Abstract
It was reported that oseltamivir (Tamiflu) absorption was mediated by human peptide transporter (hPEPT) 1. Understanding the exact mechanism(s) of absorption is important in the context of drug-drug and diet-drug interactions. Hence, we investigated the mechanism governing the intestinal absorption of oseltamivir and its active metabolite (oseltamivir carboxylate) in wild-type [Chinese hamster ovary (CHO)-K1] and hPEPT1-transfected cells (CHO-PEPT1), in pharmacokinetic studies in juvenile and adult rats, and in healthy volunteers. In vitro cell culture studies showed that the intracellular accumulation of oseltamivir and its carboxylate into CHO-PEPT1 and CHO-K1 was always similar under a variety of experimental conditions, demonstrating that these compounds are not substrates of hPEPT1. Furthermore, neither oseltamivir nor its active metabolite was capable of inhibiting Gly-Sar uptake in CHO-PEPT1 cells. In vivo pharmacokinetic studies in juvenile and adult rats showed that the disposition of oseltamivir and oseltamivir carboxylate, after oral administration of oseltamivir, was sensitive to the feed status but insensitive to the presence of milk and Gly-Sar. Moreover, oseltamivir and oseltamivir carboxylate exhibited significantly higher exposure in rats under fasted conditions than under fed conditions. In humans, oral dosing after a high-fat meal resulted in a statistically significant but moderate lower exposure than after an overnight fasting. This change has no clinical implications. Taken together, the results do not implicate either rat Pept1 or hPEPT1 in the oral absorption of oseltamivir.
Introduction
Neuraminidase inhibitors such as oseltamivir (Tamiflu; F. Hoffmann-La Roche Ltd., Basel, Switzerland) prevent viral replication by blocking the exit of the influenza virus from the host cell and are therefore active against all strains of influenza A and B. A number of studies demonstrated the effectiveness of neuraminidase inhibitors in preventing influenza in healthy volunteers when administered in a prophylactic manner (Moscona, 2005). Oseltamivir is an orally available ester prodrug of its active moiety RO0640802 (oseltamivir carboxylate) (He et al., 1999b; Hoffmann et al., 2009). After an oral dose, oseltamivir is readily absorbed and converted by the liver carboxylesterase 1 to its active carboxylate, which is detectable within 30 min in plasma, with peak levels after 3 to 5 h (He et al., 1999b; Hill et al., 2002). The carboxylate is primarily excreted by passive glomerular filtration and active secretion into the urine possibly via the human organic anion transporters (hOATs) 1 and 3 (hOAT1, SLC22A6 and hOAT3, SLC22A8) (He et al., 1999b; Hill et al., 2002; Ose et al., 2009).
The human peptide transporter (PEPT) 1 (SLC15A1) is a high-capacity, low-affinity proton-coupled cotransporter expressed on the apical membranes of enterocytes located on the microvilli in the small intestine and to a lesser degree on epithelial cells in the kidney proximal tubule (Liang et al., 1995). hPEPT1 has been implicated in the absorption of aminocephalosporins, angiotensin-converting enzyme inhibitors, β-lactam antibiotics, amino acid-conjugated antiviral drugs, l-dopa, and tri- and dipeptides such as Gly-Sar (Brandsch, 2009). Several clinically relevant hPEPT1 inhibitors such as sulfonylurea antidiabetic drugs, nateglinide, glibenclamide, tolbutamide, chlorpropamide, sartans, and ester prodrugs of angiotensin-converting enzyme inhibitors have been identified (Faria et al., 2004; Knütter et al., 2008, 2009). Dipeptides such as Gly-Sar and Val-Ala have demonstrated inhibitory potential; therefore, di- and tripeptides resulting from the digestion of milk proteins may also inhibit hPEPT1-mediated drug absorption, causing reduced exposure of the victim drug (Fujisawa et al., 2006).
The in vitro transport characteristics of oseltamivir were examined in Caco-2 and hPEPT1 transfected HeLa cells (Ogihara et al., 2009). In these studies, a significant reduction of oseltamivir uptake at 4°C and in the presence of Gly-Sar and Trp-Gly was observed. In addition, the in vivo disposition of oseltamivir in rats after the oral administration of oseltamivir in the absence or presence of cow milk, casein, and Gly-Sar (20 and 125 mM) was determined. Oseltamivir exposure was reduced in the presence of milk, casein, and Gly-Sar compared with water. Furthermore, studies were conducted in juvenile rats (1 week old) under fasted and breast-fed conditions, and oseltamivir plasma and brain concentrations were measured at a single time point (30 min) after an oral dose. Oseltamivir plasma levels in nursed animals were significantly lower than those in fasted animals (Ogihara et al., 2009). From these studies, the authors concluded that PEPT1 is involved in the absorption of oseltamivir and that this process can be reduced by milk proteins. Consequently, oseltamivir may be subject to drug and/or dietary interactions with significant implications for its disposition.
The same group reported that milk consumption affects the absorption rate of oseltamivir but not the total exposure in healthy volunteers (Morimoto et al., 2011). In previous studies, He et al. (1999b) demonstrated that coadministration of amoxicillin, a hPEPT1 substrate (Herrera-Ruiz and Knipp, 2003), had no effect on the disposition of oseltamivir carboxylate in healthy volunteers. In addition, only the prodrug oseltamivir was studied by Ogihara et al. (2009) in the pharmacokinetic rat study, in which the plasma concentration-time profile of the active metabolite was not reported.
The present in vitro and in vivo studies were conducted to investigate the discrepancy with the amoxicillin drug-drug interaction clinical study and to follow oseltamivir carboxylate exposure in rat in the presence and absence of PEPT1 inhibitors. One goal of these studies was to clarify whether oseltamivir is indeed a hPEPT1 substrate and/or inhibitor. For that reason, the intracellular accumulation of oseltamivir and its active metabolite (oseltamivir carboxylate) was tested in Chinese hamster ovary (CHO) cells (parent and hPEPT1 transfected) along with the effects of temperature (4°C), different pH values (from 4 to 8), and known hPEPT1 inhibitors (Gly-Sar, Trp-Gly, valacyclovir, and cefadroxil). Pharmacokinetic studies in juvenile and adult rats were conducted to investigate food effect (rat chow and milk) and interaction with Gly-Sar. The results of a bioequivalence study in healthy volunteers comparing fasting with a high-fat meal is discussed with regard to the results published by Morimoto et al. (2011). We present strong evidence that oseltamivir and its active metabolite are neither hPEPT1/rat Pept1 substrates nor inhibitors.
Materials and Methods
Materials.
Oseltamivir, RO0640802 (oseltamivir carboxylate), and their 14C-labeled analogs were obtained from the Department of Medicinal Chemistry (F. Hoffmann-La Roche, Basel, Switzerland), and Gly-Sar, Trp-Gly, Tyr-Phe, and cefadroxil were from Sigma-Aldrich (St. Louis, MO). [3H]Gly-Sar was acquired from American Radiolabeled Chemicals (St. Louis, MO). Valacyclovir was from Kemprotec (Middlesbrough, UK), and valsartan was from Apin Chemicals (Abingdon, Oxfordshire, UK). Pasteurized whole-fat bovine milk (3.5% fat; “milk”) was purchased from a local grocery store (Migros, Basel, Switzerland). 2,2-Dichlorovinyl dimethyl phosphate (dichlorvos) was obtained from Honeywell Riedel-de Haen (Sleeze, Germany). Polypropylene tubes containing K2EDTA were purchased from Milian AG (Basel, Switzerland). Acetonitrile was from Merck Chemicals (Zug, Switzerland). Bovine serum albumin (BSA) was from Sigma-Aldrich (Buchs, Switzerland), phosphate-buffered saline (PBS) and Triton X-100 were from U.S. Biochemical Corp. (Cleveland, OH), and scintillation fluid was from PerkinElmer (Schwerzenbach, Switzerland).
Cell Cultures.
CHO cells, such as CHO-K1 (control) and those transfected with hPEPT1 (SLC15A1) (CHO-PEPT1) or hPEPT2 (SLC15A2) (CHO-PEPT2) were obtained from Solvo Biotechnology (Budaörs, Hungary). The cells were maintained in Ham's F-12K medium (Invitrogen, Carlsbad, CA), containing 10% fetal calf serum (Sigma-Aldrich) and supplemented with 1:200 of penicillin-streptomycin solution (10,000 IU · μg−1 · ml−1; Sigma-Aldrich), at 37°C in a humidified atmosphere with 5% CO2 in tissue culture flasks (Falcon; BD Biosciences Discovery Labware, Bedford, MA).
Cells were plated in 24-well plates (Falcon; BD Biosciences Discovery Labware) for in vitro cell-based studies. The incubation medium consisted of Krebs-Henseleit (KH) buffer (4.83 mM KCl, 0.96 mM KH2PO4, 23.8 mM NaHCO3, 142 mM NaCl, 1.2 mM MgSO4, 1.53 mM CaCl2, 12.5 mM MES, 5 mM d-glucose) adjusted to pH 5 or 6 after the addition of substrate and inhibitor.
In Vitro Cell-Based Studies.
The intracellular accumulation of the model hPEPT1 substrate [3H]Gly-Sar, [14C]oseltamivir, and [14C]RO0640802 was evaluated under various conditions such as concentration, temperature (4–37°C), pH, and the absence or presence of hPEPT inhibitors using the transfected cell lines CHO-PEPT1 and CHO-PEPT2 and was compared with results obtained from CHO-K1 (control) cells. The experimental details have been described previously (Poirier et al., 2008). In brief, before initiating the experiment, cell culture medium was removed followed by a single (1-ml) wash step with KH buffer (37°C) adjusted to the corresponding pH. In vitro studies were initiated by replacing (aspiration) the wash buffer with 150 μl of incubation medium (temperature set according to incubation) containing the compound under investigation in the absence or presence of indicated inhibitors. After addition of the substrate plus/minus inhibitor in the KH buffer, the pH was readjusted to the desired value. Plates were placed on a 37°C heating block (Eppendorf AG, Hamburg, Germany) or on ice (4°C incubation). At predetermined time points (30 s to 15 min), plates were removed, and 1 ml of ice-cold PBS containing 0.2% BSA (PBS-BSA) was added to quench uptake. BSA was added to minimize nonspecific binding to plastic ware. This step was followed by two wash steps (2 ml each) with PBS-BSA (37°C) followed by a third wash step (3 ml) with PBS (37°C) alone to remove BSA. Cells were solubilized with 300 μl of 1% Triton X-100 for 15 min at 60°C on a shaking heating block. Two hundred microliters of the cell lysate was added to 4 ml of scintillation fluid, and radioactivity was measured. Protein content was determined using the BCA Protein Assay Kit with albumin as the standard (Thermo Fisher Scientific, Waltham, MA). The absence of ester hydrolysis of [14C]oseltamivir to carboxylate during the incubation was confirmed by high-performance liquid chromatography (HPLC) coupled with radiodetection.
In vitro studies were performed three times on different days with each data point being run in triplicate. For each experiment, Gly-Sar was incubated as a positive control.
Animals.
Adult (7–8 weeks old) male Sprague-Dawley rats (243–283 g each) and newborn (male and female) Sprague-Dawley pups together with their dams were obtained from Charles River Laboratories (Les Oncins, France) and were housed under a controlled environment (temperature, humidity, and 12-h light/dark cycle) with access to food and water ad libitum. For the pharmacokinetic studies in adult animals, the rats were pretreated with buprenorphine (0.05 mg/kg s.c.), and the jugular vein was cannulated under ketamine/xylazine anesthesia (90 mg/kg ketamine and 10 mg/kg xylazine 2% diluted in 0.9% aqueous NaCl, intraperitoneally). A polyethylene catheter (Portex; Smiths Medical International Ltd., Kent, UK) with a silicon tip (AMT Aromando Medizintechnik, Dusseldorf, Germany) was used for the collection of serial blood samples. Animals received meloxicam (3.0 mg/kg s.c. at 2, 24, and 48 h after surgery) and were used in the pharmacokinetic studies after a recovery period of 3 days. Juvenile animals (7 days old, 9.7–18.9 g each) were treated orally as described under Pharmacokinetic Studies in Juvenile Rats, and blood was collected by cardiac puncture under deep isoflurane anesthesia. All rodent studies were conducted in strict adherence to the Swiss federal regulations on animal protection and the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International, along with the explicit approval of the local veterinary authority.
Pharmacokinetic Studies in Adult Rats.
The effect of bovine milk or Gly-Sar (125 mM) on oseltamivir and oseltamivir carboxylate maximal plasma concentration (Cmax), time to maximal concentration (tmax), and area under the concentration-time profile (AUC) was evaluated in both fasted and fed adult animals. Fasting was initiated at night, 6 h before dosing, and was continued for the duration of the study (6 h). Three animals per group received a single oral dose of 30 mg/kg oseltamivir in the three different vehicles at 10 ml/kg as follows: group 1, compound in aqueous solution; group 2, compound dissolved in pasteurized whole bovine milk; and group 3, compound in aqueous solution containing 125 mM Gly-Sar. Serial blood samples (250 μl each) were taken from the jugular vein at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, and 6 h after dosing for the fasted condition and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 24 h after dosing for the fed condition. The blood samples were collected with a syringe and were added into K2EDTA-coated polypropylene tubes containing 2.5 μl (1% v/v) of the esterase inhibitor dichlorvos 0.7% in acetonitrile and placed on ice to prevent ester cleavage into oseltamivir carboxylate. Plasma was prepared within 30 min by centrifugation at 3000g for 5 min at 4°C and was then frozen immediately to −20°C.
Pharmacokinetic Studies in Juvenile Rats.
The influence of breast-feeding, milk, and Gly-Sar on oseltamivir and oseltamivir carboxylate pharmacokinetics was evaluated in 7-day-old rats. Animals (15 per group, males and females) were dosed with 30 mg/kg oseltamivir by oral gavage (10 ml/kg) under the following conditions: group 1, ad libitum access to mother's milk (breast-fed animals); groups 2 to 4, fasted from 8 h before the dose and for the study duration (5 h). The compound was given as aqueous solution (groups 1 and 2), as solution in milk (group 3), or as aqueous solution containing 125 mM Gly-Sar (group 4). Blood samples (300 μl each) were collected at 0.25, 0.5, 1, 2, and 5 h after dosing and were processed as described under Pharmacokinetic Studies in Adult Rats.
Compound Analysis in Rat Plasma.
Plasma concentrations of oseltamivir and its carboxylate were determined using a validated HPLC-tandem mass spectrometry method (Heinig and Bucheli, 2008). A column-switching system was employed, consisting of an autosampler, pumps for on-line solid-phase extraction and analytical gradient separation (Shimadzu, Kyoto, Japan), and a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific) with electrospray ionization in the positive mode. As internal standards, 3-fold deuterated oseltamivir and carboxylate were used. Multiple reaction monitoring was performed at m/z 313→166 for oseltamivir, m/z 316→167 for its internal standard, m/z 285→138 for oseltamivir carboxylate, and m/z 288→139 for its internal standard. Linear regression with 1/χ2 weighting was applied, and the range of quantitation was between 0.1 and 500 μg/l.
Rat Pharmacokinetic Analysis.
Pharmacokinetic parameters were estimated by noncompartmental analysis with the software program ToxKin (Entimo AG, Berlin, Germany). The concentration at time zero after oral administration was set equal to zero. Cmax and tmax were determined graphically from the plasma concentration-time profiles. AUC0-tlast was calculated by linear trapezoidal rule from time zero to the last time after the dose. AUC0-∞ was calculated by linear trapezoidal rule and extrapolated to infinity using the apparent terminal elimination rate λz. λz was obtained by log-linear regression of the terminal phase of the plasma concentration-time curve.
Human Pharmacokinetic Study.
The effect of food on the pharmacokinetics of oseltamivir and oseltamivir carboxylate was evaluated during an open-label food effect study using the market formulation of oseltamivir in healthy subjects. Details of the study have been described previously (He et al., 1999a,b). In brief, 18 healthy volunteers (nine male, nine female, mean age 26 years) received an oral dose of 150 mg of oseltamivir fasting (from midnight) and after a high-fat, high-calorie breakfast (consistent with U.S. Food and Drug Administration guidance, www.fda/gov/downloads/regulatoryinformation/guidances/ucm126833.pdf). This meal included eggs, bacon, toast with butter, hash brown potatoes, and whole milk. Treatments were separated by a 7- to 10-day washout period. Plasma and urine samples (0–72 h) were analyzed for oseltamivir and oseltamivir carboxylate by HPLC-tandem mass spectrometry. The primary statistical objective was to compare the plasma AUC and Cmax (log transformed) of oseltamivir and oseltamivir carboxylate under fasted versus fed status. Sample size was determined a priori to ensure a balanced overall crossover study design and 80% power for having the confidence interval on the metabolite AUC and Cmax fall completely between 80 and 125% if any of the treatments truly differed by 10% or less. Analyses of variance (ANOVAs) appropriate for this design were performed including terms for sequence, subjects within sequence, period, and regimen. Analysis was done on both the raw and the natural logarithm-transformed scale for AUC parameters and Cmax. Confidence intervals (90%) for the difference in computed parameter least-squares means were calculated and were expressed as a percentage of the reference. The ANOVA was performed using SAS software (version 6.12; SAS Institute, Cary, NC). Microsoft Excel (version 4 or equivalent; Microsoft, Redmond, WA) was used to calculate the pharmacokinetic parameters.
This study was conducted in full compliance with the principles of the Declaration of Helsinki or with the laws and regulations of the country in which the research was conducted, whichever afforded greater protection to the individual. All subjects gave written informed consent before enrollment in the study after adequate explanation of the aims, methods, anticipated benefits, and potential hazards of the study.
Statistical Analysis.
The in vitro experiments were analyzed with hierarchical models, where the technical replicates were nested within days. The sample size (n) was the number of independent replications of the experiment (between three and six), which were conducted on different days, with typically three technical replicates per experimental condition per day (Cumming et al., 2007). No corrections for multiple testing were performed.
A power analysis was performed to estimate the number of rats needed to obtain significant results (using α = 0.05) for various effect sizes. Based on the AUC0–6h values for the distilled water and milk groups from Table 1 of Ogihara et al. (2009), n = 3 rats per group gave sufficient power (greater than 99%) to detect a difference between the means of the two groups as large as that observed in the Ogihara et al. (2009) study. If the difference between the means were 50% smaller than that in the Ogihara et al. (2009) study (but assuming that the variance in the data is the same), power would still be 98% with n = 3 rats per group.
TABLE 1.
Summary of the mean (S.D.) pharmacokinetic parameters of oseltamivir and oseltamivir carboxylate after a single oral dose of oseltamivir (30 mg/kg) to adult fasted rats (n = 3/group)
Oseltamivir was dosed either as a water solution, as a solution in milk, or as 125 mM aqueous Gly-Sar. Statistical analysis was performed on Cmax, tmax, AUC0–6h, and AUC0-∞ values; bovine milk (group 2) and Gly-Sar (group 3) treatments were compared with water (group 1). No statistically significant difference was observed.
| Pharmacokinetic Parameter | Oseltamivir |
Oseltamivir Carboxylate |
||||
|---|---|---|---|---|---|---|
| Water Group 1 | Bovine Milk Group 2 | Gly-Sar Group 3 | Water Group 1 | Bovine Milk Group 2 | Gly-Sar Group 3 | |
| Cmax, μg/l | 1690 (452) | 1630 (200) | 2070 (101) | 2290 (283) | 1630 (236) | 2560 (533) |
| tmax, h | 0.83 (0.57) | 0.5 (0) | 0.25 (0) | 1.08 (0.38) | 1.92 (1.13) | 1.17 (0.72) |
| AUC0–6h, μg/l · h | 2680 (262) | 3010 (533) | 3780 (821) | 5910 (429) | 5810 (1280) | 8350 (1850) |
| AUC0-∞, μg/l · h | 2710 (271) | 3060 (510) | 3810 (795) | 6140 (502) | 6300 (1310) | 8860 (1420) |
Pharmacokinetic parameters from the studies in adult rats were analyzed with a one-way ANOVA (water versus milk versus Gly-Sar). In case of a significant overall effect, Tukey's Honestly Significant Difference post hoc tests were used for pair-wise comparisons, with the main comparisons of interest being milk versus water and Gly-Sar versus water. The same parameters were also analyzed with a two-way ANOVA, with status (fed versus fasted) and group (milk versus Gly-Sar versus no milk/no Gly-Sar) as the two variables. The juvenile rats provided a sample only at one time point, and therefore, pharmacokinetic parameters could not be estimated for each animal. Nevertheless, differences in AUC0–5h between groups could be estimated. It was performed with the pharmacokinetic package (http://cran.r-project.org/web/packages/PK) for R (www.r-project.org). The main comparisons of interest were fasted milk (group 3) and fasted Gly-Sar (group 4) versus fasted water (group 2) and fasted water versus fed water (group 1). Corrections for multiple testing were done with Holm's method. For all analyses, oseltamivir and oseltamivir carboxylate data were analyzed separately.
Results
Effect of hPEPT1 Expression in CHO Cells on Gly-Sar, Oseltamivir, and Oseltamivir Carboxylate Intracellular Accumulation.
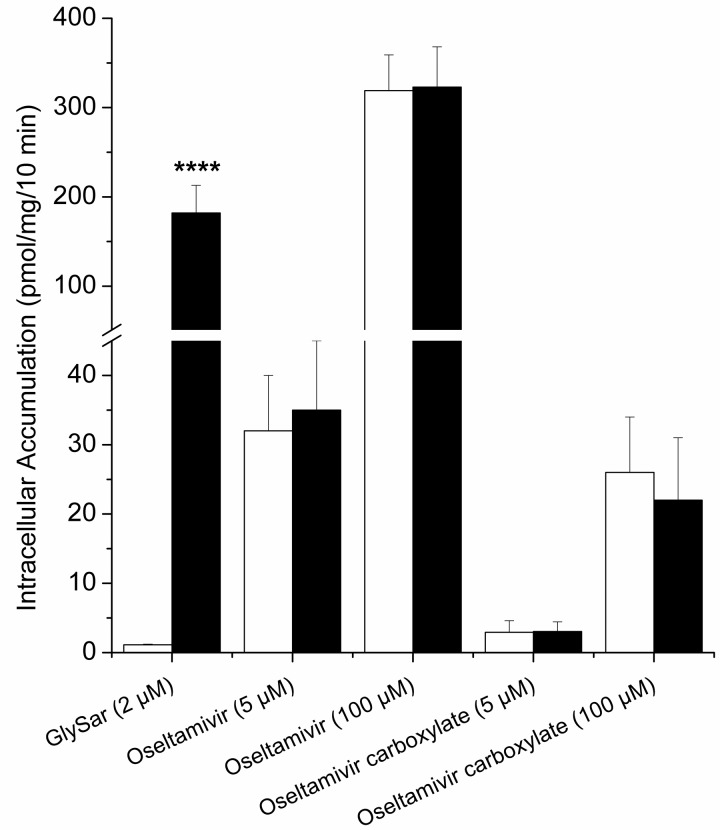
To evaluate the functionality of the cell system, active uptake of [3H]Gly-Sar was measured. In the control cells (CHO-K1), after a 10-min incubation at pH 6, [3H]Gly-Sar was barely detectable (1.1 ± 0.1 pmol/mg of protein) in the cell lysates. In contrast, in CHO-PEPT1 cells, the intracellular [3H]Gly-Sar level reached 182 ± 31 pmol/mg of protein (p < 0.0001; Fig. 1). Tested from 30 s to 15 min, [3H]Gly-Sar intracellular concentration continuously increased over time in CHO-PEPT1 cells, but not in CHO-K1 cells (data not shown). These results are consistent with a hPEPT1-mediated uptake of Gly-Sar in CHO-PEPT1 cells (Fujisawa et al., 2006). Using the same conditions, no difference in intracellular accumulation was observed between parental and transfected cells for oseltamivir or its carboxylate at 5 or 100 μM (Fig. 1). Different experimental conditions such as pH (5 and 6), incubation time (from 30 s to 15 min) and buffers (KH and Hanks' balanced salt solution) were tested in vitro (data not shown); under all conditions, no statistically significant difference was observed between CHO-PEPT1 and CHO-K1 cells for oseltamivir or oseltamivir carboxylate. These observations suggest that oseltamivir and oseltamivir carboxylate permeation into CHO cells is independent of hPEPT1. hPEPT2 substrate properties were also tested in parallel using the same conditions (data not shown), and likewise, no active transport of oseltamivir or its carboxylate could be observed.
Fig. 1.
Gly-Sar, oseltamivir, and oseltamivir carboxylate intracellular accumulation in CHO-K1 cells (white bars) and CHO-PEPT1 cells (black bars) after a 10-min incubation at 37°C, pH 6. Values are mean ± S.D. of three independent experiments (except Gly-Sar, n = 6). Statistical analysis was performed on intracellular accumulation; data for CHO-K1 cells were compared with those for CHO-PEPT1 cells. ****, p < 0.0001.
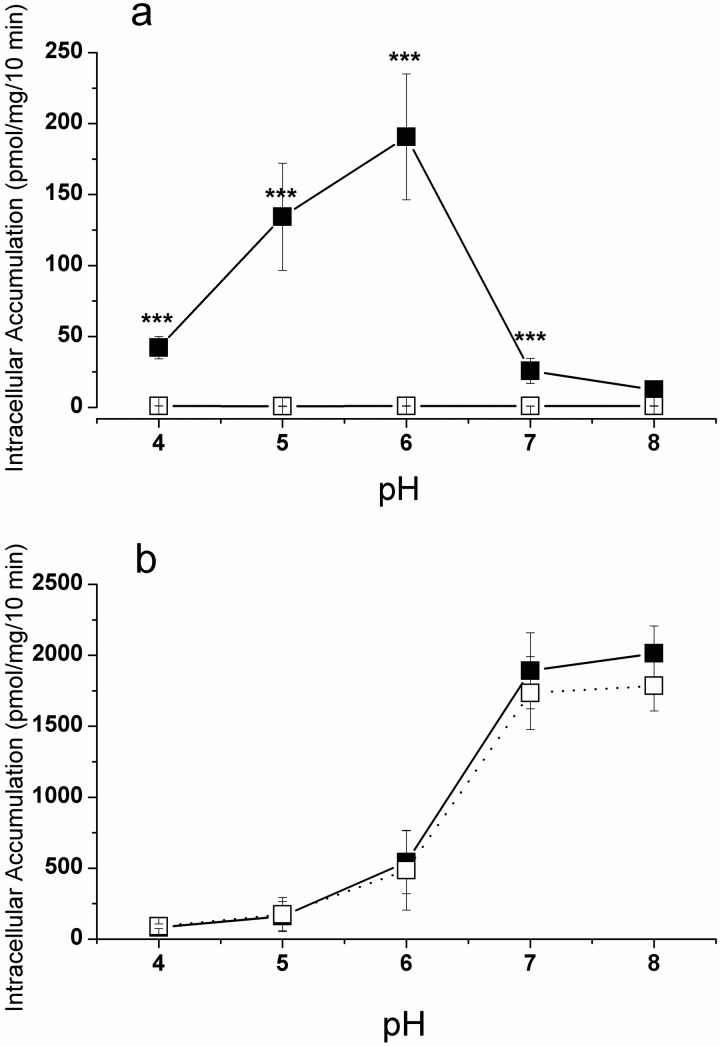
Effect of Extracellular pH on Gly-Sar and Oseltamivir Intracellular Accumulation into CHO-K1 and CHO-PEPT1 Cells.
To investigate the contribution of a proton gradient on intracellular accumulation, the pH was varied between 4 and 8. Gly-Sar permeation into control cells was independent of the pH in the incubation medium (Fig. 2a). In contrast, in hPEPT1-expressing cells, Gly-Sar active uptake was strongly pH-dependent and exhibited a bell-shaped curve with its highest uptake at pH 6, which was approximately 200-fold higher than that in control cells (p < 0.001; Fig. 2a). Our results are in excellent agreement with earlier reports (Fujisawa et al., 2006). The permeation of oseltamivir displayed a sigmoidal curve in both control and hPEPT1-expressing cells and appeared to reach a plateau value at higher pH (Fig. 2b), as is expected from passive permeation of a basic compound (Neuhoff et al., 2003). This curve was distinctively different from the bell-shaped curve for Gly-Sar and further supports the contention that oseltamivir is not a substrate of hPEPT1.
Fig. 2.
Effect of pH on Gly-Sar (2 μM) (a) and oseltamivir (100 μM) (b) intracellular accumulation in CHO-K1 cells (open squares) and CHO-PEPT1 cells (closed squares) after a 10-min incubation at 37°C. Values are mean ± S.D. of three independent experiments. Statistical analysis was performed on intracellular accumulation; data for CHO-K1 cells were compared with those for CHO-PEPT1 cells: ***, p < 0.0001.
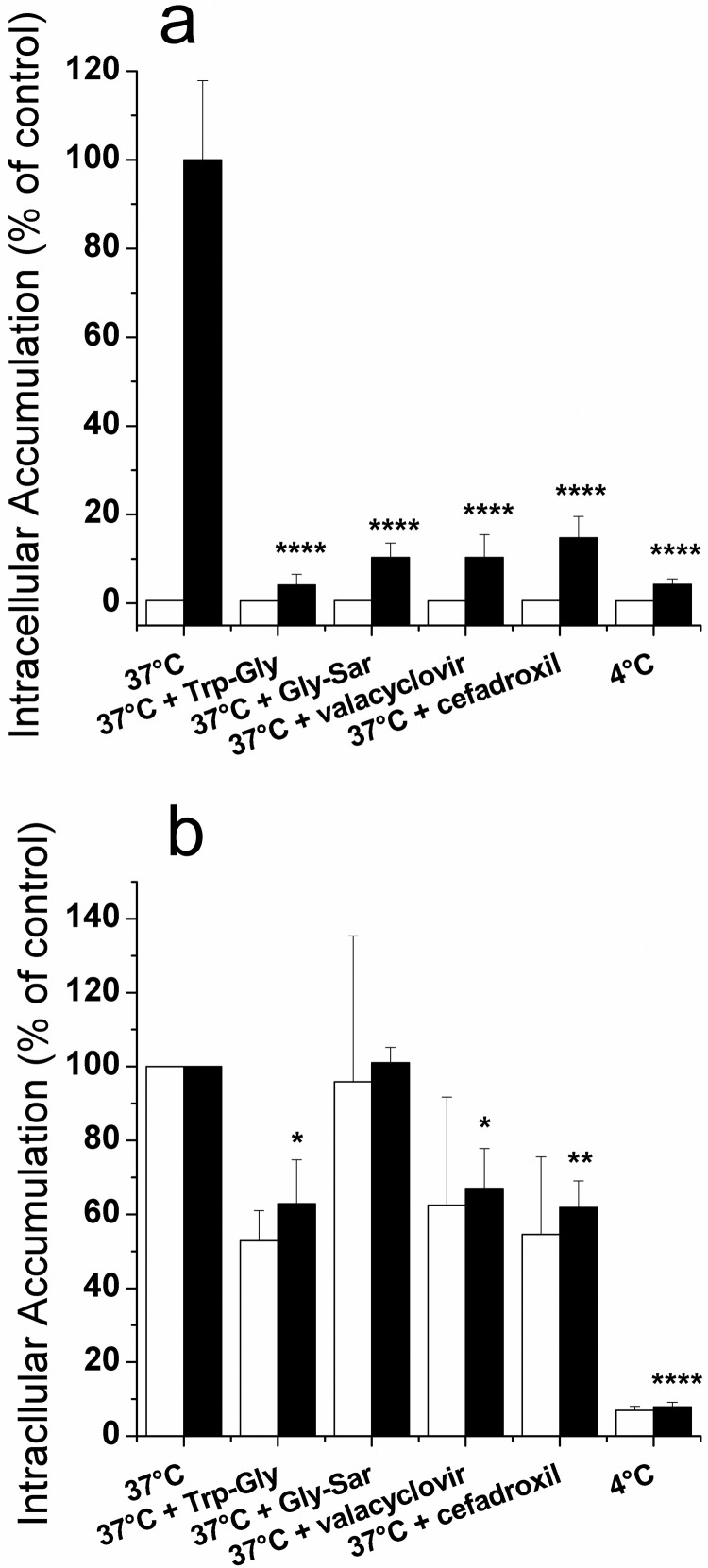
Effect of hPEPT1 Model Inhibitors on Gly-Sar and Oseltamivir Intracellular Accumulation into CHO-PEPT1 Cells.
To further establish the involvement of hPEPT1 in Gly-Sar or oseltamivir uptake, we examined the effect of known hPEPT1 inhibitors [Trp-Gly, valacyclovir, and cefadroxil (Faria et al., 2004)] at concentrations of 20 mM on Gly-Sar and oseltamivir accumulation in CHO-K1 and CHO-PEPT1 cells. As illustrated in Fig. 3a, the addition of hPEPT1 inhibitors decreased the intracellular accumulation of Gly-Sar in CHO-PEPT1 cells by 85 to 96% down to the background level in CHO-K1 cells (p < 0.0001), thereby demonstrating hPEPT1-mediated transport. Even though the addition of Trp-Gly, valacyclovir, and cefadroxil significantly decreased the oseltamivir levels in CHO-PEPT1 cells (p < 0.05), this change was similar in control and hPEPT1-transfected cells (Fig. 3b). Therefore, the effect of Trp-Gly, valacyclovir, and cefadroxil was nonspecific, providing additional evidence that hPEPT1 does not play a role in oseltamivir uptake.
Fig. 3.
Effect of Trp-Gly, Gly-Sar, valacyclovir, cefadroxil (all 20 mM), and 4°C temperature on Gly-Sar (2 μM) (a) and oseltamivir (100 μM) (b) intracellular accumulation in CHO-K1 cells (white bars) and CHO-PEPT1 cells (black bars) after a 10-min incubation at 37°C, pH 6. Values are mean ± S.D. of three independent experiments. Statistical analysis was performed on intracellular accumulation in CHO-PEPT1 where data in control condition (37°C) were compared with those in the presence of inhibitors or at 4°C: *, p < 0.05; **, p < 0.01; ****, p < 0.0001. Statistical analysis was also performed on intracellular accumulation where data for CHO-K1 cells were compared with those for CHO-PEPT1 cells: no statistically significant difference was observed for oseltamivir.
Effect of Temperature (4°C) on Gly-Sar and Oseltamivir Intracellular Accumulation into CHO-PEPT1 Cells.
The effect of low temperature (4°C) on Gly-Sar and oseltamivir accumulation was compared with a 37°C incubation in both CHO-K1 and CHO-PEPT1 cells. As illustrated in Fig. 3a, lowering the temperature to 4°C decreased the intracellular accumulation of Gly-Sar in CHO-PEPT1 cells by 96% down to the background level in CHO-K1 cells (p < 0.0001). As shown in Fig. 3b, the intracellular accumulation of oseltamivir at 4°C was also significantly decreased (p < 0.0001), compared with that at 37°C. However, the extent of change was similar in control and hPEPT1-expressing cells. Therefore, for oseltamivir this reduction was not due to reduced activity of hPEPT1 expressed in CHO-PEPT1 cells.
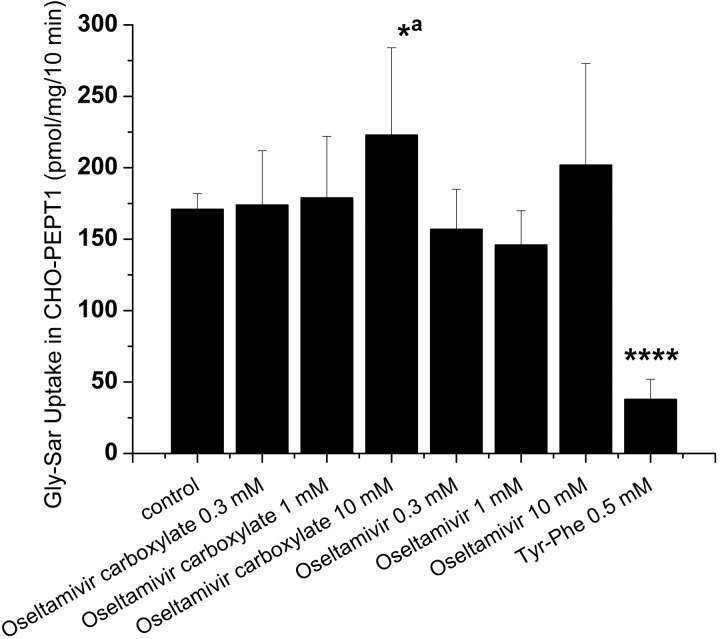
Effect of Oseltamivir and Oseltamivir Carboxylate on the Active Uptake of Gly-Sar in CHO-PEPT1 Cells.
It is known that different substrates for the same transporter will compete for the available binding site(s). Therefore, to elucidate whether oseltamivir and oseltamivir carboxylate are capable of competing with Gly-Sar for uptake by hPEPT1, we performed competition experiments across a 30-fold concentration range (0.3–10 mM). No statistically significant reduction on Gly-Sar uptake was noticeable in the presence of oseltamivir or oseltamivir carboxylate. Only a slightly significant (p = 0.045) increase of Gly-Sar uptake was observed in the presence of oseltamivir carboxylate at 10 mM. Incubation with Tyr-Phe (0.5 mM), a substrate and inhibitor of hPEPT1, resulted in a ∼4-fold reduction of Gly-Sar uptake (p < 0.0001; Fig. 4).
Fig. 4.
Effect of oseltamivir, oseltamivir carboxylate, and Tyr-Phe (as a positive control) on Gly-Sar (2 μM) intracellular accumulation in CHO-PEPT1 cells after a 10-min incubation at 37°C, pH 6. Values are mean ± S.D. of three independent experiments. Statistical analysis was performed on intracellular accumulation in CHO-PEPT1; data in the presence of oseltamivir, oseltamivir carboxylate, and Tyr-Phe were compared with those in the control condition: *, p < 0.05; a, p = 0.045; ****, p < 0.0001.
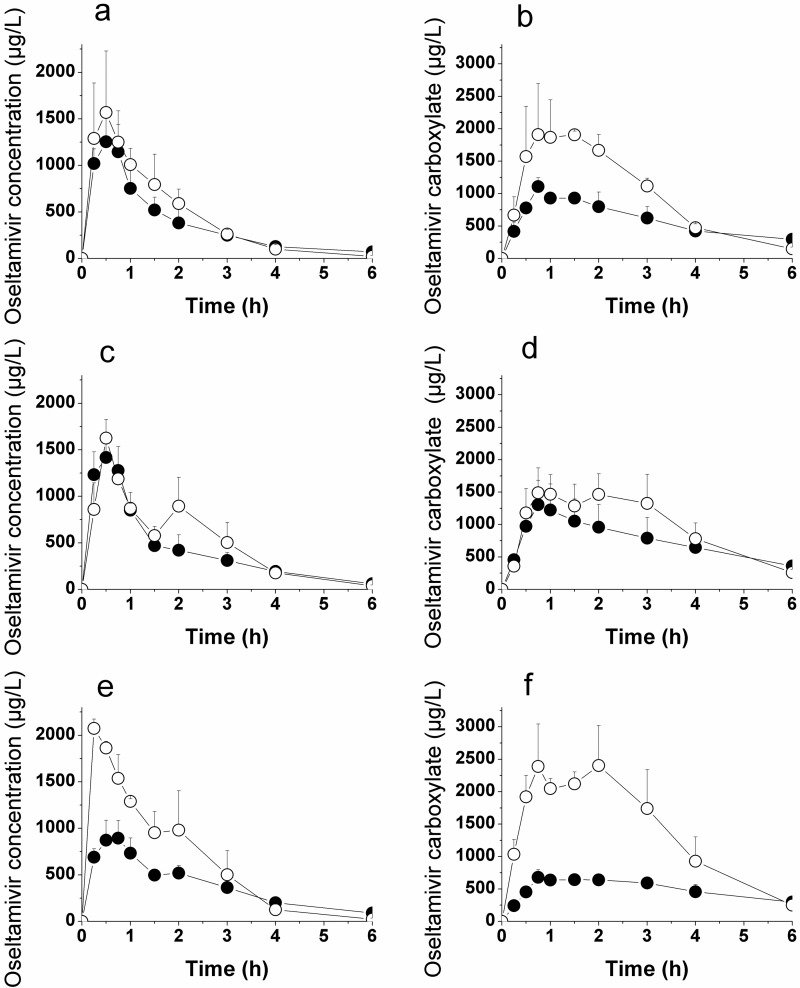
Pharmacokinetics of Oseltamivir and Oseltamivir Carboxylate in Adult Rats.
Oseltamivir was dosed orally at 30 mg/kg (calculated as a free base) to rats in various vehicles (water, bovine milk, and aqueous 125 mM Gly-Sar) under both fasted and fed conditions. The plasma concentration-time profiles and the derived pharmacokinetic parameters, including statistics, for the fasted condition are depicted in Fig. 5 and Table 1. Over 90% of the overall oseltamivir and oseltamivir carboxylate exposure (AUC0-∞) was covered by the 6-h observation interval. Comparing the different conditions, there was no statistically significant influence of milk or Gly-Sar on the Cmax, tmax, and plasma exposure (AUC) of both oseltamivir and oseltamivir carboxylate.
Fig. 5.
Mean plasma concentration-time profiles for oseltamivir (a, c, e) and oseltamivir carboxylate (b, d, f) after a single oral dose of oseltamivir (30 mg/kg) to adult rats. Oseltamivir was dosed either as a water solution (a, b), as a solution in bovine milk (c, d), or as a water solution containing 125 mM Gly-Sar (e, f). The rats were either fed (closed circles) or fasted (open circles). Values are mean ± S.D. (n = 3/group).
Plasma concentration-time profiles and pharmacokinetic parameters, including statistics, for the fed condition are depicted in Fig. 5 (truncated to 6 h for better comparison with the fasted condition) and Table 2. The 24-h observation period (AUC0–24h) covered more than 99% of the overall oseltamivir and oseltamivir carboxylate exposure (AUC0-∞). The statistical analysis of exposures after a single oral dose of 30 mg/kg oseltamivir to fed adult rats in the presence and absence of milk or Gly-Sar shows no significant differences in oseltamivir or carboxylate Cmax, tmax, and plasma exposure (AUC) between the various coadministrations.
TABLE 2.
Summary of the mean (S.D.) pharmacokinetic parameters of oseltamivir and oseltamivir carboxylate after a single oral dose of oseltamivir (30 mg/kg) to adult fed rats (n = 3/group)
Oseltamivir was dosed either as a water solution, as a solution in milk, or as 125 mM aqueous Gly-Sar. Statistical analysis was performed on Cmax, tmax, AUC0–6h, and AUC0-∞ values; bovine milk (group 2) and Gly-Sar (group 3) treatments were compared with water (group 1). No statistically significant difference was observed.
| Pharmacokinetic Parameter | Oseltamivir |
Oseltamivir Carboxylate |
||||
|---|---|---|---|---|---|---|
| Water Group 1 | Bovine Milk Group 2 | Gly-Sar Group 3 | Water Group 1 | Bovine Milk Group 2 | Gly-Sar Group 3 | |
| Cmax, μg/l | 1270 (325) | 1430 (250) | 926 (180) | 1110 (136) | 1310 (374) | 693 (104) |
| tmax, h | 0.41 (0.14) | 0.41 (0.14) | 0.66 (0.14) | 0.75 (0) | 0.75 (0) | 1.42 (0.63) |
| AUC0–6h, μg/l · h | 2200 (185) | 2510 (606) | 2280 (383) | 3540 (562) | 4510 (1440) | 2960 (469) |
| AUC0–24h, μg/l · h | 2510 (172) | 2800 (597) | 2830 (507) | 5280 (861) | 6330 (1680) | 4860 (998) |
| AUC0-∞, μg/l · h | 2510 (175) | 2810 (589) | 2840 (506) | 5290 (862) | 6340 (1670) | 4870 (999) |
Comparison of Drug Plasma Exposure in Fasted Versus Fed Adult Rats.
The effect of food intake on oseltamivir and oseltamivir carboxylate plasma exposure in rats was evaluated by a two-way ANOVA statistical analysis and is reported in Table 3. For oseltamivir, the exposure (AUC0–6h) was significantly lower in fed versus fasted rats (p = 0.0051). This negative food effect was similar across groups (interaction effect, p = 0.199). However, no statistically significant food effect was seen for AUC0-∞, likely because of the large percentage of extrapolated AUC from the 6-h time point to infinity in the fed state. A comparably larger fraction of exposure was observed after the 6-h time point (tlast in the fasted state) in fed animals than in fasted animals. Oseltamivir Cmax concentration was significantly lower in fed versus fasted rats (p = 0.0007), with an even larger negative food effect in the presence of Gly-Sar (interaction effect, p = 0.0287). The exposure of carboxylate (AUC0–6h) was lower in fed versus fasted rats (p < 0.0002), and this negative food effect was larger in the presence of Gly-Sar (interaction effect, p = 0.0243). However, for the exposure of oseltamivir carboxylate (AUC0-∞), a significant negative food effect (p = 0.049) was seen only in the presence of Gly-Sar. In addition, the carboxylate Cmax concentration was significantly lower in fed versus fasted rats (p < 0.0001), and this negative food effect was larger in the presence of Gly-Sar (interaction effect, p = 0.0038). No statistically significant discrepancy in tmax value for both oseltamivir and oseltamivir carboxylate was observed between fasted and fed conditions (group 1 fasted versus group 1 fed).
TABLE 3.
Two-way ANOVA statistical analysis of pharmacokinetic parameters with status (fed vs. fasted) and group (milk vs. Gly-Sar vs. no milk no Gly-Sar) as categorical variables
| p Values | Cmax | tmax | AUC0–6h | AUC0-∞ |
|---|---|---|---|---|
| Oseltamivir | ||||
| Status (fed vs. fasted) | 0.000669 | 0.822 | 0.00514 | 0.0745 |
| Group (milk vs. Gly-Sar vs. water) | 0.954 | 0.455 | 0.177 | 0.0959 |
| Status-group interaction | 0.0287 | 0.0467 | 0.199 | 0.380 |
| Oseltamivir carboxylate | ||||
| Status (fed vs. fasted) | 0.00000624 | 0.182 | 0.000118 | 0.0718 |
| Group (milk vs. Gly-Sar vs. water) | 0.453 | 0.467 | 0.402 | 0.418 |
| Status-group interaction | 0.00381 | 0.184 | 0.0243 | 0.0489 |
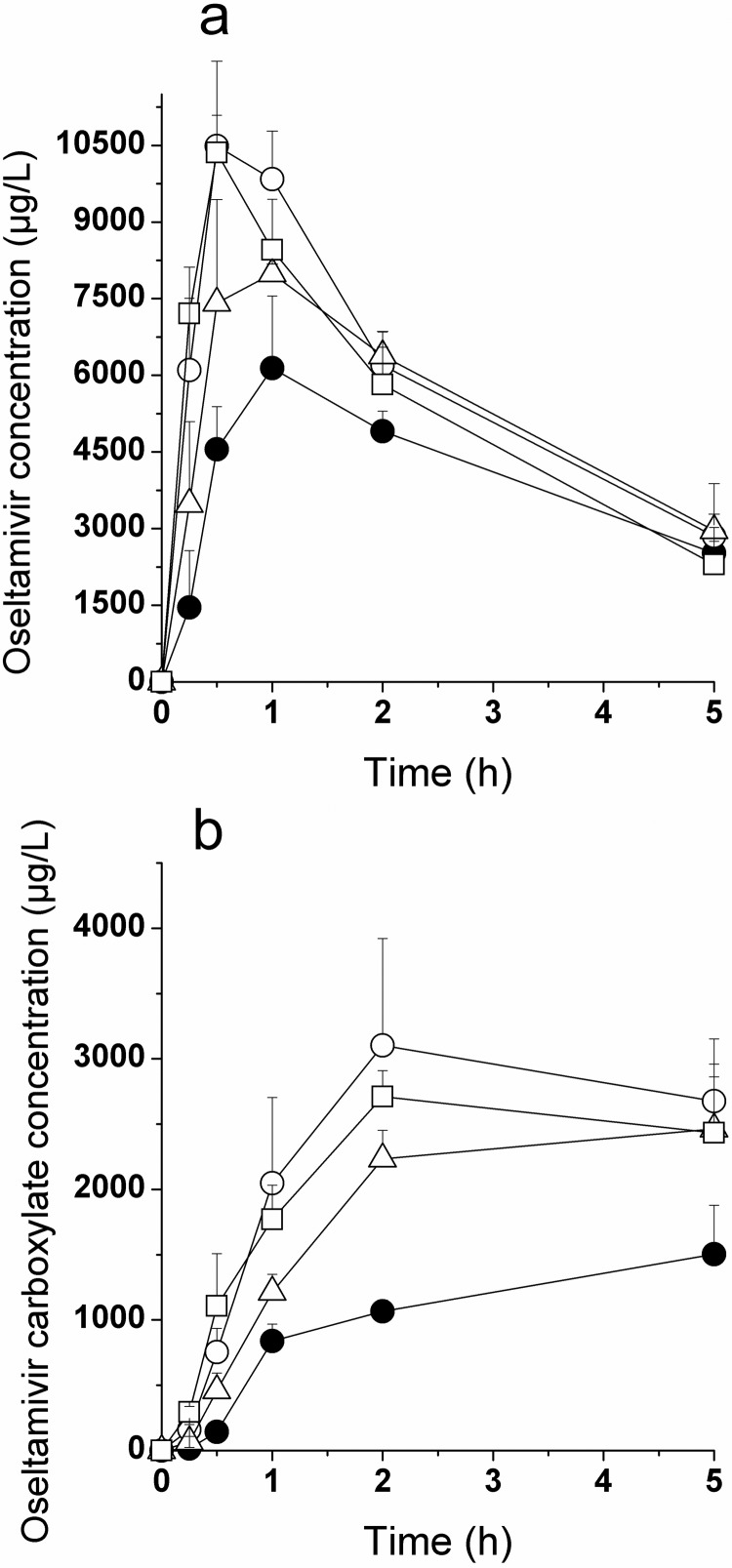
Plasma Exposure of Oseltamivir and Oseltamivir Carboxylate in Juvenile Rats.
Oseltamivir was dosed at 30 mg/kg (calculated as a free base) by oral gavage to juvenile male and female rats in various formulations (water, bovine milk, and 125 mM Gly-Sar in water) under breast-fed (residing with the dams, oseltamivir administered in water only) or fasted conditions (pups removed from the dams, all three treatments). Plasma concentration-time profiles and pharmacokinetic parameters are depicted in Fig. 6 and Table 4. The AUC0-∞ was not calculated in this study because of the limited coverage or complete absence of a terminal phase for oseltamivir and oseltamivir carboxylate, respectively. Within the 5-h observation period, breast-fed pups showed significantly lower exposures than fasted pups for oseltamivir (−30%, p = 0.002) and oseltamivir carboxylate (−60%, p < 0.001) after administration as aqueous solution. In fasted pups, no impact of Gly-Sar coadministration was observed, and coadministration of milk reduced the exposure of oseltamivir carboxylate (−24%, p = 0.028) but not oseltamivir. The presence of maternal or bovine milk in the gastrointestinal tract of juvenile rats tended to delay the tmax of oseltamivir and consequently that of the active metabolite, compared with the tmax values obtained under fasted conditions (groups 1 and 3 versus groups 2 and 4).
Fig. 6.
Mean plasma concentration-time profiles for oseltamivir (a) and oseltamivir carboxylate (b) after a single oral dose of oseltamivir (30 mg/kg) to juvenile rats. Oseltamivir was dosed either as a water solution (circles), as a solution in bovine milk (open triangles), or as a water solution containing 125 mM Gly-Sar (open squares). The juvenile rats were either breast-fed (closed circles) or fasted (open symbols). Values are mean ± S.D. (n = 3/time point).
TABLE 4.
Summary of the mean pharmacokinetic parameters of oseltamivir and oseltamivir carboxylate after a single oral dose of oseltamivir (30 mg/kg) to juvenile rats (composite design, n = 3/time point)
Oseltamivir was dissolved in either water, bovine milk, or aqueous Gly-Sar (125 mM). The juvenile rats were either breast-fed or fasted. Statistical analysis was performed on AUC0–5h values; data for fasted juvenile rats receiving bovine milk (group 3) or Gly-Sar (group 4) were compared with those of fasted animals receiving the drug in water solution (group 2). In addition, group 2 was compared with group 1 (breast-fed juvenile rats).
| Pharmacokinetic Parameter | Oseltamivir |
Oseltamivir Carboxylate |
||||||
|---|---|---|---|---|---|---|---|---|
| Breast-Fed |
Fasted |
Breast-Fed |
Fasted |
|||||
| Water |
Water |
Bovine Milk |
Gly-Sar |
Water |
Water |
Bovine Milk |
Gly-Sar |
|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 1 | Group 2 | Group 3 | Group 4 | |
| Cmax, μg/l | 6140 | 10,500 | 7990 | 10,400 | 1500 | 3100 | 2460 | 2710 |
| tmax, h | 1.0 | 0.50 | 1.0 | 0.50 | 5.0a | 2.0 | 5.0a | 2.0 |
| AUC0–5h, μg/l · h | 20,300 | 29,500b | 26,900 | 27,100 | 5070 | 12,100b | 9260c | 10,900 |
5-h time point was last sampling time point, so actual tmax may be later.
p < 0.002 versus group 1.
p = 0.028 versus group 2.
Pharmacokinetics of Oseltamivir and Oseltamivir Carboxylate in Healthy Volunteers after a Single Oral Dose under Fed or Fasted Condition.
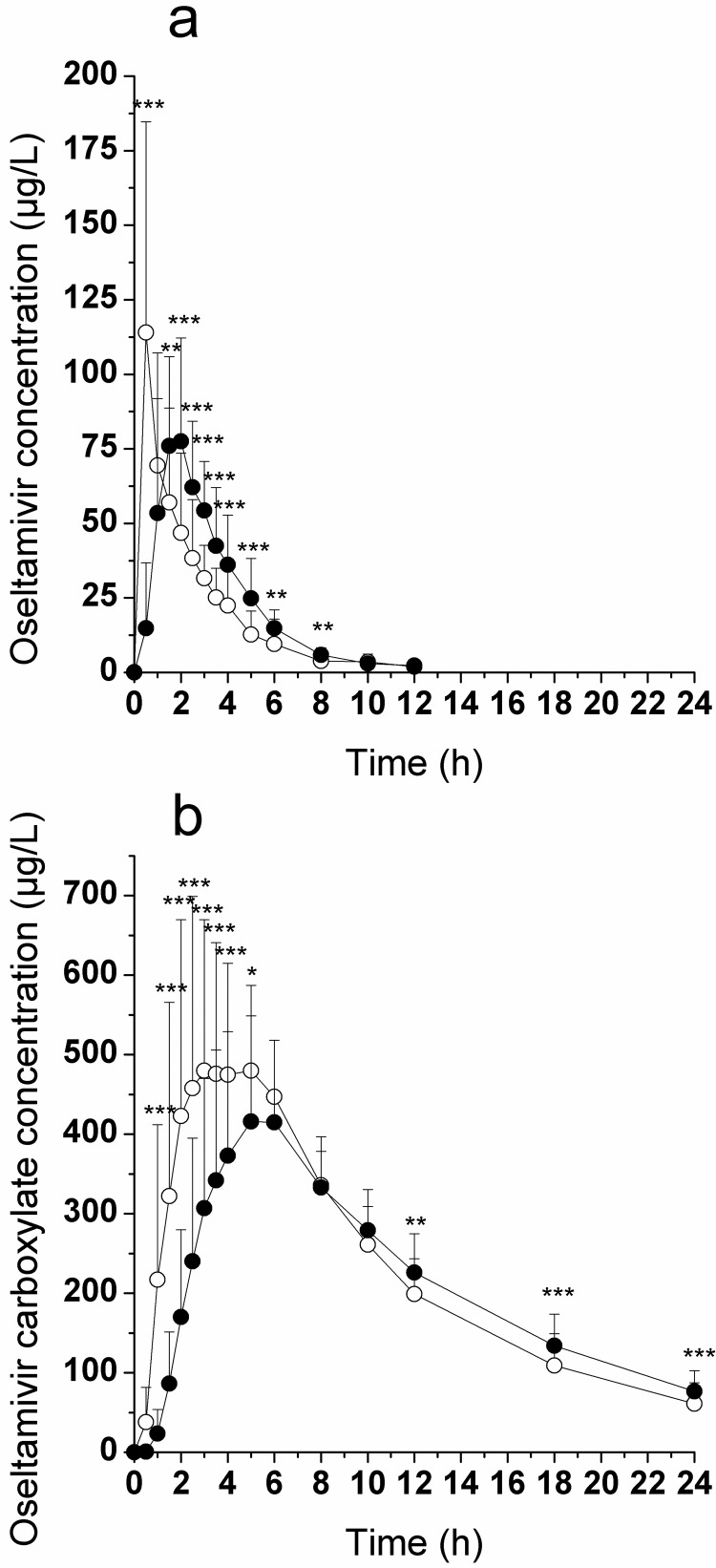
Mean plasma concentration-time profiles in healthy volunteers for oseltamivir and oseltamivir carboxylate, after administration of oseltamivir after overnight fasting and with food, are shown in Fig. 7. Mean (S.D.) pharmacokinetic parameters are summarized in Table 5. Food had a substantial effect on oseltamivir plasma concentrations, resulting in an 18% decrease in Cmax, an 18% increase in AUC0-∞, a 2.6-fold increase in tmax, and a 33% increase in half-life. Oseltamivir carboxylate plasma concentrations increased rapidly in all subjects, reaching maximal levels at approximately 4.5 h for the fasted group and 5.4 h with food. The presence of food resulted in a small reduction in oseltamivir carboxylate Cmax (approximately 20%) and AUC0–24h (approximately 9%), a delay of approximately 1 h in achieving maximal plasma concentrations, and an increase of 1 h in half-life. However, for the primary pharmacokinetic parameters of the active metabolite, the 90% confidence intervals for the ratios of means for the two treatments were within the 80 to 125% ranges for log-transformed AUC0-∞ (93.3–100.7%) and were within the 70 to 143% ranges for log-transformed Cmax (75.6–86.2%), indicating equivalence of the two treatments.
Fig. 7.
Mean plasma concentration-time profiles of oseltamivir (a) and oseltamivir carboxylate (b) after a single oral dose of oseltamivir (150 mg) to healthy volunteers before (open circles) and after (closed circles) a standardized high-fat and high-calorie breakfast. Values are mean ± S.D. (n = 18). Asterisks indicate a significant difference between treatments: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
TABLE 5.
Summary of the mean (S.D.) pharmacokinetic parameters of oseltamivir and oseltamivir carboxylate after a single oral dose of oseltamivir (150 mg) to healthy volunteers (n = 18) under fasting conditions and with food
Statistical analysis was performed on pharmacokinetic parameters; data for fasted volunteers were compared with those receiving food.
| Pharmacokinetic Parameter | Oseltamivir |
Oseltamivir Carboxylate |
||||
|---|---|---|---|---|---|---|
| Fasted | Fed | Ratio (%) Fed/Fasted | Fasted | Fed | Ratio (%) Fed/Fasted | |
| Cmax, μg/l | 124 (63.2) | 101 (32.2) | 81.4 | 551 (204) | 441 (142)*** | 80.0 |
| tmax, h | 0.806 (0.572) | 2.06 (0.937)*** | 256 | 4.37 (1.21) | 5.42 (0.772)** | 124 |
| tβ, h | 1.61 (0.529) | 2.15 (1.17) | 134 | 6.87 (1.39) | 8.20 (1.63)*** | 120 |
| CL/F, l/min | 13.0 (11.1) | 9.54 (3.96) | 73.4 | 0.371 (0.0427) | 0.385 (0.0665) | 103.9 |
| AUC0–24h, μg/l · h | 238 (82.0) | 280 (71.4)*** | 118 | 5543 (688) | 5064 (865)** | 91.3 |
| AUC0-∞, μg/l · h | 244 (81.9) | 287 (71.6)*** | 118 | 6218 (756) | 6069 (1029) | 97.6 |
| fe, % | 3.09 (1.22) | 4.32 (1.53)*** | 140 | 58.5 (10.9) | 55.5 (9.59) | 94.9 |
| CLR, l/h | 20.4 (7.20) | 23.1 (5.95) | 113 | 13.4 (2.76) | 13.1 (2.65) | 97.7 |
CL/F, clearance as a function of bioavailability; fe, fraction excreted unchanged in urine; CLR, renal clearance.
, p < 0.01;
, p < 0.001.
Food caused an increase in the amount of renally secreted oseltamivir compared with fasted, as is evidenced by an increase in excreted drug from 3.09 ± 1.22 to 4.32 ± 1.53%. The high-fat meal had no effect on apparent clearance and/or renal clearance for oseltamivir or its carboxylate (Table 5).
Discussion
Oseltamivir is an orally available ester-prodrug of RO0640802 (carboxylate), clinically used as a neuraminidase inhibitor to treat influenza. Upon absorption, oseltamivir is converted to its pharmacologically active carboxylate in the liver by carboxylesterase 1 (Shi et al., 2011). Ogihara et al. (2009) and Morimoto et al. (2011) reported that oseltamivir transport in vitro and in vivo, in rats and healthy volunteers, is mediated by PEPT1. In the present report, we provide an in-depth systematic examination of the pharmacokinetic parameters of oseltamivir and its carboxylate in adult and juvenile rats, as well as mechanistic studies in hPEPT1-expressing CHO cells. Furthermore, we compared food effect data obtained from a human bioequivalence study with the observation by Morimoto et al. (2011) and conclude the intestinal absorption of oseltamivir is not limited by rat Pept1 or hPEPT1.
Oseltamivir and Oseltamivir Carboxylate Are Not hPEPT1 Substrates In Vitro.
We previously emphasized the importance of controlling the experimental conditions to quantify active transport in vitro (Poirier et al., 2008). In the present report, we implemented thorough procedures for the evaluation of oseltamivir and oseltamivir carboxylate as hPEPT1 substrates. Instructions detailed by Brandsch (2009) to avoid false negatives and positives in PEPT in vitro experiments were strictly followed, especially with regard to pH monitoring, drug stability, comparison to control cells, and inhibitor effects.
First, we established the functionality of CHO-PEPT1 cells by demonstrating a 200-fold higher uptake of Gly-Sar in CHO-PEPT1 compared with CHO-K1 cells, an optimal pH, and a strong inhibition of Gly-Sar accumulation in the presence of typical inhibitors (Figs. 1–3). These results implicate hPEPT1-mediated uptake for Gly-Sar and are in accordance with studies by Fujisawa et al. (2006). Oseltamivir is an ethyl ester, which potentially can be hydrolyzed in the incubation medium during the experiment. We monitored for and confirmed ester stability during the incubation period.
hPEPT1 favors a pH gradient for optimal function. The efficiency of hPEPT1-mediated transport depends on an acidic extracellular pH environment, and the optimal pH seems to be substrate-specific (Steel et al., 1997). Therefore, we performed a pH-dependent experiment that clearly showed the following: 1) as opposed to Gly-Sar, oseltamivir lacks an optimal pH for accumulation in CHO-PEPT1 cells, and 2) as opposed to Gly-Sar, oseltamivir accumulation in CHO-K1 closely follows that in CHO-PEPT1 cells (Fig. 2). Because no difference between hPEPT1 and control cells was seen, our data do not support hPEPT1-mediated uptake of oseltamivir.
As observed by Ogihara et al. (2009), when incubated at 4°C, oseltamivir intracellular accumulation decreased drastically in CHO-PEPT1 cells (Fig. 3). However, a similar observation was noted in CHO-K1 cells, demonstrating a nonspecific effect, as described previously (Poirier et al., 2008). A decrease of permeation at 4°C is not proof of active uptake.
To further corroborate the lack of interaction of oseltamivir and its carboxylate with hPEPT1, a series of inhibition experiments was conducted. As suggested by Brandsch (2009), the inclusion of CHO-K1 cells is essential for correct results interpretation. Using typical hPEPT1 inhibitors, we noticed an identical degree of interference with accumulation in CHO-PEPT1 and CHO-K1 cells for oseltamivir (Fig. 3b). Care was taken in readjusting the pH after dissolving substrate and inhibitors. An additional piece of evidence for lack of interaction with hPEPT1 comes from results demonstrating that Gly-Sar uptake in CHO-PEPT1 cells was not inhibited by up to 10 mM oseltamivir or oseltamivir carboxylate (Fig. 4).
In summary, the evidence from our in vitro studies supports the validity of the CHO-PEPT1 cell line. Moreover, oseltamivir and oseltamivir carboxylate are neither substrates nor inhibitors of hPEPT1 (or hPEPT2, data not shown). These in vitro findings are supported by the independent work of Hu et al. (2012) who demonstrated, using transfected yeast, that oseltamivir was not a substrate for human, mouse, and rat PEPT1.
PEPT1 Inhibitors Do Not Significantly Affect Oseltamivir and Oseltamivir Carboxylate Rat Pharmacokinetics.
The present rat studies were designed and powered to confirm data from a previous report on effects of milk and PEPT1 inhibitors on oseltamivir pharmacokinetics (Ogihara et al., 2009). The pharmacokinetics (AUC0–6h and Cmax) in fasted rats treated with aqueous oseltamivir matched very well those reported previously (Ogihara et al., 2009). However, we found no reduction of oseltamivir exposure (AUC0-∞) in fasted rats (Fig. 5, open circles; Table 1) when administered in milk or in Gly-Sar.
The same vehicles were also investigated in fed rats (rat chow ad libitum; Fig. 5, closed circles; Table 2). This allowed a comparison of the effects of putative (bovine milk) or known (Gly-Sar) PEPT1 inhibitors in fasted and fed rats. As in the fasted rats, the different vehicles did not affect oseltamivir exposure in fed rats.
In this study, the active drug (oseltamivir carboxylate) was monitored in parallel to the prodrug in all plasma samples to evaluate the effect of the coadministered vehicles on the pharmacologically active oseltamivir carboxylate. All blood samples were treated with dichlorvos immediately after sampling to prevent ex vivo conversion of oseltamivir to oseltamivir carboxylate (Chang et al., 2009). Consistent behavior of prodrug and carboxylate across treatments indicates adequate stabilization of blood samples and corroborates the findings with the prodrug.
Administering oseltamivir in water, milk, or aqueous Gly-Sar had no effect on oseltamivir carboxylate exposure in fasted or fed rats (Fig. 5, open and closed circles, respectively; Tables 1 and 2). Overall, we confirmed previously reported exposures of oseltamivir when administered as aqueous solution to fasted rats but could not observe an effect of coadministration of milk or aqueous Gly-Sar on oseltamivir or oseltamivir carboxylate exposure in male Wistar rats.
Application of oseltamivir in the three vehicles to fasted and fed rats also allows for a direct comparison of the exposures under the two feeding conditions (Fig. 5, open versus closed circles; Table 3). In contrast to the lack of impact of the PEPT1 inhibitor coadministration, fed rats showed moderately but consistently lower exposure toward oseltamivir carboxylate compared with fasted rats, although the reduction of exposure toward oseltamivir was not statistically significant. The reduced exposure may be due to slower absorption of oseltamivir, together with a slower conversion to oseltamivir carboxylate in fed rats favoring competitive elimination (other than conversion to the active carboxylate) in the fed state of rodents. A slight trend toward a larger fraction unchanged excreted in the urine is observed in fed versus fasted humans (Table 5); this effect may be more pronounced in the preclinical species.
The pharmacokinetics of oseltamivir and its carboxylate were further examined in juvenile rats either breast-fed or fasted in the presence and absence of bovine milk or Gly-Sar (Fig. 6; Table 4). As in the adult animals, Gly-Sar had no statistically significant effect on oseltamivir and active metabolite plasma exposure in juvenile rats. In addition, the presence of bovine milk in the formulation had no significant effect on the exposure of oseltamivir but reduced the exposure of the active metabolite. Breast-feeding resulted in a reduction of oseltamivir and oseltamivir carboxylate exposure (AUC0–5h). Similar results were observed by Ogihara et al. (2009) determining oseltamivir exposure at a single time point (30 min after dosing). Apparently, breast-feeding in juvenile rats had a very similar effect on the absorption and disposition of oseltamivir and oseltamivir carboxylate as food in adult rats. Oseltamivir and oseltamivir carboxylate exposure in adult rats was lower than that in juvenile rats irrespective of feed status. A very similar age dependence for exposure of oseltamivir and oseltamivir carboxylate was reported previously for marmosets (Parrott et al., 2011).
The rodent in vivo studies show that coadministration of the PEPT1 inhibitor Gly-Sar or bovine milk has no relevant effect on oseltamivir and oseltamivir carboxylate exposure. However, a moderate food effect of rat chow in adult rats and breast-feeding in juvenile animals is observed. Taken together, the in vivo data in rodents support the results of the in vitro studies with hPEPT1 that oseltamivir is not a substrate for PEPT1.
Food Effect Study in Healthy Human Volunteers.
The relative bioavailability of oseltamivir was examined and compared in healthy volunteers under fasted and fed (standard high-fat, high-calorie breakfast including milk) conditions (Fig. 7; Table 5). Food had a significant effect on oseltamivir prodrug plasma concentrations; however, these changes are not clinically relevant. In contrast, food had a relatively small effect on the active metabolite. The active metabolite AUC0-∞ ratio after oral administration of oseltamivir with food, relative to fasting, was 98%. The AUC0-∞ values demonstrated that the fasting and fed treatment regimens were equivalent, with a 90% confidence interval of 93 to 101% on the log-transformed scale. Consequently, from a clinical perspective, oseltamivir may be given in the fed or fasted state without having an impact on its clinical efficacy. Whereas overall exposure (AUC0-∞) of oseltamivir carboxylate was not affected by high-fat food, the AUC0–24h, Cmax, tmax, and half-life were modestly but significantly altered, suggesting a mechanistic interaction with carboxylesterases responsible for prodrug conversion.
Morimoto et al. (2011) reported an initial reduction of absorption of oseltamivir in the presence of milk in six healthy volunteers as evidenced by the delayed onset of plasma concentration of oseltamivir. Striking are the similarities between the plasma concentration profiles after dosing in milk or high-fat meal (including milk) in the study by Morimoto et al. (2011) and the present study, respectively. The authors attributed the early reduction in absorption to the inhibition of hPEPT1-mediated uptake of oseltamivir. However, our observations are as follows: 1) in the present report, neither oseltamivir nor its carboxylate is a substrate of hPEPT1 in vitro; 2) a monkey physiology-based pharmacokinetic model using exclusively passive permeability to predict absorption without any active component could describe oseltamivir and oseltamivir carboxylate pharmacokinetic profiles (Parrott et al., 2011); and 3) He et al. (1999b) demonstrated that coadministration of amoxicillin, a known hPEPT1 substrate (Herrera-Ruiz and Knipp, 2003), had no effect on the disposition of the oseltamivir carboxylate. Taken together, the results point away from hPEPT1-mediated absorption of oseltamivir. Instead, the results point toward a significant but moderate food effect on oseltamivir that is mediated by milk and/or food not affecting the overall exposure (AUC0-∞) of the active drug, oseltamivir carboxylate. In concluding, we present evidence from in vitro studies, using a multitude of conditions, and in vivo pharmacokinetic (animal and human) experiments that oseltamivir and its carboxylate are neither substrates nor inhibitors of rat Pept1 or hPEPT1.
Acknowledgments
We thank Werner Rubas (PK/ADME Consulting, LLC, Redwood City, CA) for support in writing this manuscript and Andreas Weidenbach for technical assistance.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM035498] (to D.E.S.). D.E.S. is a consultant to F. Hoffmann-La Roche Ltd.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- hOAT
- human organic anion transporters
- PEPT
- peptide transporter
- CHO
- Chinese hamster ovary
- BSA
- bovine serum albumin
- PBS
- phosphate-buffered saline
- KH
- Krebs-Henseleit
- MES
- 4-morpholineethanesulfonic acid
- HPLC
- high-performance liquid chromatography
- Cmax
- maximal plasma concentration
- tmax
- time to maximal concentration
- AUC
- area under the concentration-time profile
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Poirier, Belli, Funk, Otteneder, Prinssen, Lazic, Rayner, Hoffmann, Singer, Smith, and Schuler.
Conducted experiments: Poirier, Belli, Portmann, Heinig, and Rayner.
Contributed new reagents or analytic tools: Heinig.
Performed data analysis: Poirier, Belli, Funk, Lazic, Rayner, and Schuler.
Wrote or contributed to the writing of the manuscript: Poirier, Belli, Funk, Lazic, Rayner, Smith, and Schuler.
References
- Brandsch M. (2009) Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin Drug Metab Toxicol 5:887–905 [DOI] [PubMed] [Google Scholar]
- Chang Q, Chow MS, Zuo Z. (2009) Studies on the influence of esterase inhibitor to the pharmacokinetic profiles of oseltamivir and oseltamivir carboxylate in rats using an improved LC/MS/MS method. Biomed Chromatogr 23:852–857 [DOI] [PubMed] [Google Scholar]
- Cumming G, Fidler F, Vaux DL. (2007) Error bars in experimental biology. J Cell Biol 177:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria TN, Timoszyk JK, Stouch TR, Vig BS, Landowski CP, Amidon GL, Weaver CD, Wall DA, Smith RL. (2004) A novel high-throughput pepT1 transporter assay differentiates between substrates and antagonists. Mol Pharm 1:67–76 [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Tateoka R, Nara T, Kamo N, Taira T, Miyauchi S. (2006) The extracellular pH dependency of transport activity by human oligopeptide transporter 1 (hPEPT1) expressed stably in Chinese hamster ovary (CHO) cells: a reason for the bell-shaped activity versus pH. Biol Pharm Bull 29:997–1005 [DOI] [PubMed] [Google Scholar]
- He G, Massarella J, Schulz R, Barrett J, Woodruffe-Peacock R, Dorr A, Ward P, Brown A. (1999a) The effect of food on the pharmacokinetics of the novel oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104). American Association of Pharmaceutical Scientists, Southeast Regional Meeting; 1999 June 21; Durham, NC American Association of Pharmaceutical Scientists, Arlington, VA [Google Scholar]
- He G, Massarella J, Ward P. (1999b) Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet 37:471–484 [DOI] [PubMed] [Google Scholar]
- Heinig K, Bucheli F. (2008) Sensitive determination of oseltamivir and oseltamivir carboxylate in plasma, urine, cerebrospinal fluid and brain by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 876:129–136 [DOI] [PubMed] [Google Scholar]
- Herrera-Ruiz D, Knipp GT. (2003) Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci 92:691–714 [DOI] [PubMed] [Google Scholar]
- Hill G, Cihlar T, Oo C, Ho ES, Prior K, Wiltshire H, Barrett J, Liu B, Ward P. (2002) The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab Dispos 30:13–19 [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Funk C, Fowler S, Otteneder MB, Breidenbach A, Rayner CR, Chu T, Prinssen EP. (2009) Nonclinical pharmacokinetics of oseltamivir and oseltamivir carboxylate in the central nervous system. Antimicrob Agents Chemother 53:4753–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chen X, Smith DE. (2012) Functional analysis of glycylsarcosine and oseltamivir in Pichia pastoris expressing the rat, mouse, and human intestinal peptide transporter. Drug Metab Dispos doi:10.1124/dmd.111.044263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knütter I, Kottra G, Fischer W, Daniel H, Brandsch M. (2009) High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos 37:143–149 [DOI] [PubMed] [Google Scholar]
- Knütter I, Wollesky C, Kottra G, Hahn MG, Fischer W, Zebisch K, Neubert RH, Daniel H, Brandsch M. (2008) Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther 327:432–441 [DOI] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. (1995) Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem 270:6456–6463 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Kishimura K, Nagami T, Kodama N, Ogama Y, Yokoyama M, Toda S, Chiyoda T, Shimada R, Inano A, et al. (2011) Effect of milk on the pharmacokinetics of oseltamivir in healthy volunteers. J Pharm Sci 100:3854–3861 [DOI] [PubMed] [Google Scholar]
- Moscona A. (2005) Neuraminidase inhibitors for influenza. N Engl J Med 353:1363–1373 [DOI] [PubMed] [Google Scholar]
- Neuhoff S, Ungell AL, Zamora I, Artursson P. (2003) pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res 20:1141–1148 [DOI] [PubMed] [Google Scholar]
- Ogihara T, Kano T, Wagatsuma T, Wada S, Yabuuchi H, Enomoto S, Morimoto K, Shirasaka Y, Kobayashi S, Tamai I. (2009) Oseltamivir (Tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos 37:1676–1681 [DOI] [PubMed] [Google Scholar]
- Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Hosokawa M, Schuetz JD, Sugiyama Y. (2009) Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxyl ate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos 37:315–321 [DOI] [PubMed] [Google Scholar]
- Parrott N, Davies B, Hoffmann G, Koerner A, Lave T, Prinssen E, Theogaraj E, Singer T. (2011) Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin Pharmacokinet 50:613–623 [DOI] [PubMed] [Google Scholar]
- Poirier A, Lavé T, Portmann R, Brun ME, Senner F, Kansy M, Grimm HP, Funk C. (2008) Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab Dispos 36:2434–2444 [DOI] [PubMed] [Google Scholar]
- Shi D, Yang D, Prinssen EP, Davies BE, Yan B. (2011) Surge in expression of carboxylesterase 1 during the postneonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J Infect Dis 203:937–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel A, Nussberger S, Romero MF, Boron WF, Boyd CA, Hediger MA. (1997) Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J Physiol 498 (Pt 3):563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]