Abstract
We recently reported that phosphoinositide 3-kinase γ (PI3Kγ) directly regulates airway smooth muscle (ASM) contraction by modulating Ca2+ oscillations. Because ASM contraction plays a critical role in airway hyperresponsiveness (AHR) of asthma, the aim of the present study was to determine whether targeting PI3Kγ in ASM cells could suppress AHR in vitro and in vivo. Intranasal administration into mice of interleukin-13 (IL-13; 10 μg per mouse), a key pathophysiologic cytokine in asthma, induced AHR after 48 h, as assessed by invasive tracheostomy. Intranasal administration of a broad-spectrum PI3K inhibitor or a PI3Kγ-specific inhibitor 1 h before AHR assessment attenuated IL-13 effects. Airway responsiveness to bronchoconstrictor agonists was also examined in precision-cut mouse lung slices pretreated without or with IL-13 for 24 h. Acetylcholine and serotonin dose-response curves indicated that IL-13-treated lung slices had a 40 to 50% larger maximal airway constriction compared with controls. Furthermore, acetylcholine induced a larger initial Ca2+ transient and increased Ca2+ oscillations in IL-13-treated primary mouse ASM cells compared with control cells, correlating with increased cell contraction. As expected, PI3Kγ inhibitor treatment attenuated IL-13-augmented airway contractility of lung slices and ASM cell contraction. In both control and IL-13-treated ASM cells, small interfering RNA-mediated knockdown of PI3Kγ by 70% only reduced the initial Ca2+ transient by 20 to 30% but markedly attenuated Ca2+ oscillations and contractility of ASM cells by 50 to 60%. This report is the first to demonstrate that PI3Kγ in ASM cells is important for IL-13-induced AHR and that acute treatment with a PI3Kγ inhibitor can ameliorate AHR in a murine model of asthma.
Introduction
Airway hyperresponsiveness (AHR) is exaggerated constriction of the airways in response to bronchoconstrictor stimuli (Hargreave et al., 1985). It is a key diagnostic criterion of asthma, and improvement in AHR is associated with better control of asthma (Busse, 2010). Many factors, including airway inflammation and remodeling, contribute to AHR (Fahy et al., 2000; Berend et al., 2008; Casale and Stokes, 2008), but it is increased ASM contractility that is directly responsible for AHR (Shore, 2004; An et al., 2007). G protein-coupled receptors (GPCRs) are important regulators of multiple cell types involved in asthma. Excessive activation of different bronchoconstrictor GPCRs, such as muscarinic, serotonin, endothelin B, leukotriene, and proton-sensing OGR1 receptors in ASM, contributes to AHR of asthma (Deshpande and Penn, 2006; Saxena et al., 2011). Drugs targeting specific GPCRs are used as therapies for AHR in asthma (Shore and Moore, 2003; Currie and McLaughlin, 2006; Hanania et al., 2010; Moulton and Fryer, 2011), yet asthma still affects 23 million Americans, causing significant morbidity. The strategy of inhibiting a single GPCR is limited because airway constriction can be induced by different GPCRs simultaneously, thereby having bronchoconstrictor signal redundancy. Targeting downstream molecules that mediate integrated signals from multiple GPCRs in ASM cells could provide an effective alternate strategy to attenuate excessive airway constriction in asthma.
The type I phosphoinositide 3-kinase (PI3K) family includes α, β, γ, and δ isoforms. PI3Kγ is only activated by GPCRs, whereas PI3Ks α, β, and δ are typically stimulated by receptor tyrosine kinases (Leopoldt et al., 1998; Vanhaesebroeck and Waterfield, 1999). PI3Kγ has been implicated in the pathogenesis of asthma. For example, knockout of PI3Kγ or treatment with aerosolized TG100-115, an inhibitor of PI3Ks γ and δ, markedly reduced allergen-induced asthmatic symptoms in experimental animals, including eosinophilic airway inflammation and AHR (Doukas et al., 2009; Lim et al., 2009; Takeda et al., 2009). Thus, PI3Kγ may be a novel therapeutic target in asthma and other respiratory diseases (Marwick et al., 2010). The mechanism underlying the pathological importance of PI3Kγ in asthma has been considered indirect, through release of inflammatory cell mediators. However, our recent study showed that PI3Kγ is expressed in ASM cells and controls contractility of airways through regulation of Ca2+ oscillations in ASM cells (Jiang et al., 2010). Thus, PI3Kγ in ASM cells may also exert direct effects on the airway constriction that contributes to pathologic AHR.
The T-helper type 2 cytokine interleukin-13 (IL-13) is thought to play a central role in the development of airway inflammation and AHR in asthma. IL-13 is increased in airways of asthmatics and correlates with AHR (Saha et al., 2008). IL-13-deficient mice are protected from development of allergen-induced AHR (Walter et al., 2001), whereas administration of IL-13 is sufficient to induce AHR in mice (Wills-Karp et al., 1998). In humans, anti-IL-13 monoclonal antibody has recently been shown to have positive therapeutic effects in asthma (Corren et al., 2011; Gauvreau et al., 2011).
There is compelling evidence that IL-13 may cause AHR via a direct effect on ASM. ASM cells express IL-13 receptors and respond to IL-13, resulting in augmented Ca2+ mobilization and contractility of ASM cells (Laporte et al., 2001; Deshpande et al., 2004; Eum et al., 2005; Sathish et al., 2009). We recently reported that a PI3Kγ-specific inhibitor, but not inhibitors of other PI3K isoforms, can attenuate contraction of ASM cells via modulating Ca2+ oscillations (Jiang et al., 2010). The purpose of the present study was to determine whether pharmacologic blockade or small interfering RNA (siRNA)-mediated knockdown of PI3Kγ can also suppress IL-13-induced ASM hypercontractility in vitro and AHR in vivo. Using animal, tissue, and cellular models, we demonstrated that PI3Kγ activation is important in development of AHR and that directly targeting PI3Kγ expression or activity in ASM cells can suppress the AHR associated with asthma.
Materials and Methods
Animals.
The original breeding pairs of C57BL/6J mice used in our study were a gift from Dr. Stephen J. Gold (University of Texas Southwestern Medical Center, Dallas, TX). The mice were obtained by in-house breeding. All experiments were approved by the Creighton University Institutional Animal Care and Use Committee.
Reagents.
Murine recombinant IL-13 was purchased from PeproTech (Rocky Hill, NJ). Hanks' balanced salt solution supplemented with 10 mM HEPES buffer, penicillin, streptomycin, amphotericin B, fura-2/acetoxymethyl ester, and Pluronic F-127 were all purchased from Invitrogen (Carlsbad, CA). PI3Kγ inhibitor II [5-(2,2-difluoro-benzo[1,3]dioxol-5-ylmethylene)-thiazolidine-2,4-dione]; a broad-spectrum PI3K inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002); and its inactive analog 2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one (LY303511) were obtained from EMD Biosciences (San Diego, CA). Rabbit PI3Kα and PI3Kγ antibodies and IRDye800-labeled anti-rabbit IgG were purchased from Cell Signaling Technology Inc. (Danvers, MA) and LI-COR Bioscience (Lincoln, NE), respectively. Acetylcholine (ACh) and serotonin were purchased from Sigma-Aldrich (St. Louis, MO). ON-TARGETplus SMARTpool siRNA targeting mouse PI3Kγ was purchased from Thermo Scientific Dharmacon (Lafayette, CO). Unless indicated otherwise, other reagents were purchased from either Sigma-Aldrich or Thermo Fisher Scientific (Waltham, MA).
Intranasal Challenge with Murine Recombinant IL-13 and Measurement of Airway Resistance of Mice.
A murine model of AHR was established as described previously (Yang et al., 2001; Townley et al., 2009). In brief, 8- to 10-week-old male mice (body weight, ∼20 g) were anesthetized and intranasally administered 10 μg of IL-13 dissolved in 50 μl of phosphate-buffered saline (PBS) containing 0.1% BSA or BSA in PBS solution alone (control). Methacholine-induced airway resistance (RL) was measured 48 h later. To assess the effect of PI3Kγ blockade on IL-13-induced AHR, mice were administered PI3Kγ inhibitor II, LY294002, LY303511, or 20 μl of DMSO vehicle intranasally 1 h before airway resistance measurements. Several doses of inhibitors were tested in preliminary studies, and a dose of 20 mg/kg was used in the final experiments.
Airway resistance of anesthetized mice, defined as the pressure driving respiration divided by flow, was assessed by invasive tracheostomy as described previously (Guedes et al., 2006). In brief, anesthetized mice were placed into a plethysmograph chamber (Buxco, Wilmington, NC) and ventilated mechanically at a rate of 140 breaths per minute and a tidal volume of 200 μl. Mice were challenged with 2 μl of saline (baseline) followed by cumulative doses of methacholine (6.25–100 mg/ml) without or with 10 μg of inhibitors or inactive LY303511 using an ultrasonic nebulizer. The data were collected by the FinePointe RC System (Buxco) for 3 min. The response to each methacholine dose was expressed as relative RL (cmH2O/ml/s).
Preparation of Lung Slices and Treatment with IL-13.
Lung slices were prepared as described previously (Bergner and Sanderson, 2002; Jiang et al., 2010). Lung slices of 150-μm thickness cultured in serum-free Dulbecco's modified Eagle's medium were treated without or with IL-13 (100 ng/ml) for 24 h and subjected to airway constriction measurements.
Measurement of Airway Constriction.
Lung slices with airways that were completely lined by epithelial cells with beating cilia were selected (Jiang et al., 2010). Airways with similar cross-sectional areas, which averaged 61,837 ± 1436 μm2, were used for all studies. The slices were placed on glass coverslips in a self-constructed chamber, and phase-contrast images were recorded with a digital CoolSNAP HQ2 camera (Photometrics, Tucson, AZ) using Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD). Frames were captured in time-lapse mode (one frame per 3 s), and time-dependent changes in the cross-sectional areas of the airway lumen were measured by pixel summing using Image-Pro Plus. A decrease in the cross-sectional area was considered to be airway constriction.
RNA Interference by Dual Transfection.
Mouse tracheal ASM cell isolation and culture were described previously (Jiang et al., 2010). Only α-smooth muscle actin staining positive cells with the classic “hill and valley” morphology, passages 3 to 6, were used. ASM cells (8 × 105 in 100 μl) were electroporated with 300 nM control siRNA (negative control no. 1 siRNA; Ambion, Austin, TX) or ON-TARGETplus SMARTpool siRNA for PI3Kγ using Nucleofector kits for smooth muscle and the Amaxa Nucleofector System (Lonza Walkersville Inc., Walkersville, MD). Cells were seeded on six-well plates. The following day, adherent ASM cells were retransfected with the same siRNA using Lipofectamine 2000 as described previously (Wong et al., 2011). Two days later, cells were harvested for Western blot analysis of PI3Kα and PI3Kγ, Ca2+ signaling, and cell contraction assays.
Western Blot Analysis.
Protein was extracted from ASM cells using 1× radioimmunoprecipitation assay complete lysis buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Protein concentrations were determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA). Protein samples (60 μg) were loaded on 8% SDS polyacrylamide gels, electrophoresed, and transferred onto a polyvinylidene difluoride membrane. Membranes were probed with a PI3Kα- or PI3Kγ-specific antibody followed by IRDye800-labeled anti-rabbit IgG. β-Actin was used as a loading control. The signal was visualized using an Odyssey IR imaging system (LI-COR Bioscience).
Measurement of Contraction of Mouse Tracheal ASM Cells.
Primary ASM cells were sparsely plated onto glass coverslips and cultured overnight. The coverslips were mounted in a custom-made Plexiglas chamber, and images were taken using an inverted Nikon TE200 microscope equipped with a CoolSNAP HQ2 digital camera (Photometrics). An initial recording (time, 0 min) was made to obtain the size of quiescent cells. Various concentrations of ACh or serotonin were added to the chamber at 30 s, and images were recorded at 10-s intervals for 5 min. Contractility was assessed using Image-Pro Plus as described previously (Govindaraju et al., 2006; Jiang et al., 2010). Only those cells with a distinct longitudinal axis and clearly visible cell substrate boundaries were selected for analysis. Surface area was obtained by tracing the outer boundary of the cell. Three experiments were performed on multiple days with 50 cells recorded. The extent of contraction was calculated as the ratio of the change in surface area to the initial value.
Measurement of Intracellular-Free Ca2+ and Ca2+ Oscillation Frequency of Mouse Tracheal ASM Cells.
Fura-2-loaded cells were excited at 340 and 380 nm using a DeltaRAM X monochronometer (Photon Technology International, Lawrenceville, NJ). Fluorescent images of individual cells were acquired at one frame per second for at least 300 s to characterize Ca2+ transients and Ca2+ oscillations after ACh stimulation. The intracellular-free calcium concentration in selected regions of interest (3 × 3 pixels) of individual cells is presented as the ratio of the fluorescence intensities (500-nm emission) at excitation of 340 nm compared with 380 nm. A fluorescence signal was defined as a Ca2+ oscillation only if the peak amplitude was greater than twice the background noise. Ca2+ oscillation frequency was measured as the inverse of the average time between two oscillations. Data are averages from at least 30 cells.
Data Analysis.
Data are expressed as means ± S.E. Groups were compared using a Student's t test for unpaired observations or a two-way analysis of variance (ANOVA) with the Bonferroni correction where there were multiple comparisons. A probability level (p) of 0.05 was used to determine statistical significance.
Results
The Effect of PI3Kγ Βlockade on IL-13-Induced Mouse AHR In Vivo.
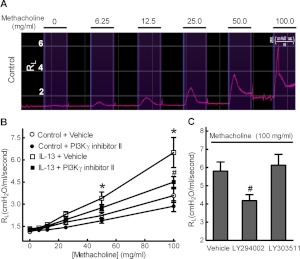
The airway resistance (RL) in response to increasing doses of methacholine challenge was assessed in anesthetized mice (Fig. 1A). In response to inhaled methacholine, mice challenged with intranasal administration of IL-13 (10 μg per mouse) showed a significantly increased RL compared with control mice (Fig. 1B). The methacholine concentration required to double the basal airway resistance was decreased from 60 mg/ml in control mice to 30 mg/ml in IL-13-challenged mice, suggesting that intranasal administration of IL-13 caused AHR. Preadministration of PI3Kγ-specific inhibitor II significantly attenuated IL-13-augmented RL in response to methacholine (Fig. 1B). Because our previous studies showed that a broad-spectrum PI3K inhibitor, LY294002, also attenuated PI3Kγ-dependent mouse airway constriction (Jiang et al., 2010), we also examined the effects of LY294002 on IL-13-augmented mouse RL in vivo. As shown in Fig. 1C, preadministration of LY294002, but not its inactive analog LY303511, attenuated IL-13-augmented RL in response to methacholine. Thus, PI3Kγ activation seems to be critical for IL-13-induced AHR in mice in vivo.
Fig. 1.
Acute blockade of PI3Kγ attenuates AHR in an IL-13-challenged murine model of asthma. Mice were challenged with 10 μg of IL-13 in PBS containing 0.1% BSA or with BSA in PBS solution alone (Control). Forty-eight hours later, airway resistance (RL) of anesthetized mice was measured in response to aerosolized methacholine using invasive tracheostomy as described under Materials and Methods. A, the airway resistance trend graph of a control mouse in response to aerosolized PBS (0) and increasing doses (6.25–100 mg/ml) of methacholine. B, control and IL-13-challenged mice were intranasally administered 20 mg/kg PI3Kγ inhibitor II or DMSO vehicle, 60 min before airway resistance measurements. Data are means ± S.E. from six mice per group. *, p < 0.05 for IL-13 versus control; #, p < 0.05 for IL-13 + PI3Kγ inhibitor II versus IL-13 + vehicle. C, intranasal administration of 20 mg/kg LY294002, but not its inactive analog LY303511, attenuated IL-13-augmented mouse RL in response to 100 mg/ml methacholine. Data are means ± S.E. from four mice. #, p < 0.05 compared with vehicle. Statistical comparisons used ANOVA with the Bonferroni correction.
IL-13 Augments Airway Contractility of Mouse Lung Slices In Vitro.
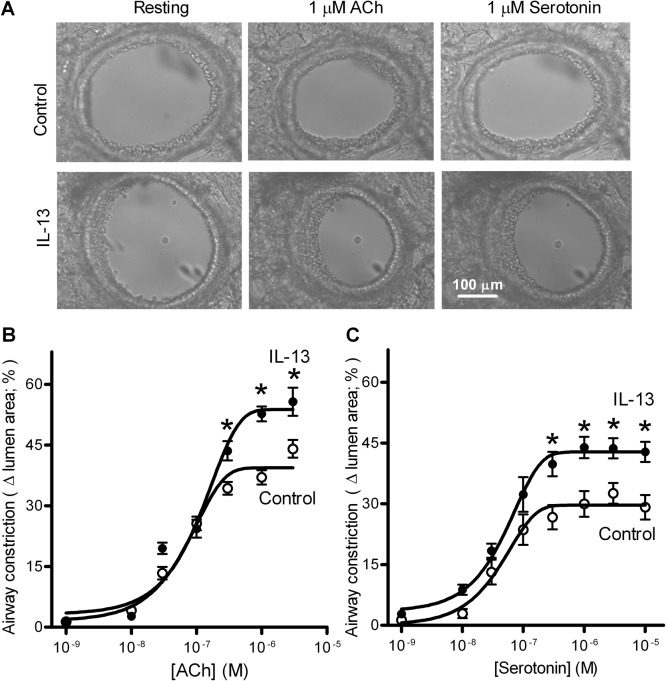
To determine whether IL-13-induced AHR is due to changes of airway contractility, precision-cut mouse lung slices were incubated without or with IL-13 (100 ng/ml) for 24 h, and airway constriction induced by ACh or serotonin was examined. As shown in Fig. 2A, IL-13 treatment increased airway constriction induced by 1 μM ACh or serotonin. The dose-response curves shown in Fig. 2, B and C, indicate that IL-13 treatment increased ACh- or serotonin-induced maximal airway constriction by 40 to 50%. However, IL-13 treatment had no effects on the median effective concentrations (EC50) for ACh (control, 74 ± 8 nM; IL-13, 83 ± 17 nM) or serotonin (control, 43 ± 4 nM; IL-13, 45 ± 6 nM). Thus, IL-13 treatment did not affect the potency of these bronchoconstrictors.
Fig. 2.
IL-13 treatment augments ACh- and serotonin-induced airway constriction of cultured mouse precision-cut lung slices. A, representative images of 1 μM ACh- or serotonin-induced airway constriction in cultured lung slices pretreated without (control) or with IL-13 (100 ng/ml) for 24 h. B and C, dose-response curves for ACh-induced (B) and serotonin-induced (C) airway constriction. Changes in the cross-sectional area of the airway lumen were monitored with phase-contrast microscopy and recorded in time-lapse mode at one frame per 3 s. Each point represents mean ± S.E., using eight lung slices from four (B) or five (C) mice. Statistical analysis was performed on the mean data from each mouse using a Student's t test. *, p < 0.01 compared with control.
Effects of PI3Kγ Βlockade on IL-13-Augmented Airway Contractility of Lung Slices.
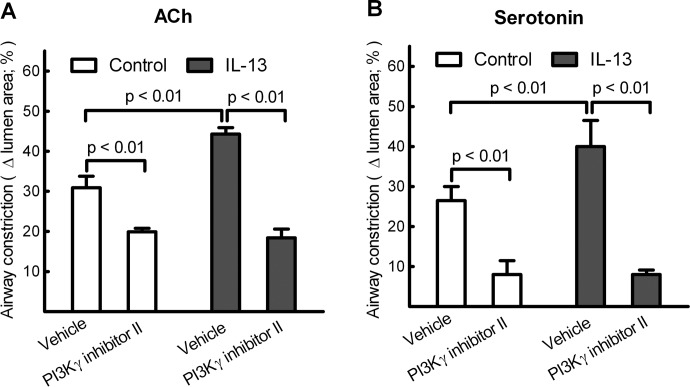
Our previous study showed that PI3Kγ directly controls contractility of airways in response to ACh in cultured lung slices (Jiang et al., 2010). To determine whether PI3Kγ is also involved in IL-13-augmented airway contractility, lung slices were treated without or with IL-13 for 24 h, and contraction of airways was then measured in the absence or presence of 10 μM PI3Kγ inhibitor II. As shown in Fig. 3, PI3Kγ inhibitor II reduced ACh- and serotonin-induced airway constriction of control lung slices by 40 and 70%, respectively, whereas airway constriction of IL-13-treated lung slices was decreased by 60 and 80%, respectively. It is interesting to note that, in the presence of PI3Kγ inhibitor II, there was no significant difference in ACh- or serotonin-induced airway constriction between control and IL-13-treated lung slices (Fig. 3), suggesting that PI3Kγ blockade abolished IL-13-augmented airway constriction. Thus, PI3Kγ pathways are involved in both normal airway constriction and in IL-13-induced airway hypercontractility.
Fig. 3.
Blockade of PI3Kγ attenuates IL-13-augmented airway constriction of cultured lung slices induced by ACh (A) or serotonin (B). Lung slices pretreated without (control) or with IL-13 (100 ng/ml) for 24 h were incubated with vehicle (0.1% DMSO), and 1 μM ACh- or serotonin-induced airway constriction was measured as described under Materials and Methods. Bronchoconstrictor ligands were then washed out, lung slices were treated with PI3Kγ inhibitor II (10 μM) for 10 min, and ACh- or serotonin-induced airway constriction was remeasured. Data are means ± S.E., using eight lung slices from four (A) or five (B) mice. Statistical analysis using ANOVA with the Bonferroni correction was performed on the mean data from each mouse.
Blockade or siRNA-Mediated Knockdown of PI3Kγ Αttenuates IL-13-Augmented Contraction of Isolated Mouse ASM Cells.
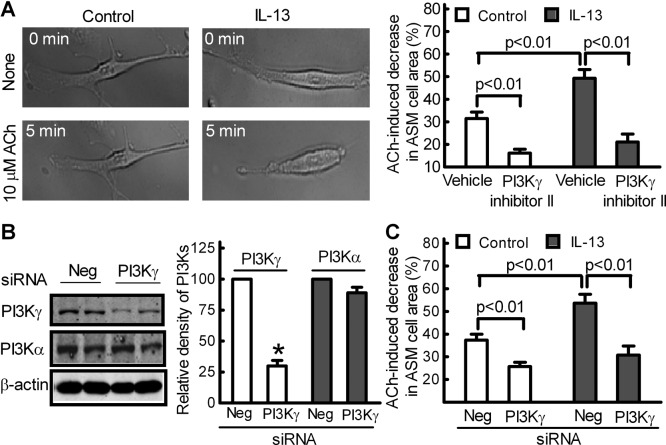
There is compelling evidence that IL-13 may cause AHR via a direct effect on ASM. Indeed, IL-13 treatment increased 10 μM ACh-induced ASM cell contractions by approximately 60% (Fig. 4A). Treatment of ASM cells with PI3Kγ inhibitor II (10 μM) largely attenuated ACh-induced contraction of control and IL-13-treated ASM cells (Fig. 4A).
Fig. 4.
Targeting PI3Kγ to attenuate IL-13-augmented contraction of primary mouse ASM cells. A, blockade of PI3Kγ attenuated IL-13-augmented contraction of mouse ASM cells. ASM cells were treated without (control) or with IL-13 (100 ng/ml) for 24 h. Cells were then exposed to 10 μM ACh in the presence of PI3Kγ inhibitor II (10 μM) or vehicle (0.1% DMSO). Representative images of an initial recording (None, 0 min) and after 5-min exposure to 10 μM ACh are shown on the left. The extent of contraction, calculated as the percentage change in cell-surface area, is shown on the right. Mouse ASM cells were also dual transfected with PI3Kγ siRNA or negative (Neg) siRNA for 48 h and treated without (control) or with IL-13 (100 ng/ml) for 24 h. B, Western blot analysis of PI3Kγ, PI3Kα, and β-actin expression levels in IL-13-treated mouse ASM cells (left), analyzed by measuring band density with β-actin as the normalizing control (right). Data represent means ± S.E. of three independent experiments performed in duplicate. C, siRNA-mediated knockdown of PI3Kγ inhibited IL-13-augmented ASM cell contraction in response to 10 μM ACh. Data in A and C are means ± S.E. and represent 50 cells from three separate experiments performed on multiple days. Statistical comparisons used ANOVA with the Bonferroni correction.
We further knocked down endogenous PI3Kγ to determine the role of PI3Kγ in IL-13-augmented ASM cell contraction. Fig.4B shows that dual transfection of a PI3Kγ-specific SMARTpool siRNA into mouse ASM cells reduced PI3Kγ by approximately 70% but not PI3Kα protein expression compared with a negative control siRNA. ACh-induced ASM cell contraction was decreased by 40 to 50% (Fig. 4C), which is consistent with our results using PI3Kγ inhibitor II (Fig. 4A) (Jiang et al., 2010). More importantly, siRNA-mediated knockdown of endogenous PI3Kγ largely blocked IL-13-augmented ASM cell contractility (Fig. 4C).
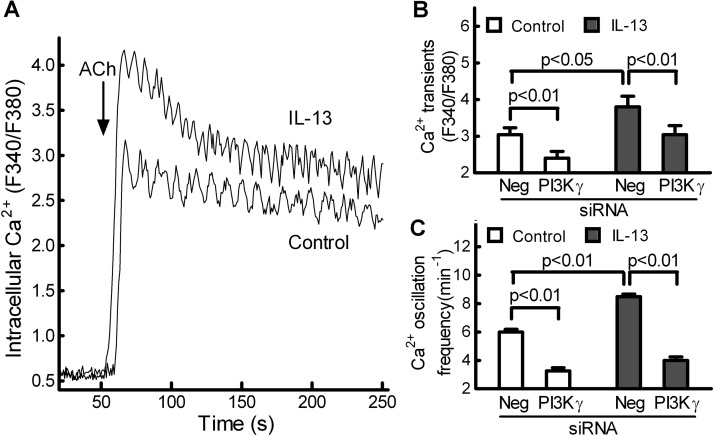
siRNA-Mediated Knockdown of PI3Kγ Ιnhibits IL-13-Augmented Intracellular Ca2+ Signaling in Isolated Mouse ASM Cells.
Intracellular Ca2+ is the key signaling molecule for ASM contraction. Consistent with previous reports (Roux et al., 1997; Jiang et al., 2010), ACh-induced increase in intracellular Ca2+ consisted of an initial Ca2+ transient that is responsible for initial contraction, followed by Ca2+ oscillations that are critical for maintenance of sustained airway constriction (Fig. 5A). Compared with control ASM cells, IL-13-treated ASM cells showed a larger initial Ca2+ transient, followed by increased Ca2+ oscillations (Fig. 5A), correlating with increased cell contraction (Fig. 4, A and C). On average, IL-13 treatment increased the Ca2+ transient and Ca2+ oscillation frequency of mouse ASM cells by approximately 40% (Fig. 5, B and C). It is interesting to note that, in both control and IL-13-treated ASM cells, siRNA-mediated knockdown of PI3Kγ only reduced the initial Ca2+ transient by 20 to 30% (Fig. 5B) but more markedly attenuated Ca2+ oscillations by 50 to 60% (Fig. 5C), correlating with decreased cell contraction (Fig. 4C).
Fig. 5.
siRNA-mediated knockdown of PI3Kγ attenuated IL-13-augmented Ca2+ oscillations in primary mouse ASM cells. A, a concentration of 1 μM ACh induced Ca2+ mobilization (Fura-2 as a probe) of mouse ASM cells. The intracellular Ca2+ concentration is presented as the ratio of the fluorescence intensities at an excitation of 340 nm compared with 380 nm (F340/F380). These data were quantified and are shown in B and C. B and C, in control and IL-13-treated ASM cells, siRNA-mediated knockdown of PI3Kγ only partially reduced the initial Ca2+ transient (B) but more markedly attenuated Ca2+ oscillations (C) of ASM cells in response to ACh. Data are means ± S.E. and represent at least 30 cells from three separate experiments performed on multiple days. Statistical comparisons used ANOVA with the Bonferroni correction. Neg, negative.
Discussion
In the present study, we report for the first time that intranasal administration of a PI3Kγ inhibitor can attenuate IL-13-induced AHR in mice. Furthermore, our studies show that targeting PI3Kγ, using a pharmacologic inhibitor or RNA interference, suppresses IL-13-augmented Ca2+ oscillations and contractility of ASM cells.
IL-13 is thought to play a central role in the development of airway inflammation and AHR in asthma. Previous studies (Wills-Karp et al., 1998; Yang et al., 2001) have shown that intranasal administration of IL-13 induced AHR as early as 6 h after administration, long before significant inflammation has occurred, followed by an amplified and sustained AHR that peaked at 48 h. Using this IL-13-challenged murine model of AHR, we demonstrate that intranasal administration of one dose of IL-13 into naive mice for 48 h produced greater airway resistance to inhaled methacholine compared with control mice, an indication of robust AHR in mice. Consistent with our previous findings in vitro, PI3Kγ inhibitor II attenuated airway constriction to the contractile agonist methacholine in naive mice. More importantly, intranasal administration of PI3Kγ inhibitor II blocked IL-13-induced AHR in mice.
PI3Kγ inhibitor II exhibits great selectivity for PI3Kγ over other PI3K isoforms (20–100-fold) and shows little effect on a large panel of receptors, unrelated enzymes, ion channels, and commonly studied kinases. In our preliminary studies, we tested several doses of PI3Kγ inhibitor II in IL-13-challenged mice at different times before methacholine challenge and found that intranasal administration of PI3Kγ inhibitor II at a dose of 20 mg/kg, 60 min before airway resistance measurements, is sufficient to attenuate IL-13-induced AHR. Because the local concentration of this inhibitor in mouse airways in vivo is unknown, whether it is still selective in vivo at the dose used remains to be determined. However, we found that preadministration of LY294002, a broad-spectrum PI3K inhibitor, but not its inactive analog LY303511, attenuated IL-13-induced AHR in mice. This is consistent with our previous results showing that LY294002 also attenuated PI3Kγ-dependent mouse airway constriction (Jiang et al., 2010). Thus, PI3Kγ activation seems to be critical for IL-13-induced AHR in mice.
The role of PI3Ks in asthma has been studied previously. These studies, using two broad-spectrum inhibitors of PI3K, wortmannin and LY294002, suggested that PI3K contributes to the pathogenesis of asthma by affecting the recruitment, activation, and apoptosis of inflammatory cells (Ezeamuzie et al., 2001; Kwak et al., 2003). More recent studies have been focused on the involvement of PI3K isoforms in asthma. Lee et al. (2006) found that inhibition of PI3Kδ attenuated allergic airway inflammation and AHR in an ovalbumin-challenged murine model of asthma. PI3Kγ has been implicated in the pathogenesis of asthma, but its mechanism has been considered indirect, through release of inflammatory cell mediators (Lim et al., 2009; Takeda et al., 2009). We recently reported that PI3Kγ controls contractility of airways through regulation of Ca2+ oscillations in ASM cells (Jiang et al., 2010), which could be important for development of AHR in asthma. Because IL-13 may cause AHR through a direct stimulatory effect on ASM cell contraction, independent of airway inflammation, we also investigated whether targeting PI3Kγ in ASM cells could attenuate IL-13-augmented airway responsiveness using both precision-cut mouse lung slices and isolated mouse ASM cells as models. As expected, in the absence of IL-13 treatment, preincubation with a PI3Kγ inhibitor reduced lung slice airway contractility caused by ACh or serotonin, consistent with our previous results that PI3Kγ directly controls airway constriction caused by bronchoconstrictors (Jiang et al., 2010). More importantly, PI3Kγ inhibitor treatment largely blocked the IL-13-induced augmentation of airway constriction to ACh or serotonin, suggesting that PI3Kγ signaling is important in controlling IL-13-augmented airway contractility. Furthermore, because ACh and serotonin activate muscarinic receptors and serotonin receptors, respectively, our results show that targeting PI3Kγ can suppress bronchoconstrictor signals from multiple GPCRs. In our studies, PI3Kγ inhibition had a greater effect in blocking serotonin compared with ACh-stimulated cell contraction; however, the mechanisms responsible for this difference are unknown. Serotonin induces airway constriction via activation of Gq-coupled serotonin receptors, whereas ACh not only activates Gq-coupled m3 muscarinic receptors but also Gi-coupled m2 muscarinic receptors to induce airway constriction. It is possible that PI3Kγ may differentially mediate responses caused by different bronchoconstrictor GPCRs and different G-proteins in mouse ASM cells.
Excessive activation of many different bronchoconstrictor GPCRs contributes to the AHR of asthma (Deshpande and Penn, 2006). In asthma, an important treatment paradigm is to block aberrant GPCR bronchoconstrictor responses. Selective antagonists of bronchoconstrictor GPCRs can be used, but given the large number of GPCRs and endogenous ligands, signaling redundancies can offset the desired inhibitory/therapeutic effects. A GPCR conveys signals by dissociating inactive G-proteins into active Gα and Gβγ subunits. PI3Kγ is known to be selectively activated by Gβγ subunits rather than Gα subunits (Leopoldt et al., 1998). Thus, PI3Kγ acts just downstream from the GPCR signaling convergence in cells, such that it can potentially be activated by multiple types of bronchoconstrictor GPCRs. Therefore, targeting PI3Kγ could be an effective alternative strategy for a broader-based suppression of the unwanted GPCR signaling in ASM that causes AHR in asthma.
Changes in intracellular Ca2+ mobilization in ASM cells has been proposed as an important mechanism underlying AHR induced by allergen sensitization or cytokines in asthma. For example, exposure of human ASM cells to IL-13 resulted in augmented intracellular Ca2+ responses to multiple contractile agonists (Deshpande et al., 2004). Indeed, compared with control cells, we found that IL-13-treated ASM cells had a larger initial Ca2+ transient, followed by increased Ca2+ oscillations, correlating with increased cell contraction. Previous studies from our laboratory using cultured lung slices and isolated primary ASM cells from mouse trachea have demonstrated that PI3Kγ controls contractility of airways through regulation of Ca2+ oscillations in ASM cells. However, whether PI3Kγ signaling is also involved in IL-13-mediated augmentation of intracellular Ca2+ mobilization has not been established. In the present study, we show that in both control and IL-13-treated ASM cells, siRNA-mediated knockdown of endogenous PI3Kγ only partially reduced the initial Ca2+ transient but markedly attenuated Ca2+ oscillations in ASM cells, which are critical for maintenance of sustained airway constriction and AHR (Roux et al., 1997). Thus, it is possible that PI3Kγ regulates ASM contraction by controlling Ca2+ oscillations in both physiological and pathological conditions. However, additional details of the molecular mechanisms underlying PI3Kγ-dependent regulation of Ca2+ signaling in ASM cells remain to be established. Sarcoplasmic reticulum Ca2+ release is a major contributor to agonist-induced Ca2+ elevation in ASM cells (Jude et al., 2008). In general, activation of inositol 1,4,5-trisphosphate receptors in the sarcoplasmic reticulum is crucial for the initiation of Ca2+ oscillations, whereas activation of ryanodine receptors maintains the Ca2+ oscillations that correlate with sustained airway contraction. Thus, it will be important to investigate the effects of PI3Kγ blockade or siRNA-mediated knockdown of PI3Kγ on the functions of inositol 1,4,5-trisphosphate and ryanodine receptors in ASM cells. These studies are currently in progress.
In summary, our data show that PI3Kγ signaling plays an important role in IL-13-induced mouse ASM hypercontraction in vitro and AHR in vivo. More importantly, targeting PI3Kγ in ASM cells can ameliorate AHR in an IL-13-challenged murine model of asthma. IL-13 is a one mediator of asthma, and studies aimed at blocking IL-13 in human asthma have recently shown some promise (Corren et al., 2011; Gauvreau et al., 2011); however, there are no data on effects of anti-IL-13 strategies on either the baseline or stimulated contractility underlying AHR. Because previous studies have shown that increased IL-13 is critical for the development of AHR in acute but not chronic ovalbumin challenge of mice (Taube et al., 2002; Leigh et al., 2004), it will be of interest to investigate effects of the PI3Kγ inhibitor on AHR in acute versus chronic ovalbumin-challenge models of asthma. Such studies could further define the PI3Kγ signaling pathway as a potential new pharmacologic target for the treatment of the AHR in multiple models of asthma.
Acknowledgments
We thank Dr. Dennis W. Wolff for analysis of Ca2+ signaling in ASM cells.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant G20-RR024001]; and in part by an American Asthma Foundation Early Excellence Award (to Y.T.) and the State of Nebraska Research Fund [Grant LB692] (to Y.T. and T.B.C.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- AHR
- airway hyperresponsiveness
- ACh
- acetylcholine
- ASM
- airway smooth muscle
- GPCR
- G protein-coupled receptor
- IL-13
- interleukin-13
- PBS
- phosphate-buffered saline
- PI3Kγ
- phosphoinositide 3-kinase γ
- BSA
- bovine serum albumin
- DMSO
- dimethyl sulfoxide
- siRNA
- small interfering RNA
- RL
- airway resistance
- ANOVA
- analysis of variance
- LY294002
- 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- LY303511
- 2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one.
Authorship Contributions
Participated in research design: Jiang, Xie, Abel, Toews, Townley, Casale, and Tu.
Conducted experiments: Jiang, Xie, and Tu.
Contributed new reagents or analytic tools: Abel and Townley.
Performed data analysis: Jiang, Xie, and Abel.
Wrote or contributed to the writing of the manuscript: Jiang, Xie, Abel, Toews, Casale, and Tu.
References
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, et al. (2007) Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 29:834–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berend N, Salome CM, King GG. (2008) Mechanisms of airway hyperresponsiveness in asthma. Respirology 13:624–631 [DOI] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ. (2002) Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 119:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse WW. (2010) The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138 (Suppl 2): 4S–10S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale TB, Stokes JR. (2008) Immunomodulators for allergic respiratory disorders. J Allergy Clin Immunol 121:288–296 [DOI] [PubMed] [Google Scholar]
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. (2011) Lebrikizumab treatment in adults with asthma. N Engl J Med 365:1088–1098 [DOI] [PubMed] [Google Scholar]
- Currie GP, McLaughlin K. (2006) The expanding role of leukotriene receptor antagonists in chronic asthma. Ann Allergy Asthma Immunol 97:731–741 [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Penn RB. (2006) Targeting G protein-coupled receptor signaling in asthma. Cell Signal 18:2105–2120 [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. (2004) Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31:36–42 [DOI] [PubMed] [Google Scholar]
- Doukas J, Eide L, Stebbins K, Racanelli-Layton A, Dellamary L, Martin M, Dneprovskaia E, Noronha G, Soll R, Wrasidlo W, et al. (2009) Aerosolized phosphoinositide 3-kinase γ/δ inhibitor TG100-115 [3-[2,4-diamino-6-(3-hydroxyphenyl) pteridin-7-yl]phenol] as a therapeutic candidate for asthma and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 328:758–765 [DOI] [PubMed] [Google Scholar]
- Eum SY, Maghni K, Tolloczko B, Eidelman DH, Martin JG. (2005) IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288:L576–L584 [DOI] [PubMed] [Google Scholar]
- Ezeamuzie CI, Sukumaran J, Philips E. (2001) Effect of wortmannin on human eosinophil responses in vitro and on bronchial inflammation and airway hyperresponsiveness in guinea pigs in vivo. Am J Respir Crit Care Med 164:1633–1639 [DOI] [PubMed] [Google Scholar]
- Fahy JV, Corry DB, Boushey HA. (2000) Airway inflammation and remodeling in asthma. Curr Opin Pulm Med 6:15–20 [DOI] [PubMed] [Google Scholar]
- Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, Deschesnes F, Duong M, Durn BL, Howie KJ, et al. (2011) Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med 183:1007–1014 [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. (2006) Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol 291:C957–C965 [DOI] [PubMed] [Google Scholar]
- Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. (2006) CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 291:L1286–L1293 [DOI] [PubMed] [Google Scholar]
- Hanania NA, Dickey BF, Bond RA. (2010) Clinical implications of the intrinsic efficacy of beta-adrenoceptor drugs in asthma: full, partial and inverse agonism. Curr Opin Pulm Med 16:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreave FE, O'Byrne PM, Ramsdale EH. (1985) Mediators, airway responsiveness, and asthma. J Allergy Clin Immunol 76:272–276 [DOI] [PubMed] [Google Scholar]
- Jiang H, Abel PW, Toews ML, Deng C, Casale TB, Xie Y, Tu Y. (2010) Phosphoinositide 3-kinase gamma regulates airway smooth muscle contraction by modulating calcium oscillations. J Pharmacol Exp Ther 334:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude JA, Wylam ME, Walseth TF, Kannan MS. (2008) Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 5:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, Lee YC. (2003) Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 111:1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA, Jr, Kinet JP, Shore SA. (2001) Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 164:141–148 [DOI] [PubMed] [Google Scholar]
- Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. (2006) Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J 20:455–465 [DOI] [PubMed] [Google Scholar]
- Leigh R, Ellis R, Wattie J, Donaldson DD, Inman MD. (2004) Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am J Respir Crit Care Med 170:851–856 [DOI] [PubMed] [Google Scholar]
- Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. (1998) Gβγ stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273:7024–7029 [DOI] [PubMed] [Google Scholar]
- Lim DH, Cho JY, Song DJ, Lee SY, Miller M, Broide DH. (2009) PI3Kγ-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling. Am J Physiol Lung Cell Mol Physiol 296:L210–L219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick JA, Chung KF, Adcock IM. (2010) Phosphatidylinositol 3-kinase isoforms as targets in respiratory disease. Ther Adv Respir Dis 4:19–34 [DOI] [PubMed] [Google Scholar]
- Moulton BC, Fryer AD. (2011) Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br J Pharmacol 163:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux E, Guibert C, Savineau JP, Marthan R. (1997) [Ca2+]i oscillations induced by muscarinic stimulation in airway smooth muscle cells: receptor subtypes and correlation with the mechanical activity. Br J Pharmacol 120:1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, Monk P, Bradding P, Wardlaw AJ, Pavord ID, et al. (2008) Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol 121:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. (2009) Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297:L26–L34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena H, Deshpande D, Tiegs B, Yan H, Battafarano R, Burrows W, Damera G, Panettieri R, Dubose T, Jr, An S, et al. (2011) The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol 166:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SA. (2004) Airway smooth muscle in asthma–not just more of the same. N Engl J Med 351:531–532 [DOI] [PubMed] [Google Scholar]
- Shore SA, Moore PE. (2003) Regulation of β-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol 137:179–195 [DOI] [PubMed] [Google Scholar]
- Takeda M, Ito W, Tanabe M, Ueki S, Kato H, Kihara J, Tanigai T, Chiba T, Yamaguchi K, Kayaba H, et al. (2009) Allergic airway hyperresponsiveness, inflammation, and remodeling do not develop in phosphoinositide 3-kinase γ-deficient mice. J Allergy Clin Immunol 123:805–812 [DOI] [PubMed] [Google Scholar]
- Taube C, Duez C, Cui ZH, Takeda K, Rha YH, Park JW, Balhorn A, Donaldson DD, Dakhama A, Gelfand EW. (2002) The role of IL-13 in established allergic airway disease. J Immunol 169:6482–6489 [DOI] [PubMed] [Google Scholar]
- Townley RG, Gendapodi PR, Qutna N, Evans J, Romero FA, Abel P. (2009) Effect of interleukin 13 on bronchial hyperresponsiveness and the bronchoprotective effect of β-adrenergic bronchodilators and corticosteroids. Ann Allergy Asthma Immunol 102:190–197 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. (1999) Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 253:239–254 [DOI] [PubMed] [Google Scholar]
- Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. (2001) Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 167:4668–4675 [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. (1998) Interleukin-13: central mediator of allergic asthma. Science 282:2258–2261 [DOI] [PubMed] [Google Scholar]
- Wong CY, Wuriyanghan H, Xie Y, Lin MF, Abel PW, Tu Y. (2011) Epigenetic regulation of phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 gene expression in prostate cancer cells. J Biol Chem 286:25813–25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. (2001) Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 25:522–530 [DOI] [PubMed] [Google Scholar]