Abstract
The interplay between cadherin- and integrin-dependent signals controls cell behavior, but the precise mechanisms that regulate the strength of adhesion to the extracellular matrix remains poorly understood. We deposited cells expressing a defined repertoire of cadherins and integrins on fibronectin (FN)-coated polyacrylamide gels (FN-PAG) and on FN-coated pillars used as a micro-force sensor array (μFSA), and analyzed the functional relationship between these adhesion receptors to determine how it regulates cell traction force. We found that cadherin-mediated adhesion stimulated cell spreading on FN-PAG, and this was modulated by the substrate stiffness. We compared S180 cells with cells stably expressing different cadherins on μFSA and found that traction forces were stronger in cells expressing cadherins than in parental cells. E-cadherin-mediated contact and mechanical coupling between cells are required for this increase in cell-FN traction force, which was not observed in isolated cells, and required Src and PI3K activities. Traction forces were stronger in cells expressing type I cadherins than in cells expressing type II cadherins, which correlates with our previous observation of a higher intercellular adhesion strength developed by type I compared with type II cadherins. Our results reveal one of the mechanisms whereby molecular cross talk between cadherins and integrins upregulates traction forces at cell-FN adhesion sites, and thus provide additional insight into the molecular control of cell behavior.
Introduction
Adhesion molecules are crucial for the development of multicellular organisms. Cadherins and integrins are the major classes of transmembrane adhesion receptors that mediate cell-cell interactions and the adhesion of cells to the extracellular matrix (ECM), respectively (1,2). The extracellular domain of integrins and cadherins interacts with ligands to mediate cell adhesion. The cadherin and integrin intracellular domain interacts with cytoplasmic partners, connecting these receptors to the cytoskeleton. Mechanically, the cytoskeletal structures that are recruited at adhesion sites act as cables supporting and transmitting stresses on a large scale through the body of the cell. The dynamics of these stresses contribute to the transmission of force at cell adhesion sites (3). Such structures can also form a physical link between two distant cell contacts, transmitting information from cell-cell junctions to focal contacts and vice versa. Integrin-mediated mechanosensing modulates cell fate, proliferation, and apoptosis. It enables cells to detect and respond to environmental stresses and applied traction forces, which are key elements in the control of biological processes such as cell adhesion, migration, survival, proliferation, and stem cell fate (4–6). Cadherins also act as mechanosensors and constitute some of the components of intercellular adhesion complexes (7,8). Mechanical forces govern the formation and maintenance of cellular assemblies and tumoral progression and metastasis (4,9).
Cross talk between integrins and cadherins regulates cell behavior, including adhesion strength, cell shape, migration, and contractility (10–17). Mechanical coupling between cell-cell and cell-matrix adhesions was recently shown to play an important role in regulating endothelial and epithelial intercellular junction size and tension (18,19). We previously showed that integrin stimulation increases the strength of cadherin-mediated intercellular adhesion in dual pipette assays measuring the separation force of cell doublets (15). The effects of intercellular adhesions on the cellular response to ECM and vice versa may be either positive or negative, depending on the cellular or environmental context. The regulation of cell adhesion strength has been shown to be highly complex, but the underlying molecular mechanisms have yet to be fully elucidated.
Here, we studied the reciprocal cross talk between cadherins and integrins in response to substrate-based mechanical cues. To that end, we first used the micro-force sensor array (μFSA) technique (20–22) to analyze the impact of cadherin-based adhesion on the traction exerted by cells at the level of individual cell-fibronectin (FN) adhesion sites. Our cellular model, S180 cells, enabled us to control the amount of cadherins expressed at cell-cell junctions (23,24) while cells were deposited on a dense array of deformable cylindrical FN-coated micropillars. Moreover, we exposed cells to various substrate stiffnesses to study the cells' response as a function of their cadherin repertoire, and the ways in which integrin and cadherin-based adhesions are coupled to ensure the modulation of the cell-matrix adhesion status and mechanical properties.
Materials and Methods
Details regarding the materials and methods used in this work are provided in the Supporting Material.
Cell lines, plasmids, stable transfections, antibodies, reagents, and immmunostaining
Stably transfected S180 clones producing chicken E-cadherin (Ecad), N-cadherin (Ncad), and cadherin-7 (Cad7) were cultured as previously described (24–26). The cells were plated overnight on μFSA arrays before experiments were conducted. Rho kinase and PI3K inhibitor (both 15 μM) and Src kinase inhibitor 1 (Src Inh 1; 200 nM) were added to the sample 30 min before measurements were obtained. The monoclonal antibodies used in the study are detailed in the Supporting Material. Samples were fixed in 4% paraformaldehyde fixation buffer in PBS+CaMg or in cold methanol. Immunodetection was performed as previously described (23).
Preparation of poly(dimethylsiloxane) micropillars for μFSA and traction force measurements
An array of cylindrical pillars was prepared as described elsewhere (21). The tops of the pillars were fluorescently labeled as previously described (27) with a mixture (1:10 molar ratio) of bovine plasma FN (Sigma, St. Louis, MO) and Alexa 594-FN (Molecular Probes, Eugene, OR). We used arrays of stiff pillars (2 μm in diameter, 4 μm center-to-center, 3.6 μm long) with a pillar spring constant k ∼ 100 nN/μm (Eeff = 72 kPa) (28). We also used arrays of softer pillars (2 μm in diameter, 4 μm center-to-center, 6.5 μm long) with k ∼ 15 nN/μm (Eeff = 12 kPa). Unless otherwise specified, 3.6-μm-long pillars were used in most experiments.
To measure traction force, we carried out time-lapse imaging of the top of the pillars as previously described (27). Image stacks were acquired with a frame delay of 30 s over a period of ∼1 h and analyzed automatically with the use of ImageJ software. The displacement of all pillars in the field of view (both those below the cells and those not covered by cells) was analyzed. The spatial resolution for pillar displacement was 220 nm, which corresponds to a traction force resolution of 5 nN. From a comparative analysis of pillar displacement on fields devoid of cells and fields containing Ecad, Cad7, Ncad, and S180 cells, we plotted forces >16 nN on graphs, to ensure clarity. The statistical significance of differences was assessed in two-tailed Mann-Whitney U-tests.
Preparation of EcadFC-coated beads and cell stimulation
EcadFC-coated beads (EcadFC beads) and control beads were prepared as described in the Supporting Material. EcadFC beads or control beads were added to Ecad cells plated overnight on μFSA arrays at very low density to avoid cell-cell contacts on the FN-pillars array, as described in the Supporting Material. The nonadherent beads were removed before time-lapse imaging was performed.
Polyacrylamide gels
We prepared FN-coated polyacrylamide gels (FN-PAG), as described previously (29,30) and in the Supporting Material, with ratios of bis-acrylamide/acrylamide of 0.06, 0.14, 0.26, and 0.52. These ratios produced gels with a Young's modulus of 2.8, 7.5, 16.7, and 23.4 kPa, respectively (hereafter referred to as stiffnesses 1, 2, 3, and 4, respectively).
Results
Modulation of cadherin-expressing cell spreading, aggregation, and adhesion-site formation by substratum stiffness
To analyze the influence of substrate rigidity on our cell model, we first investigated the behavior of cadherin-expressing cells on FN-PAGs of different stiffnesses (as described in Materials and Methods). Cad7 and Ecad cells expressed similar integrin repertoires and classical Ecad and type II Cad7, respectively (15). The behavior of these two types of cells was significantly and similarly affected by the elasticity of the FN-PAG (Fig. S1). Most cells failed to adhere to soft gels (stiffness 1) with an elastic modulus of 2.8 kPa. The cells that did adhere to such gels were round or elongated, and the mean area of spread was similar for Ecad (9732 arbitrary units) and Cad7 (10863 arbitrary units) cells. No adhesion complexes were formed, and it was easy to detach the cells from the gel. Stiffer gels were associated with a higher percentage of cells being able to adhere to the surface and spread. For instance, Ecad cells deposited onto gels of stiffnesses 2, 3, and 4 had spreading areas that were 1.5, 2, and 2.4 times larger, respectively (Fig. S1 B), compared with those deposited onto gels of stiffness 1. A similar effect was observed for Cad7 cells (Fig. S1 A). This phenomenon was even more marked for the stiffest substrate, which was made of glass, on which the spreading areas of Ecad and Cad7 cells were 2.7 and 3.4 times larger, respectively, than that on a gel of stiffness 1. The adhesion sites that formed on the stiffest FN-PAG were smaller than those that formed on FN-coated glass, and were not observed on softer gels (stiffnesses 1 and 2; Fig. S2).
Cadherin expression induced a marked tendency of cells to form three-dimensional (3D) aggregates on the softest gel (Fig. S3 A, right panel), consistent with the findings of a previous study on normal rat kidney epithelial cells (31). By contrast, these cells mostly formed well-spread-out, two-dimensional (2D) clusters on stiffer gels and glass (Fig. S3 A, middle and left panels).
By focusing on cells adherent to the surface, we analyzed the effect of intercellular adhesion on cell spreading area on FN-PAG and its response to stiffness. Ecad cells that adhered to other cells had a larger spreading area on FN-coated substrata compared with isolated cells (Fig. S3 B). This effect was modulated by the external rigidity, because it was more pronounced when the cells were deposited onto FN-PAG of greater stiffness. Thus, cadherin-expressing sarcoma cells are able to sense environmental stiffness and respond to it by modulating their intercellular and cell-matrix adhesion properties. We did not specifically measure adhesion strength in these conditions. However, under the assumption that cells have a tendency to maximize their adhesion, we would expect cells with compromised adhesion to the substrate to adhere more strongly with each other if they express cadherins.
Ecad cells adhere to, spread, and form focal and intercellular adhesions on FN pillars
We used the μFSA technique to analyze the impact of the intercellular adhesion receptor repertoire on the cell traction forces acting on FN-contact sites. Ecad cells were deposited onto dense arrays of FN-coated pillars (FN pillars) and incubated for 16 h. Each pillar in this system acts as a separate detector of the pulling force at the cell-FN contact site. We used two arrays of pillars corresponding to stiff and soft parameters (k ∼ 100 nN/μm (Eeff = 72 kPa) and 15 nN/μm (Eeff = 12 kPa), respectively). Cells adhered to and moved on the two types of FN pillars, but they spread over a larger area on the stiffer pillars. Both isolated Ecad cells and those in contact with other cells developed focal adhesions on the FN pillars (Fig. 1 A), where activated focal adhesion kinase (FAK) was recruited. α5β1 integrin was localized to these sites together with paxillin and activated Fes kinase. α5β1 integrin was mostly detected at the peripheral contacts, whereas other β1 integrins tended to be recruited more centrally. The adhesion complexes were distributed mostly around the edge of the FN pillars (2 μm in diameter). This localization is in agreement with results described in a recent study (32). By contrast, β3 integrins were not detected at these sites (data not shown). The actin cytoskeleton was highly dynamic at the site of cell adhesion to FN pillars (Fig. 1 B and Movie S1). Ecad cells formed cell-cell contacts with their neighbors on the FN pillars (Fig. 1 C).
Figure 1.
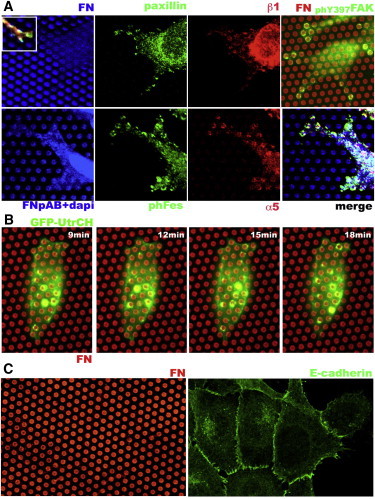
Localization of focal adhesion proteins, actin, and Ecad for Ecad cells deposited on μFSA arrays coated with FN. (A) Top: Confocal pictures showing FN deposition (blue) at the top of the pillar and the immunolocalization of paxillin, β1 integrins, and phY397FAK. The inset shows a merge image of a focal adhesion at the tip of a cellular protrusion. Bottom: FN deposition (blue) and staining of the nuclei with DAPI, phFes (green), α5 integrin (red), and a merge image. (B) Images extracted from a movie showing actin dynamics (green), as revealed with the GFP-UtrCH probe, at the level of cell adhesion to cell-FN pillar (red). (C) Intercellular contacts formed between Ecad cells in cluster on FN pillars (red, left panel), as demonstrated by Ecad immunostaining (green, right panel).
Cadherin-mediated adhesion stimulates traction force at FN pillars
Isolated cells typically spread over 35–45 pillars, but a large deflection was observed only over 10 pillars located at the periphery of the cell, as previously observed for other cell lines (33) (Fig. S4). The vector of the pulling force was always directed toward the center of the cell, consistent with the findings of previous studies. We determined the distribution of forces developed by isolated Ecad cells on FN pillars (Eeff = 72 kPa), together with the maximal force applied to the FN pillar, for each cell analyzed. Pulling forces of up to 75 nN were measured (Fig. 2 A, left panel, black bar), and the mean maximal force exerted by an isolated Ecad cell on a pillar was 51 ± 3 nN (Fig. 2 B, left panel, black bar). Then, we studied the impact of cadherin-mediated adhesion on cell traction to FN for cells engaged in contact with a limited number of neighbors, mimicking the processes observed during individual cell migration and cells moving as thin chains within tissues. We analyzed the behavior of small clusters of cells (three to five cells interacting together) to minimize the impact of collective behavior on the cell traction force (34). We observed that in Ecad clusters, displacements generally occurred only at the periphery of the cluster, but smaller movements occurred below the cells in all of the cell types studied. Ecad cells in contact with other cells pulled more strongly on FN pillars, with forces of up to 130 nN (Fig. 2 A, left panel, gray bars) and a mean maximal force of 83 ± 4 nN (Fig. 3 B, left panel, gray bar), versus only 75 nN and 51 nN, respectively, for isolated Ecad cells. The incubation of isolated Ecad cells on FN pillars with the medium produced by dense cell cultures had no effect on the pulling force, demonstrating the need for direct contact between cells rather than a paracrine effect of a soluble factor for modulation of the cell traction force in this assay (data not shown). Indeed, parental S180 cells that did not express cadherins at their surface exerted traction forces (Fig. 2 A, right panel, black bars) similar to those exerted by isolated Ecad cells (Fig. 2 A, left panel, black bars), with forces of up to 75 nN recorded and a mean maximal force of 54 ± 1 nN (Fig. 2 B, right panel, black bar).
Figure 2.
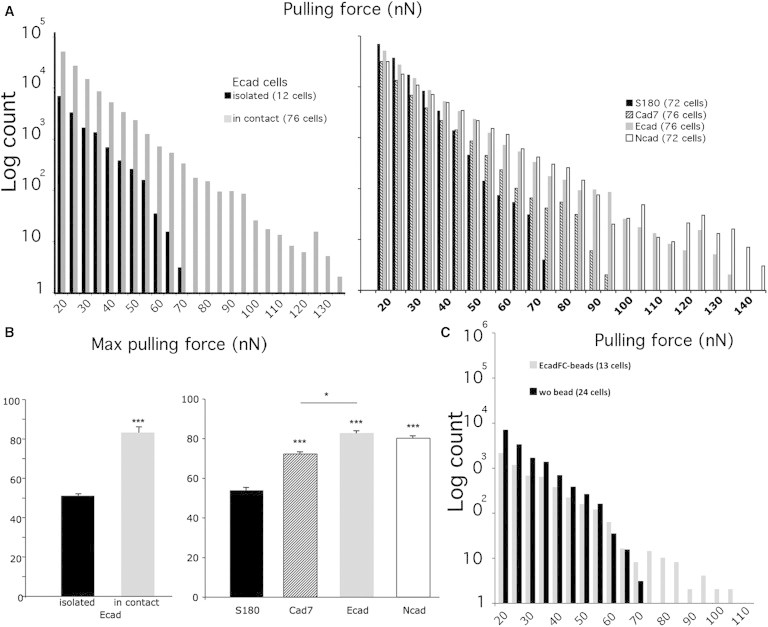
Cadherin-dependent intercellular adhesion increases the pulling force exerted on cell-FN pillars. (A) Distribution of forces (left panel) for isolated Ecad cells (black bars) and Ecad cells displaying cell-cell contacts (gray bars). Distribution of forces (right panel) for parental S180 cells (black bars) and cells expressing Cad7 (hatched bars), Ecad (gray bars), and Ncad (white bars). (B) Histograms of mean maximal force (left panel) for isolated Ecad cells (black bar) and Ecad cells displaying cell-cell contacts (gray bar). Histograms of mean maximal force (right panel) for parental S180 cells (black bars) and small clusters of Cad7 cells (hatched bars), Ecad (gray bars), and Ncad cells (white bars). (C) Distribution of forces for isolated Ecad cells (black bars) and isolated Ecad cells stimulated by EcadFC-coated beads (gray bars). Error bars indicate the mean ± SE. ∗∗∗p < 0.001, ∗p < 0.05 (two-tailed).
Figure 3.
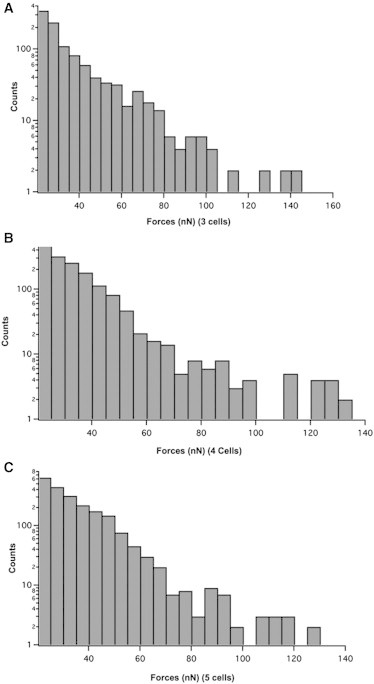
Cluster size does not modify the pulling force on FN pillars. Distribution of pulling force for clusters composed of three (A), four (B), and five (C) Ecad cells.
The extent to which cells deflected softer pillars (Eeff = 12 kPa) was similar to that observed for stiffer pillars, and this deflection required a much smaller force, in the range of 15–20 nN. We compared the maximal pulling force developed by cells on these softer pillars and found that S180 cells pulled less strongly than Ecad cells, with forces of 16 ± 1 and 21 ± 2 nN, respectively, recorded.
Thus, the presence of cadherins at the cell surface is not sufficient to increase the pulling force exerted by the cell on FN pillars, but the engagement of cadherins in intercellular adhesion is required to induce an increase of traction force at cell-FN adhesion sites. We investigated the role of mechanical coupling between cells on traction force by analyzing the effect of cluster size on traction force. We found that clusters of three, four or five Ecad cells displayed a similar distribution of pulling force up to 140 nN (Fig. 3), indicating that for small Ecad clusters, the effect of Ecad-mediated adhesion on traction force does not vary with the cluster size. The increase in pulling force observed in contacting Ecad cells could be due to the activation of cytoplasmic signaling cascades upon cadherin-mediated adhesion or to the transmission of force across cells of the small cluster, or to both processes. To ascertain the effect of cadherin-based adhesion on pulling force independently of the transmission of force across cell-cell contact, we incubated isolated Ecad cells with EcadFC beads (see Materials and Methods). Ecad cells deposited onto FN pillars and incubated with EcadFC beads (with a maximum of one or two beads per cell) exhibited a distribution of pulling force up to 105 nN, compared with 70 nN observed for nonstimulated cells (Fig. 2 C). The mean maximal pulling force for stimulated versus nonstimulated cells was 62 nN ± 3 and 52 ± 2 nN, respectively (errors are the mean ± standard error (SE) and considered statistically significant at p < 0.005 (two-tailed)). The interaction of cells with one or two beads did not influence the effect on pulling force. In addition, we checked the effect of control beads that did not contain Ecad-FC at their surface. We found that these control beads did not interact with cells in the course of the experiments, indicating that the effect of EcadFC beads on cell traction force at FN pillars is specific to Ecad-mediated adhesion. Taken together, our results indicate that the activation of cytoplasmic events downstream of cadherin-mediated contact positively regulate pulling force. However, the increase in pulling force measured in isolated cells interacting with EcadFC beads was lower than that obtained for small cell clusters, suggesting that the mechanical coupling between cells also stimulates traction force at cell-FN adhesions.
We then investigated whether different types of cadherins modulate the cell-FN traction force in a specific manner. We compared the traction force exerted by Ecad, Ncad, and cad7 cells expressing type I (Ecad and Ncad) and type II (Cad7) cadherins. The distribution of the traction force on FN pillars for Cad7, Ecad, and Ncad cells showed that these cells pulled more strongly on the FN pillars than the parental S180 cells, with forces of up to 145 nN (Fig. 2 A, right panel (hatched, gray, and white bars, respectively)) and a mean maximal force of 72, 83, and 88 nN (Fig. 2 B, right panel) for Cad7, Ecad, and Ncad, respectively.
Src and PI3 kinase activity is required to promote an Ecad-dependent increase in the pulling force on FN pillars
We previously showed that integrin-dependent stimulation of the cadherin-mediated adhesion strength requires Src kinase and actomyosin contractility (15). Here, we sought to determine whether these signaling pathways and PI3K are involved in the reciprocal cross talk between these adhesion receptors leading to the cadherin-dependent increase in integrin-based pulling force.
Experiments were first carried out in the presence of a dimethyl sulfoxide solvent that was used to prepare the stock solution of Src, PI3K, and ROCK inhibitors and added to the culture medium at a working dilution (1/1000) to ensure that the solvent had no pertubation effect on cell behavior (data not shown). After incubation with Src and PI3K inhibitors (Fig. 4 A) for 30 min, Ecad cells in culture displayed similar cell-cell and cell-FN adhesions compared with solvent-treated cells, as shown by the immunodetection of Ecad and paxillin staining.
Figure 4.
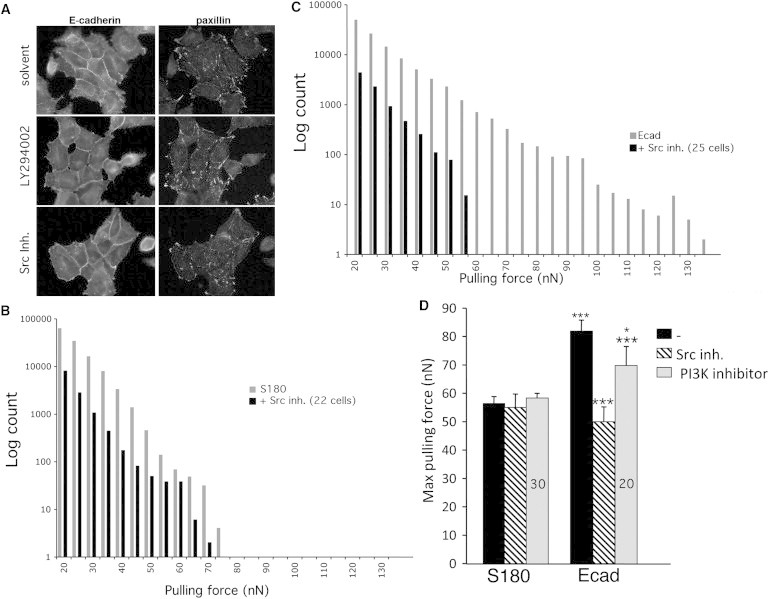
Inhibitors of Src and PI3K decrease the pulling force of cells. (A) Immunostaining for Ecad and paxillin in solvent-treated (top panels), PI3K inhibitor (LY294002)-treated (middle panels), and Src Inh 1-treated (lower panels) Ecad cells. (B) Representative distribution of pulling force for S180 cells (gray bars) and S180 cells treated with Src Inh 1 (black bars). (C) Representative distribution of pulling force for Ecad cells (gray bars) and Ecad cells treated with Src Inh 1 (black bars). (D) Histograms of the mean maximal pulling force of S180 and Ecad cells with and without inhibitor treatments. Error bars indicate the mean ± SE. Small asterisks indicate statistical significance for differences between untreated (−) and PI3K inhibitor-treated S180 cells. Large asterisks indicate statistically significant differences with respect to untreated Ecad cells. ∗∗∗p < 0.001, ∗p < 0.05 (two-tailed).
Ecad cells were deposited onto FN pillars and treated with ROCK inhibitor (Y27632), Src Inh 1, and PI3K inhibitor (LY294002) for 30 min before traction force measurements were obtained.
The inhibition of ROCK by Y27632 greatly decreased the maximal pulling force to 23 ± 1 nN for isolated Ecad cells (n = 20 cells) deposited onto FN pillars, compared with 51 ± 3 nN measured for untreated isolated Ecad cells. This effect highlights the role of ROCK in the integrin-dependent traction force generated at cell-FN contact sites by increases in cell contractility. ROCK inhibition is also known to have a direct effect on cadherin adhesion strength (15). These observations did not allow us to test the involvement of ROCK in the cadherin-mediated stimulation of integrin-driven cell traction.
We conducted control experiments to examine the effect of Src and PI3K inhibitors on S180 cells, because S180 cells and isolated Ecad cells exhibit similar traction forces, and to ensure that the effects of drugs on various signaling processes do not alter the intrinsic cell traction force. In the presence of Src Inh 1, S180 cells exerted forces of up to 70 nN and a mean maximal force of 55 ± 5 nN, which is similar to that exerted by untreated cells (54 ± 3 nN; Fig. 4, B and D). In addition, an analysis of isolated Ecad cells treated with this inhibitor showed that they exhibited forces up to 60 nN (Fig. S5) and a mean maximal pulling force of 41 nN ± 4 (mean ± SE) that was not statistically different from than observed for untreated isolated Ecad cells (p > 0.1 (two-tailed); Fig. 3 B). By contrast, the greater traction force exerted by Ecad cells in contact with other cells was abolished in the presence of this inhibitor (Fig. 4 C). Treated Ecad cells exhibited forces up to 55 nN and a lower mean maximal force of 50 ± 5 nN on FN pillars (Fig. 4 D). This value is similar to that obtained for treated parental S180 cells and isolated Ecad cells (Figs. 3 B and 4 D). Thus, the cadherin-dependent stimulation of pulling force requires Src kinase activity. The inhibition of PI3K by LY294002 also significantly decreased the pulling force of Ecad cells, with the mean maximal force decreasing from 82 ± 4 nN to 66 ± 7 nN for untreated and LY294002-treated cells, respectively (Fig. 4 D). By contrast, this drug had no effect on the pulling force of S180 cells, with a force of 60 ± 2 nN being measured in these conditions. Taken together, our results show that Src kinase and PI3K play a role in cadherin-based regulation of the integrin-dependent traction of cell-FN contact sites (Fig. 5).
Figure 5.
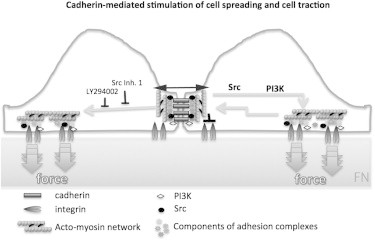
Model showing how cadherin-mediated stimulation of integrin-based adhesion increases cell traction force (gray arrows) at the cell periphery via an Src- and PI3K-dependent mechanism. Mechanical coupling across cells also contributes to the positive stimulation of traction force at FN-adhesion sites (double-headed black arrow). Integrin-dependent adhesion and cadherin-based adhesion have reciprocal positive effects on each other that also involve Src-dependent mechanisms (15). In addition, intercellular adhesion can also have a negative on cell traction in close vicinity to cell-cell contacts (black lines) (18,19). Src (small black dot) and PI3K (small white lozenge) are also involved in regulating integrin-based adhesion itself.
Discussion
The molecular mechanisms that regulate the interactions of cells with their environment and their responses to external stimuli, thereby modulating their behavior and fate, are not fully understood. In particular, it is unknown how cells integrate signals downstream from cell adhesion receptors, such as cadherins and integrins, to regulate mechanosensing and mechanotransduction. In this study, we analyzed in detail the functional relationship between these adhesion receptors and its role in the regulation of cell mechanics.
We found that the adhesion receptor repertoire and substrate stiffness regulate cell spreading and intercellular adhesion properties. Cells that are in contact through cadherin-mediated adhesion spread more than isolated cells on FN-coated surfaces (Fig. S1, Fig. S2, and Fig. S3). We investigated the effect of cadherins on regulation of the traction force exerted by a cell at a focal adhesion site in a μFSA assay. Cells that expressed cadherins at their surface exerted traction forces up to 2.5 times stronger than the corresponding parental cells that did not produce detectable amounts of cadherin. This effect requires the involvement of cadherins in intercellular contacts, because isolated cells did not display such a large increase in traction forces. Our results show that traction forces at the cell-ECM interface are strengthened when cells form intercellular contacts through cadherins (Figs. 2 and 3). Two recent studies investigated the modulation of tugging force between two endothelial cells held in close contact in a surface-patterned μFSA assay (18), and the cell-ECM-dependent regulation of intercellular adhesion on freely moving endothelial cells deposited onto a deformable flat substrate with embedded fluorescent beads (19). Another study showed the modulation of MCF7 cell-cell and cell-matrix adhesion properties in response to dual-component soft and rigid surfaces coated with FN and EcadFC patterns (13). In this work, we obtained addidtional insight into the reciprocal interplay between integrin and cadherin, and its role in regulating cell traction, by focusing on the changes in traction force at FN-contact sites induced by cadherin-mediated adhesion.
The stimulation of Ecad cells with EcadFC beads also increased traction force at cell-FN adhesion, but to a lesser extent than that observed for contacting cells. There are several possible explanations for this effect: 1), The density of EcadFC fragments coated onto the bead did not properly mimic cadherin density at the interacting cell-cell interface in clusters. 2), EcadFC beads could not activate the signaling cascades upon contact to the same extent. (However, previous studies using cadFC-coated beads showed that they can stimulate anchoring of cadherins at the cell surface to the actin cytoskeleton and signaling (35–38). 3), Cadherins upon bead stimulation were less efficiently connected to the underlying cell cytoskeleton, or may not favor the formation of cortical actomyosin bundles, a process known to contribute to epithelial morphogenesis (39,40). 4), The link between the cytoskeleton of the two contacting cells through trans-interactions of cadherins is missed when cells interact with beads where mechanical coupling does not occur (41). The results obtained from the cell-bead interaction and traction force versus cluster size indicate that the mechanical coupling between cells and force transmission across cells in cluster also participates in the positive regulation of cell traction force at FN pillars. Clusters of three to five interacting cells produce similar maximal traction forces, indicating that the mechanical coupling among three cells does not significantly vary with a small increase in cluster size.
We have shown that cells exert quantitatively different traction forces on FN pillars as a function of the cadherins they express at their surface (Fig. 2). The involvement of cadherin in intercellular contacts resulted in cellular traction forces 1.8, 1.9, and 1.3 times higher than those observed for isolated Ecad, Ncad, and Cad7 cells, respectively. These observations probably reflect the different intercellular adhesion properties of the various types of cadherin (42,43). Indeed, in previous studies we characterized Ecad, Ncad, and Cad7 cells for their respective cadherin expression levels and intercellular adhesion strengths (23,24). We showed that Ecad and Ncad cells adhered more rapidly and had a higher adhesion strength than Cad7 cells, favoring the strong cohesion of clusters. This phenomenon reflects the poor incorporation of Cad7 into the cytoskeleton insoluble fraction (24). Differences in the stability of the intercellular bonds formed between cadherin extracellular domains due to differences in the adhesive interface have been observed between type II and classical type I cadherins (44,45). These differences may affect the stability and sensitivity of mechanotransduction occurring downstream from adhesion receptors, the reciprocal cross talk between cadherin- and integrin-based adhesions, and the transfer of force across cells depending on the type of cadherin expressed. This hypothesis is supported by our finding that Ecad and Ncad cells, which express classical type I cadherins, displayed a significantly larger increase in traction force when they were in contact with other cells than did Cad7 cells expressing type II Cad7.
Cell adhesion to ECM and the formation of intercellular contacts trigger numerous signaling events, including regulation of kinases and small Rho family GTPases activity, cytoskeleton remodeling, and actomyosin contractility. Cell adhesion acts in concert with growth factor stimulation in regulating these events, which play a crucial role during morphogenesis and are often deregulated in pathologies (16,46,47). These molecular events can contribute to the cadherin-dependent regulation of cellular traction forces. Indeed, VEGF-dependent stimulation of endothelial cell traction (48) and epithelial cell architecture, intercellular adhesion strength, and wound closure require ROCK (39,40). Isolated Ecad cells exhibited lower traction force to FN pillars in serum-free conditions compared with cells in a low (1%) serum condition (A. Jasaitis, unpublished results), suggesting that growth-factor downstream events stimulate cell traction by increasing cell contractility, as previously shown (20). In our study, we analyzed the traction force of isolated cells or cells in contact in a similar culture medium that contained 1% serum to minimize the growth-factor-dependent downstream effect on the regulation of cellular traction. In these conditions, the increment of cell traction observed in contacting cells depends on signals downstream from the intercellular adhesion process. However, the presence of reduced serum levels may help to potentiate the effect of cadherin-integrin cross talk in regulating traction force.
Cadherin-mediated adhesions transfer tension to integrin-based adhesions, thereby promoting FN assembly and regulation intercellular adhesion, the strength of which depends on cell contractility (11,15,49). In this context, we show that ROCK is required for individual cell traction on FN pillars. Our previous study showed that this kinase is also involved in cadherin-mediated strengthening of intercellular adhesion and its regulation upon integrin stimulation. These results strongly support a role for cadherin-dependent stimulation of the ROCK pathway, which in turn induces the integrin-dependent traction of cell-FN adhesion sites.
Src and PI3K are crucial for the cellular response to external stimuli. These proteins regulate cytoskeleton remodeling through activation of Rac (47,50). Indeed, Src kinase plays an important role in controlling integrin functions (51–53) and is activated by the mechanical stretching induced by FN- or anti-integrin-coated beads (54,55). This kinase also acts as a downstream signaling protein in the development of intercellular junctions (56) and in the integrin-dependent regulation of cadherin-based adhesion strength (15). The PI3K pathway is activated downstream from integrins (1) and regulates the formation and function of adherens junctions (57). PI3K is activated by the cadherin-dependent stimulation of Src, generating a positive feedback loop that regulates cadherin function (56). The PI3K pathway plays a role downstream from cadherin in the epithelial–mesenchymal transition (58,59). These two signaling proteins are also involved in the mechanical stimulation of carcinoma cell adhesion to the matrix (60).
We have shown here that the increase in cellular traction force at cell-FN contacts after cadherin-mediated intercellular adhesion requires the Src and PI3K signaling pathways (Figs. 4 and 5). We conducted a control experiment to examine the effects of Src and PI3K inhibitors on S180 cells, and ensure that their effects on various signaling processes did not alter the intrinsic cell traction force at the FN pillar or the cell-matrix adhesion stability. Inhibition of Src and PI3K did not impede cell-cell contact and thus allowed transmission of force across Ecad cells in a cluster; however, these inhibitions abolished or affected the increase in the pulling force observed in contacting cells. This supports the conclusion that in addition to the mechanical coupling between cytoskeleton of neighboring cells, the cadherin-activated Src-PI3K pathway significantly contributes to stimulate mechanotransduction at focal adhesions and cell traction force that may involve the modulation of actin dynamics through regulation of Rac activity. However, the PI3K inhibitor was less effective than the Src kinase inhibitor at inhibiting the cadherin-dependent increase in the pulling force exerted on FN pillars. This suggests that a PI3K-independent pathway downstream from Src is probably involved via the stimulation of cellular contractility.
Conclusions
Cadherin expression and substrate stiffness regulate cell spreading and intercellular adhesion properties. This positive effect and reciprocity of the molecular and mechanical cross talk between cadherins and integrins in regulating the strength of intercellular adhesion, adhesion, and spreading over the ECM are likely to have profound implications for the sensing behavior and responses of cellular assemblies as a function of the properties of the environment, including rigidity, and the adhesive repertoire expressed by cells at their surface. We have shown that cadherin-mediated adhesion increases cell traction at FN-adhesion sites, and that Src and PI3K pathways are at the crossroads of the reciprocal interplay between cadherins and integrins in the regulation of adhesion strength. Our results provide additional insight into some of the molecular mechanisms that regulate the balance between cell cohesion and interactions with the ECM, and the mechanotransduction processes that regulate cellular traction forces. This phenomenon is crucial for regulation of the adhesion and migration properties of single cells or cell layers during the epithelial–mesenchymal transition, morphogenesis, regeneration, and both normal and pathological processes.
Acknowledgments
S.D. and B.L. designed the study; A.J. and M.E. performed the research; J.H. performed cell culture; A.J., B.L. and S.D. analyzed the data; and S.D wrote the article. The authors thank A. Bershadsky, W. M. Bement, and M. Arpin for generously providing plasmids and antibodies; F. Gallet and W. Thomas for critical readings of the manuscript; the Nikon Imaging Centre at Institut Curie-CNRS, and V. Fraisier, F. Waharte, and L. Sengmanivong for their assistance with imaging and computerized video microscopy; C. Carlier for technical help; and M. C. Leong for help with image analysis.
This work was supported by grants from the Agence Nationale de la Recherche (PCVI program, grant number PCV07-186757, and Programme Blanc 2010 (MECANOCAD)), Association Française contre la Myopathie, and Association de la Recherche contre le Cancer. A.J. was supported by the Agence Nationale de la Recherche (PCVI program), Institut Curie, and the Fondation Pierre-Gilles de Gennes. The research was conducted within the scope of the International Associated Laboratory Cell Adhesion France Singapore.
Supporting Material
References
- 1.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 3.Wang N., Suo Z.G. Long-distance propagation of forces in a cell. Biochem. Biophys. Res. Commun. 2005;328:1133–1138. doi: 10.1016/j.bbrc.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 4.Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz U.S., Bischofs I.B. Physical determinants of cell organization in soft media. Med. Eng. Phys. 2005;27:763–772. doi: 10.1016/j.medengphy.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert P.M., Havenstrite K.L., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.le Duc Q., Shi Q., de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taguchi K., Ishiuchi T., Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J. Cell Biol. 2011;194:643–656. doi: 10.1083/jcb.201104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz D., Konstantopoulos K., Searson P.C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montero J.-A., Heisenberg C.-P. Adhesive crosstalk in gastrulation. Dev. Cell. 2003;5:190–191. doi: 10.1016/s1534-5807(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij J., Kerstens A., Waterman-Storer C.M. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Gumbiner B.M. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J. Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai J., Kam L. Rigidity-dependent cross talk between integrin and cadherin signaling. Biophys. J. 2009;96:L39–L41. doi: 10.1016/j.bpj.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghi N., Lowndes M., Nelson W.J. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc. Natl. Acad. Sci. USA. 2010;107:13324–13329. doi: 10.1073/pnas.1002662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Rico C., Pincet F., Dufour S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J. Cell Sci. 2010;123:712–722. doi: 10.1242/jcs.047878. [DOI] [PubMed] [Google Scholar]
- 16.Papusheva E., Heisenberg C.P. Spatial organization of adhesion: force-dependent regulation and function in tissue morphogenesis. EMBO J. 2010;29:2753–2768. doi: 10.1038/emboj.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber G.F., Bjerke M.A., DeSimone D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011;124:1183–1193. doi: 10.1242/jcs.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z., Tan J.L., Chen C.S. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruthamuthu V., Sabass B., Gardel M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J.L., Tien J., Chen C.S. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.du Roure O., Saez A., Ladoux B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. (Erratum in Proc. Natl. Acad. Sci. USA. 2005. 27;102:14122.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladoux B., Anon E., Mège R.M. Strength dependence of cadherin-mediated adhesions. Biophys. J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Y.S., Thomas W.A., Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y.S., Eder O., Dufour S. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J. Biol. Chem. 2006;281:2901–2910. doi: 10.1074/jbc.M506185200. [DOI] [PubMed] [Google Scholar]
- 25.Mege R.M., Matsuzaki F., Edelman G.M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc. Natl. Acad. Sci. USA. 1988;85:7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufour S., Beauvais-Jouneau A., Thiery J.P. Differential function of N-cadherin and cadherin-7 in the control of embryonic cell motility. J. Cell Biol. 1999;146:501–516. doi: 10.1083/jcb.146.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saez A., Ghibaudo M., Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghibaudo M., Saez A., Ladoux B. Traction forces and rigidity sensing regulate cell functions. Soft Matter. 2008;4:1836. [Google Scholar]
- 29.Tse J.R., Engler A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010 doi: 10.1002/0471143030.cb1016s47. Jun;Chapter 10:Unit 10.16. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y.L., Pelham R.J., Jr. Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 31.Guo W.H., Frey M.T., Wang Y.L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghassemi S., Meacci G., Hone J. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl. Acad. Sci. USA. 2012;109:5328–5333. doi: 10.1073/pnas.1119886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saez A., Buguin A., Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 2005;89:L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saez A., Anon E., Ladoux B. Traction forces exerted by epithelial cell sheets. J. Phys. Condens. Matter. 2010;22:194119. doi: 10.1088/0953-8984/22/19/194119. [DOI] [PubMed] [Google Scholar]
- 35.Lambert M., Padilla F., Mege R.M. Immobilized dimers of N-cadherin-fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. J. Cell Sci. 2000;113:2207–2219. doi: 10.1242/jcs.113.12.2207. [DOI] [PubMed] [Google Scholar]
- 36.Baumgartner W., Schütz G.J., Drenckhahn D. Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J. Cell Sci. 2003;116:1001–1011. doi: 10.1242/jcs.00322. [DOI] [PubMed] [Google Scholar]
- 37.Thoumine O., Lambert M., Choquet D. Regulation of N-cadherin dynamics at neuronal contacts by ligand binding and cytoskeletal coupling. Mol. Biol. Cell. 2006;17:862–875. doi: 10.1091/mbc.E05-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabdanov E., Borghi N., Thiery J.P. Role of E-cadherin in membrane-cortex interaction probed by nanotube extrusion. Biophys. J. 2009;96:2457–2465. doi: 10.1016/j.bpj.2008.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamada M., Perez T.D., Sheetz M.P. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J. Cell Biol. 2007;176:27–33. doi: 10.1083/jcb.200609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaezi A., Bauer C., Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 41.Gomez G.A., McLachlan R.W., Yap A.S. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Duguay D., Foty R.A., Steinberg M.S. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev. Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 43.Panorchan P., Thompson M.S., Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J. Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 44.Patel S.D., Ciatto C., Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 45.Shimoyama Y., Tsujimoto G., Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem. J. 2000;349:159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamora C., Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 47.Guo W., Giancotti F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 48.Yang M.T., Reich D.H., Chen C.S. Measurement and analysis of traction force dynamics in response to vasoactive agonists. Integr. Biol. (Camb) 2011;3:663–674. doi: 10.1039/c0ib00156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dzamba B.J., Jakab K.R., DeSimone D.W. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev. Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huveneers S., Danen E.H.J. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 51.Mitra S.K., Schlaepfer D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Playford M.P., Schaller M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 53.Avizienyte E., Frame M.C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Botvinick E.L., Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 55.Browe D.M., Baumgarten C.M. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J. Gen. Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLachlan R.W., Kraemer A., Yap A.S. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol. Biol. Cell. 2007;18:3214–3223. doi: 10.1091/mbc.E06-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivard N. Phosphatidylinositol 3-kinase: a key regulator in adherens junction formation and function. Front. Biosci. 2009;14:510–522. doi: 10.2741/3259. [DOI] [PubMed] [Google Scholar]
- 58.Larue L., Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 59.Nalla A.K., Estes N., Patel J., Rao J.S. N-cadherin mediates angiogenesis by regulating monocyte chemoattractant protein-1 expression via PI3K/Akt signaling in prostate cancer cells. Exp. Cell Res. 2011;317:2512–2521. doi: 10.1016/j.yexcr.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Thamilselvan V., Craig D.H., Basson M.D. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 61.Naba A., Reverdy C., Arpin M. Spatial recruitment and activation of the Fes kinase by ezrin promotes HGF-induced cell scattering. EMBO J. 2008;27:38–50. doi: 10.1038/sj.emboj.7601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burkel B.M., von Dassow G., Bement W.M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


