Abstract
Several long-term temporal analyses of the structure of Robertsonian (Rb) hybrid zones in the western house mouse, Mus musculus domesticus, have been performed. Nevertheless, the detection of gradual or very rapid variations in a zone may be overlooked when the time elapsed between periods of study is too long. The Barcelona chromosomal polymorphism zone of the house mouse covers about 5000, km2 around the city of Barcelona and is surrounded by 40 chromosome telocentric populations. Seven different metacentrics and mice with diploid numbers between 27 and 40 chromosomes and several fusions in heterozygous state (from one to seven) have been reported. We compare the present (period 2008–2010) and past (period 1996–2000) structure of this zone before examining its dynamics in more detail. Results indicate that there is not a Rb race in this area, which is consistent with the proposal that this zone was probably originated in situ, under a primary intergradation scenario. The lack of individuals with more than five metacentrics in heterozygous state in the current period suggests that selection acted against such mice. By contrast, this situation did not occur for mice with fewer than five fusions in heterozygous condition. Changes in human activity may affect the dynamics of gene flow between subpopulations, thus altering the chromosomal composition of certain sites. Although these local variations may have modified the clinal trend for certain metacentrics, the general staggered structure of the zone has not varied significantly in a decade.
Keywords: chromosomal polymorphism zone, gene flow, Mus musculus domesticus, spatio-temporal analysis, Robertsonian (Rb) fusion
Introduction
Chromosomal hybrid zones are regions in which differentiated chromosomal populations come into contact, reproduce and give rise to descendants with mixed karyotypes (Barton and Hewitt, 1985). These zones are of particular interest because they constitute natural scenarios in which to investigate the mechanisms involved in chromosomal speciation. Classical evolutionary models consider hybrid sterility as the main factor involved in these processes, as individuals that are heterozygous for chromosomal rearrangements are partially or totally infertile, due either to segregation problems or to their recombinant products that generate unbalanced gametes. However, a different point of view has been suggested by recent theoretical models, which assume that the reduction of recombination between chromosomes, that are carriers of different rearrangements, is the decisive factor in speciation (see, for example, review in Faria and Navarro, 2010).
The western house mouse, Mus musculus domesticus, is a good model to study these evolutionary mechanisms in mammals because of the structural predisposition of its chromosomes to originate Robertsonian (Rb) translocations, centromeric fusions of pairs of acrocentric or telocentric chromosomes to form metacentrics (Gropp and Winking, 1981). Although the species has a standard (St) karyotype of 40 telocentric chromosomes, individuals with 22 to 39 chromosomes have been detected in several natural populations (see, for example, Piálek et al., 2005). After the arrival of M. musculus domesticus in eastern Europe, about 3000 years ago (Cucchi et al., 2005), numerous chromosomal races (contiguous populations that share the same chromosomal composition in the homozygous state; Hausser et al., 1994), or metacentric populations (geographical groupings characterized by the same set of fusions in a fixed or nearly fixed state; Piálek et al., 2005) were described in areas ranging from the Orkney islands (Scotland) to the north of Africa and the Middle East (Adolph and Klein, 1981; Gazave et al., 2003; Piálek et al., 2005; Gündüz et al., 2010).
Contact zones between two races are an interesting scenario for studying evolutionary dynamics in chromosomal rearrangements in a hybrid zone. Although some authors suggest that, theoretically, these zones are ephemeral and unstable (Harrison, 1990), numerous hybrid areas persist spatially and temporally in dynamic equilibrium as tension zones between selection against hybrids and migration (Endler, 1977; Barton and Hewitt, 1985; Barton and Gale, 1993). Thus, spatio-temporal analyses are essential to detect possible time-scale movements that could alter evolutionary outcomes inside the contact zone (Buggs, 2007) and to provide insight into spatial structure and dispersal patterns, low hybrid fitness and selection against those hybrids (Barton and Gale, 1993).
When there is contact between two chromosomal races, differing in only a few metacentrics, gene flow can occur without restrictions (Wallace et al., 2002). By contrast, the accumulation of numerous Rb translocations is related to hybrid hypofertility or sterility as a result of alterations in meiotic processes (Wallace et al., 1992; Castiglia and Capanna, 2000). Therefore, high heterozygosity for rearrangements is expected to reduce the fitness of hybrid mice, thus limiting the gene flow between races (Hauffe and Searle, 1998; Nunes et al., 2011).
A Rb polymorphism zone of the western house mouse is found in the vicinity of Barcelona (NE Spain). It spreads over 5000, km2 and is surrounded by standard populations (Gündüz et al., 2001; Sans-Fuentes et al., 2007). Diploid numbers (2n) range between 27 and 39 chromosomes, with the lowest 2n values observed at sites located about 30 km west of the city of Barcelona. This area was first described by Adolph and Klein (1981), and studied more extensively by Gündüz et al. (2001) and Sans-Fuentes et al. (2007). Seven different metacentrics, Rb(3.8), (4.14), (5.15), (6.10), (7.17), (9.11) and (12.13), with a staggered clinal pattern have been reported. However, no parental Rb race, as defined by Hausser et al. (1994), has been found. Therefore, Sans-Fuentes, (2004) proposed that this Rb area should be considered as a polymorphism zone rather than a typical chromosomal hybrid zone. Hence, we can observe an exceptional evolutionary scenario in which Rb individuals not belonging to a chromosomal race, make contact and disperse throughout a region bounded by St mice. This unusual metacentric pattern has not been reported in other Rb contact zones, and our knowledge of the area of Barcelona is relatively limited. Morphological (Muñoz-Muñoz et al., 2003, 2006, 2011; Sans-Fuentes et al., 2009), ethological (Sans-Fuentes et al., 2005) and reproductive (Sans-Fuentes et al., 2010) studies have revealed differences between St and Rb animals in the area. Nonetheless, dynamics and temporary changes of structure have not been described.
Several long-term spatio-temporal studies of Rb hybrid zones have been reported (Hauffe and Searle, 1993; Castiglia and Capanna, 1999; Faria and Navarro, 2010). However, the detection of gradual variations or rapid changes in a zone may be overlooked when the time scale is too long. Hence, we carry out mid-term analysis focused on the detection of possible structural variations of the polymorphism area of Barcelona over the past 13 years. This period, in a temperate Mediterranean region, corresponds to nearly 50 generations of mice because of their reproductive characteristics (Castiglia and Capanna, 1999). Thus, we assume that this is time enough to reveal detailed variations, which will allow us to obtain a more accurate vision of the dynamics of the polymorphism area at longer time scales.
Taking all this into account, our research has the following aims: (i) to report information on the past and present structure of the zone regarding the chromosomal composition by site; (ii) to determine local variations, if any, in the chromosomal characteristics and gene diversity over one decade; and (iii) to compare the past and present geographic distribution of each metacentric.
Materials and methods
New data
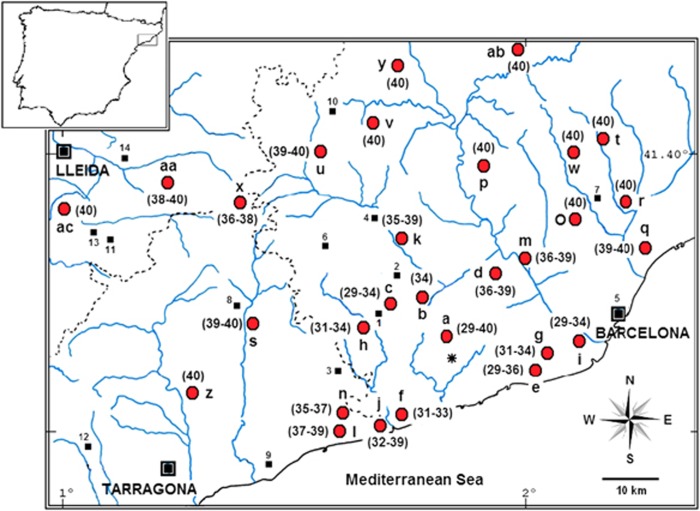
A total of 304 wild mice were live-trapped between 2008 and 2010 in commensal habitats from 29 villages in the Rb polymorphism zone of Barcelona (Figure 1). Karyotypes were determined from bone marrow plates (Ford, 1966) and stained using Wright Staining for G bands (Mandahl, 1992). Chromosomes were identified under optic microscope according to the Committee on Standardized Genetic Nomenclature for Mice (1972).
Figure 1.
Localities studied from the polymorphism zone of Barcelona (see details in Table 1). Range of diploid numbers (2n) found for each site are shown in parenthesis. Sites sampled in 2008–2010 period and those from 1996–2000 survey, used for temporal comparisons, are labelled with a letter. Numbered labels indicate farms sampled in 1996–2000 period, which are deserted in 2008–2010. Asterisk indicates the polymorphism centre of the area.
Mean and range of diploid number per site, and metacentric frequencies per site and fusion were calculated. We tested for deviations from Hardy–Weinberg equilibrium using Fisher's method for each locus in each population (Raymond and Rousset, 1995).
The heterozygosity per site was determined by the mean number of heterozygous chromosomes per individual (H index), the mean number of alleles in heterozygosity per locus (A/l) at each site, and the percentage of mice with one to five metacentrics in heterozygous state throughout the polymorphism zone. The population characteristics of chromosomal heterozygosity per fusion and among all fusions were estimated by Wright's F-statistics (FIS, FST and FIT) by the method described in Weir and Cockerham (1984). Endogamy coefficient (FIS) ranges from −1 to 1 and is a measure of deviation between genotypic and panmictic frequencies, in terms of excess or deficiency of heterozygotes caused by the overcrossing between related individuals. The standardized variance of allele frequency (FST) ranges from 0 to 1 and evaluates the effect of the genetic subdivision in terms of heterozygous reduction in a population. Total consanguinity coefficient (FIT) ranges from −1 to 1 and is associated with the inbreeding degree of individuals related to the whole population. A null distribution of FST was generated from 5000 permutations. The observed value was considered significant when it exceeded the 95th percentile of the simulated distribution.
Mean value and s.e. of these parameters were estimated by the Jackknife method (Weir, 1990). A global score (U) test among populations for each fusion was performed to quantify the departure of heterozygote deficiency related to the H–W expectations (Rousset and Raymond, 1995).
GENEPOP software version 4.0 (Raymond and Rousset, 1995) was used to obtain metacentric frequencies, H–W equilibrium and global score (U) tests. Wright's F-statistics (estimated values, significance by permutations and jackknifing) were computed with the GENETIX software version 3.3 (Belkhir et al., 1996).
Temporal analyses
Data reported by Gündüz et al. (2001) and Sans-Fuentes (2004), corresponding to the structure of the Rb polymorphism zone of Barcelona during the period 1996–2000, were pooled in order to compare: (i) the chromosomal characteristics by site (frequencies, diploid numbers and heterozygosity); (ii) the population subdivision among fusions (F-statistics); and (iii) the clinal pattern for each fusion.
Sites with Rb mice were selected for both temporal samples to contrast: first, the mean and range of diploid numbers, the H index, the proportion of individuals with one to five metacentrics in heterozygous condition and the mean A/l for each site; and second, the F-statistics (FIS, FST, FIT) for each metacentric. In order to test temporal differences in the frequency per fusion and site, a gene variation exact test (G test) was computed for the same polymorphic sites sampled for both periods (Garraf, Gavà, Vilanova i la Geltrú, Viladecans, La Granada, Sant Martí Sarroca, Sant Sadurní d'Anoia, Calafell, Bellaterra, Les Pobles and Santa Coloma de Queralt) by means of GENEPOP software version 4.0 (Raymond and Rousset, 1995).
As mice from villages lying to the north of Barcelona presented St or close to St karyotype, only the sites from the west of the distribution area were used to perform the clinal comparisons between 1996–2000 and 2008–2010 periods (following the criterion described in Gündüz et al., 2001). The centre of the polymorphism area was calculated as the mean point between localities with the highest level of polymorphism (Garraf, Viladecans, La Granada, El Prat de Llobregat, Gavà and Sant Martí Sarroca). A logistic function (f(x)=es(x−c)/1+es(x−c)) was calculated to fit the metacentric clines, where x is the geographic distance from the polymorphism centre, and s and c the maximum slope and the centre of the cline, respectively. The width (w) was calculated as the absolute value of 4 divided by the maximum slope (s) according to Endler (1977). The software package C-Fit 6 (devised by T Lenormand) was used to obtain the parameters c and s and to compute those corresponding maximum likelihood values for each pair of clines using the metropolis algorithm (Szymura and Barton, 1986). To examine differences between clines, it was assumed that twice the difference in loge likelihood values between constrained and unconstrained model (on cline centre for coincidence and on cline slope for concordance) follows a χ2 distribution with df equal to the difference of the parameters estimated (Fel-Clair et al., 1996). We used Bonferroni corrections to account for concordance and coincidence multiple testing (described in Sokal and Rohlf, 1995). Individuals with incomplete karyotype information and sites with <4 individuals were excluded from all the computed analyses.
Results
New data
The chromosomal characteristics of mice from the polymorphism zone of Barcelona corresponding to the period 2008–2010 are summarized in Table 1 and Figure 1. The seven Rb metacentrics described to date [Rb(3.8), (4.14), (5.15), (6.10), (7.17), (9.11) and (12.13)] were detected throughout the area studied. Diploid numbers ranged from 29 to 40 chromosomes and no individual with the seven metacentrics in the homozygous state (2n=26 chromosomes) was found. A total of 106 different karyotypes were observed. The lowest mean diploid numbers were detected in animals from La Granada (2n=31.56), Gavà (2n=30.50), Viladecans (2n=31.66) and El Prat de Llobregat (2n=31.79). The range of 2n was broad at sites located near the centre of the polymorphism area (Garraf: 2n=29–40; Gavà: 2n=29–36; Vilanova i la Geltrú: 2n=31–38). Rb(4.14) and (12.13) were the most widely distributed throughout the zone with a frequency of 0.52 and 0.51, respectively. Rb(9.11) and (5.15) also showed relatively high frequencies (0.49 and 0.41), whereas Rb(6.10), (3.8) and, particularly, Rb(7.17) had a more restricted distribution (frequencies: 0.20, 0.12 and 0.01, respectively). Rb(7.17) was detected at low frequencies and only in Garraf (0.08) and Sant Martí Sarroca (0.11). No significant deviations from Hardy–Weinberg equilibrium were found (P>0.05 for all sites and fusions). This result was supported by the FIS estimator, close to 0 for all Rbs (Table 2).
Table 1. Sites and chromosomal characteristics of mice from the polymorphism area of Barcelona corresponding to the periods 2008–2010 and 1996–2000 (see also Figure 1).
| Sitea | km | Nb | Mean 2n | Range 2n | Hc |
Frequencies of metacentric chromosomes |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.8 | 4.14 | 5.15 | 6.10 | 7.17 | 9.11 | 12.13 | ||||||
| 2008–2010 period | ||||||||||||
| a. Garraf | 3.5 | 13 | 32.76 | 29–40 | 2.61 | 0.51 | 0.73 | 0.42 | 0.35 | 0.08 | 0.74 | 0.78 |
| b. Sant Pau d'Ordal | 8.4 | 1 | 34.00 | 34 | 4 | 0 | 0.50 | 0.50 | 1 | 0 | 0.50 | 0.50 |
| c. La Granada | 12.4 | 16 | 31.56 | 29–34 | 2.56 | 0.46 | 0.87 | 0.81 | 0.44 | 0 | 0.81 | 0.81 |
| d. Corbera de Llobregat | 12.8 | 8 | 37.00 | 36–39 | 1.25 | 0 | 0.62 | 0.13 | 0 | 0 | 0.44 | 0.31 |
| e. Gavà | 13.1 | 13(1) | 30.50 | 29–36 | 1.46 | 0.31 | 0.88 | 0.88 | 0.73 | 0 | 0.96 | 0.96 |
| f. Vilanova i la Geltrú | 14.2 | 6 | 33.66 | 31–38 | 1.33 | 0.16 | 0.75 | 0.83 | 0 | 0 | 0.67 | 0.75 |
| g. Viladecans | 14.3 | 9 | 31.66 | 30–34 | 2.55 | 0.33 | 0.72 | 0.78 | 0.72 | 0 | 0.89 | 0.72 |
| h. Sant Martí Sarroca | 16.4 | 9 | 32.77 | 31–34 | 1 | 0 | 1 | 0.67 | 0 | 0.11 | 0.94 | 0.89 |
| i. El Prat de Llobregat | 19.5 | 27 | 31.79 | 29–34 | 2.03 | 0.13 | 0.70 | 0.91 | 0.65 | 0 | 0.81 | 0.91 |
| j. Cubelles | 19.6 | 14(3) | 34.85 | 32–39 | 2.28 | 0.04 | 0.42 | 0.64 | 0.18 | 0 | 0.68 | 0.61 |
| k. Sant Sadurní d'Anoia | 19.9 | 19(2) | 37.21 | 35–39 | 1.42 | 0 | 0.61 | 0 | 0 | 0 | 0.37 | 0.42 |
| l. Calafell | 20.0 | 19 | 37.76 | 37–39 | 1.84 | 0.07 | 0.28 | 0 | 0.08 | 0 | 0.16 | 0.53 |
| m. El Papiol | 20.1 | 8 | 37.62 | 36–39 | 0.87 | 0 | 0.13 | 0.25 | 0 | 0 | 0.69 | 0.06 |
| n. Bellvei | 24.4 | 6 | 36.16 | 35–37 | 1.83 | 0 | 0.501 | 0.16 | 0.25 | 0 | 0.42 | 0.58 |
| o. Bellaterra | 28.1 | 7 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p. Vacarisses | 31.6 | 7 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| q. Badalona | 36.0 | 11(2) | 39.84 | 39–40 | 0.15 | |||||||
| r. Sta. Perpètua de la Mogoda | 36.8 | 10 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| s. Les Pobles | 37.4 | 13(2) | 39.83 | 39–40 | 0.15 | 0 | 0 | 0.08 | 0 | 0 | 0 | 0 |
| t. Castellar del Vallès | 37.7 | 8 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| u. Jorba | 39.4 | 3 | 39.66 | 39–40 | 0.33 | 0 | 0.16 | 0 | 0 | 0 | 0 | 0 |
| v. Castellfollit del Boix | 39.9 | 14 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| w. Caldes de Montbuí | 42.8 | 2 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| x. Santa Coloma de Queralt | 45.1 | 7 | 36.86 | 36–38 | 1.71 | 0 | 0.71 | 0.50 | 0 | 0 | 0 | 0.36 |
| y. Fals | 48.2 | 3(2) | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| z. Nulles | 49.6 | 7 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| aa. Ametlla de Segarra | 57.7 | 9 | 39.44 | 38–40 | 0.33 | 0 | 0.27 | 0 | 0 | 0 | 0 | 0 |
| ab. Olost | 75.7 | 16(1) | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ac. Arbeca | 81.1 | 6 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1996–2000 period | ||||||||||||
| a. Garraf | 3.5 | 34 | 30.22 | 28–35 | 1.82 | 0.69 | 0.86 | 0.77 | 0.72 | 0.07 | 0.97 | 0.79 |
| 1. Avinyonet del Penedès | 7.4 | 4 | 31.20 | 30–33 | 0.25 | 0 | 1 | 0.88 | 0.50 | 0 | 1 | 1 |
| b. Sant Pau d'Ordal | 8.7 | 55 | 30.15 | 28–34 | 1.22 | 0.44 | 0.95 | 1 | 0.69 | 0 | 0.90 | 0.93 |
| 2. Lavern | 10.0 | 24 | 32.12 | 31–33 | 1.46 | 0 | 0.79 | 0.81 | 0.37 | 0 | 1 | 0.95 |
| c. La Granada | 12.4 | 67 | 30.79 | 27–35 | 1.79 | 0.44 | 0.98 | 0.89 | 0.32 | 0.14 | 0.84 | 0.89 |
| e. Gavà | 13.1 | 9 | 30.22 | 29–32 | 1.33 | 0.55 | 1 | 1 | 0.38 | 0 | 1 | 0.94 |
| f. Vilanova i la Geltrú | 14.2 | 36 | 31.89 | 30–34 | 1.33 | 0.24 | 0.90 | 0.82 | 0.12 | 0.04 | 0.93 | 0.94 |
| g. Viladecans | 14.3 | 4 | 30.20 | 29–31 | 0.75 | 0.12 | 1 | 0.75 | 1 | 0 | 1 | 1 |
| h. Sant Martí Sarroca | 16.4 | 4 | 32.50 | 32–33 | 0.50 | 0 | 1 | 1 | 0 | 0 | 0.75 | 1 |
| k. Sant Sadurní d'Anoia | 19.9 | 7 | 34.70 | 33–37 | 1.57 | 0 | 0.71 | 0.64 | 0.07 | 0 | 0.57 | 0.50 |
| l. Calafell | 20.0 | 20 | 36 | 35–37 | 1.30 | 0 | 0.75 | 0.15 | 0 | 0.02 | 0.17 | 0.90 |
| 3. Llorenç del Penedès | 24.6 | 5 | 36.60 | 36–38 | 2.20 | 0 | 0.50 | 0.50 | 0 | 0 | 0.30 | 0.40 |
| 4. Vallbona d'Anoia | 25.1 | 6 | 39.33 | 37–40 | 0.20 | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 |
| 5. Barcelona | 27.8 | 13 | 34.07 | 32–36 | 1.46 | 0.15 | 0.31 | 0.53 | 0 | 0 | 1 | 0.96 |
| o. Bellaterra | 28.1 | 31 | 39 | 38–40 | 0.32 | 0 | 0.06 | 0.08 | 0 | 0 | 0 | 0.03 |
| 6. La Llacuna | 31.1 | 5 | 35.80 | 35–37 | 1.50 | 0 | 0.25 | 0.25 | 0 | 0 | 0.25 | 1 |
| 7. Sabadell | 33.2 | 9 | 38.55 | 38–39 | 0.56 | 0 | 0 | 0 | 0 | 0 | 0 | 0.72 |
| s. Les Pobles | 37.4 | 23 | 33.12 | 37–40 | 1.17 | 0.02 | 0.22 | 0.26 | 0 | 0 | 0 | 0.17 |
| 8. Les Ordes | 38.3 | 1 | 38 | 38 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 9. La Riera | 44.3 | 11 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| x. Santa Coloma de Queralt | 45.1 | 7 | 38.30 | 37–40 | 1.14 | 0 | 0.57 | 0.07 | 0 | 0 | 0 | 0.14 |
| 10. Calaf | 49.6 | 8 | 39.50 | 38–40 | 0.17 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 |
| 11. Fulleda | 70.4 | 22 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12. Les Borges del Camp | 71.2 | 5 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13. L'Espluga Calba | 73.0 | 9 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14. Anglesola | 73.6 | 5 | 39.60 | 39–40 | 0.40 | 0 | 0.20 | 0 | 0 | 0 | 0 | 0 |
Sites are ordered according to distance (km) of each site (a–ac, 1–14) from the centre of polymorphism area. Sites numbered from 1 to 14 correspond to farms departed or which no mice were trapped during 2008–2010 period.
N refers to numbers of mice for which the full karyotype (diploid number and metacentrics identification) has been determined. Numbers in parentheses refer to additional animals for which karyotype was not completely identified.
H is the mean number of fusions in heterozygous state per individual.
Table 2. F-statistics FIS, FST and FIT, estimated according to Weir and Cockerham (1984), and Mean F-statistics and s.e., obtained by jackknifing bootstrap, for each and for overall loci in 2008–2010 and 1996–2000 periods.
| Rb | FIS | Mean FIS | s.e. | FST | Mean FST | s.e. | FIT | Mean FIT | s.e. |
|---|---|---|---|---|---|---|---|---|---|
| 2008–2010 | |||||||||
| 3.8 | −0.0544 | −0.0562 | 0.0227 | 0.2345* | 0.2369 | 0.0638 | 0.1929* | 0.1942 | 0.0716 |
| 4.14 | 0.0078 | −0.0009 | 0.0755 | 0.2789* | 0.2783 | 0.0968 | 0.2845* | 0.2787 | 0.1162 |
| 5.15 | 0.0749 | 0.0697 | 0.0759 | 0.5372* | 0.5506 | 0.1023 | 0.5719* | 0.5809 | 0.0961 |
| 6.1 | −0.1186 | −0.1249 | 0.0519 | 0.4063* | 0.4095 | 0.0746 | 0.3358* | 0.3375 | 0.1013 |
| 7.17 | −0.0594 | −0.0594 | 0.0288 | 0.0523 | 0.0472 | 0.0287 | 0.0040 | −0.0103 | 0.008 |
| 9.11 | 0.0770 | 0.0788 | 0.0787 | 0.4377* | 0.4369 | 0.0959 | 0.4810* | 0.4795 | 0.0891 |
| 12.13 | −0.0315 | −0.0361 | 0.1096 | 0.3909* | 0.3919 | 0.1143 | 0.3717* | 0.3712 | 0.1388 |
| All | −0.0034 | −0.0025 | 0.0283 | 0.3925* | 0.3946 | 0.0438 | 0.3904* | 0.3936 | 0.0539 |
| 1996–2000 | |||||||||
| 3.8 | 0.0104 | 0.0121 | 0.0512 | 0.2999* | 0.2799 | 0.1033 | 0.3072* | 0.2916 | 0.1274 |
| 4.14 | −0.0191 | 0.0202 | 0.0907 | 0.5633* | 0.5871 | 0.1351 | 0.5549* | 0.5834 | 0.1592 |
| 5.15 | 0.0499 | 0.0540 | 0.0715 | 0.5194* | 0.5276 | 0.1153 | 0.5434* | 0.5509 | 0.1053 |
| 6.1 | 0.0406 | 0.0460 | 0.0916 | 0.3860* | 0.3791 | 0.1115 | 0.4109* | 0.4104 | 0.1338 |
| 7.17 | −0.0289 | −0.1182 | 0.1933 | 0.0596 | 0.0831 | 0.0446 | 0.0325 | 0.0354 | 0.1466 |
| 9.11 | 0.1437 | 0.1809 | 0.1531 | 0.7041* | 0.7055 | 0.1298 | 0.7466* | 0.7499 | 0.1025 |
| 12.13 | −0.0659 | −0.0681 | 0.0572 | 0.5498* | 0.5859 | 0.1798 | 0.5202* | 0.5572 | 0.1926 |
| All | 0.0205 | 0.0206 | 0.0204 | 0.4937* | 0.4963 | 0.0595 | 0.5041* | 0.5713 | 0.0630 |
Abbreviations: FIS, endogamy coefficient; FIT, total consanguinity coefficient; FST, standardized variance of allele frequency.
Asterisk indicates significant values at P<0.05.
Results demonstrated that mice with high structural heterozygosity were commonly present throughout the area studied. Only 20 out of the 205 individuals with metacentrics were homozygous for all fusions, whereas the rest were heterozygous for at least one fusion. The mean number of heterozygous metacentrics per individual (H; Table 1) and the mean number of alleles per locus (A/l; Table 3) showed that mice with the highest number of metacentrics in heterozygosity were located close to the polymorphism centre (Garraf: H=2.61, A/l=2.00; La Granada: H=2.56, A/l=1.86; Viladecans: H=2.55, A/l=1.86; El Prat de Llobregat: H=2.03, A/l=1.86, and Cubelles: H=2.28, A/l=1.86). Heterozygous mice for one (28.64%), two (30.81%), three (24.86%) or four (18.91%) fusions were common within the area, while those carrying five fusions in heterozygous state were scarce (1.85%). No individuals heterozygous for more than five metacentrics were found.
Table 3. Genetic diversity indexes for the Rb sites studied.
| Site | Hexp | Hobs | P(0.95)a | P(0.99)a | A/l |
|---|---|---|---|---|---|
| 2008–2010 period | |||||
| a. Garraf | 0.3838 | 0.3736 | 1 | 1 | 2 |
| c. La Granada | 0.3033 | 0.3661 | 0.8571 | 0.8571 | 1.8571 |
| d. Corbera de Llobregat | 0.2299 | 0.1786 | 0.5714 | 0.5714 | 1.5714 |
| e. Gavà | 0.1965 | 0.2088 | 0.5714 | 0.5714 | 1.5714 |
| f. Vilanova i la Geltrú | 0.25 | 0.1905 | 0.7143 | 0.7143 | 1.7143 |
| g. Viladecans | 0.3131 | 0.3651 | 0.8571 | 0.8571 | 1.8571 |
| h. Sant Martí Sarroca | 0.1349 | 0.1746 | 0.7143 | 0.7143 | 1.7143 |
| i. El Prat de Llobregat | 0.2481 | 0.2857 | 0.8571 | 0.8571 | 1.8571 |
| j. Cubelles | 0.3178 | 0.3265 | 0.7143 | 0.8571 | 1.8571 |
| k. Sant Sadurní d'Anoia | 0.2044 | 0.203 | 0.4286 | 0.4286 | 1.4286 |
| l. Calafell | 0.2157 | 0.2707 | 0.7143 | 0.7143 | 1.7143 |
| m. El Papiol | 0.1629 | 0.0714 | 0.4286 | 0.4286 | 1.4286 |
| n. Bellvei | 0.3036 | 0.2619 | 0.7143 | 0.7143 | 1.7143 |
| s. Les Pobles | 0.0203 | 0.022 | 0.1429 | 0.1429 | 1.1429 |
| x. Santa Coloma de Queralt | 0.1953 | 0.2245 | 0.4286 | 0.4286 | 1.4286 |
| aa. Ametlla de Segarra | 0.0573 | 0.0476 | 0.1429 | 0.1429 | 1.1429 |
| 1996–2000 period | |||||
| a. Garraf | 0.2748 | 0.2647 | 0.8571 | 1 | 2 |
| b. Sant Pau d'Ordal | 0.1890 | 0.1740 | 0.5714 | 0.7143 | 1.7143 |
| c. La Granada | 0.2646 | 0.2559 | 0.8571 | 1 | 2 |
| e. Gavà | 0.1534 | 0.1905 | 0.4286 | 0.4286 | 1.4286 |
| f. Vilanova i la Geltrú | 0.2029 | 0.1931 | 1 | 1 | 2 |
| g. Viladecans | 0.0848 | 0.1071 | 0.2857 | 0.2857 | 1.2857 |
| h. Sant Martí Sarroca | 0.0536 | 0.0714 | 0.1429 | 0.1429 | 1.1429 |
| k. Sant Sadurní d'Anoia | 0.2843 | 0.2245 | 0.7143 | 0.7143 | 1.7143 |
| l. Calafell | 0.1639 | 0.1857 | 0.5714 | 0.7143 | 1.7143 |
| o. Bellaterra | 0.0473 | 0.0415 | 0.2857 | 0.4286 | 1.4286 |
| s. Les Pobles | 0.1508 | 0.1677 | 0.4286 | 0.5714 | 1.5714 |
| x. Santa Coloma de Queralt | 0.1254 | 0.1633 | 0.4286 | 0.4286 | 1.4286 |
| 1. Avinyonet del Penedès | 0.1027 | 0.0357 | 0.2857 | 0.2857 | 1.2857 |
| 2. Lavern | 0.1690 | 0.2083 | 0.4286 | 0.5714 | 1.5714 |
| 3. Llorenç del Penedès | 0.2714 | 0.3143 | 0.5714 | 0.5714 | 1.5714 |
| 4. Vallbona d'Anoia | 0.0457 | 0.0571 | 0.1429 | 0.1429 | 1.1429 |
| 5. Barcelona | 0.1796 | 0.2088 | 0.4286 | 0.5714 | 1.5714 |
| 7. Sabadell | 0.0536 | 0.0714 | 0.1429 | 0.1429 | 1.1429 |
| 10. Calaf | 0.0218 | 0.0238 | 0.1429 | 0.1429 | 1.1429 |
| 14. Anglesola | 0.0457 | 0.0571 | 0.1429 | 0.1429 | 1.1429 |
Abbreviations: A/l, Mean number of alleles in heterozygosity per locus in each site; Hexp and Hobs, expected and observed heterozygosity for all locus in each Rb population.
Polymorphism of each site for all locus at 95% or at 99% of confidence threshold.
Values of F-statistics are shown in Table 2. For overall Rbs, FST and FIT statistics were significant (all P<0.05) indicating a moderate deficit of heterozygotes among subpopulations and within the whole Rb population. Nevertheless, the U test for heterozygote deficiency did not show significant departures from equilibrium assumptions (P>0.05 for all sites and each fusion).
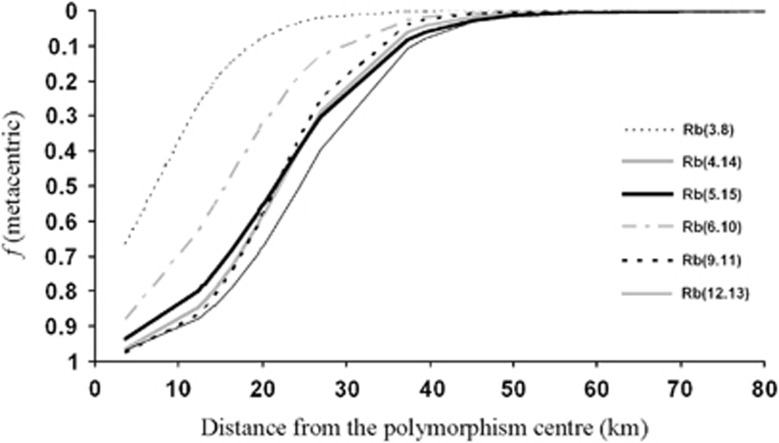
Despite the absence of a Rb race, chromosomal clines were defined from the region with the highest metacentric frequencies towards the periphery with St animals. The cline fitted for each fusion is shown in Figure 2. Rb(7.17) was not considered because of its limited geographic distribution. Polymorphic sites with the highest metacentric frequencies were mainly located along 25 km from the polymorphism centre. Further from the centre these frequencies diminished and, consequently, diploid numbers increased. Mice trapped at a distance of 30 km from the centre carried a moderate number of fusions, and those trapped more than 35 km away generally had standard karyotypes. Concordance likelihood tests did not reveal significant differences in distribution pattern among metacentrics, whereas Rb(3.8) and Rb(6.10) clines were significantly non-coincident (P<0.01), showing the centres of distribution displaced (Table 4).
Figure 2.
Metacentric clinal pattern fitted for the 2008–2010 period. The variation in frequencies across the western area of the polymorphism zone of Barcelona was represented for all fusions except Rb(7.17).
Table 4. Tests of concordance (above diagonal) and coincidence (below diagonal) of metacentric clines corresponding to the period 2008–2010.
| Metacentric | 3.8 | 4.14 | 5.15 | 6.10 | 9.11 | 12.13 | All |
|---|---|---|---|---|---|---|---|
| 3.8 | – | 0.15 | 1.31 | 0.38 | 0.06 | 0.61 | 2.17 |
| 4.14 | 119.48** | – | 0.76 | 0.08 | 0.56 | 0.19 | 1.65 |
| 5.15 | 84.81** | 0.17 | – | 0.23 | 2.66 | 0.19 | 0.02 |
| 6.10 | 19.98** | 37.48** | 25.69** | – | 0.92 | 0.01 | 0.51 |
| 9.11 | 141.39** | 0.11 | 0.019 | 37.76** | – | 1.46 | 5.16 |
| 12.13 | 147.43** | 4.19 | 5.28 | 64.15** | 6.26 | – | 0.55 |
| All | 160.74** | 27.07** | 15.01** | 7.13 | 28.36** | 58.03** | – |
These are the χ2 values obtained in the comparison between constrained and unconstrained maximum likelihood models for cline slopes (concordance) and cline centres (coincidence). The significance level was obtained after Bonferroni correction.
Double asterisk indicates significant values at P<0.01.
Temporal analyses
In order to perform comparisons by site, chromosomal characteristics and gene diversity statistics corresponding to the earlier period (1996–2000) were calculated (Tables 1, 2 and 3). Chromosomal variations occurred in several of the sites resampled. An increase in the mean and ranges of 2n in almost all localities was found in the period 2008–2010. Comparisons of metacentric frequencies between subpopulations (G-test) revealed temporal changes in animals from Calafell (Rb(4.14), P<0.001; Rb(5.15), P=0.025; Rb(6.10), P=0.011; Rb(12.13), P<0.001), Garraf (Rb(5.15), P=0.002; Rb(6.10), P=0.001; Rb(9.11), P=0.001), Sant Sadurní d'Anoia (Rb(5.15), P<0.001; Rb(9.11), P=0.032), Les Pobles (Rb(4.14), P=0.007; Rb(12.13), P=0.023), La Granada (Rb(4.14), P=0.011), Gavà (Rb(6.10), P=0.031), Vilanova i la Geltrú (Rb(9.11), P=0.019) and Santa Coloma de Queralt (Rb(5.15), P=0.032). Individuals from Viladecans, Garraf and La Granada showed a high increase in H index in the present period (from 0.75 to 2.55, 1.82 to 2.61 and 1.79 to 2.56, respectively; Table 1). Although data from Sant Martí Sarroca, Calafell and Santa Coloma de Queralt indicated an increase in H values, the trend was quite moderate. A reduction in mean heterozygous chromosomes was found in animals from Bellaterra and Les Pobles (Table 1). In Bellaterra, mice carrying several fusions (Rb(4.14), (5.15) and (12.13)) were described in the 1996–2000 period, whereas no metacentrics were found in our survey.
The inter-sample karyotypic heterogeneity per metacentric obtained for both periods was high, indicating differentiation between subpopulations (Table 2). However, Rb(12.13), (4.14) and, especially, Rb(9.11) FST values indicated a stronger differentiation between subpopulations in the earlier period.
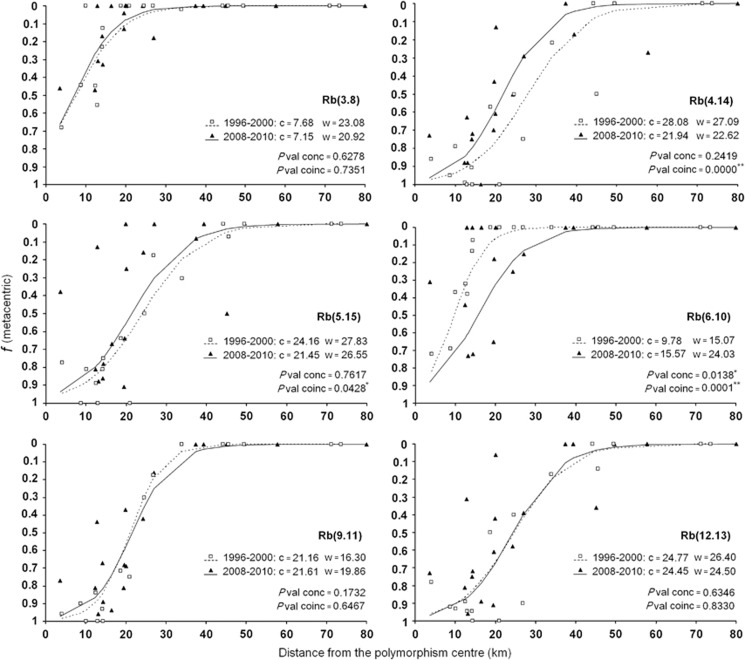
The clinal parameters (centres and widths) fitted for the two periods studied (1996–2000 and 2008–2010) are illustrated in Figure 3. Concordance likelihood tests did not show significant changes in the general trend of the width between periods, while coincidence tests revealed noticeable differences in centres of Rb(4.14), (6.10) and in lesser extent, Rb(5.15), (Figure 3). These results indicated that slight temporal changes in the geographic distribution of some metacentrics had occurred, although the Rb structure of the zone had remained relatively stable between both periods.
Figure 3.
Comparison of clinal patterns for each metacentric between 1996–2000 and 2008–2010 periods. Asterisk indicates significant values at P<0.05; Double asterisk designates significant values at P<0.01 after Bonferroni correction. Abbreviations: c, centre of the clines fitted; Pval conc, probability of concordance obtained in the maximum likelihood test between constrained and unconstrained models for clinal widths; Pval coinc, probability of coincidence obtained in the maximum likelihood test between constrained and unconstrained models for clinal centres; w, width of the clines fitted.
Discussion
Previous studies of the polymorphism zone of Barcelona (Adolph and Klein, 1981; Nachman et al., 1994; Gündüz et al., 2001; Muñoz-Muñoz et al., 2003) hypothesized the presence of a chromosomal race with all fusions in the homozygous state. The data examined in these studies together with the results reported here represent a general survey, covering about 45 sites and the capture of more than 600 specimens. This information treated as a whole allows us to indicate that in the Barcelona zone there is no parental Rb race, as defined by Hausser et al. (1994). Further, metacentric combinations obtained in each locality (Appendix) also fail to support the hypothesis of a specific metacentric population with a set of fusions fixed in the homozygous state, sensu Piálek et al. (2005), a less strict definition of a chromosomal evolutionary entity than the Rb race proposed by Hausser et al., 1994. Therefore, as suggested by Sans-Fuentes (2004), it seems more appropriate to define this area as a polymorphism zone rather than a hybrid zone between two chromosomal races.
Laboratory studies in the house mouse demonstrate that Rb translocations appear spontaneously at high frequencies because of the presence of DNA sequences that make such rearrangements possible (Nachman and Searle, 1995). The presence of one fusion could increase the mutation rate, facilitating the appearance of new fusions (Gündüz et al., 2001). These high mutation rates supported by the effect of genetic drift, inbreeding or the meiotic drift in small and isolated subpopulations, might explain the high fixation ratio for the Rb fusions in the whole population (Hedrick, 1981; Michalakis and Olivieri, 1993; Piálek et al., 2005). Consequently, metacentric populations may be originated in situ by the successive accumulation of Rb fusions in the same geographical area (Britton-Davidian et al. 1989). However, it must be kept in mind that selection does not act equally for all metacentrics. Recent studies demonstrate that factors, such as genomic characteristics or the morphology of the chromosomes involved in the fusions, may affect the formation of some combinations of metacentrics negatively, reducing their fixation rate in the population (see Sans-Fuentes et al., 2007; and references therein).
Chromosomal data showed that, in both periods analysed, the animals with the highest number of fusions were confined to the same area, close to the sites of Garraf and Viladecans, indicating that the polymorphism centre is located in the surrounding areas. However, the mean and range of diploid numbers increased around this centre from one period (1996–2000) to the next (2008–2010), and one animal with the standard karyotype was even found in Garraf (see Table 1). These results suggest that processes of introgression of animals, carrying diverse chromosomal combinations, towards the centre of polymorphism may alter the expected metacentric frequencies in the area. The arrival of allochthonous mice in the centre of a metacentric zone has also been reported in Rb populations from Italy (Franchini et al., 2007; Castiglia and Capanna, 1999) and Madeira (Nunes et al., 2005). In those cases, as may also occur in the Rb zone of Barcelona, the action of humans on the natural landscape has influenced the dynamics of commensal mice (Chatti et al., 1999; Castiglia and Capanna, 1999). Passive transport via vehicles, especially in farming areas, may have promoted introgression events independently of the common mouse dispersion (Cucchi et al., 2005; Franchini et al., 2007). Hence, interaction between mice with differing degrees of polymorphism could explain the increase in the heterozygosity per site (H index and A/l) as well as the percentage of individuals with several heterozygous chromosomes detected in the last period.
FST values for both temporal samples (1996–2000 and 2008–2010) indicate significant levels of genetic fragmentation within populations in the area studied. Even, the values were lower for the latest period (2008–2010) suggesting a slight genotypic homogenization between sites.
According to Franchini et al. (2007) genetic fragmentation can be explained by the typical population dynamics of the house mouse, where commensalism has an important role limiting dispersal between sites. Hence, an increase in the heterogeneity of the distribution of some agricultural zones, related to the replacement of farms by urban zones, (as occurs in the vicinity of the cities of Barcelona, Tarragona and Lleida), and the conversion of traditional agriculture to intensive practices may have produced the isolation of specific polymorphic populations, allowing the fixation of fusions by genetic drift or inbreeding effects. This would explain the unexpectedly high frequencies of some fusions for animals trapped far from the centre of the area (for example, sites x, aa) as well as the subdivision detected in the Rb area of Barcelona.
Fluctuations in genetic subdivision between the two time periods could arise as a consequence of the recent apparition of some of the chromosomal mutations detected in the area. If so, it could be assumed that the Rb population has not already reached its own migration–drift balance and that the differences in FST detected between the two time periods could simply reflect the system approaching equilibrium. Nevertheless, if migration–drift equilibrium has been reached, passive transport of animals associated with human activities could produce a genotypic homogenization between demes, thus varying the FST values in the population (see for example, Castiglia and Capanna, 1999).
Classical theories of hybrid fitness suggest that mice with some heterozygous translocations tend to be eliminated from the hybrid zones because of their adverse effect on fertility due to the high percentage of aneuploid gametes (Wallace et al., 1992; Hauffe and Searle, 1998). Conversely, in the Rb polymorphism zone of Barcelona, mice with six or even seven heterozygous fusions were reported in the 1996–2000 period. Indeed, individuals with one to four metacentrics in the heterozygous state were commonly detected in the current sample. A priori, this situation suggests that selection against hybrids was not as strong as expected. However, individuals with more than five heterozygous fusions were not found in the period 2008–2010. This situation may indicate that selection acts against hybrids only when individuals present more than five fusions in the heterozygous state. This is consistent with the results reported in other Rb systems of the house mouse, according to which the presence of one to three fusions in the heterozygous state was not related to significant alterations in reproduction (Wallace et al., 1992, 2002).
In models of selection against hybrids, the width of a metacentric cline, assumed as a parameter of the extent of gene flow at the centre of the zone, may provide supplementary information about the reproductive isolation of a hybrid zone (Barton and Gale, 1993). Especially, when genetic flow occurs and there is no strong effect against hybrids, clines tend to be wide (Sage et al., 1993). Analyses from the general clinal trend in the polymorphism zone of Barcelona showed several broad metacentric clines (Figure 2), indicating that gene flow between St and Rb mice occurs. This finding concurs with the results for the chromosomal parameters (range and mean of 2n, H, A/l per site and frequencies for each metacentric), in which genetic exchange between populations may have an important role.
Comparative analyses among clines corresponding to the 2008–2010 period indicated that all fusions follow a concordant pattern across the area studied. By contrast, the non-coincidence of the centres for Rb(3.8) and (6.10) significantly displaced from the rest of clines indicated a staggered structure. When substantial chromosomal differences between St and Rb races are found, as in mice from Italy (Franchini et al., 2010) and Tunisia (Saïd et al., 1999), selective forces tend to preserve the clines together due to hybrid unfitness (Searle, 1993). By contrast, staggering can occur when those chromosomal differences are fewer and constraining forces are weaker, for example, in primary contact between St and Rb animals with few fusions. Considering that the presence of a Rb translocation leads to the appearance of new chromosomal fusions (Winking, 1986), under a primary contact scenario, the staggering of the clines could reflect the order of appearance of the fusions. The most widespread (that is, Rb(12.13)) would be the earliest formed, and those with the smallest distribution (Rb(3.8) and (7.17)) would be the most recent (Figure 3). The primary contact hypothesis is supported by the fact that the combination of metacentrics of the Barcelona polymorphism zone has not been reported elsewhere (Piálek et al., 2005) and that metacentrics shared with other Rb systems have an independent origin (Riginos and Nachman, 1999). Although staggered clines have also been detected in other Rb zones of M. musculus domesticus, the presence of metacentric races in close geographic regions supports the hypothesis of secondary contact in these areas (Gündüz et al., 2010).
Clinal comparison for each fusion indicated that the general clinal structure of the metacentrics did not change significantly between 1996–2000 and 2008–2010. Nevertheless, the Rb(4.14), (6.10) and (5.15) showed variation in the position of their cline centres. Although the current trend shows a backward movement for Rb(4.14) and Rb(5.15) towards the centre of polymorphism, the clines of Rb(6.10) indicate that this fusion has spread over the last decade. Hybrid zones can move when ecological (Bull and Burzacott, 2001), behavioural (Cabrero et al., 1999; Leaché and Cole, 2007) or some other kind of advantage for one hybridizing taxa (Dorken and Pannell, 2007) occurs. In this case the zone would move in favour of the superior taxon (Barton and Hewitt, 1985; Yannic et al., 2009). Otherwise, the movement of these zones could also take place to local regions of low dispersal or low population density (Barton and Hewitt, 1985). In the Rb polymorphism zone around Barcelona, the disequilibrium between anthropogenic activities (urbanization, commercial transport or desertion of rural zones) and dynamics on mice raciation may be responsible for the movement of the centre of metacentric clines. The magnitude and frequency of these events, as well as the fixation degree of each fusion in each population, may indirectly alter the selection/dispersal balance in the tension zone. Therefore, intrinsic variations in local chromosomal composition may reverberate under the global structure of the contact zone. Nevertheless, it is necessary to continue evaluating the effects of complex local variations under the global clinal pattern to avoid overestimation of the changes detected between periods.
Data archiving
There were no data to deposit.
Acknowledgments
We are grateful to Josep Medarde, María Casado and Ramón Solís for their help in the field work; Laia Capilla, Marta Blanc and Álex Sánchez for support in the laboratory and Maria Assumpció Sans-Fuentes for technical assistance. We also thank three anonymous reviewers for their helpful comments and suggestions. This work was funded by the Spanish Ministerio de Ciencia y Tecnologia (project number CGL2007–62111) and by a PIF grant from the Universitat Autònoma de Barcelona to NM. Thanks also to Robin Rycroft (Servei d'Assessorament Lingüístic, Universitat de Barcelona) for revising the English.
Appendix
Individual karyotypes corresponding to the 2008–2010 period
Table A1.
| Site | N | 2n | 3.8 | 4.14 | 5.15 | 6.10 | 7.17 | 9.11 | 12.13 |
|---|---|---|---|---|---|---|---|---|---|
| a. Garraf | 1 | 29 | H | M | M | M | A | M | M |
| 1 | 29 | M | M | H | H | H | M | M | |
| 1 | 30 | M | M | H | H | H | H | M | |
| 1 | 31 | H | M | H | H | A | M | M | |
| 1 | 32 | H | H | H | H | A | M | M | |
| 1 | 32 | M | M | H | A | A | M | H | |
| 1 | 32 | H | M | H | H | A | M | H | |
| 1 | 32 | H | M | A | H | A | M | M | |
| 1 | 32 | H | H | M | H | A | H | M | |
| 1 | 33 | H | M | A | A | A | M | M | |
| 1 | 36 | A | H | H | A | A | H | H | |
| 1 | 38 | A | H | A | A | A | A | H | |
| 1 | 40 | A | A | A | A | A | A | A | |
| b. Sant Pau d'Ordal | 1 | 34 | A | H | H | M | A | H | H |
| c. La Granada | 1 | 29 | M | M | M | M | A | H | M |
| 1 | 30 | M | M | M | A | A | M | M | |
| 1 | 31 | H | M | H | M | A | M | H | |
| 1 | 31 | H | M | M | H | A | M | H | |
| 1 | 31 | H | H | M | H | A | M | M | |
| 1 | 31 | A | M | M | H | A | M | M | |
| 1 | 31 | H | M | M | A | A | M | M | |
| 1 | 32 | H | M | M | H | A | H | H | |
| 1 | 32 | H | H | M | A | A | M | M | |
| 3 | 32 | H | M | H | H | A | H | M | |
| 1 | 32 | M | M | H | A | A | M | H | |
| 1 | 32 | A | M | H | H | A | M | M | |
| 1 | 33 | A | H | M | H | A | M | H | |
| 1 | 34 | A | H | M | H | A | H | H | |
| d. Corbera de Llobregat | 1 | 36 | A | A | A | A | A | M | M |
| 1 | 36 | A | M | A | A | A | H | H | |
| 1 | 36 | A | M | H | A | A | H | A | |
| 1 | 37 | A | H | H | A | A | H | A | |
| 1 | 37 | A | M | A | A | A | H | A | |
| 1 | 37 | A | A | A | A | A | H | M | |
| 1 | 38 | A | M | A | A | A | A | A | |
| 1 | 39 | A | H | A | A | A | A | A | |
| e. Gavà | 1 | 29 | M | M | M | H | A | M | M |
| 4 | 30 | A | M | M | M | A | M | M | |
| 3 | 30 | H | M | M | H | A | M | M | |
| 1 | 30 | H | M | H | M | A | M | M | |
| 1 | 30 | H | H | M | M | A | M | M | |
| 1 | 31 | H | H | H | M | A | M | M | |
| 1 | 31 | A | M | M | H | A | M | M | |
| 1 | 36 | A | H | H | A | A | H | H | |
| 1 | – | – | – | – | – | – | – | – | |
| f. Vilanova i la Geltrú | 1 | 31 | H | M | M | A | A | M | M |
| 2 | 33 | A | M | M | A | A | H | M | |
| 1 | 33 | A | H | M | A | A | M | M | |
| 1 | 34 | A | M | M | A | A | H | H | |
| 1 | 38 | H | A | A | A | A | H | A | |
| g. Viladecans | 2 | 30 | H | M | M | H | A | M | M |
| 1 | 30 | H | H | M | M | A | M | M | |
| 1 | 31 | A | M | M | M | A | H | M | |
| 1 | 32 | M | H | M | H | A | H | H | |
| 2 | 32 | A | M | H | M | A | M | H | |
| 1 | 34 | H | H | H | A | A | M | H | |
| 1 | 34 | A | H | H | H | A | M | H | |
| h. Sant Martí Sarroca | 1 | 31 | A | M | M | A | H | M | M |
| 1 | 32 | A | M | M | A | A | M | M | |
| 2 | 32 | A | M | M | A | A | M | M | |
| 1 | 32 | A | M | H | A | H | M | M | |
| 2 | 34 | A | M | H | A | A | M | H | |
| 1 | 34 | A | M | A | A | A | M | M | |
| 1 | 34 | A | M | H | A | A | H | M | |
| i. El Prat de Llobregat | 1 | 29 | H | M | M | M | A | M | M |
| 2 | 30 | H | M | M | H | A | M | M | |
| 1 | 30 | A | M | M | M | A | M | M | |
| 1 | 31 | H | M | M | H | A | M | H | |
| 1 | 31 | A | M | M | H | A | M | M | |
| 3 | 31 | A | M | M | M | A | H | M | |
| 1 | 31 | H | M | M | A | A | M | M | |
| 2 | 31 | A | H | M | M | A | M | M | |
| 1 | 31 | H | H | M | M | A | H | M | |
| 3 | 32 | A | H | M | H | A | M | M | |
| 1 | 32 | A | A | M | M | A | M | M | |
| 1 | 32 | A | H | M | M | A | H | M | |
| 1 | 33 | A | M | H | H | A | M | H | |
| 1 | 33 | A | H | M | H | A | M | H | |
| 1 | 33 | A | H | M | H | A | H | M | |
| 2 | 33 | A | H | H | H | A | M | M | |
| 1 | 33 | A | M | M | H | A | H | H | |
| 1 | 34 | A | H | M | H | A | A | M | |
| 1 | 34 | A | H | H | H | A | M | H | |
| 1 | 34 | H | H | H | A | A | H | M | |
| j. Cubelles | 1 | 32 | A | H | M | H | A | M | M |
| 1 | 33 | H | H | H | H | A | M | H | |
| 1 | 33 | A | H | M | A | A | M | M | |
| 1 | 33 | A | M | M | H | A | M | A | |
| 1 | 34 | A | H | H | A | A | M | M | |
| 1 | 34 | A | M | M | A | A | A | M | |
| 1 | 34 | A | H | M | A | A | M | H | |
| 1 | 35 | A | H | H | H | A | H | H | |
| 1 | 35 | A | A | M | A | A | M | H | |
| 1 | 36 | A | A | H | A | A | H | M | |
| 1 | 36 | A | H | A | H | A | H | H | |
| 1 | 36 | A | H | H | A | A | H | H | |
| 1 | 38 | A | A | A | A | A | H | H | |
| 1 | 39 | A | A | H | A | A | A | A | |
| 3 | – | – | – | – | – | – | – | – | |
| k. Sant Sadurní d'Anoia | 1 | 35 | A | H | A | A | A | M | M |
| 1 | 35 | A | M | A | A | A | H | M | |
| 1 | 35 | A | M | A | A | A | M | H | |
| 1 | 36 | A | H | A | A | A | H | M | |
| 1 | 36 | A | M | A | A | A | H | H | |
| 1 | 37 | A | M | A | A | A | A | H | |
| 1 | 37 | A | H | A | A | A | M | A | |
| 2 | 37 | A | H | A | A | A | H | H | |
| 1 | 37 | A | M | A | A | A | H | A | |
| 1 | 38 | A | M | A | A | A | A | A | |
| 2 | 38 | A | H | A | A | A | H | A | |
| 2 | 38 | A | H | A | A | A | A | H | |
| 1 | 38 | A | A | A | A | A | A | M | |
| 2 | 39 | A | H | A | A | A | A | A | |
| 1 | 39 | A | A | A | A | A | A | H | |
| 2 | – | – | – | – | – | – | – | – | |
| l. Calafell | 1 | 37 | A | H | A | H | A | H | A |
| 1 | 37 | H | H | A | A | A | A | H | |
| 1 | 37 | H | A | A | A | A | A | M | |
| 1 | 37 | A | M | A | A | A | A | H | |
| 1 | 37 | A | H | A | A | A | A | M | |
| 1 | 37 | A | H | A | H | A | A | H | |
| 1 | 37 | H | H | A | A | A | A | H | |
| 1 | 37 | A | H | A | A | A | A | M | |
| 1 | 38 | A | H | A | A | A | H | A | |
| 4 | 38 | A | A | A | A | A | H | H | |
| 1 | 38 | A | H | A | A | A | A | H | |
| 1 | 38 | A | A | A | H | A | A | H | |
| 1 | 38 | A | H | A | A | A | A | H | |
| 3 | 39 | A | A | A | A | A | A | H | |
| m. El Papiol | 1 | 36 | A | A | M | A | A | M | A |
| 2 | 37 | A | A | H | A | A | M | A | |
| 2 | 38 | A | H | A | A | A | A | H | |
| 2 | 38 | A | A | A | A | A | M | A | |
| 1 | 39 | A | A | A | A | A | H | A | |
| n. Bellvei | 1 | 35 | A | A | M | A | A | H | M |
| 1 | 35 | A | H | A | H | A | M | H | |
| 1 | 36 | A | H | A | A | A | H | M | |
| 1 | 37 | A | M | A | A | A | A | H | |
| 1 | 37 | A | A | M | H | A | A | A | |
| 1 | 37 | A | A | A | H | A | H | H | |
| o. Bellaterra | 7 | 40 | A | A | A | A | A | A | A |
| p. Vacarisses | 7 | 40 | A | A | A | A | A | A | A |
| q. Badalona | 11 | 40 | A | A | A | A | A | A | A |
| 2 | 39 | – | – | – | – | – | – | – | |
| r. Sta. Perpètua de la Mogoda | 10 | 40 | A | A | A | A | A | A | A |
| s. Les Pobles | 11 | 40 | A | A | A | A | A | A | A |
| 2 | 39 | A | A | H | A | A | A | A | |
| 2 | – | – | – | – | – | – | – | – | |
| t. Castellar del Vallès | 8 | 40 | A | A | A | A | A | A | A |
| u. Jorba | 1 | 39 | A | H | A | A | A | A | A |
| 2 | 40 | A | A | A | A | A | A | A | |
| v. Castellfollit del Boix | 14 | 40 | A | A | A | A | A | A | A |
| w. Caldes de Montbuí | 2 | 40 | A | A | A | A | A | A | A |
| x. Santa Coloma de Queralt | 2 | 36 | A | M | H | A | A | A | H |
| 1 | 36 | A | M | M | A | A | A | A | |
| 1 | 37 | A | H | H | A | A | A | H | |
| 1 | 37 | A | H | M | A | A | A | A | |
| 2 | 38 | A | H | A | A | A | A | H | |
| y. Fals | 3 | 40 | A | A | A | A | A | A | A |
| 2 | – | – | – | – | – | – | – | – | |
| z. Nulles | 7 | 40 | A | A | A | A | A | A | A |
| aa. Ametlla de Segarra | 1 | 38 | A | M | A | A | A | A | A |
| 3 | 39 | A | H | A | A | A | A | A | |
| 5 | 40 | A | A | A | A | A | A | A | |
| ab. Olost | 16 | 40 | A | A | A | A | A | A | A |
| 1 | – | – | – | – | – | – | – | – | |
| ac. Arbeca | 6 | 40 | A | A | A | A | A | A | A |
Abbreviations: A, homozygote acrocentric; H, heterozygote metacentric; M, homozygote metacentric; N, number of individuals.
The authors declare no conflict of interest.
References
- Adolph S, Klein J. Robertsonian variation in Mus musculus from Central Europe, Spain, and Scotland. J Hered. 1981;72:219–221. doi: 10.1093/oxfordjournals.jhered.a109478. [DOI] [PubMed] [Google Scholar]
- Barton NH, Hewitt GM. Analysis of hybrid zones. Annu Rev Ecol Systematics. 1985;16:113–148. [Google Scholar]
- Barton NH, Gale KS.1993Genetic analysis of hybrid zonesIn: Harrison RG (ed.)Hybrid Zones and the Evolutionary Process Oxford University Press: New York; 13–45. [Google Scholar]
- Belkhir K, Borsa P, Goudet J, Chikhi L, Bonhomme F. GENETIX, logiciel sous Windows TM pour la génétique des populations. Laboratoire du Génome et Populations, CNRS UPR 9060. Université de Montpellier II: Montpellier, France; 1996-1998. [Google Scholar]
- Britton-Davidian J, Nadeau JH, Croset H, Thaler L. Genic differentiation and origin of Robertsonian populations of the house mouse (Mus musculus domesticus Rutty) Genet Res. 1989;53:29–44. doi: 10.1017/s0016672300027841. [DOI] [PubMed] [Google Scholar]
- Buggs RJA. Empirical study of hybrid zone movement. Heredity. 2007;99:301–312. doi: 10.1038/sj.hdy.6800997. [DOI] [PubMed] [Google Scholar]
- Bull CM, Burzacott D. Temporal and spatial dynamics of a parapatric boundary between two Australian reptile ticks. Mol Ecol. 2001;10:639–648. doi: 10.1046/j.1365-294x.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- Cabrero J, Shapiro LH, Camacho JPM. Male sterility in interespecific Meadow Katydid hybrids. Hereditas. 1999;131:79–82. [Google Scholar]
- Castiglia R, Capanna E. Contact zones between chromosomal races of Mus musculus domesticus. 1. Temporal analysis of a hybrid zone between the CD chromosomal race (2n=22) and populations with the standard karyotype. Heredity. 1999;83:341–359. doi: 10.1038/sj.hdy.6885820. [DOI] [PubMed] [Google Scholar]
- Castiglia R, Capanna E. Contact zone between chromosomal races of Mus musculus domesticus. 2. Fertility and segregation in laboratory-reared and wild mice heterozygous for multiple Robertsonian rearrangements. Heredity. 2000;85:147–156. doi: 10.1046/j.1365-2540.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Chatti N, Ganem G, Benzekri K, Catalan J, Britton-Davidian J, Saïd K. Microgeographical distribution of two chromosomal races of house mice in Tunisia: pattern and origin of habitat partitioning. Proc R Soc Lond. 1999;266:1561–1569. doi: 10.1098/rspb.1999.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Standarized Genetic Nomenclature for Mice Standard karyotype of the mouse, Mus musculus. Heredity. 1972;63:69–72. [PubMed] [Google Scholar]
- Cucchi T, Vigne JD, Auffray JC. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: a zooarchaeological revision of subfossil occurrences. Biol J Linnean Soc. 2005;84:429–445. [Google Scholar]
- Dorken ME, Pannell JR. The maintenance of hybrid zones along a disturbance gradient. Heredity. 2007;99:89–101. doi: 10.1038/sj.hdy.6800969. [DOI] [PubMed] [Google Scholar]
- Endler JA. Geographic Variation, Speciation and Clines. Princeton University Press: Princeton; 1977. [PubMed] [Google Scholar]
- Faria R, Navarro A. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol Evol. 2010;25:660–669. doi: 10.1016/j.tree.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Fel-Clair F, Lenormand T, Catalan J, Grobert J, Orth A, Boursot P, Viroux MC, Britton-Davidian J. Genomic incompatibilies in the hybrid zone between house mice in Denmark: evidence from steep and non-coincident chromosomal clines for Robertsonian fusions. Genet Res. 1996;67:123–134. doi: 10.1017/s0016672300033589. [DOI] [PubMed] [Google Scholar]
- Ford CE. Tissue Grafting and Radiation. Academic Press: New York; 1966. The use of chromosome markers; pp. 197–206. [Google Scholar]
- Franchini P, Castiglia R, Capanna E. Reproductive isolation between chromosomal races of the house mouse Mus musculus domesticus in a parapatric contact area revealed by an analysis of multiple unlinked loci. J Evol Biol. 2007;21:502–513. doi: 10.1111/j.1420-9101.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- Franchini P, Colangelo P, Solano E, Capanna E, Verheyen E, Castiglia R. Reduced gene flow at pericentromeric loci in a hybrid zone involving chromosomal races of the house mouse. Mus musculus domesticus Evol. 2010;64:2020–2032. doi: 10.1111/j.1558-5646.2010.00964.x. [DOI] [PubMed] [Google Scholar]
- Gazave E, Catalan J, Ramalhinho MG, Mathias ML, Nunes C, Dumas D, Britton-Davidian J, Auffray JC. The non-random occurrence of Robertsonian fusion in the house mouse. Genet Res. 2003;81:33–42. doi: 10.1017/s001667230200602x. [DOI] [PubMed] [Google Scholar]
- Gündüz I, López-Fuster MJ, Ventura J, Searle JB. Clinal analysis of a chromosomal hybrid zone in the house mouse. Genet Res. 2001;77:41–51. doi: 10.1017/s0016672300004808. [DOI] [PubMed] [Google Scholar]
- Gündüz I, Pollock CL, Giménez MD, Förster DW, White TA, Sans-Fuentes MA, Hauffe HC, Ventura J, López-Fuster MJ, Searle JB. Staggered Chromosomal Hybrid Zones in the House Mouse: Relevance to Reticulate Evolution and Speciation. Genes. 2010;1:193–209. doi: 10.3390/genes1020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp A, Winking H.1981Robertsonian translocations: cytology, meiosis, segregation pattern and biological consequences of heterozygosity. Symposia of the Zoological Society of LondonIn: Berry RJ (ed.)Biology of the House Mouse Academic press: London; 141–181. [Google Scholar]
- Harrison RG.1990Hybrid zones: windows on evolutionary processIn: Futuyma D, Antonovics J (eds)Evolutionary Biology Oxford University Press: Oxford; Vol. 769–128. [Google Scholar]
- Hauffe HC, Searle JB. Extreme karyotypic variation in a Mus musculus domesticus hybrid zone: The tobacco mouse story revisited. Evolution. 1993;47:1374–1395. doi: 10.1111/j.1558-5646.1993.tb02161.x. [DOI] [PubMed] [Google Scholar]
- Hauffe HC, Searle JB. Chromosomal heterozygosity and fertility in house mice (Mus musculus domesticus) from northern Italy. Genetics. 1998;150:1143–1154. doi: 10.1093/genetics/150.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser J, Fedyk S, Fredga K, Searle JB, Volobouev V, Wójcik JM, Zima J. Definition and nomenclature of the chromosome races of Sorex araneus. Folia Zoologica. 1994;43:1–9. [Google Scholar]
- Hedrick PW. The establishment of chromosomal variants. Evolution. 1981;35:322–332. doi: 10.1111/j.1558-5646.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Leaché AD, Cole CJ. Hybridization between multiple fence lizard lineages in an ecotone: locally discordant variation in mitocondrial DNA, chromosomes, and morphology. Mol Ecol. 2007;16:1035–1054. doi: 10.1111/j.1365-294X.2006.03194.x. [DOI] [PubMed] [Google Scholar]
- Mandahl N. Methods in solid tumour cytogenetics. IRL Press: London; Human Cytogenetics. A Practical Approach. 1992;Vol. II:155–187. [Google Scholar]
- Michalakis Y, Olivieri I. The influence of local extinctions on the probability of fixation of chromosomal rearrangements. J Evol Biol. 1993;6:153–170. [Google Scholar]
- Muñoz-Muñoz F, Sans-Fuentes MA, López-Fuster MJ, Ventura J. Non-metric morphological divergence in the western house mouse, Mus musculus domesticus, from the Barcelona chromosomal hybrid zone. Biol J Linnean Soc. 2003;80:313–322. [Google Scholar]
- Muñoz-Muñoz F, Sans-Fuentes MA, López-Fuster MJ, Ventura J. Variation in fluctuating asymmetry levels across a Robertsonian polymorphic zone of the house mouse. J Zoolog Syst Evol Res. 2006;44:236–250. [Google Scholar]
- Muñoz-Muñoz F, Sans-Fuentes MA, López-Fuster MJ, Ventura J. Evolutionary modularity of the mouse mandible: dissecting the effect of chromosomal reorganizations and isolation by distance in a Robertsonian system of Mus musculus domesticus. J Evol Biol. 2011;24:1763–1776. doi: 10.1111/j.1420-9101.2011.02312.x. [DOI] [PubMed] [Google Scholar]
- Nachman MW, Boyer SN, Searle JB, Aquadro CF. Mitochondrial DNA variation and the evolution of Robertsonian chromosomal races of house mice. Mus domesticus Genet. 1994;136:1105–1120. doi: 10.1093/genetics/136.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman MW, Searle JB. Why is the mouse karyotype so variable. Trends Ecol Evol. 1995;10:397–402. doi: 10.1016/s0169-5347(00)89155-7. [DOI] [PubMed] [Google Scholar]
- Nunes AC, Britton-Davidian J, Catalan J, Ramalhinho MG, Capela R, Mathias ML, Ganem G. Influence of physical environmental characteristics and anthropogenic factors on the position and structure of contact zone between two chromosomal races of the house mouse on the island of Madeira (North Atlantic, Portugal) J Biogeography. 2005;32:2123–2134. [Google Scholar]
- Nunes AC, Catalan J, Lopez J, Ramalhinho MG, Mathias ML, Britton-Davidian J. Fertility assessment in hybrids between monobrachially homologous Rb races of the house mouse from the island of Madeira: implications for modes of chromosomal evolution. Heredity. 2011;106:348–356. doi: 10.1038/hdy.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piálek J, Hauffe HC, Searle JB. Chromosomal variation in the house mouse. Biol J Linnean Soc. 2005;84:535–563. [Google Scholar]
- Raymond M, Rousset F. GENEPOP software: Population genetics software for exact tests and ecumeni-cism. J Hered. 1995;86:248–249. [Google Scholar]
- Riginos C, Nachman MW. The origin of a Robertsonian chromosomal translocations in house mice inferred from linked microsatellite markers. Mol Biol Evol. 1999;16:1763–1773. doi: 10.1093/oxfordjournals.molbev.a026088. [DOI] [PubMed] [Google Scholar]
- Rousset F, Raymond M. Testing heterozygotes excess and deficiency. Genetics. 1995;140:1413–1419. doi: 10.1093/genetics/140.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RD, Atchley WR, Capanna E. House mice as models in systematic biology. Syst Biol. 1993;42:523–561. [Google Scholar]
- Saïd K, Auffrey JC, Boursot P, Britton-Davidian J. Is chromosomal speciation ocurryng in house mice in Tunisia. Biol J Linnean Soc. 1999;68:387–399. [Google Scholar]
- Sans-Fuentes MA.2004Estudio biológico de Mus domesticus Rutty, 1772 en una zona de polimorfismo RobertsonianoPhD ThesisUniversitat de Barcelona: Barcelona [Google Scholar]
- Sans-Fuentes MA, López-Fuster MJ, Ventura J, Diez-Noguera A, Cambras T. Effect of Robertsonian translocations on circadian rhythm of motor activity in house mouse. Behav Genet. 2005;35:603–613. doi: 10.1007/s10519-005-5375-5. [DOI] [PubMed] [Google Scholar]
- Sans-Fuentes MA, Muñoz-Muñoz F, Ventura J, López-Fuster MJ. Rb(7.17), a rare Robertsonian fusion in wild populations of the house mouse. Genet Res. 2007;89:207–213. doi: 10.1017/S0016672307008993. [DOI] [PubMed] [Google Scholar]
- Sans-Fuentes MA, Ventura J, López-Fuster MJ, Corti M. Morphological variation in house mice from the Robertsonian polymorphism area of Barcelona. Biol J Linnean Soc. 2009;97:555–570. [Google Scholar]
- Sans-Fuentes MA, García-Valero J, Ventura J, López-Fuster MJ. Spermatogenesis in house mouse in a Robertsonian polymorphism zone. Reproduction. 2010;140:560–581. doi: 10.1530/REP-10-0237. [DOI] [PubMed] [Google Scholar]
- Searle JB.1993Chromosomal hybrid zones in eutherian mammalsIn: Harrison RG (ed.)Hybrid Zones and Evolutionary Process Oxford University Press: Oxford; 309–353. [Google Scholar]
- Sokal RR, Rohlf FJ.1995Biometry: The Principles and Practice of Statistics in Biological Research3rd edn.Freeman WH, New York; p887 [Google Scholar]
- Szymura JM, Barton NH. Genetic analysis of a hybrid zone between the fire-bellied toads, Bombina bombina and B. variegata, near Cracow in southern Poland. Evolution. 1986;40:1141–1159. doi: 10.1111/j.1558-5646.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- Wallace BM, Searle JB, Everett CA. Male meiosis and gametogenesis in wild house mouse (Mus musculus domesticus) from a chromosomal hybrid zone. A comparison between ‘simple' Robertsonian heterozygotes and homozygotes. Cytogenet Cell Genet. 1992;6:211–220. doi: 10.1159/000133410. [DOI] [PubMed] [Google Scholar]
- Wallace BMN, Searle JB, Everett CA. The effect of multiple simple Robertsonian heterozygosity on chromosome pairing and fertility of wild-stock house mice (Mus musculus domesticus) Cytogenet Genome Res. 2002;96:276–286. doi: 10.1159/000063054. [DOI] [PubMed] [Google Scholar]
- Weir BS. Genetic data analysis. Sinauer Associates: Sunderland, Massachusetts; 1990. [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Winking H. Some aspects of Robertsonian karyotype in European wild mice. Curr Top Microbiol Immunol. 1986;127:68–74. doi: 10.1007/978-3-642-71304-0_8. [DOI] [PubMed] [Google Scholar]
- Yannic G, Basset P, Hausser J. Cromosomal rearrangements and gene flow over time in an inter-specific hybrid zone of the Sorex araneus group. Heredity. 2009;102:616–625. doi: 10.1038/hdy.2009.19. [DOI] [PubMed] [Google Scholar]