Abstract
Mechanosensors are important for many life functions, including the senses of touch, balance, and proprioception; cardiovascular regulation; kidney function; and osmoregulation. Many channels from an assortment of families are now candidates for eukaryotic mechanosensors and proprioception, as well as cardiovascular regulation, kidney function, and osmoregulation. Bacteria also possess two families of mechanosensitive channels, termed MscL and MscS, that function as osmotic emergency release valves. Of the two channels, MscL is the most conserved, most streamlined in structure, and largest in conductance at 3.6 nS with a pore diameter in excess of 30 Å; hence, the structural changes required for gating are exaggerated and perhaps more easily defined. Because of these properties, as well as its tractable nature, MscL represents a excellent model for studying how a channel can sense and respond to biophysical changes of a lipid bilayer. Many of the properties of the MscL channel, such as the sensitivity to amphipaths, a helix that runs along the membrane surface and is connected to the pore via a glycine, a twisting and turning of the transmembrane domains upon gating, and the dynamic changes in membrane interactions, may be common to other candidate mechanosensors. Here we review many of these properties and discuss their structural and functional implications.
Introduction
Many membrane proteins appear to sense mechanical forces. Some channels appear to be membrane-tension-gated and are often referred to as mechanosensitive (MS) channels, whereas other channels, specifically the ligand- and even voltage-gated channels, have been found to be modulated by membrane tension (1). Voltage and MS channels may even have a common evolutionary origin (2). Results from studies of amphipaths suggest that many of the characterized MS proteins, including microbial sensors and pumps, sense the biophysical properties of the membrane, specifically the tension within it (3–6). Given that protein-lipid interactions are of prime importance for these families of channels, surprisingly little is known about the dynamics of protein-lipid interactions upon membrane protein activation or channel gating. However, one molecule, the bacterial MS channel of large conductance (MscL), is now evolving as a paradigm of the dynamics of protein-lipid interactions upon channel gating.
Two crystal structures have been obtained for MscL. The first shows a homopentameric structure that appears to be a closed or nearly closed state of the Mycobacterium tuberculosis MscL (Tb-MscL) channel. Several lines of evidence suggest that this structure may reflect a channel that is close to but does not completely reflect the in vivo closed state (7). The second structure shows a C-terminal-truncated Staphylococcus aureus channel (Sa-MscL) with a homotetrameric oligomerization rather than a pentameric one as expected (8). Although the structure was originally thought to reflect an intermediate-gating state, it did not fit well with some expectations for structural changes that should occur. Specifically, several lines of evidence suggested that a corkscrew motion of the first transmembrane domain (TMD1) occurs early in the gating process (7,9), but this was not seen in this putative gating intermediate. Indeed, in subsequent studies, no significant amount of Sa-MscL tetramer was observed in vivo (9,10). The reorganization of the pentamer into a tetrameric complex appears to be a detergent-dependent process. Specifically, the detergent LDAO, which was used in the crystallization process, appears to induce this oligomeric shift, whereas the detergents Triton X-100 and C5E8 do not (9). Interestingly, the oligomeric rearrangement appears to be reversible (9). Although there are some indications that the truncation of the protein may have played a role in amplifying this oligomeric reorganization (11), the observation that even the truncated Sa-MscL protein oligomerizes into pentamers in vivo strongly supports the notion that tetramerization of this channel is largely a detergent-dependent phenomenon (10). Perhaps MscL, as a mechanosensor, is more sensitive to changes in its environment because of its intimate connection to the lipid bilayer. This observation of detergent-dependent reorganization of the protein not only serves as a cautionary tale for interpreting crystallographic results, it may also underscore the importance of using a normal lipid-bilayer environment, or detergents that better mimic this milieu, to determine physiological structural elements.
Because it appears to be closer to a physiological state, we show the nearly closed structure derived from the Tb-MscL (Fig. 1, top). The protein starts with an amphipathic α-helix that appears to lie along the membrane. A glycine hinge is seen at position 12 (14 in Escherichia coli), leading to TMD1, which as can be seen from above forms the pore constriction site, followed by a periplasmic loop region, and then the second transmembrane domain (TMD2), which is in contact with the lipid bilayer. The protein ends with a pentameric α-helix bundle to which each of the five subunits contributes, and a linker connects TMD2 with this helical bundle. Because of its tractability, almost all functional studies have focused on the E. coli MscL (Eco-MscL). In orchestration with another channel family, MscS, the MscL channel functions as a biological emergency release valve that opens upon acute decreases in the osmotic environment to protect the cell from lysis (12). Upon stimulation, the channel opens a massive pore that has been predicted by molecular sieving experiments to exceed 30 Å (13). The channel is predicted to decrease its thickness within the membrane and to open like the iris of an old-fashioned camera, with the first TMD largely forming the pore (14,15). A model for the type of rearrangement that occurs among the TMDs for the Eco-MscL, as determined by EPR and other methods (15), is shown in the bottom panel of Fig. 1. Early mutagenesis work with Eco-MscL suggested that residues along both the periplasmic and cytoplasmic aqueous/lipid boundaries are important for normal function (16,17); however, the periplasmic region of the pore forms a vestibule, whereas the cytoplasmic region better defines the pore constriction. Indeed, as discussed below, recent evidence centers more on the cytoplasmic subdomains and the importance of the dynamics of protein-lipid interactions in this region.
Figure 1.
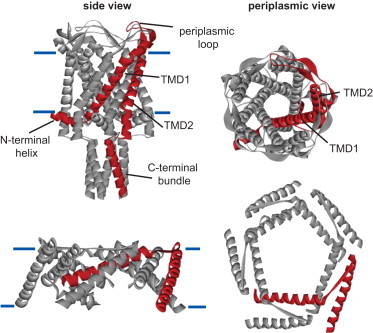
Structure of the homopentameric MS channel MscL from M. tuberculosis and a model for the structure of the TMDs of E. coli MscL in the open structure. The top panels show the structure of the nearly closed Tb-MscL as resolved in the crystal structure. The relatively simple topology of each MscL subunit can be observed in the side view (left), with a single subunit highlighted in red for clarity. The approximate position of the lipid membrane is indicated by the horizontal blue lines. The N-terminus forms a helix that lies along the membrane and connects with TMD1. TMD1 crosses the membrane lining the pore of the channel, as can be clearly observed in the periplasmic view (right). TMD1 is connected to TMD2 by a periplasmic loop. TMD2 surrounds the TMD1 helix and is in contact with the membrane. Finally, a cytoplasmic loop connects TMD2 with a cytoplasmic helix that forms a bundle at the C-terminal end of the channel. The bottom panels show similar side (left) and periplasmic (right) views of a theoretical model based on EPR and other experimental data (15) for what the TMDs might look like in the open structure. Note that the N- and C-terminal domains and periplasmic loop are not shown; the TMDs are connected with a straight line for orientation purposes.
In this review, we first concentrate on what we refer to as lipid-protein interactions (specifically, the important and not-so-important properties of the lipid membrane) and discuss what the channel may actually be sensing. We assess whether the channel senses the pressure across the membrane or the tension within it. We then discuss whether hydrophobic mismatch or changes in the lateral pressure profile of the membrane have a vital role in transducing the energy of membrane tension to the protein to induce conformational changes. Next, we address the issue of whether specific lipid headgroups interact with the protein and modify channel activity. Finally, we discuss what is known about protein-lipid interactions in different regions of the protein, including recent studies on domains within the cytoplasmic aqueous-lipid interface.
Pressure across the membrane or the tension within it, hydrophobic mismatch, or changes in the lateral pressure profile: what is actually sensed?
Perhaps the first issue to address when discussing what is actually sensed is whether the channel senses pressure across a membrane (analogously to a trashcan lid that opens upon pressure within the can) or the tension lateral to and within the membrane. By La Place's law, tension (T) within a membrane is proportional to the pressure across it (P) and the radius of curvature (r) of the matrix in the following relationship: T = 1/2 P · r. The pressure can be measured by a pressure transducer, and with good optics one can observe the radius of curvature of a patched membrane and thus calculate the tension. Moe and Blount (18) studied several membrane patches with quite different radius-of-curvature values, and found that although they yielded different pressure profiles for Eco-MscL gating, when tension was calculated and plotted, they all gated at the same calculated tension. Hence, it appears that pressure across the membrane plays no detectable role in MscL channel gating, and membrane tension is by far the primary stimulus.
We are left, then, with one or more of the biophysical properties of the membrane contributing to the sensed stimulus. The first property to consider is membrane thinning. The best estimates are that a normal, naked biological membrane can compress by only a few percent, depending on its composition (19). It seems unlikely that this would be enough to induce large protein shifts in conformation. On the other hand, when a protein is within a membrane, it can influence the membrane thickness around it through hydrophobic mismatch (20). This suggests a potential interactive dynamics between membrane thickness and protein structure, i.e., each can influence the other. Indeed, when a Tb-MscL F80W channel was studied by quenching of Trp fluorescence by brominated phospholipids in lipids of different lengths, the results showed that lipid-binding constants changed by only a factor of 1.5 in the chain length range from C12 to C24, which is much less than expected from theories of hydrophobic mismatch in which the protein is treated as a rigid body (21). This suggests that MscL distorts to match changes in bilayer thickness. Consistent with this proposed protein-lipid interplay, as discussed above and shown in Fig. 1 (bottom), the Eco-MscL protein is thought to thin in the plane of the membrane upon gating; thus, it is undoubtedly changing the thickness of the membrane surrounding it. To directly determine whether membrane thickness plays a role in Eco-MscL channel gating, Perozo et al. (6) reconstituted the protein into lipids of varying carbonyl chain length. Thinning of the membrane did not lead to channels that gated spontaneously, but did make them easier to gate, suggesting it may modulate gating but was not the primary stimulus. On the other hand, the same study demonstrated that the amphipath lysophosphatidylcholine (LPC), which would intercalate into the membrane asymmetrically, gated the channel. Thus, it appears that distortion of the membrane's lateral pressure profile is a stimulus for gating. These data are consistent with several other mechanosensors that appear to be activated by such amphipaths (1). However, it is important to note that changes within the lateral pressure profile, rather than the curving of the membrane per se, are the stimulus for MscL gating (18).
Are negatively charged lipids required for normal MscL function?
For several membrane proteins, including osmotically regulated transporters (22), negatively charged lipids appear to play a vital role in their function. For MscL, the data are complicated. One of the standard assays for MscL activity in a reconstituted system is the flux of calcein from liposomes. The calcein dye is quenched when at high concentration in the liposomes, and only gives signal when fluxed. One can activate the channel chemically using the amphipath LPC, or by inducing a charge in the pore by treating an MscL cysteine mutant (usually Eco-MscL G22C) with the charged sulfhydryl reagent MTSET+. When incorporated into liposomes containing only the zwitterionic headgroup PC, Eco-MscL was shown to have a blunted calcein flux response compared with liposomes containing negatively charged lipids (23). This finding was interpreted to mean that a significant number of channels are in an inactive conformation when they are incorporated into membranes devoid of anionic lipids. Indeed, basic calculations suggest that the incorporation rate of active channels into liposomes, even those containing anionic lipids, is only 0.7%. However, previous findings obtained by patch-clamp assay showed that when Eco-MscL is incorporated into pure PC lipids, its function is indistinguishable from that observed when it is incorporated into mixed lipid bilayers containing anionic lipids (18). One of the major differences between the flux and patch-clamp studies is the process used to incorporate the purified MscL into the lipid bilayers. MscL was incorporated directly into the liposomes for the flux studies, whereas a dehydration step, which allows the lipids to form a multibilayer lattice, was used for the patch-clamp studies. In the latter approach, liposomes, or blisters, were encouraged to form after rehydration by the addition of Mg2+ (24). Could the differences in results then be due to the efficiency of incorporation into the lipids, or even the clustering of channels into a subpopulation of vesicles? The MscL protein does appear to incorporate at reasonable levels in the flux system (21); however, a direct and quantitative comparison of incorporation into different lipids has not been performed to date. Furthermore, although the observation that brominated lipids efficiently quench the fluorescence of a tryptophan MscL mutant (F93W) suggests that MscL channels are not highly clustered (23), MscL channel clustering has been proposed by another study (25). Regardless, it is clear that in one liposome reconstitution system, fewer functional channels are incorporated when anionic lipids are absent. The dehydration/rehydration approach may bypass this by either forcing incorporation or selecting for bilayers bearing functional channels in the blister formation process. In summary, although anionic lipids appear to play a role in the incorporation of functional MscL channels in one reconstituted system, in another system the patch-clamp data demonstrate that anionic lipids are not required for normal activity, and thus the hypothesis that anionic lipids regulate MscL function in vivo remains speculative.
The N-terminal amphipathic α-helix: S1 stabilizer, anchor domain, and slide helix
In the original M. tuberculosis crystal structure, the N-terminal region of the protein (specifically, the first nine amino acids) was not resolved (26). However, a reevaluation of the structure resolved the placement of this region as an amphipathic α-helix lying along the cytoplasmic membrane (27). This region is highly conserved, suggesting its importance. In particular, there are two phenylalanines. According to the E. coli register for amino acid location, the phenylalanines at positions 7 and 10 are 97% and 100% conserved, respectively, in an alignment of 232 species; in the few outliers, they are substituted only by leucines (28). The S1 domain motif of XXYYFYYFXX (with X being hydrophobic and Y polar amino acids) is conserved in 79%, showing only small variations. This motif is even conserved in species that have as many as 30 additional amino acids N-terminal to this helix. In addition, early mutagenic and deletion studies touted the importance of this region of the protein for channel function. Even small deletions in this region of three, eight, or 11 amino acids, or substitution of nine amino acids of the S1 domain with a random sequence, can lead to channels with either decreased sensitivity or total loss of activity (29,30).
Iscla et al. (28) serially mutated every residue in the Eco-MscL S1 helix to cysteine, and evaluated the probability of disulfide bridging between subunits. They found that several of the mutant channel activities under variable redox potentials were consistent with the crystal structure showing this region running along the cytoplasmic membrane. In evaluating the potential function of the S1 domain, it may be important to note that all current models for the gating of MscL predict a significant tilting of the TMD1 membrane upon opening (15,31–33), and several lines of evidence also suggest a clockwise corkscrewing of TMD1 of almost 180°, as would be observed from the periplasm (14,15,28,33,34). It therefore seems likely that the S1 domain helps to define the tilt and turns of TMD1 by maintaining its interaction with the membrane, thus serving as an anchor (Fig. 2 A). Interestingly, the two most conserved residues within the S1 domain, F7 and F10 (represented as red hexagons in Fig. 2 A), have a high affinity for the lipid environment. Given this functional role, the highly conserved glycine at position 14 in E. coli would appear to be a hinge between TMD1 and S1 domains (Fig. 2 A). This structure, a helical membrane running along the cytoplasmic membrane and connected to the pore domain via a flexible glycine, is found in several other channels where its perturbation can often have functional consequences. For example, the bacterial inward rectifying K+ channel KirBac harbors a cytoplasmic α-helix running parallel to the membrane (slide helix) (35), which was found to directly interact with the phospholipids headgroups and thus regulate channel gating (36). In another study, the aromatic nature of a residue at the cytoplasmic end of the putative pore-forming TMD6 domain of the MS yeast TRPY1 channel was shown to be important for gating, and has been referred to as a gate anchor (37). This region could potentially form an amphipathic α-helix, although the structure of this channel has not yet been determined. A crystallographic structure of MscS, another bacterial MS channel from an independent channel family, also appears to contain an α-helix along the cytoplasmic/membrane surface just adjacent to the pore (27,38). In similarity to MscL, current models of MscS gating predict a tilting of the pore domain (39), and a glycine (Gly-113) is also present between these two domains, which probably serves as a hinge (27,38). Finally, a recently solved structure of the mammalian lipid-MS channel TRAAK shows a cytoplasmic amphipathic helix after the first pore domain. The ability of this inner helix to interact with both the hydrophobic tails and acidic headgroups of the membrane lipids has been proposed to be related to the channel's mechanosensitivity (40). This is only a partial list; several other examples of channels containing cytoplasmic α-helix running parallel to the membrane also exist. Thus, MscL may be using a common strategy among channels, i.e., using a slide helix as an anchor domain to stabilize the opening transmembrane pore within the bilayer.
Figure 2.
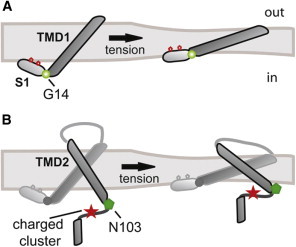
Schematic representation of the interactions between MscL TMDs and the lipid membrane. (A) TMD1 and the N-terminal helix (S1) are represented in the closed (left) and open (right) states. The position of Gly-14 between the S1 and TMD1 domains is shown as a green sphere. The conserved phenylalanine residues at positions 7 and 10 in the S1 domain are shown as red hexagons. (B) A single MscL subunit is represented in closed (left) and open (right) states. TMD2, the cytoplasmic loop, and the C-terminal helix are highlighted in a darker color. The positions of residue N103 and the charged cluster (RKKEE) are shown as a green hexagon and a red star, respectively.
Dynamics of the cytoplasmic end of TMD2
Early random mutagenesis studies not only indicated the importance of the pore domain in channel function but also revealed a handful of gain-of-function and loss-of-function mutations lining the aqueous-lipid interface at the cytoplasmic end of TMD2 (41,42). The cytoplasmic end of the TMD2 has been defined experimentally. Probe accessibility data and electron paramagnetic resonance (EPR) studies determined a membrane span for Eco-MscL of 25 residues from His-74 to Leu-98 (43), and tryptophan fluorescence spectra of Tb-MscL showed a transmembrane span comparable to that observed by EPR (44).
Five amino acids after the predicted end of TMD2, there is a cluster of charged residues (RKKEE) from residues 104–108 in Eco-MscL (Fig. 2 B). An early mutagenesis study of Eco-MscL showed that a C-terminal deletion at this point leads to nonfunctional channels (29), and another study suggested that changing the composition of the region can change the pH modulation of the channel (45). The RKK within the sequence has also been suggested to interact with anionic phospholipids, and mutation of these residues to glutamines influences channel conformation and function (46).
In a recent study, Iscla et al. (47) investigated the potential dynamics of the region. They screened a library of individual cysteine mutations on the cytoplasmic side of TMD2 from residues L98C–E108C before and after posttranslational modification with hydrophobic MTS reagents. Importantly, the screen involved an in vivo assessment of the viability of the cells expressing the modified protein, precluding the need for any protein purification or in vitro assays. The scan revealed a hot spot at position N103 in which changes in polarity showed the greatest effects in channel function with multiple reagents. Patch-clamp analysis, site-directed mutagenesis, and tryptophan fluorescence measurements confirmed these findings. In summary, the data strongly suggest that this region is critical for MscL gating, and that N103 transiently enters the headgroup region of the bilayer during the normal gating process, as shown in Fig. 2 B. Interestingly, a series of charged amino acids distal to a cytoplasmic transmembrane helix were noted in other channel families predicted to have MS channel members (see Kloda et al. (45) for a discussion), suggesting that here again, MscL may be utilizing conserved or analogous molecular mechanisms.
Conclusions
The bacterial MscL channel is the largest conducting gated pore and functions as an osmotic emergency release valve. By exploiting the exaggerated conformational changes that occur in the MscL channel, as well as its tractable nature, investigators have been able to use an assortment of approaches including bacterial genetics involving in vivo screens, biochemical screens, and electrophysiological characterization in both native and synthetic membranes to obtain a detailed assessment of the structure-function relationships within this channel. It thus serves as a model for how a protein can sense and respond to a mechanical force. Many of the findings regarding how this channel functions now appear to be common themes. We now know that MscL senses the tension in the membrane—more specifically, changes within the lateral pressure profile (6,18). MS currents from yeast (48) and chick skeletal muscle (49) have also shown to respond to tension, suggesting that this may be the stimulus sensed by eukaryotic mechanosensors as well. Indeed, when exposed to amphipaths that intercalate into the membrane and thus cause stress within the bilayer, many eukaryotic channels show lower thresholds or spontaneous activity, suggesting that membrane tension is a common stimulus for MS channel activation (1). Furthermore, the observation that the channel can function in a bilayer composed of the zwitterionic phosphatidylcholine, which is not synthesized by E. coli, demonstrates that interactions with negatively charged lipids or native lipid headgroups are not required for normal MS channel activity (18). Both TMDs tilt within the membrane, and TMD1 rotates in a corkscrew fashion clockwise, as observed from the periplasm (14,15,33,34). The S1 amphipathic helix that runs along the cytoplasmic membrane and is attached to this gating pore domain via a flexible glycine appears to serve as a stabilizer for the twists and turns this pore-forming TMD1 must undergo, and such an anchor or slide helix appears to be a common feature among many channels (28). Finally, we now have evidence that at least one region of the protein near the lipid-interacting TMD2 is extremely dynamic and embeds itself within the membrane during the gating process. Future experiments will show whether this feature is shared with other mechanosensors. Undoubtedly, given its tractable nature, the MscL channel will continue to reveal how it senses and responds to membrane stretch. Investigators must now determine whether these gating principles are also common among mechanosensors and other channels.
Acknowledgments
P.B. was supported by grants I-1420 from the Welch Foundation, NNH08ZTT003N NRA from NASA, RP100146 from the Cancer Prevention and Research Institute of Texas, and AI080807 and GM061028 from the National Institutes of Health. I.I. was supported by grant 12SDG8740012 from the National American Heart Association.
References
- 1.Blount P., Li Y., Iscla I. Mechanosensitive channels gated by membrane tension: bacteria and beyond. In: Kamkin A., Kiseleva I., editors. Mechanosensitive Ion Channels. Springer Press; New York: 2008. pp. 71–101. [Google Scholar]
- 2.Kumánovics A., Levin G., Blount P. Family ties of gated pores: evolution of the sensor module. FASEB J. 2002;16:1623–1629. doi: 10.1096/fj.02-0238hyp. [DOI] [PubMed] [Google Scholar]
- 3.Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 4.Glaasker E., Heuberger E.H., Poolman B. Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactobacillus plantarum. J. Bacteriol. 1998;180:5540–5546. doi: 10.1128/jb.180.21.5540-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Heide T., Poolman B. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA. 2000;97:7102–7106. doi: 10.1073/pnas.97.13.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perozo E., Kloda A., Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 7.Blount P., Iscla I., Li Y. MscL: the bacterial mechanosensitive channel of large conductance. In: Hamill O.P., editor. Mechanosensitive Ion Channels. Elsevier Press; St. Louis, MO: 2007. pp. 202–233. [Google Scholar]
- 8.Liu Z., Gandhi C.S., Rees D.C. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorwart M.R., Wray R., Blount P. S. aureus MscL is a pentamer in vivo but of variable stoichiometries in vitro: implications for detergent-solubilized membrane proteins. PLoS Biol. 2010;8:e1000555. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iscla I., Wray R., Blount P. The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Protein Sci. 2011;20:1638–1642. doi: 10.1002/pro.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi C.S., Walton T.A., Rees D.C. OCAM: a new tool for studying the oligomeric diversity of MscL channels. Protein Sci. 2011;20:313–326. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levina N., Tötemeyer S., Booth I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruickshank C.C., Minchin R.F., Martinac B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 1997;73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlett J.L., Levin G., Blount P. An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 2004;101:10161–10165. doi: 10.1073/pnas.0402040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perozo E., Cortes D.M., Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 16.Maurer J.A., Dougherty D.A. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J. Biol. Chem. 2003;278:21076–21082. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura K., Nomura T., Sokabe M. Loss-of-function mutations at the rim of the funnel of mechanosensitive channel MscL. Biophys. J. 2004;86:2113–2120. doi: 10.1016/S0006-3495(04)74270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe P., Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 19.Koenig B.W., Strey H.H., Gawrisch K. Membrane lateral compressibility determined by NMR and x-ray diffraction: effect of acyl chain polyunsaturation. Biophys. J. 1997;73:1954–1966. doi: 10.1016/S0006-3495(97)78226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janmey P.A., Kinnunen P.K. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Powl A.M., East J.M., Lee A.G. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- 22.Poolman B., Blount P., van der Heide T. How do membrane proteins sense water stress? Mol. Microbiol. 2002;44:889–902. doi: 10.1046/j.1365-2958.2002.02894.x. [DOI] [PubMed] [Google Scholar]
- 23.Powl A.M., East J.M., Lee A.G. Anionic phospholipids affect the rate and extent of flux through the mechanosensitive channel of large conductance MscL. Biochemistry. 2008;47:4317–4328. doi: 10.1021/bi702409t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blount P., Sukharev S.I., Kung C. Mechanosensitive channels of bacteria. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- 25.Grage S.L., Keleshian A.M., Martinac B. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys. J. 2011;100:1252–1260. doi: 10.1016/j.bpj.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang G., Spencer R.H., Rees D.C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 27.Steinbacher S., Bass R., Rees D.C. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Hamill O.P., editor. Mechanosensitive Ion Channels. Elsevier Press; St. Louis, MO: 2007. pp. 1–20. [Google Scholar]
- 28.Iscla I., Wray R., Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys. J. 2008;95:2283–2291. doi: 10.1529/biophysj.107.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blount P., Sukharev S.I., Kung C. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1996;93:11652–11657. doi: 10.1073/pnas.93.21.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Häse C.C., Le Dain A.C., Martinac B. Molecular dissection of the large mechanosensitive ion channel (MscL) of E. coli: mutants with altered channel gating and pressure sensitivity. J. Membr. Biol. 1997;157:17–25. doi: 10.1007/s002329900212. [DOI] [PubMed] [Google Scholar]
- 31.Sukharev S., Betanzos M., Guy H.R. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 32.Sukharev S., Durell S.R., Guy H.R. Structural models of the MscL gating mechanism. Biophys. J. 2001;81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Wray R., Blount P. An open-pore structure of the mechanosensitive channel MscL derived by determining transmembrane domain interactions upon gating. FASEB J. 2009;23:2197–2204. doi: 10.1096/fj.09-129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iscla I., Levin G., Blount P. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys. J. 2004;87:3172–3180. doi: 10.1529/biophysj.104.049833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo A.L., Gulbis J.M., Doyle D.A. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 36.Enkvetchakul D., Jeliazkova I., Nichols C.G. Control of inward rectifier K channel activity by lipid tethering of cytoplasmic domains. J. Gen. Physiol. 2007;130:329–334. doi: 10.1085/jgp.200709764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X., Su Z., Saimi Y. Yeast screens show aromatic residues at the end of the sixth helix anchor transient receptor potential channel gate. Proc. Natl. Acad. Sci. USA. 2007;104:15555–15559. doi: 10.1073/pnas.0704039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bass R.B., Strop P., Rees D.C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 39.Edwards M.D., Li Y., Booth I.R. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat. Struct. Mol. Biol. 2005;12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 40.Brohawn S.G., del Mármol J., MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurer J.A., Dougherty D.A. A high-throughput screen for MscL channel activity and mutational phenotyping. Biochim. Biophys. Acta. 2001;1514:165–169. doi: 10.1016/s0005-2736(01)00390-x. [DOI] [PubMed] [Google Scholar]
- 42.Ou X., Blount P., Kung C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perozo E., Kloda A., Martinac B. Site-directed spin-labeling analysis of reconstituted Mscl in the closed state. J. Gen. Physiol. 2001;118:193–206. doi: 10.1085/jgp.118.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powl A.M., Wright J.N., Lee A.G. Identification of the hydrophobic thickness of a membrane protein using fluorescence spectroscopy: studies with the mechanosensitive channel MscL. Biochemistry. 2005;44:5713–5721. doi: 10.1021/bi047338g. [DOI] [PubMed] [Google Scholar]
- 45.Kloda A., Ghazi A., Martinac B. C-terminal charged cluster of MscL, RKKEE, functions as a pH sensor. Biophys. J. 2006;90:1992–1998. doi: 10.1529/biophysj.105.075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powl A.M., East J.M., Lee A.G. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 2005;44:5873–5883. doi: 10.1021/bi047439e. [DOI] [PubMed] [Google Scholar]
- 47.Iscla I., Wray R., Blount P. An in vivo screen reveals protein-lipid interactions crucial for gating a mechanosensitive channel. FASEB J. 2011;25:694–702. doi: 10.1096/fj.10-170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustin M.C., Zhou X.L., Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- 49.Sokabe M., Sachs F., Jing Z. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys. J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


