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19(21):4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 4.Hoff PM, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19(8):2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy J, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006;33(5) Suppl 10:S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Giantonio BJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 11.Tabernero J, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25(33):5225–5232. doi: 10.1200/JCO.2007.13.2183. [DOI] [PubMed] [Google Scholar]
- 12.Bokemeyer C, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 13.Douillard JY, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOL-FOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, et al. A meta-analysis of the CRYSTAL and OPUS studies combining cetuximab with chemotherapy (CT) as 1st-line treatment for patients (pts) with metastatic colorectal cancer (mCRC): results according to KRAS and BRAF mutation status. Eur J Cancer Suppl. 2009;7(2):345. Abstract # P-6077. [Google Scholar]
- 15.Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 16.Ciardiello F, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6(9):3739–3747. [PubMed] [Google Scholar]
- 17.Saltz LB, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25(29):4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Tol J, et al. Chemotherapy, bevacizumab, and cetuximab in meta-static colorectal cancer. N Engl J Med. 2009;360(6):563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 20.Hecht JR, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 21.Wong NS, et al. A phase II study of capecitabine, oxaliplatin, bevacizumab and cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res. 2011;31(1):255–261. [PMC free article] [PubMed] [Google Scholar]
- 22.Sanoff HK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26(35):5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punt CJ, Tol J. More is less—combining targeted therapies in metastatic colorectal cancer. Nat Rev Clin Oncol. 2009;6(12):731–733. doi: 10.1038/nrclinonc.2009.168. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JL. Vascular endothelial growth factor plus epidermal growth factor receptor dual targeted therapy in metastatic colorectal cancer: synergy or antagonism? J Oncol. 2009;2009:937305. doi: 10.1155/2009/937305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvakumaran M, et al. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem Pharmacol. 2008;75(3):627–638. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, et al. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol Cancer Ther. 2008;7(5):1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- 27.Zeng M, et al. Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells. PLoS One. 2010;5(6):e10966. doi: 10.1371/journal.pone.0010966. [DOI] [PMC free article] [PubMed] [Google Scholar]