Abstract
Lipid lateral segregation in the plasma membrane is believed to play an important role in cell physiology. Sphingomyelin (SM) and cholesterol (Chol)-enriched microdomains have been proposed as liquid-ordered phase platforms that serve to localize signaling complexes and modulate the intrinsic activities of the associated proteins. We modeled plasma membrane domain organization using Langmuir monolayers of ternary POPC/SM/Chol as well as DMPC/SM/Chol mixtures, which exhibit a surface-pressure-dependent miscibility transition of the coexisting liquid-ordered and -disordered phases. Using Brewster angle microscopy and Langmuir monolayer compression isotherms, we show that the presence of an oxidatively modified phosphatidylcholine, 1-palmitoyl-2-azelaoyl-sn-glydecero-3-phosphocholine, efficiently opposes the miscibility transition and stabilizes micron-sized domain separation at lipid lateral packing densities corresponding to the equilibrium lateral pressure of ∼32 mN/m that is suggested to prevail in bilayer membranes. This effect is ascribed to augmented hydrophobic mismatch induced by the oxidatively truncated phosphatidylcholine. To our knowledge, our results represent the first quantitative estimate of the relevant level of phospholipid oxidation that can potentially induce changes in cell membrane organization and its associated functions.
Introduction
Thousands of different lipids comprise eukaryotic cell membrane systems and play a crucial role in maintaining cell integrity and organelle compartments, signal transduction, and embedded protein functionalities (1). Glycerophospholipids enriched in unsaturated acyl chains are thought to be balanced by cholesterol (Chol) and sphingolipids to modulate lipid lateral packing and bilayer morphological dynamics. Colocalization of Chol and sphingolipids is responsible for their lateral segregation into liquid-ordered (Lo) phase domains (2,3), allegedly providing a mechanism for lipid and protein sorting in the plasma membrane (4). Under ambient conditions, ternary mixtures consisting of a low main-transition temperature (Tm) lipid, high-Tm sphingomyelin (SM), and Chol segregate into a low-Tm lipid-enriched liquid-disordered phase (Ld) and SM- and Chol-enriched Lo phase (5–7). Monolayer compression isotherms have shown that this liquid-liquid phase coexistence is surface pressure dependent, and upon compression the components undergo a miscibility transition to produce one homogeneous phase (8). Intriguingly, oxidation has been found to stabilize phase segregation, preventing the miscibility transition in monolayers and vesicle systems (8–10). A nanoscale analysis of such mixed lipid monolayers revealed that oxygen-induced lipid oxidation can cause condensed domain size evolution at surface pressures well above the expected miscibility transition (11). Similarly, the formation of large domains was observed in giant unilamellar vesicles upon their photooxidation and production by electroswelling (12). These findings raise questions concerning the validity of experimental data obtained in the presence of potential peroxide sources. Moreover, nonconformity in the oxidation techniques used and the complexity of the oxidized system impose ambiguity regarding the exact nature and extent of the induced chemical modification. Therefore, it is essential to achieve full control over the type of changes that are induced and the degree of lipid oxidation to further elaborate the consequences of oxidative stress.
Here, we used for the first time (to our knowledge) a specific oxidatively modified phospholipid to examine the effect of oxidative stress on the lipid miscibility transition in a lipid monolayer model of plasma membrane. We were able to determine the effective concentration that is sufficient for the breakdown of the miscibility transition and the formation of micron-sized domains. Taken together, our findings indicate a potential consequence of oxidative stress in membrane microdomain segregation mechanisms.
Materials and Methods
Materials
1-Palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (PazePC), 1-palmitoyl-2-oleyl-sn-glycero-3-phosphatidylcholine (POPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-ditridecanoyl-sn-glycero-3-phosphocholine (DTPC), Chol, and bovine brain SM (bSM) were obtained from Avanti Polar Lipids (Alabaster, AL). Phosphate-buffered saline tablets were obtained from Sigma (St. Louis, MO). Phospholipid concentrations were determined gravimetrically with a high-precision electrobalance (Cahn Instruments, Cerritos, CA). All buffers were prepared with deionized water that was glass distilled and purified further by a Milli-Q system (Millipore, Milford, MA).
Methods
Monolayer measurements
A computer-controlled Langmuir-type film balance (μTrough XL; Kibron, Helsinki, Finland) equipped with a Precision Plus trough was used to simultaneously measure π-A and Δψ-A isotherms, using the embedded features of the control software (FilmWare 3.52; Kibron). The indicated lipid mixtures were made in chloroform and spread onto the air-aqueous phase interface with a Hamilton microsyringe. After a 10 min equilibration (to ensure evaporation of the solvent), the film compression was started using two symmetrically moving barriers. In all measurements, the compression rate was 10 Å2/chain/min so as to allow for reorientation and relaxation of the lipids during the course of the compression. Surface pressure (π) was monitored with a metal alloy probe hanging from a high-precision microbalance (KBN 315; Kibron) connected to a computer. We measured the monolayer dipole potential ψ using the vibrating-plate method (μSpot; Kibron). The isotherms shown in the figures are representative of three experimental runs and were reproducible within ±2 Å2· molecule−1.
Brewster angle microscopy
An in-house-made Brewster angle microscope mounted on a Langmuir film balance was used to observe the microscopic structures in situ. The light source of the microscope was a diode laser emitting at 532 nm and an output power 50 mW in a collimated beam. Brewster angle microscopy (BAM) images were recorded with a CCD camera (DCU223M; Thorlabs, Newton, NJ). The scanner objective was a Nikon superlong working distance objective with a nominal 20× magnification and diffraction-limited lateral resolution of 2 μm. The images were corrected to eliminate side ratio distortion originating from the nonperpendicular line of vision of the microscope.
Results
We examined the effect of oxidative stress on the model lipid rafts monolayer system by analyzing ternary lipid mixtures containing POPC, bSM, and Chol. We also monitored changes in phase distribution and miscibility transition in monolayers where POPC was gradually replaced with its oxidatively truncated derivative, PazePC (Fig. 1 A). Several studies have identified PazePC as a primary product of POPC oxidation in monolayers at the air-water interface (13,14) and in unilamellar phospholipid vesicles (15). This PC derivative also exerts a number of effects in cells and promotes apoptosis (16,17).
Figure 1.
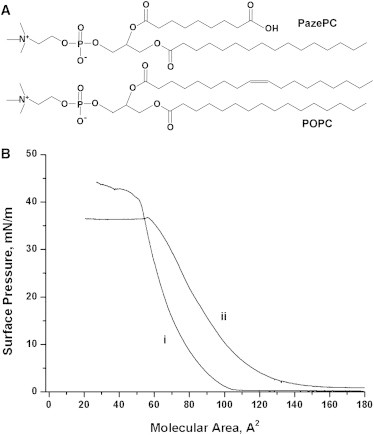
(A) Chemical structures of POPC and its oxidized analog, PazePC. (B) Compression isotherm of POPC (i) and PazePC (ii) at 25°C.
The choice of lipids was dictated by their physiological relevance and relative resistance to oxidation by ambient air, which was further confirmed in our experiments. First, the monolayers were allowed to equilibrate at the air-water interface for prolonged times (up to 2 h) before compression. The surface pressures of the miscibility transitions of the monolayers were found to match the values obtained from monolayers that were compressed immediately after solvent evaporation (viz., 10 min) after the mixture was spread on the air-water interface. Surface pressure stability was previously reported to be a good measure of monolayer oxidation resistance (18). Air-exposed monolayers of polyunsaturated lipids undergo oxidative deterioration, whereas the monounsaturated POPC is quite resistant to oxidation (13,18). Thus, air-induced peroxidation of 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) monolayers has been reported to result in a fast drop in surface pressure and trough area coverage on a timescale of tens of minutes (8,18). Of note, truncative peroxidation of both unsaturated hydrophobic moieties is required to effectively dissolve DOPC into the aqueous subphase (18). Apparently, the unsaturated and loosely packed DOPC membranes are more susceptible to oxidative deterioration than analogous systems composed of monounsaturated POPC. Unfortunately, a direct comparison of POPC and DOPC peroxidation in our experimental setup is impossible because peroxidation of POPC is not associated with a loss of material from the film, which would cause a drop in surface pressure. Corresponding mixed monolayers used in our experiments were tested for surface pressure stability at 20 mN/m and were found to sustain minimal pressure change (∼0.5 mN/m) for at least 2 h. Taken together, these results conform with previous studies in which monounsaturated lipids were found to exhibit augmented stability in ambient conditions, and more powerful oxidizing agents were generally needed to facilitate their chemical modification (13,19).
The recorded compression isotherms for pure compound monolayers (Figs. 1 B and 2 A) were in line with previously reported behavior (20–22). Data for POPC and PazePC monolayers reveal several interesting features (Fig. 1 B). First, PazePC extends over an ∼35% larger molecular area than POPC, which readily complies with a model in which the hydrophilic group bearing the sn-2 azelaoyl chain of PazePC would reverse its direction so as to accommodate the polar moiety in the vicinity of the lipid headgroups at the air-water interface. This is also in keeping with previous results (23,24) indicating that oxidatively modified acyl chains may locate closer to the interface than the corresponding unoxidized chains. This increase in molecular area may provide an explanation for the observed initial monolayer expansion during lipid oxidative deterioration at the air-water interface (18). Local truncative oxidation of one of the acyl chains in polyunsaturated lipids will apparently increase surface pressure under the area-restrictive conditions; however, further oxidation of the second chain, such as in DOPC, would vastly increase the water solubility of the oxidation products and subsequently lead to a drop in surface pressure (8). A sharp inflection followed by a plateau region at 38 mN/m in the PazePC compression isotherm is evident before the monolayer collapses, and most probably is associated with continuous dissolving of the oxidized lipid from a monolayer into the aqueous phase.
Figure 2.
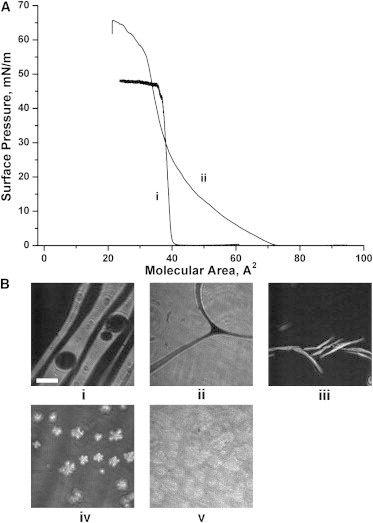
(A) Compression isotherms of Chol (i) and brain SM (ii) at 25°C. (B) BAM images recorded at the indicated surface pressures within the Chol isotherm ((i) 0, (ii) 10, and (iii) 47 m/Nm) and the SM isotherm ((iv) 10 and (v) 30 m/Nm). The bar represents 50 μm.
We then proceeded to study the Lo phase components, Chol and SM. The pressure-area isotherm of Chol shows a sharp increase in surface pressure corresponding to an extremely low compressibility, yielding highly packed monolayers. BAM detected the bright regions of the Chol liquid-condensed phase constituting the foam-like-patterned monolayer, which was already evident at the beginning of compression before the pressure rise (Fig. 2 B, i). Upon further compression, Chol formed homogeneous, optically isotropic, evenly reflective films (Fig. 2 B, ii) that eventually collapsed at ∼42 mN/m to form needle-shaped structures (Fig. 2 B, iii), in keeping with previous observations (21).
bSM was observed to form high-contrast, bright domains at 5 mN/m. The BAM images (Fig. 2 B, iv) show a domain fingering instability that reflects the competing effects of line tension and long-range dipolar interactions (25). These domains merge to produce a highly reflective film (Fig. 2 B, v) as the surface pressure approaches the collapse.
The impact of a gradual exchange of POPC to its oxidation product in PC/bSM/Chol (1.5:1.5:1) mixtures is presented in Fig. 3. Monolayers containing PazePC show noticeably higher average molecular areas, up to the pressure where the oxidized lipid dissolves into the aqueous phase. Importantly, all tested mixtures that contained more than ∼25% of PazePC (out of the total glycerophospholipid content) consistently showed a characteristic dissolution plateau region at 38 mN/m (Fig. 3 A). This suggests an immiscibility of the oxidized lipid in all of the above compositions, in keeping with previous studies in which the addition of oxidatively modified lipids to monolayers and vesicles was shown to involve phase separation and formation of domains exclusively populated by phospholipid oxidation products (23,26). In contrast, however, surface pressure-area isotherms of mixtures with lower PazePC content (up to 25%) showed an elevation in the dissolution plateau (Fig. 3 A), suggesting a possible miscibility of the oxidized lipid with other film components. These observations are further supported by surface potential measurements (Fig. 3 B). An inclination in compression isotherms associated with PazePC subphase dissolution was reflected in a concomitant drastic rise in surface potential values of ∼50 mV. Upon a decrease in the content of PazePC, the rise became more moderate at 12.5% PazePC and eventually completely disappeared (Fig. 3 B).
Figure 3.
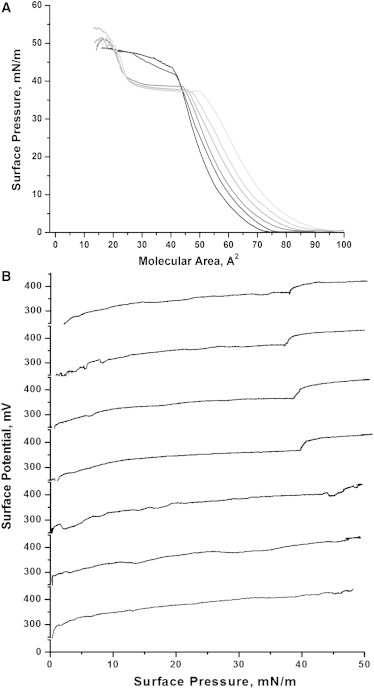
(A) Compression isotherms of (POPC + PazePC)/bSM/Chol mixtures (1.5/1.5/1 molar ratio). PazePC content in glycerophospholipid fraction increases (left to right) from 0.0, 12.5, 18.75, 25.0, 50.0 to 100% at 25°C. (B) Surface potentials of (POPC + PazePC)/bSM/Chol mixtures (1.5/1.5/1 molar ratio) with the indicated PazePC content within the glycerophospholipid fraction, which increases (bottom to top) from 0.0, 6.25, 12.5, 18.75, 25.0, and 50.0 to 100% at 25°C.
BAM images (Fig. 4) provide additional information about the monolayer phase distribution in the two mixtures. For POPC-containing monolayers, we observe a lateral segregation at low pressures into bright domains mostly comprised of SM and Chol, and a dark phase composed of the unsaturated POPC (Fig. 4, i). When the surface pressure approaches ∼10 mN/m, the lipid mixture passes through a miscibility transition and a homogeneous monolayer is observed at the lateral resolution of the setup (Fig. 4, ii). Intriguingly, this miscibility transition was completely absent in monolayers where all POPC was exchanged with PazePC, and segregated micron-sized bright domains were observed at all pressures (Fig. 4, iii and iv). Recently, a similar effect was shown to be modulated by air exposure of analogous mixtures, imparted by more readily oxidizable brain PC and DOPC lipids (8,10). Visual inspection of the mixed films further revealed a decrease in the size of the Lo domains, apparently induced by PazePC.
Figure 4.
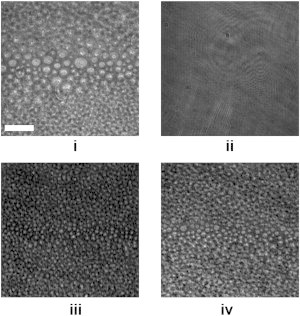
BAM images recorded at the indicated surface pressures within the POPC/bSM/Chol mixture isotherm ((i) 5 and (ii) 15 m/Nm) and the PazePC/bSM/Chol mixture isotherm ((iii) 5 m/Nm and (iv) 32 m/Nm). The bar represents 50 μm.
A surface analysis of a series of mixtures in which POPC was gradually exchanged with PazePC revealed a linear increase in the miscibility transition pressure with an increase in the content of PazePC (Fig. 5). According to these data, ∼20 mol % of the POPC (corresponding to 7.5 mol % of the total lipid) should be exchanged to its oxidized analog to induce transition at 32 mN/m, i.e., at a surface pressure that is considered to prevail in cellular bilayer membranes (27,28). Furthermore, this effect of PazePC on the miscibility transition can be used to test the lipid monolayer stability upon exposure to air. Visual inspection of the POPC/bSM/Chol monolayer did not show phase segregation for a system maintained at 15 mN/m in ambient conditions for at least 1 h. This phenomenon may attest for relative system stability, because according to our results, the addition of only 2.3 mol % (out of total lipids) of the oxidized lipid would induce phase segregation in the lipid mixtures employed at that pressure.
Figure 5.
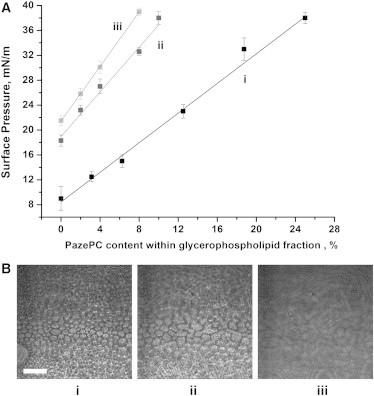
(A) Increase in miscibility transition surface pressures of (i) (POPC + PazePC)/bSM/Chol, (ii) (DMPC + PazePC)/bSM/Chol, and (iii) (DTPC + PazePC)/bSM/Chol monolayers (1.5/1.5/1 molar ratio) as a function of PazePC content within the glycerophospholipid fraction at 25°C. (B) BAM images recorded in the vicinity of the critical surface pressure (∼39 mN/m) within the DMPC/PazePC/bSM/Chol (34.5/3.0/37.5/25 molar ratio) mixture isotherm. Images i, ii, and iii are placed in order of appearance upon monolayer compression. The bar represents 50 μm.
To further address the effect of the oxidized lipid derivative (PazePC) on the miscibility transition in ternary lipid systems, we conducted additional experiments in which we varied the hydrophobic mismatch by using the shorter, fully saturated DMPC or DTPC instead of the Ld phase-forming POPC. Miscibility transitions in DMPC/bSM/Chol and DTPC/bSM/Chol mixtures were observed at elevated surface pressures of ∼18.3 and 23.5 mN/m, respectively (Fig. 5 A). In similarity to our previous results, the presence of PazePC further elevated the miscibility transition pressure in lipid mixtures containing DMPC or DTPC (Fig. 5 A). Although in these mixtures PazePC is not an oxidation product of any of the components, its concentration-dependent impact on the miscibility transition suggests an intrinsic property, i.e., the ability to modulate membrane physical parameters in manner that influences the two-phase coexistence.
Visual inspection of compressed monolayers revealed a characteristic domain size and shape transformation in close proximity to the miscibility transition, which was common for all mixtures. Fig. 5 B (i–iii) depicts BAM images of a DMPC/PazePC/bSM/Chol (34.5/3.0/37.5/25) monolayer at surface pressures close to the miscibility transition at ∼39 mN/m. At this pressure, closely packed, discrete circular domains (Fig. 5 B, i) begin to merge, forming large, randomly shaped structures (Fig. 5 B, ii) that finally undergo a miscibility transition observed as a sudden loss of contrast between Lo and Ld phases (Fig. 5 B, iii).
Discussion
Polyunsaturated lipids comprising the plasma membrane are particularly vulnerable to oxidation, a prominent hallmark of disease and apoptosis that is associated with elevated production of intracellular mitochondrial reactive oxygen species (29,30). In situ modification of lipid chemical structures as a result of oxidation damage can be expected to alter the lipid-packing efficiency, phase distribution, and transbilayer movement (31,32). Here, we assessed the impact of the presence of an oxidatively modified lipid on the phase behavior of a raft model monolayer system in which a low-Tm lipid (POPC) was exchanged for PazePC. Changes were immediately evident even at low PazePC contents (3 mol %). The miscibility transition pressure was shown to be particularly sensitive to the presence of PazePC. Just 3 mol % of POPC substituted by PazePC resulted in a marked elevation of the miscibility transition pressure to 12 mN/m, and 25 mol % PazePC was enough to completely eliminate the miscibility transition and stabilize phase segregation up to the collapse pressure of ∼48 mN/m. We also detected significant changes in the surface potential profiles and surface pressure-area isotherm in the same PazePC concentration range, with a linear increase in miscibility transition pressure. Both the increase in surface pressure of PazePC subphase dissolution and the concomitant disappearance of the pronounced rise in surface potential with a decrease in PazePC concentration definitely point to the miscibility of POPC and its oxidized analog above the molar ratio of 3:1. No significant changes in monolayer state were evident upon a further increase in the content of PazePC until all POPC was substituted by PazePC. The latter is most probably indicative of PazePC segregation into a separate phase. Taken together, these findings suggest that the oxidized lipid plays a significant role in altering the physicochemical properties of the Ld phase, which in turn affects Ld-Lo phase separation.
To further elucidate the underlying mechanism for PazePC-induced alterations, we considered the pioneering works of McConnell and De Koker (33) and McConnell (34), which suggested that a balance between lipid dipole repulsion and domain line tension controls the domain size and shape. Line tension reduces the domain boundary length and thus favors large, circularly shaped domains, whereas dipolar interactions favor small and striped domains. Although the addition of PazePC may alter the dipole density difference between Ld and Lo phases, no detectable changes in average dipole density were observed upon the miscibility transition, which suggests that no reorientation of molecular dipoles is taking place. No difference in surface potential has been reported for DLPC/pSM/Dchol mixtures with compositions corresponding to the same tie-line, which implies that coexisting liquid phases have similar dipolar properties (35). Moreover, a significant difference in dipole density would be expected to result in interfacial boundary destabilization associated with domain deformation and a decrease in miscibility transition pressure; however, the opposite is evident from our experiments. These considerations lead to the conclusion that dipole repulsion is not a critical determinant of domain line boundary destabilization upon phase mixing. This suggests that line tension is a major factor in the regulation of domain shape and stability. One of the notable parameters contributing to the line tension at the boundary between Lo and Ld phases is hydrophobic mismatch (36–38). Domains containing SM with saturated long acyl chains, associated with Chol, appear to be thicker than the surrounding glycerophospholipid-enriched Ld phase (39,40). This height mismatch in bilayer systems allows lipid molecule deformations at the interface to cope with energetically unfavorable hydrophobic tail exposure to a hydrophilic environment (41). Kuzmin et al. developed a theoretical model that directly translates the energetic cost of hydrophobic mismatch into the energy per unit length of boundary, i.e., line tension (42). This model predicts that line tension will increase quadratically with the initial difference in thickness between the Lo and its surrounding lipid phase.
In contrast to bilayers, hydrophobic mismatch in monolayers is primarily associated with acyl chain exposure at the hydrocarbon-air interface due to the unmatched thicknesses of Ld and Lo phases. This phenomenon remained largely unexplored in the literature until it was recently examined by Lee et al. (43), who elaborated on the dependence of the Lo domain size distribution on line tension, dipole moment density, and mixing entropy. These authors suggested that the mixing entropy promotes a greater number of domains, with sizes significantly smaller than would be predicted by the energy alone. Furthermore, the notion that domain size distribution simply reflects the magnitude of the aforementioned factors is doubtful given the significant height mismatch between phases. Monolayer deformations at a firm Lo phase boundary were proposed to stabilize small Lo domains by setting a repulsive barrier, thereby preventing domain merging under large hydrophobic mismatch conditions, and therefore the possibility that domains can be trapped kinetically cannot be excluded (42,44).
Chemical modification associated with oxidative damage (i.e., oxidative truncation of the longest acyl chain in the POPC molecule) can be readily expected to thin the Ld phase in a concentration-dependent manner. This assumption is supported by molecular-dynamics (MD) simulations and x-ray studies (24,31). MD studies have shown that incorporating oxidized lipids (similar to those studied here) and peroxidation products of linoleic acid into phospholipid bilayers increase the area per molecule and decrease the bilayer thickness. X-ray diffraction data showed a marked reduction from 36 Å to 32 Å in the hydrocarbon core width of dilinoleoyl phosphocholine bilayers upon Fe2+/ascorbate-induced peroxidation of ∼14.5% of the total lipid, and a decrease in overall membrane thickness, including surface hydration, from 48.7 Å to 44.6 Å (45). Similarly, a 4 Å reduction in hydrocarbon core width from 40 Å to 36 Å was observed in membranes reconstituted from bovine cardiac phosphatidylcholine, where only 1.1% of the available polyunsaturated fatty acyl chains underwent peroxidation. Additionally, alterations in intermolecular packing after oxidative stress have been shown to facilitate interdigitation of the terminal methyl segments, which favors a further decrease in membrane thickness.
Together, the above findings indicate that an augmented height mismatch between Ld and Lo phases induced by lipid peroxidation would lead to an increase in line tension, which in turn would oppose the miscibility transition. Indeed, phase separation is governed by a preferential interaction of the molecules composing the same phase. Line tension is practically representing the same phenomenon, as molecules accommodating along an interfacial boundary are exposed to have less favorable contacts with neighboring phase and consequently experience unequilibrium forces directed inside to the domain. Accordingly, line tension is primarily responsible for domain relaxation in the energy-minimizing circular configuration (46–48). A decrease in the thickness of the surrounding domain will make interphase contacts even more unfavorable, resulting in augmented line tension. Phase separation and domain formation usually take place upon a decrease in the temperature of a homogeneeous lipid phase (lowering the energy barrier for line tension to be significant for separation) (44). Logically, one would expect an augmented line tension associated with a phase thickness mismatch to result in a higher energy barrier for the miscibility transition. In support of this notion, an augmented height mismatch modulated by changing the length of PC acyl chains was shown to stabilize domain formation in supported lipid bilayers at higher temperatures (37).
Both line tension and hydrophobic mismatch have been shown to diminish with an increase in surface pressure (35,43,49), suggesting that monolayer compression can balance the effect of the oxidized lipid. Indeed, our findings show that higher surface pressures are required for monolayers to undergo miscibility transitions in mixtures where augmented height mismatch has been induced either by shorter lipids (DMPC or DTPC) or by the presence of PazePC (Fig. 5 A). Our hypothesis gains additional support from visual inspection of lipid monolayers in the vicinity of the miscibility transition pressure (Fig. 5 B). Thus, compression of the monolayers up to the critical pressure attenuated the effect of the hydrophobic mismatch, allowing domain merging, but also caused a loss of their circular shape due to the decrease in total line tension.
Although it is too early to generalize these results to physiologically relevant conditions, available experimental evidence indicates that oxidative stress may induce lipid phase segregation and stabilize Lo domain formation in cell plasma membrane.
Acknowledgments
This study was financed by the Finnish Academy (European Science Foundation EuroMEMBRANE project MEM/09/E006); the Department of Biomedical Engineering and Computational Science, Aalto University; and the Sigrid Juselius Foundation.
References
- 1.Mouritsen O. Springer; Berlin/Heidelberg: 2004. Life—As a Matter of Fat. [Google Scholar]
- 2.van Duyl B.Y., Ganchev D., Killian J.A. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 2003;547:101–106. doi: 10.1016/s0014-5793(03)00678-1. [DOI] [PubMed] [Google Scholar]
- 3.London E., Brown D.A. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim. Biophys. Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 4.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich C., Bagatolli L.A., Gratton E. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane J.M., Tamm L.K. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 2004;86:2965–2979. doi: 10.1016/S0006-3495(04)74347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida R.F.M., Loura L.M.S., Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Stottrup B.L., Stevens D.S., Keller S.L. Miscibility of ternary mixtures of phospholipids and cholesterol in monolayers, and application to bilayer systems. Biophys. J. 2005;88:269–276. doi: 10.1529/biophysj.104.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benvegnu D.J., McConnell H.M. Line tensions between liquid domains in lipid monolayers. J. Phys. Chem. 1992;96:6820–6824. [Google Scholar]
- 10.Stottrup B.L., Veatch S.L., Keller S.L. Nonequilibrium behavior in supported lipid membranes containing cholesterol. Biophys. J. 2004;86:2942–2950. doi: 10.1016/S0006-3495(04)74345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coban O., Popov J., Johnston L.J. Transition from nanodomains to microdomains induced by exposure of lipid monolayers to air. Biophys. J. 2007;92:2842–2853. doi: 10.1529/biophysj.106.088419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayuyan A.G., Cohen F.S. Lipid peroxides promote large rafts: effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys. J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai C.C., Yang S.H., Finlayson-Pitts B.J. Interactions of monolayers of unsaturated phosphocholines with ozone at the air-water interface. Langmuir. 1994;10:4637–4644. [Google Scholar]
- 14.Wadia Y., Tobias D.J., Finlayson-Pitts B.J. Real-time monitoring of the kinetics and gas-phase products of the reaction of ozone with an unsaturated phospholipid at the air-water interface. Langmuir. 2000;16:9321–9330. [Google Scholar]
- 15.Santrock J., Gorski R.A., O'Gara J.F. Products and mechanism of the reaction of ozone with phospholipids in unilamellar phospholipid vesicles. Chem. Res. Toxicol. 1992;5:134–141. doi: 10.1021/tx00025a023. [DOI] [PubMed] [Google Scholar]
- 16.Uhlson C., Harrison K., Murphy R.C. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem. Res. Toxicol. 2002;15:896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 17.Chen R., Feldstein A.E., McIntyre T.M. Suppression of mitochondrial function by oxidatively truncated phospholipids is reversible, aided by bid, and suppressed by Bcl-XL. J. Biol. Chem. 2009;284:26297–26308. doi: 10.1074/jbc.M109.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liljeblad J.F.D., Bulone V., Johnson C.M. Phospholipid monolayers probed by vibrational sum frequency spectroscopy: instability of unsaturated phospholipids. Biophys. J. 2010;98:L50–L52. doi: 10.1016/j.bpj.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slotte J.P. Enzyme-catalyzed oxidation of cholesterol in mixed phospholipid monolayers reveals the stoichiometry at which free cholesterol clusters disappear. Biochemistry. 1992;31:5472–5477. doi: 10.1021/bi00139a008. [DOI] [PubMed] [Google Scholar]
- 20.Vaknin D., Kelley M.S., Ocko B.M. Sphingomyelin at the air-water interface. J. Chem. Phys. 2001;115:7697–7704. [Google Scholar]
- 21.Cadena-Navaa R.D., Martin-Mironesa J.M., Ruiz-Garcıa J. Direct observations of phase changes in Langmuir films of cholesterol. Rev. Mex. Fis. E. 2006;52:32–40. [Google Scholar]
- 22.Brown R.E., Brockman H.L. Using monomolecular films to characterize lipid lateral interactions. Methods Mol. Biol. 2007;398:41–58. doi: 10.1007/978-1-59745-513-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabatini K., Mattila J.-P., Kinnunen P.K. Characterization of two oxidatively modified phospholipids in mixed monolayers with DPPC. Biophys. J. 2006;90:4488–4499. doi: 10.1529/biophysj.105.080176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong-Ekkabut J., Xu Z., Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys. J. 2007;93:4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConnell H.M. Harmonic shape transitions in lipid monolayer domains. J. Phys. Chem. 1990;94:4728–4731. [Google Scholar]
- 26.Megli F.M., Russo L., Sabatini K. Oxidized phospholipids induce phase separation in lipid vesicles. FEBS Lett. 2005;579:4577–4584. doi: 10.1016/j.febslet.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Feng S. Interpretation of mechanochemical properties of lipid bilayer vesicles from the equation of state or pressure-area measurement of the monolayer at the air-water or oil-water interface. Langmuir. 1999;15:998–1010. doi: 10.1021/la051216n. [DOI] [PubMed] [Google Scholar]
- 28.Demel R.A., Geurts van Kessel W.S.M., van Deenen L.L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim. Biophys. Acta. 1975;406:97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- 29.Newmeyer D.D., Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 30.Simon H.-U., Haj-Yehia A., Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 31.Vernier P.T., Levine Z.A., Tieleman D.P. Electroporating fields target oxidatively damaged areas in the cell membrane. PLoS ONE. 2009;4:e7966. doi: 10.1371/journal.pone.0007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volinsky R., Cwiklik L., Kinnunen P.K. Oxidized phosphatidylcholines facilitate phospholipid flip-flop in liposomes. Biophys. J. 2011;101:1376–1384. doi: 10.1016/j.bpj.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell H.M., De Koker R.J. Note on the theory of the sizes and shapes of lipid domains in monolayers. J. Phys. Chem. 1992;96:7101–7103. [Google Scholar]
- 34.McConnell H.M. Structures and transitions in lipid monolayers at the air-water interface. Annu. Rev. Phys. Chem. 1991;42:171–195. [Google Scholar]
- 35.Fanani M.L., Maggio B. Liquid-liquid domain miscibility driven by composition and domain thickness mismatch in ternary lipid monolayers. J. Phys. Chem. B. 2011;115:41–49. doi: 10.1021/jp107344t. [DOI] [PubMed] [Google Scholar]
- 36.Akimov S.A., Kuzmin P.I., Chizmadzhev Y.A. An elastic theory for line tension at a boundary separating two lipid monolayer regions of different thickness. J. Electroanal. Chem. 2004;564:13–18. [Google Scholar]
- 37.García-Sáez A.J., Chiantia S., Schwille P. Effect of line tension on the lateral organization of lipid membranes. J. Biol. Chem. 2007;282:33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- 38.Holopainen J.M., Metso A.J., Kinnunen P.K. Evidence for the lack of a specific interaction between cholesterol and sphingomyelin. Biophys. J. 2004;86:1510–1520. doi: 10.1016/S0006-3495(04)74219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinia H.A., Snel M.M.E., de Kruijff B. Visualizing detergent resistant domains in model membranes with atomic force microscopy. FEBS Lett. 2001;501:92–96. doi: 10.1016/s0014-5793(01)02636-9. [DOI] [PubMed] [Google Scholar]
- 40.Holopainen J.M., Brockman H.L., Kinnunen P.K. Interfacial interactions of ceramide with dimyristoylphosphatidylcholine: impact of the N-acyl chain. Biophys. J. 2001;80:765–775. doi: 10.1016/S0006-3495(01)76056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fattal D.R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys. J. 1993;65:1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuzmin P.I., Akimov S.A., Cohen F.S. Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys. J. 2005;88:1120–1133. doi: 10.1529/biophysj.104.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D.W., Min Y., Zasadzinski J.A. Relating domain size distribution to line tension and molecular dipole density in model cytoplasmic myelin lipid monolayers. Proc. Natl. Acad. Sci. USA. 2011;108:9425–9430. doi: 10.1073/pnas.1106368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frolov V.A.J., Chizmadzhev Y.A., Zimmerberg J. “Entropic traps” in the kinetics of phase separation in multicomponent membranes stabilize nanodomains. Biophys. J. 2006;91:189–205. doi: 10.1529/biophysj.105.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason R.P., Walter M.F., Mason P.E. Effect of oxidative stress on membrane structure: small-angle X-ray diffraction analysis. Free Radic. Biol. Med. 1997;23:419–425. doi: 10.1016/s0891-5849(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 46.Alexander J.C., Bernoff A.J., Zou L. Domain relaxation in Langmuir films. J. Fluid Mech. 2007;571:191–219. [Google Scholar]
- 47.Goldstein R.E., Jackson D.P. Domain shape relaxation and the spectrum of thermal fluctuations in Langmuir monolayers. J. Phys. Chem. 1994;98:9626–9636. [Google Scholar]
- 48.Wintersmith J.R., Zou L., Mann E.K. Determination of interphase line tension in Langmuir films. Phys. Rev. E. 2007;75:061605. doi: 10.1103/PhysRevE.75.061605. [DOI] [PubMed] [Google Scholar]
- 49.Heinrich M.C., Levental I., Baumgart T. Critical exponents for line tension and dipole density difference from lipid monolayer domain boundary fluctuations. J. Phys. Chem. B. 2008;112:8063–8068. doi: 10.1021/jp7116246. [DOI] [PubMed] [Google Scholar]


