Abstract
Short-range ice binding and long-range solvent perturbation both have been implicated in the activity of antifreeze proteins and antifreeze glycoproteins. We study these two mechanisms for activity of winter flounder antifreeze peptide. Four mutants are characterized by freezing point hysteresis (activity), circular dichroism (secondary structure), Förster resonance energy transfer (end-to-end rigidity), molecular dynamics simulation (structure), and terahertz spectroscopy (long-range solvent perturbation). Our results show that the short-range model is sufficient to explain the activity of our mutants, but the long-range model provides a necessary condition for activity: the most active peptides in our data set all have an extended dynamical hydration shell. It appears that antifreeze proteins and antifreeze glycoproteins have reached different evolutionary solutions to the antifreeze problem, utilizing either a few precisely positioned OH groups or a large quantity of OH groups for ice binding, assisted by long-range solvent perturbation.
Antifreeze proteins (AFPs) enable polar fish to survive in seawater at its freezing point of −1.9°C. AFP activity differs from osmolyte activity by exhibiting hysteresis between freezing and melting points (1,2). The observation of hysteresis requires a kinetically frustrated mechanism of action, rather than a purely thermodynamic one. The 37-residue α-helical type I wf-AFP1 from the winter flounder is a well-developed model system for antifreeze activity (1,3,4) with the wild-type sequence “TATA”, 1 D TASDAAAAAAL TAANAKAAAEL TAANAAAAAAA TAR 37, named after the four underlined residues to be mutated here.
We recently reported that antifreeze glycoproteins, whose carbohydrate coat has a less precise hydroxyl arrangement than AFP, act by perturbing long-range solvent interactions (the long-range hypothesis) (5). In contrast, the short-range hypothesis for wf-AFP1 posits hydrogen bonding of polar threonine hydroxyl groups directly to ice surfaces (2) to arrest ice growth. Mutation studies question the short-range hypothesis because the double serine mutant SASA that preserves OH groups resulted in a large loss of activity, whereas the double valine mutant VAVA retained significant activity (3,6). An A17L mutant showed little activity (6), but that could be due to disruption of global structure.
To further investigate both hypotheses for wf-AFP1, we studied the pseudo-wild-type TATW (Ala33Trp) and its three mutants (Table 1). Mutant choice is discussed in the Supporting Material. Measurements were carried out in ammonium bicarbonate buffer (7). Trp provides a fluorescent probe for Förster resonance energy transfer (FRET) experiments and preserves high activity even at low peptide concentrations (Fig. 1). We proceeded in four steps: First, hysteresis of the mutants was used to rank their antifreeze activity. Then circular dichroism and end-to-end FRET determined how much helicity and overall rigidity were perturbed. These structural data were supported by molecular dynamics (MD) simulations. Finally, terahertz absorption was measured to quantify collective hydrogen-bond dynamics. Our results show that all active mutants have a long-range effect on the solvent dynamics, as did one less-active mutant. Our results are consistent with antifreeze activity requiring finely tuned hydrogen bonding to ice, assisted by long-range solvent perturbation that makes AFPs effective even at low concentrations. Solvent perturbation alone does not predict the strength of activity in our wf-AFP1 data set.
Table 1.
Abbreviated name, mutations from TATA wild-type, and FRET efficiency E
| Name | Mutations | E | Name | Mutations | E |
|---|---|---|---|---|---|
| TATW | A33W | <3% | SASW | T13, 24S, A33W | 8% |
| TLTW | A17L, A33W | 11% | VAVW | T13, 24V, A33W | 51% |
Figure 1.
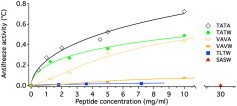
Freezing point hysteresis in 100 mM NH4HCO3 buffer. See the Supporting Material for fits and hysteresis in water versus buffer.
Hysteresis activities are ranked TATA > TATW > VAVA > VAVW > TLTW > SASW (Fig. 1 and see the Supporting Material). TATW retains ≈75% activity at 2 mg/mL. Two added valines reduce activity 10-fold, L17 25-fold (in agreement with Harding et al. (6)), and two serines over 50-fold. VAVA based on the wild-type has ≈30% activity at 2 mg/mL because its hysteresis is low at small concentrations. Its high-concentration activity is consistent with the literature (6).
Are the reductions in activity directly correlated with helix disruption? We quantified the latter by circular dichroism (Fig. 2), ranking helix stability as TATA ≈ SASW >> TATW ≈ TLTW ≈ VAVA ≈ VAVW. SASW shows that large helix content at room temperature does not guarantee activity. TATW and TLTW, as well as VAVA, gain significant helix content at 0°C (see Fig. S1 in the Supporting Material), and all have higher activity than SASW. Of the less-helical mutants, VAVA and VAVW have more random coil signal than TATW or TLTW (more negative circular dichroism (CD) <210 nm).
Figure 2.
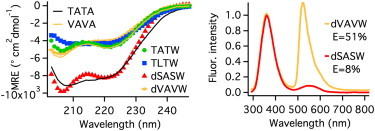
(Left) CD mean residue ellipticities. (Right) FRET comparison. In 100 mM NH4HCO3 buffer at 22°C.
To differentiate mutants further, we measured end-to-end FRET efficiency E. Trp33 (C-terminus) was the donor. We linked a Dansyl group (d) to the N-terminus (see the Supporting Material). E < 3% was observed for dTATW. More FRET was observed for dSASW despite its higher helical content. E = 11% was observed for dTLTW. A large E = 51% was observed for the dVAVW mutant (Fig. 2).
We simulated the FRET efficiency for two simple models systems for comparison: a rigid helix with a flexible quencher attached, and a helix kinked in the middle. The Dansyl-Trp distances sampled from each model were used to calculate an average E (see the Supporting Material). The simulation yielded E = 1% for the rigid helix, and E = 37% for the kinked helix. Thus dVAVW is more structurally disrupted than allowed by a single kink. dTATW is structurally rigid. dTLTW and dSASW occupy a middle ground: they could be in equilibrium between a rigid and single-kink state, or they could be sampling a frayed state.
To gain further insight into the structural fluctuations of wf-AFP1 peptides, we carried out explicit solvent MD simulation (see the Supporting Material) of the TATA wild-type and our TATW, TLTW, SASW, and VAVW mutants using AMBER and SPC/E water (8,9). Simulations were started in fully helical structures taken from the TATA crystal structure (inset a of Fig. 3), equilibrated for 30 ns, and sampled up to either 170 ns (single trajectories) or 50 ns (replica-exchange using 48 replicas). The α-helical probability was analyzed along the sequence (Fig. 3). SASW and TATW had the most helix content. Both remained extended helices throughout the single trajectory simulation. TLTW formed a persistent kink immediately adjacent to the L17 mutation (inset b of Fig. 3). The replica exchange MD data (Fig. 3, bottom) show that kinking affected helix content near the C-terminus. VAVW formed a coil structure at residues 12–17 and 22–27 during the simulation, giving it the most labile helices, in agreement with CD.
Figure 3.
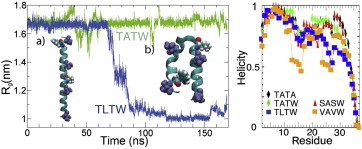
(Left) Radius of gyration Rg of TATW and TLTW from single-trajectory MD simulation. TLTW switches from extended (a) to kinked (b). (Right) Residue-specific average helix content (10) from replica-exchange MD simulations. VAVW is most disrupted.
Finally we investigated the correlation between antifreeze activity and long-range effects of our wf-AFP1 mutants on water dynamics. THz absorption spectroscopy probes such long-range dynamical hydration shells (11). Bare peptides absorb less than bulk water from 2.4 to 2.7 THz. Thus increasing their concentration would decrease the THz absorption coefficient, a THz defect (dashed line in Fig. 4). Conversely, an initial increase of the THz absorption or THz excess up to a characteristic protein concentration cmax indicates that the peptide surrounds itself with a strongly absorbing hydration shell, which is attributed to a retardation of solvation water hydrogen-bond dynamics (11). To measure the THz excess or defect, we used a p-Germanium laser to compare the absorption coefficient of the peptide sample to a bulk water reference (12). Fig. 4 reports the difference Δα as a function of peptide concentration.
Figure 4.
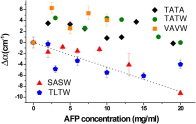
AFP1 THz absorption relative to bulk water (Δα) integrated between 2.4 and 2.7 THz. (Dotted line) Expected absorbance of aqueous AFP solution if the protein does not induce changes in bulk water dynamics.
We observed a THz excess for the strongly active wild-type TATA, for the active pseudo-wild-type TATW, and for the weakly active VAVW. We observed a THz defect for the nearly inactive TLTW and SASW mutants. This is consistent with the correlation between long-range water dynamics and activity proposed in our previous study of AFGPs (5), although there is no simple linear relationship: VAVW is only weakly active, but has the same THz excess as TATA and TATW. For our data set of five peptides, long-range solvent perturbation is therefore a necessary condition, but does not predict the strength of the activity.
Our results are consistent with precise short-range positioning of hydroxyl groups by AFPs, assisted by long-range hydration dynamics that enhances activity at low concentration.
TATW has ∼75% of the wild-type activity, expected if the C-terminal threonine positioning is disrupted by the nearby tryptophan, but the other three Thr are left intact on a fairly rigid helix. The latter is likely the case because TATW has low FRET (Table 1) and its CD spectrum approaches the wild-type at low temperature (see the Supporting Material). Likewise, VAVA retains ≈30% activity at 2 mg/mL, consistent with two out of four threonines missing and with its slightly higher random coil content by CD. In contrast, the following have low activity: VAVW, with only one threonine still present and/or undisturbed by W, and the highest flexibility by FRET and MD; TLTW, which kinks the peptide structure and completely rearranges the spatial orientation of its Thr residues; and SASW, which moves the accurate positioning of three hydroxyls, and may kink like TLTW (compare FRET data in Table 1). The observation that Ser is not a good substitution for Thr and that Trp may deactivate its nearest Thr neighbor indicates that quite-accurate Thr positioning is required for maximum activity.
Fig. 1 shows that the activity of two AFPs increases rapidly at low concentration, as fitted by the Hill equation (see the Supporting Material). The wild-type and secondmost active TATW also have a large THz excess. These observations are consistent with long-range solvent perturbation increasing the range of activity of AFPs, as was also observed for AFGPs (5). Long-range interactions are less effective in the very flexible VAVW mutant, whose hysteresis is very small at low concentrations. Likewise, the wild-type-based VAVA mutant has a much more slowly increasing activity as a function of concentration. The mechanism by which long-range solvent perturbation affects hysteresis activity remains to be elucidated: perhaps long-range alteration of the hydrogen-bonding rearrangement dynamics impedes locking of water molecules to the nascent ice surface bound to the AFP, thus preventing ice growth.
Evolution has produced two structurally different solutions to the antifreeze problem, the AFPs and AFGPs, which have similar THz excess and antifreeze activity on a mass-scaled basis. The AFGPs display many saccharide OH groups in a disordered manner (13), whereas wf-AFP-1 displays a few threonine OH groups projecting from one side of a relatively rigid helix. Given that all AFPs including the hyperactive insect antifreeze (hysteresis as high as 6°C) bind irreversibly to ice, we predict that a long-range dynamical hydration shell plays an important role in insect antifreeze proteins, enhancing their activity at low concentration.
Acknowledgments
We thank E. Bründermann for initial set-up of the expriment, and S. Funkner, G. Niehues, and S. Vachharajani for valuable assistance.
This work was partially funded by the Volkswagen Stiftung, with additional funding provided by National Science Foundation grants MCB-1019958 and OPP-0231006, and by Bochum University. J.D. acknowledges funding from the Deutsche Forschungsgemeinschaft Emmy-Noether-Program and computing time from the Leibniz Rechenzentrum.
Supporting Material
References and Footnotes
- 1.Devries A.L., Lin Y. Structure of a peptide antifreeze and mechanism of adsorption to ice. Biochim. Biophys. Acta. 1977;495:388–392. doi: 10.1016/0005-2795(77)90395-6. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J.A., DeVries A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA. 1977;74:2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao H.M., Houston M.E., Jr., Sönnichsen F.D. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry. 1997;36:14652–14660. doi: 10.1021/bi970817d. [DOI] [PubMed] [Google Scholar]
- 4.Patel S.N., Graether S.P. Structures and ice-binding faces of the alanine-rich type I antifreeze proteins. Biochem. Cell Biol. 2010;88:223–229. doi: 10.1139/o09-183. [DOI] [PubMed] [Google Scholar]
- 5.Ebbinghaus S., Meister K., Havenith M. Antifreeze glycoprotein activity correlates with long-range protein-water dynamics. J. Am. Chem. Soc. 2010;132:12210–12211. doi: 10.1021/ja1051632. [DOI] [PubMed] [Google Scholar]
- 6.Harding M.M., Ward L.G., Haymet A.D. Type I ‘antifreeze’ proteins. Structure-activity studies and mechanisms of ice growth inhibition. Eur. J. Biochem. 1999;264:653–665. doi: 10.1046/j.1432-1327.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 7.Li N., Andorfer C.A., Duman J.G. Enhancement of insect antifreeze protein activity by solutes of low molecular mass. J. Exp. Biol. 1998;201:2243–2251. doi: 10.1242/jeb.201.15.2243. [DOI] [PubMed] [Google Scholar]
- 8.Case D.A., Darden T.A., Kollman P.A. University of California; San Francisco, CA: 2006. AMBER9. [Google Scholar]
- 9.Berendsen H.J.C., Grigera J.R., Straatsma T.P. The missing term in effective pair potentials. J. Phys. Chem. A. 1987;91:6269. [Google Scholar]
- 10.Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 11.Ebbinghaus S., Kim S.J., Havenith M. An extended dynamical hydration shell around proteins. Proc. Natl. Acad. Sci. USA. 2007;104:20749–20752. doi: 10.1073/pnas.0709207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergner A., Heugen U., Haller E.E. New p-Ge THz laser spectrometer for the study of solutions: THz absorption spectroscopy of water. Rev. Sci. Instrum. 2005;76:63110. [Google Scholar]
- 13.Tsvetkova N.M., Phillips B.L., Yeh Y. Dynamics of antifreeze glycoproteins in the presence of ice. Biophys. J. 2002;82:464–473. doi: 10.1016/S0006-3495(02)75411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


