Abstract
Stress can trigger the relapse of drug use in recovering cocaine addicts and reinstatement in rodent models through mechanisms that may involve norepinephrine release and β-adrenergic receptor activation. The present study examined the role of β-adrenergic receptor subtypes in the stressor-induced reinstatement of extinguished cocaine-induced (15 mg/kg i.p.) conditioned place preference in mice. Forced swim (6 min at 22°C) stress or activation of central noradrenergic neurotransmission by administration of the selective α2 adrenergic receptor antagonist 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole (BRL-44,408) (10 mg/kg i.p.) induced reinstatement in wild-type, but not β- adrenergic receptor-deficient Adrb1/Adrb2 double-knockout, mice. In contrast, cocaine administration (15 mg/kg i.p.) resulted in reinstatement in both wild-type and β-adrenergic receptor knockout mice. Stress-induced reinstatement probably involved β2 adrenergic receptors. The β2 adrenergic receptor antagonist -(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol (ICI-118,551) (1 or 2 mg/kg i.p.) blocked reinstatement by forced swim or BRL-44,408, whereas administration of the nonselective β-adrenergic receptor agonist isoproterenol (2 or 4 mg/kg i.p.) or the β2 adrenergic receptor-selective agonist clenbuterol (2 or 4 mg/kg i.p.) induced reinstatement. Forced swim-induced, but not BRL-44,408-induced, reinstatement was also blocked by a high (20 mg/kg) but not low (10 mg/kg) dose of the β1 adrenergic receptor antagonist betaxolol, and isoproterenol-induced reinstatement was blocked by pretreatment with either ICI-118,551 or betaxolol, suggesting a potential cooperative role for β1 and β2 adrenergic receptors in stress-induced reinstatement. Overall, these findings suggest that targeting β-adrenergic receptors may represent a promising pharmacotherapeutic strategy for preventing drug relapse, particularly in cocaine addicts whose drug use is stress related.
Introduction
In many cocaine addicts, stress serves as a potent trigger for drug use. Reports that stressful stimuli can precipitate craving for cocaine in human subjects in a laboratory setting (Sinha et al., 2000) are paralleled by findings that stressors can induce reinstatement in rodent models of drug relapse (Shaham et al., 2000a). In abstinent cocaine addicts, the ability of stress to promote drug use is particularly problematic because, unlike many triggers for relapse (e.g., drug use and exposure to many drug-associated contexts and cues), the occurrence of stress is usually unavoidable. For this reason, understanding the receptor mechanisms through which stressors evoke drug-seeking behavior probably is important for the identification of better pharmacotherapeutic strategies for relapse prevention.
Noradrenergic signaling in the brain has been implicated in stress-induced relapse (Koob, 1999; Weinshenker and Schroeder, 2007). In rodent and nonhuman primate models, central delivery of norepinephrine (Brown et al., 2011) or activation of central noradrenergic neurotransmission via antagonism of α2 adrenergic receptors (Lee et al., 2004; Feltenstein and See, 2006) reinstates extinguished cocaine seeking. Likewise, functional antagonism of noradrenergic neurotransmission through the administration of α2 adrenergic receptor agonists blocks stress-induced reinstatement in preclinical models (Erb et al., 2000) and attenuates stress-induced craving in human cocaine addicts (Jobes et al., 2011; Fox et al., 2012). The apparent involvement of noradrenergic systems in stress-induced drug use poses adrenergic receptors as potential medicinal targets for relapse prevention.
One method for the preclinical investigation of drug relapse is the conditioned place preference/reinstatement approach, in which the ability of stimuli to re-establish cocaine-induced conditioned place preference after extinction is used to model drug relapse (Mueller and Stewart, 2000). An advantage of this method is that it can be used to evaluate reinstatement in mice, thus permitting assessment in available genetically modified rodent models. We and others have reported that exposing mice to a variety of stressors, including forced swim in 22°C water (Kreibich and Blendy, 2004; Mantsch et al., 2010), social stress (Ribeiro Do Couto et al., 2006), and electric foot shock (Redila and Chavkin, 2008), reinstates extinguishing drug-induced conditioned place preference, suggesting that this procedure can be used to examine the neurobiological processes that contribute to stress-induced relapse.
Using the conditioned place preference/reinstatement approach, we reported previously that reinstatement by a stressor, forced swim, or by activation of central noradrenergic neurotransmission via administration of the α2 adrenergic receptor antagonist yohimbine, is blocked by pretreatment with the nonselective β-adrenergic receptor antagonist propranolol, but not the α1-adrenergic receptor antagonist prazosin, implicating β, but not α1, adrenergic receptors in stress-induced relapse (Mantsch et al., 2010). A preliminary experiment suggested the involvement of β2 adrenergic receptors in reinstatement in response to forced swim (Mantsch et al., 2010). It is noteworthy that although these findings are indicative of a selective role for β-adrenergic receptors in stress-induced reinstatement, it has been reported that prazosin can block yohimbine- or foot shock stress-induced reinstatement of extinguished food or alcohol seeking in rats (Lê et al., 2011). In the present study we further investigate the role of β-adrenergic receptors in stress-induced relapse by testing for reinstatement of extinguished cocaine-induced conditioned place preference in β-adrenergic receptor-deficient mice. In addition, we examine the role of β- adrenergic receptor subtypes in stress-induced reinstatement by testing for the abilities of β-adrenergic receptor agonists to induce reinstatement and β-adrenergic receptor antagonists to block reinstatement after forced swim in wild-type (WT) mice. Our data support previous findings demonstrating β-adrenergic receptor involvement in reinstatement in response to stress but not cocaine administration and suggest that activation of β2 adrenergic receptors is both necessary for stress-induced cocaine seeking and sufficient for reinstatement, although the precise role of β1 adrenergic receptors remains unclear.
Materials and Methods
Subjects.
A total of 103 male mice were used. All mice were 8 to 9 weeks old at the start of the study. Mice were housed singly in a temperature- and humidity-controlled, Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility under a 12-h light/dark cycle (lights on at 7:00 AM). They had access to food and water at all times, except when in the experimental chambers. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the National Institutes of Health.
The role of β-adrenergic receptors in place conditioning, extinction, and reinstatement was initially examined by using β-adrenergic receptor-deficient Adrb1/Adrb2 double-knockout (KO) mice purchased from The Jackson Laboratory (Bar Harbor, ME). These mice were created by Rohrer et al. (1999) by mating Adrb1 homozygous knockout mice with Adrb2 homozygous knockout mice to generate compound heterozygotes, the offspring of which were then mated to obtain compound homozygotes. Backcrossed mice (seven generations) were maintained by The Jackson Laboratory by mating double homozygote null mice (eight generations). These mice have no gross physical or behavioral abnormalities and show no deficits in basal metabolic rate but display attenuated effects of exercise and lack the tachycardia and hypotensive responses to the β-adrenergic receptor agonist isoproterenol (Rohrer et al., 1999). A number of strains have contributed to the background (C57BL/6J, DBA/2, 129, FVB/N, and CD-1) of these mice. Because of the lack of availability of hybrid controls, C57BL/6J mice (Harlan, Indianapolis, IN) were used as wild-type control mice in the experiments conducted with the double-knockout mice. C57BL/6J mice were also used for the remainder of the experiments in the study.
Drugs.
Cocaine HCl was acquired from the National Institute on Drug Abuse (Bethesda, MD) through its drug supply program. The selective β1 adrenergic receptor antagonist betaxolol HCl, the selective β2 adrenergic receptor antagonist -(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol (ICI-118551) HCl, and the selective β2 adrenergic receptor agonist clenbuterol HCl were purchased from Sigma-Aldrich (St. Louis, MO). The nonselective β-adrenergic receptor agonist isoproterenol hydrochloride and the highly selective (relative to yohimbine) α2 adrenergic receptor antagonist 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole (BRL-44,408) maleate were acquired from Tocris Bioscience (Ellisville, MO). All drugs were dissolved in saline (0.9% NaCl solution) and administered intraperitoneally in a volume of 0.1 ml/20 g body mass.
Equipment.
Behavioral testing was conducted by using six ENV-3013 mouse place preference chambers from MED Associates (St. Albans, VT). The stainless-steel and polyvinyl chloride chambers consisted of three distinct compartments separated by 5 cm wide × 5.9 cm high manual guillotine doors. The two 46.5 × 12.7 × 12.7-cm side compartments consisted of a white compartment with a 6.35 × 6.35-mm stainless-steel mesh floor and a black compartment with a stainless-steel grid rod floor consisting of 3.2-mm rods spaced 7.9 mm apart. The side compartments were attached via a gray-colored 7.2-cm-long center compartment with a smooth floor. The clear tops of the compartments were hinged to permit placement and removal of the mice. Ceiling lights were attached to each top. To balance unconditioned side preference, only the light in the black compartment was illuminated during training/testing. Automated data collection was accomplished by using photobeams (six beams for the white and black test areas and two beams for the center gray area) that were evenly spaced across the length of the chamber and interfaced with a computer containing MED-PC software (MED Associates). Using this automated photobeam system, entry into a side compartment was defined as consecutive breaks of the first two photocell beams in that compartment located adjacent to the door separating that compartment from the center compartment. Exiting of a side compartment (and entry into the center compartment) was indicated by occlusion of the beams in the center compartment.
Cocaine-Induced Conditioned Place Preference.
Cocaine-induced conditioned place preference was established by using a design in which one of the side compartments was randomly designated as the cocaine compartment and the other as the saline compartment as reported previously (Mantsch et al., 2010). On the first day of the procedure, mice were placed into the center compartment of the chamber and provided free access to both side compartments for 30 min in the absence of saline or cocaine pretreatment to determine preconditioning preference. During the 8-day conditioning phase of the experiment, mice received cocaine (15 mg/kg i.p.) and saline injections on alternating days after which time they were confined to the randomly designated treatment-appropriate compartment for 30 min. After the final conditioning session, mice were tested for the expression of cocaine-induced conditioned place preference by once again placing them into the center compartment of the chamber and providing them with free access to the side compartments for 30 min. Conditioned place preference was defined as the change in time spent (seconds) within the cocaine-paired compartment after conditioning compared with the initial preconditioning session.
Extinction.
After conditioning, daily extinction training was conducted. During the extinction sessions, mice were placed into the center compartment and once again provided with free access to the side compartments for 30 min. Mice underwent daily extinction training until the preference for the cocaine-paired compartment during the session (i.e., change in time spent in the cocaine side relative to preconditioning values) was reduced by at least 50% compared with the postconditioning test session, at which time reinstatement testing was conducted.
Reinstatement Testing.
The reinstatement sessions were identical to the extinction sessions except that mice underwent forced swim and/or received drug injections before the session. Reinstatement was defined according to the time spent in the compartment previously paired with cocaine. In most cases, mice were tested more than once for reinstatement. In these cases, the sequence of reinstatement test conditions were counterbalanced to address concerns related to altered responsiveness with repeated testing. Reinstatement sessions were separated by additional extinction sessions. Mice were required to reach the extinction criterion once again before the next reinstatement test was conducted. To confirm that alterations in locomotor activity secondary to receptor deficiency or drug administration did not contribute to the observed effects by increasing or decreasing exploration of the paired compartments in the three-chamber apparatus, the total combined amount of time spent in the compartments previously paired with cocaine or saline was also examined during reinstatement.
Experiment 1: Stress and Cocaine-Induced Reinstatement in β-Adrenergic Receptor-Deficient and Wild-Type Control Mice.
We previously reported that pharmacological blockade of β-adrenergic receptors prevents stress-, but not cocaine-, induced reinstatement of extinguished conditioned place preference in mice (Mantsch et al., 2010). To further investigate the role of β-adrenergic receptors in stress-induced relapse, reinstatement after stress (forced swim), administration of a priming injection of cocaine, or activation of central adrenergic neurotransmission via delivery of the selective α2 adrenergic receptor antagonist BRL-44,408 was tested in β-adrenergic receptor-deficient Adrb1/Adrb2 double-knockout mice (n = 11) and C57BL/6J wild-type control mice (n = 9). These mice underwent cocaine-induced place conditioning and extinction before testing for reinstatement. In most cases, mice were tested for reinstatement by all three stimuli in a counterbalanced sequence.
Stress-Induced Reinstatement.
Stress-induced reinstatement was tested by using a forced swim protocol as reported previously (Kreibich and Blendy, 2004; Mantsch et al., 2010). Mice were placed into a 30 cm high × 20 cm deep cylindrical polypropylene container filled with water (20–25°C) for 6 min. After forced swim, mice were placed back into their home cages for 3 to 4 min before introduction into the center compartment of the place conditioning chamber with free access to both of the side compartments for reinstatement testing, as described above. In cases where reinstatement was measured, swimming behavior was videotaped, and the amount of time spent immobile (versus actively swimming) was determined.
Cocaine- and BRL-44,408-Induced Reinstatement.
Cocaine-induced reinstatement was tested by injecting mice with 15 mg/kg i.p. cocaine and then immediately placing them into the chamber for reinstatement testing. Reinstatement by the α2 adrenergic receptor antagonist BRL-44,408 (10 mg/kg i.p.) was tested 30 min after administration. BRL-44,408 is more selective for α2 adrenergic receptors than other commonly used antagonists (e.g., yohimbine) and has been reported to increase central norepinephrine levels in rats (Dwyer et al., 2010). We have reported previously that this dose of BRL-44,408 is optimal for reinstatement (Mantsch et al., 2010).
Experiment 2: Reinstatement of Conditioned Place Preference by Isoproterenol.
To determine whether the activation of β-adrenergic receptors is sufficient for the reinstatement of extinguished cocaine-induced conditioned place preference, mice (n = 11) were tested for reinstatement after administration of the nonselective β-adrenergic receptor agonist isoproterenol. Mice received injections of isoproterenol (1, 2, or 4 mg/kg i.p.) or vehicle 30 min before placement into the chambers and testing for reinstatement. Each mouse was tested with each dose of isoproterenol in a counterbalanced sequence.
Experiment 3: Effects of β-Adrenergic Receptor Antagonists on Reinstatement.
To determine the role of β1 and β2 adrenergic receptors in stress-induced reinstatement, mice were tested for reinstatement of extinguished place preference in response to forced swim or BRL-44,408 (10 mg/kg i.p.), as described in experiment 1, after pretreatment with the selective β1 adrenergic receptor antagonist betaxolol or the selective β2 adrenergic receptor antagonist ICI-118,551. Seven mice were tested for the effects of betaxolol (10 or 20 mg/kg i.p.; 30-min pretreatment) and vehicle on reinstatement after forced swim in a counterbalanced sequence. All seven mice were tested for the effects of 10 mg/kg betaxolol and vehicle. Six of the mice were also tested for the effects of 20 mg/kg betaxolol. Eight mice were tested for the effects of ICI-118,551 (1 or 2 mg/kg i.p.; 30-min pretreatment) on swim-induced reinstatement. All eight mice were tested for the effects of 1 mg/kg ICI-118,551 and vehicle. Seven of the mice were also tested for the effects of 2 mg/kg ICI-118,551. Seven mice were tested for the effects of betaxolol (10 mg/kg) or ICI-118,551 (1 mg/kg) and vehicle on reinstatement after administration of BRL-44,408 in a counterbalanced sequence. Five of these mice were tested for the effects of betaxolol, ICI-118,551, and vehicle. One mouse was tested only for the effects of betaxolol and vehicle, and one mouse was tested only for the effects of ICI-118,551 and vehicle. In addition, six mice were tested for the effects of 20 mg/kg betaxolol, 2 mg/kg ICI-118,551, and vehicle on BRL-44,408-induced reinstatement in a counterbalanced sequence. Each of these mice was tested under each pretreatment condition.
Finally, 11 mice were tested for the effects of betaxolol (10 mg/kg only) or ICI-118,551 (1 mg/kg only) and vehicle on reinstatement after administration of isoproterenol (4 mg/kg i.p.). Ten of these mice were tested for the effects of betaxolol, ICI-118,551, and vehicle. One mouse was tested only for the effects of betaxolol on isoproterenol-induced reinstatement.
Experiment 4: Reinstatement of Conditioned Place Preference by Clenbuterol.
Because reinstatement by stress and BRL-44,408 was blocked by the β2 adrenergic receptor antagonist ICI-188,551, mice (n = 7) were tested for reinstatement after administration of the selective β2 adrenergic receptor agonist clenbuterol to determine whether the activation of β2 adrenergic receptors is sufficient for reinstatement. These mice received injections of clenbuterol (1, 2, or 4 mg/kg i.p.) or vehicle 30 min before placement into the chambers and testing for reinstatement. Each mouse was tested with each dose of clenbuterol in a counterbalanced sequence. In addition, 11 β-adrenergic receptor knockout and five C57BL/6J wild-type control mice were tested for reinstatement by 4 mg/kg clenbuterol to confirm that clenbuterol was producing reinstatement through actions at β-adrenergic receptors. The knockout mice were also used to test reinstatement in response to stress, cocaine, and BRL-44,408 in experiment 1.
Locomotor Testing.
To assist with dose selection, the effects of various doses of betaxolol, ICI-118,551, clenbuterol, and isoproterenol on locomotor activity were tested in 26 mice by using an automated AccuScan activity system (AccuScan Instruments, Inc., Columbus, OH) consisting of a frame containing photocells (eight per cage) in which clear Plexiglas 29.5 cm long × 19 cm wide × 12.7 cm high cages were placed. Activity was measured as total photobeam breaks. During the week before locomotor testing, mice were habituated to the test environment during two 2-h sessions. On the test day, mice were placed into the chambers for 2 h before drug administration after which activity was measured over a 2-h period. The effects of betaxolol (0, 5, 10, and 20 mg/kg i.p.), clenbuterol (0, 1, 2, and 4 mg/kg i.p.), ICI-118,551 (0, 0.5, 1, and 2 mg/kg i.p.), and isoproterenol (0, 1, 2, and 4 mg/kg i.p.) were tested in separate groups of mice.
Statistical Analyses.
Place preference during testing, extinction, and reinstatement was defined as the time spent (seconds) in the designated cocaine compartment. In the experiments comparing conditioning, extinction, and reinstatement between wild-type and β-adrenergic-deficient mice, the significance of differences was determined by using two-way ANOVA with genotype as a between-subjects factor and time spent in the cocaine side during conditioning (before conditioning vs. after conditioning), extinction (day 1 versus day 2), or reinstatement (extinction versus reinstatement session) as a repeated measure followed by post hoc testing using two-tailed Student's t tests. The significance of the effects of drug pretreatments (betaxolol or ICI-118,551) on reinstatement by each of the stimuli was determined by using two-way repeated-measures reinstatement × drug pretreatment ANOVA followed by post hoc testing using two-tailed Student's t tests. The abilities of various doses of isoproterenol or clenbuterol to induce reinstatement and the dose-dependent effects of various drugs on locomotor activity were determined by using one-way repeated measures ANOVA followed by post hoc testing using the Dunnett's test. For all analyses, significance was defined as p < 0.05.
Results
Experiment 1: Stress and Cocaine-Induced Reinstatement in β-Adrenergic Receptor-Deficient and Wild-Type Control Mice
Place Conditioning and Extinction in β-Adrenergic Receptor-Deficient Mice.
To examine the role of β-adrenergic receptors in stress-induced relapse, a total of 11 β-adrenergic receptor-deficient Adrb1/Adrb2 double-knockout mice and nine wild-type mice underwent place conditioning and extinction and were tested for reinstatement. Both wild-type and double-knockout mice developed conditioned place preference, and no difference in the magnitude of cocaine-induced place preference was observed between genotypes (Fig. 1A). A two-way place conditioning (time spent in cocaine side preconditioning versus postconditioning; repeated measure) × genotype ANOVA showed that, overall, conditioned place preference was observed (significant main effect of conditioning; F1,18 = 14.812; p = 0.001). However, no effect of genotype or conditioning × genotype interaction was found.
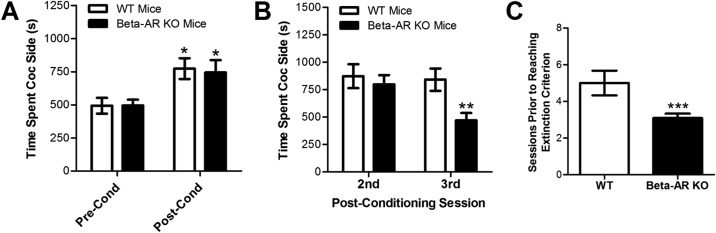
Fig. 1.
Induction and extinction of cocaine-induced conditioned place preference in β-adrenergic receptor (AR)-deficient Adrb1/Adrb2 double-KO and WT C57BL/6J control mice. Data represent the time spent (s ± S.E.) in the cocaine-paired compartment before (Pre-Cond) and after (Post-Cond) conditioning (A) and during the two extinction sessions after testing for the expression of conditioning (postconditioning days 2 and 3; B) as well as the mean number of extinction sessions before reaching the extinction criterion (C). A, both wild-type and knockout mice developed conditioned place preference. *, p < 0.05 versus preconditioning. B and C, knockout mice showed reduced preference on postconditioning day 3 (B; **, p < 0.05 versus WT) and extinguished more rapidly (C; ***, p < 0.05 versus WT) than wild-type mice.
Extinction of conditioned place preference in β-adrenergic receptor-deficient and wild-type mice is shown in Fig. 1, B and C. β-Adrenergic receptor knockout mice extinguished more quickly than wild-type controls (Fig. 1B). Wild-type mice reached the extinction criteria in 5.0 ± 0.68 sessions compared with 3.1 ± 0.23 for knockout mice (t14 = 2.167; p < 0.01). This observation is consistent with reports that pharmacological inhibition of β-adrenergic receptors produces postretrieval disruption of conditioned place preference, thereby reducing subsequent time spent in the cocaine-paired environment (Bernardi et al., 2009; Otis and Mueller, 2011). To further examine this possibility, we compared time spent in the cocaine compartment between genotypes on the 2 days after testing for the expression of place preference (i.e., on postconditioning days 2 and 3). A two-way ANOVA examining differences in time spent on the cocaine paired side on these days between genotypes showed significant main effects of genotype (F1,18 = 5.665; p < 0.05) and session/day (repeated measure F1,18 = 4.772; p < 0.05) and a nonsignificant trend toward a genotype × session/day interaction (p = 0.065). Despite the lack of a significant interaction, planned comparisons examining the amount of time spent in the cocaine-paired compartment on days 2 and 3 postconditioning in each genotype were performed. A significant reduction in the time spent in the cocaine-paired compartment on day 3 versus day 2 was observed only in knockout mice (t10 = 2.697; p < 0.05), and on day 3 postconditioning knockout mice spent less time in the cocaine-paired compartment compared with wild-type controls (t18 = 3.149; p < 0.01).
Stress- and Cocaine-Induced Reinstatement in β-Adrenergic Receptor-Deficient Mice.
The ability of various stimuli to reinstate extinguished cocaine-induced conditioned place preference in β-adrenergic receptor-deficient and wild-type mice is shown in Fig. 2. Reinstatement of extinguished cocaine-induced place preference by a stressor, forced swim, was tested in 10 β-adrenergic receptor knockout mice and five wild-type mice (Fig. 2A). Swim-induced reinstatement was observed in wild-type, but not knockout, mice. A two-way ANOVA showed a significant interaction between genotype and reinstatement condition (F1,13 = 10.815; p < 0.01). Post hoc testing showed that swim stress increased the time spent in the cocaine compartment relative to extinction in wild-type mice (paired t4 = 4.722; p < 0.01) but not in knockout mice. In addition, the amount of time spent in the cocaine compartment after forced swim was significantly increased in wild-type versus knockout mice (unpaired t13 = 3.431; p < 0.01). There was no significant difference between genotypes during the preceding extinction session. Immobility time during the 6-min swim sessions did not significantly differ between wild-type and β-adrenergic receptor-deficient mice (Table 1).
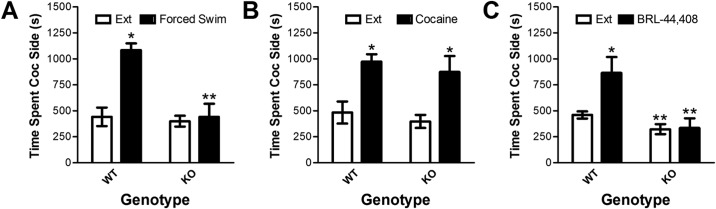
Fig. 2.
Reinstatement after forced swim (A), cocaine (15 mg/kg i.p.) administration (B), or delivery of the selective α2 adrenergic receptor antagonist BRL-44,408 (10 mg/kg i.p.; C) in β-adrenergic receptor-deficient Adrb1/Adrb2 double-KO and WT C57BL/6J control mice. Data represent the time spent in the cocaine-paired side (s ± S.E.) during reinstatement testing or the preceding extinction session (Ext). A and C, forced swim (A) and BRL-44,408 (C) induced reinstatement in WT but not KO mice. B, in contrast, cocaine induced reinstatement in both WT and KO mice. *, p < 0.05 versus extinction. **, p < 0.05 KO versus WT.
TABLE 1.
Immobility time (seconds ± S.E.) during a 6-min period of forced swim in β-adrenergic receptor-deficient Adrb1/Adrb2 double-knockout mice and wild-type C57BL/6J mice pretreated with vehicle, the β1 adrenergic receptor anta gonist betaxolol (10 or 20 mg/kg i.p.), or the β2 adrenergic receptor antagonist ICI-118,551 (1 or 2 mg/kg)
| Immobility Time | |
|---|---|
| s | |
| WT/vehicle | 231.08 ± 22.28 |
| β-Adrenergic KO | 195.25 ± 14.72 |
| Betaxolol, 10 mg/kg | 176.55 ± 26.73 |
| Betaxolol, 20 mg/kg | 218.92 ± 16.26 |
| ICI-118,551, 1 mg/kg | 163.15 ± 20.41 |
| ICI-118,551, 2 mg/kg | 218.00 ± 18.65 |
Reinstatement in response to the administration of cocaine (15 mg/kg i.p.) was determined in 11 knockout and six wild-type mice (Fig. 2B). In contrast to swim stress, cocaine administration reinstated extinguished place preference in both knockout and wild-type mice. Two-way ANOVA showed a significant overall reinstatement effect (reinstatement versus extinction; F1,15 = 13.184; p < 0.01) but no main effect of genotype or reinstatement × genotype interaction.
Because we reported previously that the activation of central noradrenergic neurotransmission through the administration of the α2 adrenergic receptor antagonist BRL-44,408 reinstates extinguished place preference (Mantsch et al., 2010), we determined the ability of 10 mg/kg i.p. BRL-44,408 to induce reinstatement in eight wild-type and 10 β-adrenergic receptor knockout mice (Fig. 2C). Similar to stress, reinstatement after BRL-44,408 administration was observed in wild-type, but not knockout, mice. A two-way ANOVA showed a significant interaction between genotype and reinstatement condition (F1,16 = 5.409; p < 0.05). Post hoc testing revealed that BRL-44,408 increased the amount of time spent in the cocaine-paired compartment in wild-type, but not knockout, mice (paired t7 = 2.768; p < 0.05) during reinstatement testing compared with the preceding extinction session. In addition, time spent in the cocaine compartment after BRL 44,408 was significantly increased in wild-type versus knockout mice (unpaired t16 = 3.050; p < 0.01). It is noteworthy that post hoc testing also showed that the time spent in the cocaine compartment during the extinction sessions before testing for BRL 44,408-induced reinstatement was reduced in knockout mice (p < 0.05).
To confirm that differences in locomotor activity between genotypes under extinction and reinstatement conditions did not significantly contribute to the observed effects of β-adrenergic receptor deficiency on reinstatement, the total combined amount of time spent in the cocaine and saline compartments of the three-chamber apparatus was also measured. In all cases (swim, cocaine, and BRL-44,408), two-way ANOVA failed to show significant overall effects of genotype or reinstatement condition or significant genotype × reinstatement condition interactions (data not shown), suggesting that the observed effects on reinstatement were not associated with differences in the amount of time that the mice spent outside of the center compartment in which they were placed at the start of the test session.
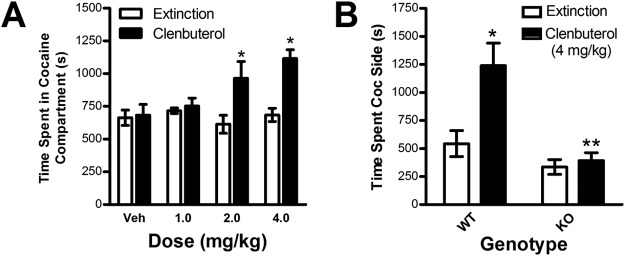
Experiment 2: Reinstatement of Conditioned Place Preference by Isoproterenol
To determine whether the activation of β-adrenergic receptors is sufficient to reinstate extinguished place preference, nine mice were tested with the nonselective β-adrenergic agonist isoproterenol. These mice showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 698.70 ± 45.801 s; postconditioning time spent = 1012.99 ± 52.59 s; paired test; t8 = 4.581; p < 0.01). Isoproterenol dose-dependently reinstated extinguished place preference (Fig. 3). Two-way repeated-measures ANOVA showed a significant interaction (F3,24 = 4.922; p < 0.01) between isoproterenol dose (0, 1, 2, and 4 mg/kg) and test condition (extinction versus reinstatement). Post hoc analysis showed that significant reinstatement was observed at the 2 mg/kg dose (t8 = 2.382; p < 0.05 versus extinction) and 4 mg/kg dose (t8 = 3.983; p < 0.01 versus extinction) but not after administration of vehicle or the lowest (i.e., 1 mg/kg) isoproterenol dose tested. Isoproterenol did not increase the total amount of time spent in the cocaine and saline compartments combined during reinstatement testing, suggesting the reinstatement was not an artifact of increased locomotor activity (data not shown).
Fig. 3.
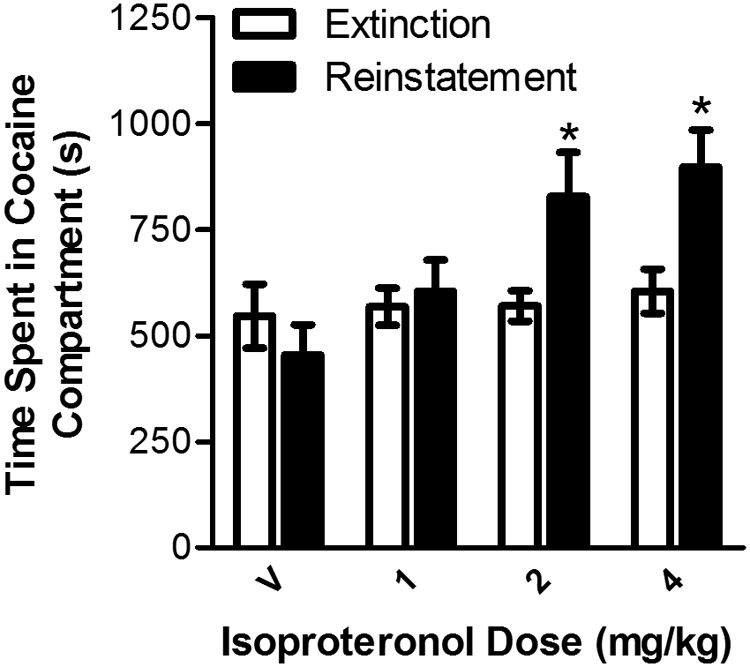
Dose-dependent reinstatement by the β-adrenergic receptor agonist isoproterenol in wild-type C57BL/6J mice. Data represent time spent in the cocaine-paired side (s ± S.E.) during reinstatement testing after pretreatment with isoproterenol (0, 1, 2, or 4 mg/kg i.p.) or during the preceding extinction session (Ext). Isoproterenol induced reinstatement at the 2 and 4 mg/kg doses. *, p < 0.05 versus extinction.
Experiment 3: Effects of β-Adrenergic Receptor Antagonists on Reinstatement
Role of β1 and β2 Adrenergic Receptors in Stress-Induced Reinstatement.
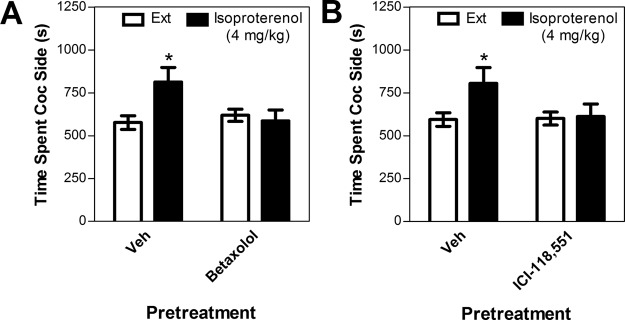
To determine which β-adrenergic receptor subtype mediates stress-induced reinstatement of extinguished cocaine-induced conditioned place preference, the effects of the selective β1 receptor antagonist betaxolol (10 or 20 mg/kg) or the selective β2 antagonist ICI-118,551 (1 or 2 mg/kg) on reinstatement after forced swim were tested (Fig. 4). For this experiment, mice were tested for stress-induced reinstatement multiple times in a counterbalanced sequence. Seven mice were tested for the effects of betaxolol on reinstatement. All of these mice were tested for the effects of 10 mg/kg betaxolol, and six of them were tested for the effects of the 20 mg/kg betaxolol dose. These mice showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 531.66 ± 95.89 s; postconditioning time spent = 828.36 ± 92.03 s; paired test; t6 = 5.196; p < 0.01). Reinstatement was not blocked by the 10 mg/kg betaxolol dose (Fig. 4A). A two-way repeated measures ANOVA showed that forced swim produced reinstatement (significant overall main effect of swim versus extinction; F1,5 = 10.729; p < 0.05). However, no main effect of 10 mg/kg betaxolol and no significant betaxolol × reinstatement interaction were observed. In contrast, a two-way ANOVA showed a significant interaction between betaxolol pretreatment and reinstatement condition in mice that received 20 mg/kg betaxolol (F1,6 = 8.210; p < 0.05; Fig. 4B). In this group, post hoc testing revealed significant reinstatement in mice pretreated with vehicle (paired t6 = 2.512; p < 0.05) but not 20 mg/kg betaxolol.
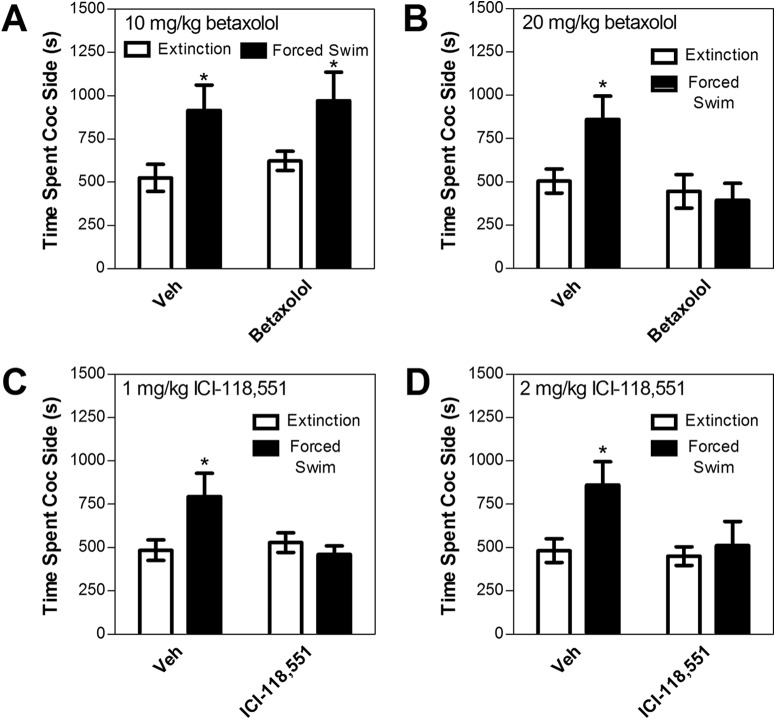
Fig. 4.
Effects of the selective β1 adrenergic receptor antagonist betaxolol [10 mg/kg i.p. (A) or 20 mg/kg i.p. (B)] or the selective β2 adrenergic receptor antagonist ICI-118,551 [1 mg/kg i.p. (C) or 2 mg/kg i.p. (D)] on reinstatement after forced swim in wild-type C57BL/6J mice. Data represent the amount of time spent in the cocaine (Coc)-paired side (s ± S.E.) during reinstatement testing after 6 min of forced swim after drug or vehicle pretreatment (Veh) or during the preceding extinction session (Ext). Betaxolol blocked swim-induced reinstatement at the 20 mg/kg, but not 10 mg/kg, dose, whereas ICI-118,551 blocked swim-induced reinstatement at both doses tested. *, significant reinstatement; p < 0.05 versus extinction.
In contrast to betaxolol, both doses of ICI-118,551 that were tested blocked swim-induced reinstatement. Eight mice were tested for the effects of ICI-118.551 on reinstatement. All eight of these mice were tested for the effects of the 1 mg/kg ICI-118,551 dose (Fig. 4C), whereas only seven were tested for the effects of the 2 mg/kg dose (Fig. 4D). These mice showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 535.22 ± 83.12 s; postconditioning time spent = 847.94 ± 82.07 s; paired test; t7 = 6.016; p = 0.001). For both doses of ICI-118,551, significant interactions between ICI-118,551 treatment (drug versus vehicle) and reinstatement conditions (swim versus extinction) were observed (F1,7 = 5.659 for the 1 mg/kg dose; F1,6 = 7.065 for the 2 mg/kg dose; p < 0.05 for both). In both cases, post hoc testing revealed that forced swim produced reinstatement in mice pretreated with vehicle (paired t7 = 2.350, p = 0.05 for the 1 mg/kg dose vehicle; paired t6 = 2.925, p < 0.05 for the 2 mg/kg dose vehicle), but not ICI-118,551.
Despite a tendency to reduce immobility during forced swim testing at the lower doses, neither betaxolol (10 or 20 mg/kg) nor ICI-118,551 (1 or 2 mg/kg) significantly altered immobility time during the 6-min sessions relative to vehicle treatment (Table 1). In addition, neither betaxolol (10 or 20 mg/kg) nor ICI-118,551 (1 or 2 mg/kg) reduced the total amount of time spent in the cocaine and saline compartments combined during reinstatement testing, suggesting that the inhibition of reinstatement was not attributable to a general reduction of locomotor activity and decreased movement out of the center start compartment (data not shown).
Role of β1 and β2 Adrenergic Receptors in Reinstatement by BRL-44,408.
The effects of betaxolol (10 and 20 mg/kg) and ICI-118,551 (1 and 2 mg/kg) on reinstatement by the selective α2 adrenergic receptor antagonist BRL-44,408 are shown in Fig. 5. Mice tested for reinstatement in response to BRL-44,408 (n = 13) showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 650.66 ± 59.13 s; postconditioning time spent = 981.67 ± 645.118.00 s; paired test; t12 = 5.170; p < 0.01). Five of these mice were tested for the effects of 10 mg/kg betaxolol and 1 mg/kg ICI-118,551. One mouse was tested only for the effects of 10 mg/kg betaxolol, and one mouse was tested only for the effects of 1 mg/kg ICI-118,551. An additional six mice were tested for the effects of both 20 mg/kg betaxolol and 2 mg/kg ICI-118,551 on reinstatement by BRL-44,408.
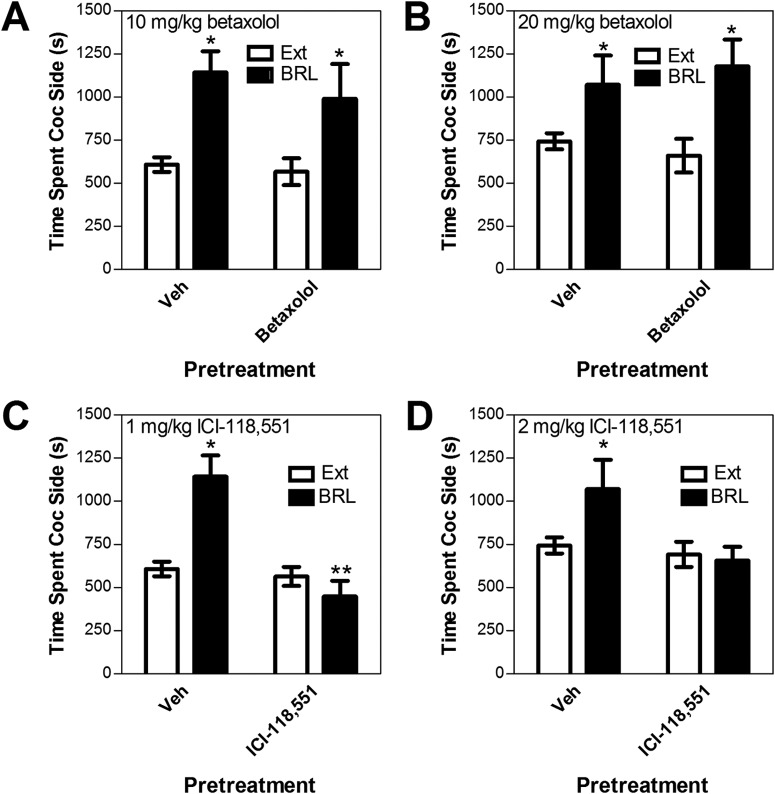
Fig. 5.
Effects of the selective β1 adrenergic receptor antagonist betaxolol [10 mg/kg i.p. (A) or 20 mg/kg i.p. (B)] or the selective β2 adrenergic receptor antagonist ICI-118,551 [1 mg/kg i.p. (C) or 2 mg/kg i.p. (D)] on reinstatement after administration of BRL-44,408 (BRL; 10 mg/kg i.p.) in wild-type C57BL/6J mice. Data represent the amount of time spent in the cocaine (Coc)-paired side (s ± S.E.) during reinstatement testing after BRL administration after antagonist or vehicle pretreatment (Veh) or during the preceding extinction session (Ext). ICI-118,551, but not betaxolol, blocked BRL-induced reinstatement. *, significant reinstatement, p < 0.05 versus extinction. **, p < 0.05 ICI-118,551 versus vehicle.
Neither 10 mg/kg i.p. (Fig. 5A) nor 20 mg/kg i.p. (Fig. 5B) betaxolol blocked BRL-44,408-induced reinstatement. Two-way repeated measures ANOVA showed significant reinstating effects of BRL-44,408 (F1,5 = 11.196, p < 0.05, 10 mg/kg betaxolol group; F1,5 = 22.117, p = 0.005, 20 mg/kg betaxolol group) but no significant main effects of betaxolol (10 or 20 mg/kg) and no betaxolol × reinstatement condition interactions. In contrast, both 1 mg/kg i.p. (Fig. 5C) and 2 mg/kg i.p. (Fig. 5D) ICI-118,551 blocked reinstatement by BRL-44,408. At the 1 mg/kg ICI-118,551 dose, a two-way repeated measures ANOVA showed a significant interaction between ICI-118,551 pretreatment and BRL-44,408-induced reinstatement (F1,5 = 25.175; p < 0.01). Post hoc testing revealed that BRL-44,408 reinstated place preference in mice after vehicle, but not ICI-118,551, pretreatment (BRL 44,408 versus extinction; paired t5 = 3.589; p < 0.05). At the 2 mg/kg ICI-118,551 dose, a two-way repeated measures ANOVA showed a near-significant interaction between ICI-118,551 pretreatment and BRL-44,408-induced reinstatement (F1,5 = 6.115; p = 0.056). A planned comparison t test revealed that BRL-44,408 reinstated place preference in mice after vehicle, but not ICI-118,551, pretreatment (BRL 44,408 versus extinction; paired t5 = 2.553; p = 0.05). As was the case with forced swim, neither betaxolol nor ICI-118,551 reduced the total amount of time spent in the cocaine and saline compartments combined during reinstatement testing, suggesting that general reductions in locomotor activity and decreased movement out of the center start compartment did not contribute to the effects on reinstatement (data not shown).
Role of β1 and β2 Adrenergic Receptors in Isoproterenol-Induced Reinstatement.
To examine the role of β1 and β2 adrenergic receptor subtypes in reinstatement in response to the nonselective β-receptor agonist isoproterenol, mice were tested for reinstatement by 4 mg/kg isoproterenol after pretreatment with the β1 adrenergic receptor-selective antagonist betaxolol (10 mg/kg i.p.; Fig. 6A) or the β2 receptor-selective antagonist ICI-118,551 (1 mg/kg i.p.; Fig. 6B). These mice (n = 11) showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 579.75 ± 59.87 s; postconditioning time spent = 944.08 ± 39.28 s; paired test; t10 = 6.704; p < 0.001). We were surprised to find that isoproterenol-induced reinstatement was blocked by either betaxolol (n = 11) or ICI-118,551 (n = 10) pretreatment. A two-way repeated measures ANOVA examining the effects of betaxolol on isoproterenol-induced reinstatement showed a significant interaction between betaxolol pretreatment (10 mg/kg betaxolol versus vehicle) and reinstatement (F1,10 = 10.981; p < 0.01). Likewise, a two-way repeated measures ANOVA examining the effects of ICI-118,551 on reinstatement showed a significant interaction between ICI-118,551 pretreatment (1 mg/kg ICI-118,551 versus vehicle) and reinstatement (F1,9 = 5.420; p < 0.05). In both cases, significant reinstatement of place preference was observed after pretreatment with vehicle (t10 = 3.338, p < 0.01 for betaxolol experiment; t9 = 2.890, p < 0.05 for ICI-118,551 experiment) but not after administration of either β-adrenergic receptor antagonist. Effects on the total amount of time spent in the cocaine and saline compartments combined during reinstatement testing were not observed (data not shown).
Fig. 6.
Effects of the selective β1 adrenergic receptor antagonist betaxolol (10 mg/kg i.p.; A) or the selective β2 adrenergic receptor antagonist ICI-118,551 (1 mg/kg i.p.; B) on reinstatement after administration of isoproterenol (4 mg/kg i.p.) in wild-type C57BL/6J mice. Data represent the amount of time spent in the cocaine (Coc)-paired side (s ± S.E.) during reinstatement testing after isoproterenol administration after antagonist or vehicle pretreatment (Veh) or during the preceding extinction session (Ext). Both ICI-118,551 and betaxolol blocked isoproterenol-induced reinstatement. *, significant reinstatement, p < 0.05 versus extinction.
Experiment 4: Reinstatement of Conditioned Place Preference by Clenbuterol
To determine whether β2 adrenergic receptor activation alone is sufficient to reinstate extinguished place preference, seven mice were tested for reinstatement after administration of the selective β2 receptor agonist clenbuterol. These mice showed significant cocaine-induced place preference (preconditioning time spent in the cocaine side = 603.03 ± 53.88 s; postconditioning time spent = 987.06 ± 37.52 s; paired test; t6 = 5.675; p < 0.01). Clenbuterol dose-dependently reinstated extinguished place preference (Fig. 7A). Two-way repeated-measures ANOVA showed a significant interaction (F3,18 = 5.206; p < 0.01) between clenbuterol dose (0, 1, 2, and 4 mg/kg) and test condition (extinction versus reinstatement). Post hoc analysis showed that significant reinstatement was observed at the 2 mg/kg dose (t6 = 3.18; p < 0.05 versus extinction) and 4 mg/kg dose (t6 = 4.667; p < 0.01 versus extinction) but not after administration of vehicle or the lowest (i.e., 1 mg/kg) clenbuterol dose. As was the case with isoproterenol, clenbuterol did not increase the total amount of time spent in the cocaine and saline compartments combined during reinstatement testing, suggesting the reinstatement was not an artifact of increased locomotor activity (data not shown). As expected, clenbuterol-induced reinstatement was not observed in β-adrenergic receptor-deficient mice (Fig. 7B). A two-way reinstatement × genotype ANOVA showed a significant interaction between genotype (knockout versus wild type) and clenbuterol-induced reinstatement (F1,14 = 15.930; p < 0.01). Clenbuterol (4 mg/kg i.p.) reinstated place preference in wild-type mice (t4 = 6.474; p < 0.05; n = 5) but not Adrb1/Adrb2 double-knockout mice (n = 11).
Fig. 7.
Dose-dependent reinstatement by the β2 adrenergic receptor agonist clenbuterol in WT C57BL/6J mice (A) and reinstatement after administration of 4 mg/kg clenbuterol in WT and β-adrenergic receptor KO mice (B). Data represent time spent in the cocaine-paired side (s ± S.E.) during reinstatement testing after pretreatment with clenbuterol or during the preceding extinction session (Ext). Clenbuterol produced reinstatement at the 2 and 4 mg/kg doses but failed to produce reinstatement in KO mice. *, p < 0.05 versus extinction. **, p < 0.05 KO versus WT.
Effects of Drugs on Locomotor Activity
To aid in data interpretation, the unconditioned locomotor (ambulatory) responses to isoproterenol, betaxolol, ICI-118,551, and clenbuterol were examined in separate groups of mice (n = 5 per group) and are shown in Table 2. Each of the drugs dose-dependently reduced ambulatory activity compared with their respective vehicle controls (repeated-measures ANOVA; F3,12 = 18.406, p < 0.001 for betaxolol; F3,12 = 16.315, p < 0.001 for ICI-118,551; F3,12 = 4.264, p < 0.05 for isoproterenol; F3,12 = 5.291, p < 0.05 for clenbuterol). Post hoc testing using a Dunnett's test showed that betaxolol significantly reduced ambulatory activity compared with vehicle at all doses tested (5, 10, and 20 mg/kg; p < 0.05), and ICI-118,551 significantly reduced activity at the two higher doses tested (1 and 2 mg/kg; p < 0.05), whereas isoproterenol and clenbuterol reduced activity only at the highest tested dose (4 mg/kg; p < 0.05).
TABLE 2.
Effects of adrenergic drugs on locomotor activity
Data represent the mean number of ambulatory counts (± S.E.) recorded over a 2-h period after intraperitoneal administration of various doses of betaxolol, ICI-118,551, isoproterenol, and clenbuterol.
| Betaxolol |
ICI-118,551 |
Isoproterenol |
Clenbuterol |
||||
|---|---|---|---|---|---|---|---|
| Dose | Ambulatory Counts | Dose | Ambulatory Counts | Dose | Ambulatory Counts | Dose | Ambulatory Counts |
| ± S.E. | mg/kg | ± S.E. | mg/kg | ± S.E. | mg/kg | ± S.E. | |
| 0 mg/kg | 3501.60 ± 385.63 | 0 | 2455.76 ± 248.19 | 0 | 2047.50 ± 466.84 | 0 | 2433.20 ± 633.59 |
| 5.0 mg/kg | 1927.60 ± 368.37* | 0.5 | 2408.40 ± 346.86 | 1.0 | 1437.8 ± 583.28 | 1.0 | 1685.40 ± 442.49 |
| 10.0 mg/kg | 1519.40 ± 304.82* | 1.0 | 1316.20 ± 152.39* | 2.0 | 959.75 ± 107.66 | 2.0 | 1234.80 ± 556.03 |
| 20.0 mg/kg | 279.80 ± 84.53* | 2.0 | 806.60 ± 110.01* | 4.0 | 527.75 ± 90.15* | 4.0 | 537.40 ± 186.78* |
p < 0.05 versus 0 mg/kg dose.
Discussion
We reported previously that disinhibition of central noradrenergic neurotransmission via α2 adrenergic receptor antagonism using yohimbine or the more selective antagonist BRL-44,408 is sufficient to reinstate extinguished cocaine-induced conditioned place preference, whereas functional antagonism of noradrenergic activity via administration of the α2 adrenergic receptor agonist clonidine prevents reinstatement by a stressor, forced swim, in mice (Mantsch et al., 2010). Furthermore, we reported that stress- and yohimbine-induced reinstatement is prevented by propranolol, but not the α1 adrenergic receptor antagonist prazosin (Mantsch et al., 2010). In this study, we confirm the role of β-adrenergic receptors in stress-induced reinstatement by demonstrating that reinstatement by forced swim or BRL-44,408 is not observed in β-receptor-deficient Adrb1/Adrb2 double-knockout mice, and that β-receptor activation using isoproterenol, a nonselective agonist, is sufficient to induce reinstatement in wild-type mice. Although suggestive of a role for β-adrenergic receptors, the absence of reinstatement in knockout mice should be interpreted with caution because of the lack of hybrid background strain controls. However, it should be noted that stress-induced reinstatement of cocaine-induced place preference has been observed in a number of mouse strains, including other hybrid background strains (Kreibich and Blendy, 2004; Redila and Chavkin, 2008; Land et al., 2009; Briand et al., 2010; Mantsch et al., 2010; Bruchas et al., 2011).
Our findings suggest that β2 adrenergic receptor activation is both necessary for stress-induced reinstatement and sufficient to reinstate and that stress-induced reinstatement may also involve β1 receptors. The selective β2 receptor antagonist ICI-118,551 blocked reinstatement by forced swim or BRL-44,408 at all doses tested, whereas the selective β2 receptor agonist clenbuterol dose-dependently reinstated. Because the β1 receptor antagonist betaxolol also interfered with swim-induced (but not BRL-44,408- induced) reinstatement at a high dose, we can not rule out a contribution of β1 receptors to stress-induced reinstatement. In contrast to ICI-118,551, which has a 300-fold higher affinity for β2 relative to β1 receptors, the selectivity for betaxolol for β1 versus β2 receptors is much lower, approximately a 35-fold affinity difference (Wellstein et al., 1986). Thus, it is possible that at the higher betaxolol dose there was some β2 receptor antagonism. However, considering that it has been reported that the same betaxolol dose can alter other stress-induced behaviors in mice on which ICI-118,551 has no effect (Stone et al., 1996), coordinated roles for both receptors are possible. Accordingly, reinstatement by isoproterenol was blocked by either betaxolol or ICI-188,551. Because activation of β2 receptors alone is sufficient for reinstatement (clenbuterol reinstates), the requirement for β1 receptors for reinstatement is intriguing and may imply that tonic levels of β1 receptor activation, possibly by basal levels of norepinephrine, are necessary for the activation of β2 receptors and/or their effects on cocaine seeking.
Both ICI-118,551 and betaxolol inhibited locomotor activity at doses tested for effects on reinstatement. Because reduced locomotor activity could produce effects that resemble decreased reinstatement in the three-compartment apparatus caused by potential reductions in the overall time spent outside of the start chamber, we also examined the total time spent in the saline and cocaine compartments combined during reinstatement testing. In all cases, antagonist-induced reductions in the time spent in the cocaine compartment were accompanied by increases in time spent in the saline compartment such that the total time spent outside of the start chamber was no different from vehicle control conditions. Thus, we consider it unlikely that locomotor-suppressing effects contributed to the reductions in reinstatement.
Based on the findings by Leri et al. (2002) that combined antagonism of β1 and β2 adrenergic receptors in the bed nucleus of the stria terminalis (BNST) or amygdala using a cocktail of betaxolol and ICI-118,551 blocks reinstatement of extinguished cocaine self-administration by foot shock stress in rats, we assume that our findings are attributable to the blockade or loss of receptors in the brain. However, a role for peripheral receptors cannot be ruled out, especially considering evidence that epinephrine released from the adrenal medulla can regulate a number of behavioral processes, despite its inability to cross the blood-brain barrier (see e.g., McGaugh and Roozendaal, 2002). Although our earlier finding that surgical adrenalectomy fails to prevent acute stress-induced reinstatement in rats argues against a role for peripheral epinephrine in stress-induced cocaine seeking (Graf et al., 2011), the role of peripheral β2 receptors remains to be determined.
β-Adrenergic receptor-mediated cocaine seeking probably involves the BNST. The ventral noradrenergic bundle, comprised of projections from medullary cell groups, heavily innervates the BNST and is critical for stress-induced reinstatement (Shaham et al., 2000b). Both β1 and β2 receptors are highly expressed in the mouse BNST (Lorton and Davis, 1987), and intra-BNST delivery of a cocktail of ICI-118,551 and betaxolol blocks stress-induced reinstatement in rats (Leri et al., 2002). In the BNST, norepinephrine enhances excitatory neurotransmission through actions at β receptors (Egli et al., 2005) via a mechanism that may involve corticotrophin-releasing factor (CRF) (Nobis et al., 2011), consistent with the finding that intra-BNST CRF delivery induces cocaine seeking, whereas CRF receptor antagonism in the BNST prevents stress-induced reinstatement (Erb and Stewart, 1999), and with reports that CRF is downstream from norepinephrine in the pathway that mediates stress-induced reinstatement (Brown et al., 2011). Adrenergic receptor-regulated GABAergic inputs to the ventral tegmental area (VTA) have been characterized (Dumont and Williams, 2004). Alternatively, β-adrenergic receptors may regulate efferents from the BNST that release CRF into the VTA (Rodaros et al., 2007). We and others have reported that delivery of CRF into the VTA can reinstate cocaine seeking in rats and VTA CRF receptor activation is necessary for stress-induced reinstatement (Wang et al., 2005; Blacktop et al., 2011). Determination of the ability of BNST β-adrenergic receptors to regulate CRF release into the VTA awaits further investigation.
β-Adrenergic receptors have also been implicated in anxiety-related responses associated with acute cocaine delivery (Schank et al., 2008) and cocaine withdrawal (Harris and Aston-Jones, 1993). In the case of withdrawal, these effects seem to be mediated by β1 receptors, probably in the amygdala (Rudoy and Van Bockstaele, 2007). The relationship between withdrawal symptoms and stress-induced relapse is unclear. However, a study involving cocaine-dependent individuals undergoing outpatient treatment found that propranolol improved treatment outcomes only in subjects who displayed severe withdrawal symptoms (Kampman et al., 2001), suggesting that medications targeting β-adrenergic receptors may be more effective for relapse prevention in subpopulations of addicts whose use is stress related.
Despite preventing stress-induced reinstatement, β-adrenergic receptor deficiency and ICI-118,551 did not significantly alter immobility time during the forced swimming bouts. This finding was unexpected and may imply that there is a disconnection between stress-induced cocaine seeking and other stress-related behaviors. Surprisingly, it has been reported that clenbuterol decreases immobility during forced swim (Finnegan et al., 1987), whereas ICI-118,551 and propranolol increase it (Stone et al., 1995), suggesting that reduced drug seeking could be counterintuitively related to a depression phenotype.
β-Adrenergic receptor-deficient mice developed conditioned place preference and did not show reductions in its expression relative to wild-type controls, suggesting that β-adrenergic receptor activation is not necessary for cocaine's rewarding effects or the retrieval of cocaine-associated memories. Cocaine-induced reinstatement was also unaffected in knockout mice, consistent with our earlier finding that pharmacological antagonism failed to alter reinstatement in response to cocaine (Mantsch et al., 2010). These findings are inconsistent with one report that β-receptor antagonism prevented the expression/retrieval of cocaine-induced conditioned place preference in rats (Otis and Mueller, 2011). Although conditioned place preference was not altered in knockout mice, differences relative to wild-type controls were observed during extinction, with no difference on the first test day after conditioning and decreased time spent in the cocaine compartment thereafter. Several groups have reported that antagonism of β receptors either before (Otis and Mueller, 2011) or after (Fricks-Gleason and Marshall, 2008; Bernardi et al., 2009) testing for place preference reduces subsequent preference, potentially because of interference with memory reconsolidation processes. Disruption of memory reconsolidation could explain the reductions in place preference with repeated testing during extinction in knockout mice. To the extent that reconsolidation of cocaine-associated memories was impaired, it did not affect subsequent cocaine-induced reinstatement, in contrast to a report by Fricks-Gleason and Marshall (2008) showing that pharmacological blockade of these receptors after retrieval produced deficits in later reinstatement by cocaine. Thus, it is unlikely that impaired memory reconsolidation contributed to the loss of stress-induced reinstatement in knockout mice. In support, acute pretreatment with ICI-118,551 selectively inhibited reinstatement by forced swim or BRL-44,408, an effect that can not be attributed to earlier disruption of reconsolidation-related processes.
To summarize, these data indicate that β-adrenergic receptor activation is both necessary for stress-induced reinstatement of cocaine seeking and sufficient to reinstate. Although our findings demonstrate a role for β2 receptors in this effect, they do not rule out a role for β1 receptors and, in fact, suggest that stress-induced cocaine seeking may involve coordination between the two receptor subtypes. Future studies will further examine the mechanism through which this potential coordination occurs as well as the brain regions and neurocircuitry through which these receptors contribute to stress-induced relapse.
Acknowledgments
We thank Katia De La Cruz and Malia Thao for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA015758, DA017328].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- WT
- wild type
- KO
- knockout
- ANOVA
- analysis of variance
- BNST
- bed nucleus of the stria terminalis
- CRF
- corticotrophin-releasing factor
- VTA
- ventral tegmental area
- BRL-44,408
- 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole
- ICI-118,551
- -(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol.
Authorship Contributions
Participated in research design: Vranjkovic, Baker, and Mantsch.
Conducted experiments: Vranjkovic, Hang, and Mantsch.
Performed data analysis: Mantsch.
Wrote or contributed to the writing of the manuscript: Mantsch.
References
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM. (2009) Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala β2- and α1-adrenergic antagonists. Learn Mem 16:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. (2011) Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci 31:11396–113403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. (2010) Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci 30:16149–16159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Nobrega JN, Erb S. (2011) Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res 217:472–476 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, et al. (2011) Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71:498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. (2004) Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24:8198–8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Platt BJ, Rizzo SJ, Pulicicchio CM, Wantuch C, Zhang MY, Cummons T, Leventhal L, Bender CN, Zhang J, et al. (2010) Preclinical characterization of BRL 44408: antidepressant- and analgesic-like activity through selective α2A-adrenoceptor antagonism. Int J Neuropsychopharmacol 13:1193–1205 [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. (2005) Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657–868 [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. (2000) α-2 Adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23:138–150 [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19:RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res 174:1–8 [DOI] [PubMed] [Google Scholar]
- Finnegan KT, Terwilliger MM, Berger PA, Hollister LE, Csernansky JG. (1987) A comparison of the neurochemical and behavioral effects of clenbuterol and desipramine. Eur J Pharmacol 134:131–136 [DOI] [PubMed] [Google Scholar]
- Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. (2012) Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol http://dx.doi.org/10.1177/0269881111430746 [DOI] [PMC free article] [PubMed]
- Fricks-Gleason AN, Marshall JF. (2008) Post-retrieval β-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem 15:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. (2011) Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology 36:1444–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. (1993) β-Adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology 113:131–136 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. (2011) Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology 218:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, et al. (2001) Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend 63:69–78 [DOI] [PubMed] [Google Scholar]
- Koob GF. (1999) Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46:1167–1180 [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. (2004) cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci 24:6686–6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. (2009) Activation of the κ opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106:19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. (2011) Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 218:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. (2004) Pharmacological blockade of α2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29:686–693 [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton D, Davis JN. (1987) The distribution of β-1- and β-2-adrenergic receptors of normal and reeler mouse brain: an in vitro autoradiographic study. Neuroscience 23:199–210 [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. (2010) Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology 35:2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. (2002) Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12:205–210 [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. (2000) Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:39–47 [DOI] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. (2011) β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry 69:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Mueller D. (2011) Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology 36:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. (2008) Stress-induced reinstatement of cocaine seeking is mediated by the κ opioid system. Psychopharmacology 200:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. (2006) Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology 185:459–470 [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150:8–13 [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. (1999) Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem 274:16701–16708 [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. (2007) Betaxolol, a selective β1-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry 31:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. (2008) Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. (2000a) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. (2000b) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12:292–302 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. (2000) Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152:140–148 [DOI] [PubMed] [Google Scholar]
- Stone EA, Manavalan SJ, Zhang Y, Quartermain D. (1995) β Adrenoceptor blockade mimics effects of stress on motor activity in mice. Neuropsychopharmacology 12:65–71 [DOI] [PubMed] [Google Scholar]
- Stone EA, Rhee J, Quartermain D. (1996) Blockade of effect of stress on risk assessment behavior in mice by a β-1 adrenoceptor antagonist. Pharmacol Biochem Behav 55:215–217 [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25:5389–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451 [DOI] [PubMed] [Google Scholar]
- Wellstein A, Palm D, Belz GG. (1986) Affinity and selectivity of β-adrenoceptor antagonists in vitro. J Cardiovasc Pharmacol 8 (Suppl 11):S36–S40 [DOI] [PubMed] [Google Scholar]