Abstract
Chronic nicotine produces up-regulation of α4β2* nicotinic acetylcholine receptors (nAChRs) (* denotes that an additional subunit may be part of the receptor). However, the extent of up-regulation to persistent ligand exposure varies across brain regions. The aim of this work was to study the cellular distribution and function of nAChRs after chronic nicotine treatment in primary cultures of mouse brain neurons. Initially, high-affinity [125I]epibatidine binding to cell membrane homogenates from primary neuronal cultures obtained from diencephalon and hippocampus of C57BL/6J mouse embryos (embryonic days 16–18) was measured. An increase in α4β2*-nAChR binding sites was observed in hippocampus, but not in diencephalon, after 24 h of treatment with 1 μM nicotine. However, a nicotine dose-dependent up-regulation of approximately 3.5- and 0.4-fold in hippocampus and diencephalon, respectively, was found after 96 h of nicotine treatment. A significant fraction of total [125I]epibatidine binding sites in both hippocampus (45%) and diencephalon (65%) was located on the cell surface. Chronic nicotine (96 h) up-regulated both intracellular and surface binding in both brain regions without changing the proportion of those binding sites compared with control neurons. The increase in surface binding was not accompanied by an increase in nicotine-stimulated Ca2+ influx, suggesting persistent desensitization or inactivation of receptors at the plasma membrane occurred. Given the differences observed between hippocampus and diencephalon neurons exposed to nicotine, multiple mechanisms may play a role in the regulation of nAChR expression and function.
Introduction
Nicotinic acetylcholine receptors (nAChRs) expressed in mammalian brain are ligand-gated ion channels assembled as pentamers composed of α (α2-α7) and β (β2-β4) subunits. Major receptor subtypes in the central nervous system are homomeric α7*-nAChRs and heteromeric α4β2*-nAChRs (* denotes that an additional subunit may be part of the receptor) (Lukas et al., 1999). A lower density of α3β4*- and α6β2*- nAChR subtypes is also found in some brain regions (Baddick and Marks, 2011).
Chronic nicotine exposure induces up-regulation of nAChRs in mice (Marks et al., 1983), rats (Schwartz and Kellar, 1983), transfected oocytes (Fenster et al., 1999), stably transfected fibroblasts (Peng et al., 1994, 1997; Bencherif et al., 1995; Warpman et al., 1998; Whiteaker et al., 1998; Gentry et al., 2003), and embryonic neurons in culture (Bencherif et al., 1995; Dávila-García et al., 1999; Nashmi et al., 2003; Lomazzo et al., 2011; Govind et al., 2012). [3H]nicotine binding sites are also increased in smokers. This increase is caused by an increase in Bmax with no change in KD (Benwell et al., 1988; Breese et al., 1997; Perry et al., 1999). Some brain regions (e.g., thalamus) are less responsive to chronic nicotine-induced up-regulation than regions (e.g., hippocampus or cortex) where up-regulation occurs even after treatment with low doses of nicotine (Pauly et al., 1991; Flores et al., 1997; Sparks and Pauly, 1999; Nguyen et al., 2003; Marks et al., 2004).
Even though up-regulation of nAChRs occurs after chronic nicotine treatment of mice, function measured by 86Rb+ efflux or [3H]dopamine release from synaptosomal preparations remains at basal control levels or even decreases in a dose-dependent manner (Marks et al., 1993, 2004). The apparent decrease in receptor function produced by chronic ligand exposure may arise from ligand-induced persistent desensitization or inactivation of nAChRs (Peng et al., 1994; Gentry et al., 2003). However, it is also possible that the population of receptors that are increased by nicotine treatments remain in intracellular compartments such that receptors at the plasma membrane are unaltered or even reduced. This last hypothesis seems unlikely because of a recent report that demonstrates that receptors at the plasma membrane of neurons in primary culture are also up-regulated by chronic nicotine treatments (Lomazzo et al., 2011), similar to the observation with cells transfected with α4 and β2 nAChR subunits (Peng et al., 1994; Whiteaker et al., 1998). However, it has also been reported that chronic nicotine treatment increases total nAChR function as a consequence of the increased receptor expression in cell lines (Gopalakrishnan et al., 1996; Buisson et al., 2000), rat synaptosomes (Nguyen et al., 2004), and midbrain neurons (Nashmi et al., 2003, 2007).
Most of the studies on nAChR cellular distribution used cell lines expressing native or transfected receptors. An alternative approach, using embryonic neurons in culture that express native nAChRs, has the advantage of investigating cells that undergo differentiation and express several markers of mature neurons (Kaech and Banker, 2006). For example, primary cultures of hippocampal neurons express α7*- and α4β2*-nAChRs and show nicotinic-induced currents after 10 days in culture (Zarei et al., 1999). Furthermore, primary neurons in culture chronically treated with nicotine up-regulate nAChRs (Bencherif et al., 1995; Dávila-García et al., 1999; Nashmi et al., 2003; Lomazzo et al., 2011; Govind et al., 2012). Few studies have addressed the resulting functionality of the receptors expressed on neurons in primary culture after prolonged agonist exposure, although nAChR function has been measured after chronic nicotine exposure in cells expressing α4β2-nAChRs heterologously (Peng et al., 1994; Gopalakrishnan et al., 1996; Buisson et al., 2000; Gentry et al., 2003).
The current study investigates the regulation of the distribution and function of nAChRs in primary neuronal cultures isolated from hippocampus and diencephalon of mouse embryos. The effect of nicotine treatments on receptor density and distribution at surface and intracellular membranes was measured. We report here that cells prepared from hippocampus and diencephalon exhibit differences in nicotine-induced up-regulation measured by [125I]epibatidine binding. The ratio between intracellular and surface receptors in both brain regions is unchanged by nicotine treatment. Moreover, receptor function assessed by fluorometric intracellular Ca2+ detection is not increased by chronic nicotine treatment, indicating that the up-regulated receptors are less functional, consistent with the results obtained in mice chronically treated with nicotine (Marks et al., 1993, 2004). Differences in the regulation of ligand binding and receptor function obtained for hippocampus and diencephalon neurons after chronic nicotine treatment indicate that multiple mechanisms are responsible for the regulation of nAChRs.
Materials and Methods
Materials.
[125I]epibatidine (2200 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). 2-(2-Bromoacetyloxy)-N,N,N-trimethylethanaminium bromide (BrACh), cytisine, (−)-nicotine hydrogen tartrate, polyethyleneimine, cytosine-β-d-arabino-furanoside, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 1,4-dithio-dl-threitol (DTT), methylcarbachol hydrochloride (MCC), and poly-l-lysine (>30,000 kDa) were purchased from Sigma (St. Louis, MO). HEPES half-sodium salt was from Roche Diagnostics (Indianapolis, IN). Neurobasal media, minimal essential medium, B27 supplement, Fluo4-NW calcium assay, Glutamax supplement, heat-inactivated horse serum, and TrypLE were purchased from Invitrogen (Carlsbad, CA).
Primary Neuronal Cultures.
All experiments were approved by and carried out in accordance with the University of Colorado's Guide for the Care and Use of Experimental Animals. Primary cultures from embryonic mouse brains (embryonic days 16–18) were established as described previously (Kaech and Banker, 2006) with some modifications. The whole brain was placed in HBSS (Ca2+ and Mg2+ free) buffer (0.407 mM; 5.33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 137.93 mM NaCl, 0.338 mM Na2HPO4, and 5.56 mM d-glucose) and separated from the meninges. Hippocampus and diencephalon (subcortical areas including thalamus, hypothalamus, and caudate nucleus) were dissected and minced into small pieces. Brain tissue was rinsed once with HBSS (Ca2+ and Mg2+ free) buffer and incubated with 0.5× TrypLE diluted in HBSS (Ca2+ and Mg2+ free) buffer for 15 min at 37°C. Tissue was mechanically dissociated through a heat-polished Pasteur pipette at room temperature. The cell suspension was centrifuged at 800g for 2 min and then resuspended in minimal essential medium supplemented with 10% horse serum, 100 U/ml penicillin, 100 U/ml streptomycin, and 0.25 mg/ml amphotericin B. Isolated neurons were seeded at a density of 50,000 to 75,000 cells/cm2 over polystyrene plates coated with 0.1 mg/ml poly-l-lysine prepared in 0.1 M borate buffer, pH 8.5. After 24 h at 37°C, media were changed to maintenance media (neurobasal media supplemented with B27, 100 U/ml penicillin, 100 U/ml streptomycin, 0.25 mg/ml amphotericin B, and 2 mM l-glutamine). On the second day of culture, 10 μM cytosine-β-d-arabino-furanoside was added for 72 h at 37°C incubation to control the proliferation of glial cells. Cultures were kept in a humidified 5% CO2-95% air incubator at 37°C. A nicotine stock (10 mM) was prepared fresh for every experiment by using maintenance medium and for dilutions. Chronic nicotine treatments were begun 12 to 14 days after plating. Depending on the experiment, cells received nicotine concentrations of 0.001 to 10 μM only once at the beginning of either a 24- or 96-h chronic treatment period at 37°C incubation.
Preparation of Total Membranes.
After treatments were completed, neurons in culture were rinsed once with KRH buffer (144 mM NaCl, 2.2 KCl, 2 mM CaCl2, 1 mM MgSO4, and 25 mM HEPES, pH 7.5) and then collected in 0.1× hypotonic KRH buffer by scraping the plate surface, triturated with an ultra turrax, and centrifuged at 25,000g for 15 min at 4°C. The pellets were washed four times by resuspension in ice-cold hypotonic KRH buffer followed by centrifugation. Cell membranes were resuspended in distilled-deionized water for the binding reaction (if carried out immediately) or hypotonic binding buffer and frozen at −70°C until assayed.
[125I]Epibatidine Binding to Cell Membrane Homogenates.
[125I]epibatidine binding was measured as described previously (Whiteaker et al., 2000). Frozen washed pellets were resuspended in the overlying buffer and centrifuged at 25,000g for 15 min at 4°C. The supernatant was discarded, and the pellet was resuspended in ice-cold water. Resuspension volumes varied among samples cultured to adjust protein concentrations such that less than 10% of the [125I]epibatidine was bound to the protein at the highest ligand concentration. Samples (5–20 μg of protein) were incubated in 96-well polystyrene plates for 2 h at 22°C in KRH buffer. Final incubation volume was 30 μl. At the completion of the binding reaction, samples were diluted with 200 μl of ice-cold KRH buffer and filtered under vacuum (0.2 atm.) at 4°C onto glass fiber filters that had been treated for 10 min with 0.5% polyethelenimine [top filter, MFS Type B (Advantec, Dublin, CA); bottom filter, Pall type A/E (Pall Life Sciences, Port Washington, NY)]. An Inotech Cell Harvester (Inotech Biosystems, Rockville, MD) was used to collect the samples, which were subsequently washed five times with ice-cold buffer. Filters containing the washed samples were transferred to glass culture tubes, and radioactivity was counted at 80% efficiency by using a Packard Cobra Auto-Gamma Counter (PerkinElmer Life and Analytical Sciences). For all experiments, nonspecific binding was measured by including 100 μM cytisine in the incubation medium. All cultured brain regions from different treatment groups were assayed for binding by using 200 pM [125I]epibatidine. Because [125I]epibatidine binds with high affinity to several different nAChR subtypes (Whiteaker et al., 2000), differential inhibition by cytisine (50 and 150 nM) was used to distinguish two binding sites: cytisine-sensitive sites comprising α4β2* and cytisine-resistant sites representing a mixed population of receptors including α3β4* (for review see Marks et al., 2010).
Alkylation of Cell Surface nAChR.
To evaluate the effect of chronic nicotine treatment on surface and intracellular binding sites, cells were treated as described above with 0 or 1 μM nicotine for 24 or 96 h, followed by alkylation of surface nAChR as described previously with some modifications (Free et al., 2005). After experimental treatment, primary neurons in culture were rinsed once with HBSS buffer, pH 7.4 (1.26 mM CaCl2, 0.493 mM MgCl2, 0.407 mM MgSO4, 5.33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 137.93 mM NaCl, 0.338 mM Na2HPO4, and 5.56 mM d-glucose), supplemented with 20 mM HEPES and 5 mM glucose, and then treated for 15 min at 37°C with 1 mM DTT prepared in the same buffer. Cultures were rinsed once with HBSS followed by 6-min incubation with 100 μM BrACh prepared in HBSS at 22°C. After rinsing with HBSS, the reaction was completed by adding 1 mM DTNB in HBSS for 15 min at 37°C. After the alkylation reaction, the neurons were rinsed once with HBSS, lysed with hypotonic ice-cold KRH buffer, and scraped from the plate. A set of cultures treated as described above, but omitting the BrACh incubation, was used to measure total [125I]epibatidine binding. Whole particulate membranes were prepared as described above, and [125I]epibatidine binding was subsequently measured by using the radioligand binding assay. Surface binding was calculated as the difference between total binding (no incubation with BrACh) and binding after receptor alkylation (intracellular).
[125I]Epibatidine Binding to Intact Neurons in Culture.
MCC, a quaternary amine that is poorly permeable across membranes, was used to quantify the density of receptors present on the surface of neurons in culture (Whiteaker et al., 1998). Neurons were assayed for total and MCC-resistant [125I]epibatidine binding, which represents remaining binding at the internal pool of receptors. Surface binding was calculated by subtracting MCC-resistant from total specific [125I]epibatidine binding. To establish optimal binding conditions several preliminary experiments were conducted. For the association kinetics, intact cells were incubated with 200 pM [125I]epibatidine at 22°C for 0.5-, 1-, 2.5-, 5-, 10-, 20-, 40-, and 60-min reaction to determine time of attainment of equilibrium binding. MCC competition binding was done to establish a concentration of this ligand that produces maximal inhibition of [125I]epibatidine binding. MCC concentrations used were 0.001, 0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 μM in a 20-min binding reaction. Counts were obtained by harvesting the cells and washing the filtered material with ice-cold KRH buffer. Nonspecific binding values were obtained by adding 100 μM nicotine to the binding reaction. Total binding was measured for samples containing no MCC. To evaluate the effect of chronic nicotine treatment on MCC-sensitive (surface) and MCC-resistant (intracellular) binding sites, cells were treated with 0, 0.001, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, or 30 μM nicotine for 96 h at 37°C. At the completion of the chronic treatment, cells were washed four times by incubation with maintenance medium for 10 min at 37°C. Subsequently, cells were incubated for 20 min with 200 pM [125I]epibatidine and either 0 or 1 μM MCC at room temperature. Cells were harvested and radioactivity was measured as described above.
Ca2+ Influx Determination.
Neurons prepared as described above were plated in 96-well plates coated with 1 mg/ml poly-l-lysine (optical bottom, black plates; Nalge Nunc International, Rochester, NY) and maintained in culture at 37°C for 12 to 14 days until drug treatments began. Cells were treated with 0, 0.001, 0.01, 0.1, 1, or 10 μM nicotine for 96 h at 37°C. After the nicotine treatment, cells were rinsed four times with maintenance media at 37°C for 10 min. Cells were then loaded with 80 μl of Fluo4-NW by incubation for 1 h at 37°C with 5% CO2 (cell culture incubator) followed by a 15-min incubation in the dark at 22°C according to the manufacturer's instructions. HBSS buffer supplemented with 20 mM HEPES and 5.5 mM glucose was used to prepare Fluo4-NW and nicotine solutions. Fluorescence was measured in a plate reader model VICTOR (PerkinElmer Life and Analytical Sciences). Samples were excited at 485 nm, and emitted fluorescence was recorded at 535 nm every second after nicotine addition. Data are presented as area under the curve during 60-s readings and normalized by total Ca2+ influx after cell lysis with 1% Triton X-100. Nicotine-stimulated fluorescence was determined by subtracting basal fluorescence values, obtained by adding 20 μl of HBSS-HEPES-glucose buffer, from the values obtained by adding 20 μl of a 5× nicotine stock. Dose-response curves were generated by using 0.001, 0.01, 0.1, 1, and 10 μM nicotine. A concentration of 10 μM nicotine achieved maximal response and was used to test the effect of chronic nicotine treatment.
Data Calculations.
Cytisine-sensitive and cytisine-resistant [125I]epibatidine binding sites were calculated by using a two-site inhibition model: BI = B1/(1 + I/IC50–1) + B2/(1 + I/IC50–2), where BI is the [125I]epibatidine binding at either cytisine concentration, I, B1 and B2 are the [125I]epibatidine binding with IC50 values IC50–1 (cytisine-sensitive sites) and IC50–2 (cytisine-resistant sites), respectively. IC50–1 and IC50–2 values used were 3.75 and 300 nM. Densities of cytisine-sensitive and cytisine-resistant sites were calculated from the [125I]epibatidine binding measured with 0, 50, and 150 nM cytisine.
[125I]Epibatidine displacement binding assays were performed in situ and in cell membrane preparations. Inhibition of binding to membranes was calculated by using a one-site inhibition model: BL = B/(1 + L/IC50). For MCC inhibition binding assays with intact cells, the following formula was used to calculate inhibitable and residual binding: BL = (B/(1 + L/IC50)) + BR, where BL is [125I]epibatidine binding measured at any concentration of inhibitor, L, and B represents binding sites sensitive to the inhibition with an apparent IC50 value. BR corresponds to the uninhibited residual binding fraction. The inhibition constant Ki was determined by using the Cheng and Prussoff equation (IC50 = Ki*(1 + L/Kd) (Cheng and Prusoff, 1973), where L is the concentration of [125I]epibatidine, and Kd is the average high-affinity binding constant calculated from saturation binding experiments for the concentration of [125I]epibatidine used in a specific experiment.
The association of [125I]epibatidine binding in intact neurons was modeled by a double exponential increase as described previously (Lippiello et al., 1987) by using the following equation: Bt = Bfast(1 − e−kfastt) + Bslow(1 − e−kslowt) where Bfast and Bslow are the two states of the receptor with high and low rates of association, and Bt is the total binding at time t.
The Hill equation was used to determine EC50 and Hill coefficients for Ca2+ influx experiments. SigmaPlot 8.0 (Systat Software, Inc., San Jose, CA) was used for calculations and graphical presentation of the data. Statistical analyses were conducted by using Sigma Stat (Systat Software, Inc.). Specific analyses are described in the figure legends.
Results
Binding to Membrane Homogenates after Chronic Nicotine Treatment.
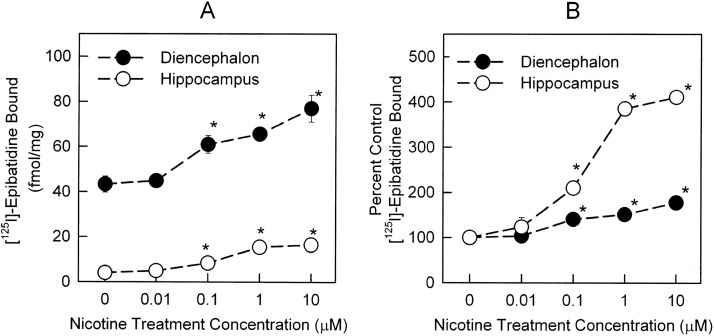
Neurons in culture were allowed to differentiate for at least 12 days before any drug treatment. This was done to assure that functional receptors were being expressed (Zarei et al., 1999). Binding of [125I]epibatidine in untreated diencephalon cells (44.4 ± 1.7 fmol/mg protein) was significantly higher than in hippocampal cells (4.0 ± 0.3 fmol/mg protein). Chronic nicotine treatment for 96 h elicited a dose-dependent increase in total specific [125I]epibatidine binding in both hippocampus neurons (F4,34 = 45.5; p < 0.001) and diencephalon neurons (F4,33 = 95.8; p < 0.001) (Fig. 1A; one-way ANOVA). EC50 values for the nicotine-induced up-regulation of total specific [125I]epibatidine binding in hippocampus and diencephalon were 180.7 ± 62.9 and 96.7 ± 39.8 nM, respectively. However, as illustrated in Fig. 1B, hippocampal cells exhibited significantly more up-regulation than did diencephalon cells (310.7 ± 42.1 and 77.2 ± 6.4% of control for 10 μM treatments in hippocampus and diencephalon, respectively).
Fig. 1.
Total specific [125I]epibatidine binding in total membranes prepared from hippocampus and diencephalon cultures treated with nicotine for 96 h. Primary cultures of hippocampus (○) or diencephalon (●) were treated with nicotine concentrations of 0, 0.01, 0.1, 1, and 10 μM for 96 h. Binding was measured by using 200 pM [125I]epibatidine in a 2-h binding reaction. Nonspecific binding was determined by including 100 μM cytisine in the binding reaction. A, total specific [125I]epibatidine binding to the total membrane fractions prepared from the cultures. B, binding data transformed to percentage of control. Data represent mean ± S.E.M. of seven replicates from three independent experiments. Significant differences with respect to control (untreated) neurons were calculated by using one-way ANOVA, followed by Student-Newman-Keuls post hoc analysis. *, p < 0.001.
Differential sensitivity to inhibition by cytisine can distinguish between the sites with relatively high affinity for cytisine (primarily α4β2*-nAChR sites) and those with relatively low affinity for cytisine (mixed nAChR sites) (Marks et al., 1998; Zoli et al., 1998; Whiteaker et al., 2000). The high-affinity [3H]epibatidine binding sites in thalamus and hippocampus of mouse brain represent mostly α4β2*-nAChRs (Marks et al., 2010). To determine the effects of chronic treatment on these two components of [125I]epibatidine binding sites, cultures of hippocampus and diencephalon cells were treated with 1 μM nicotine for 24 or 96 h.
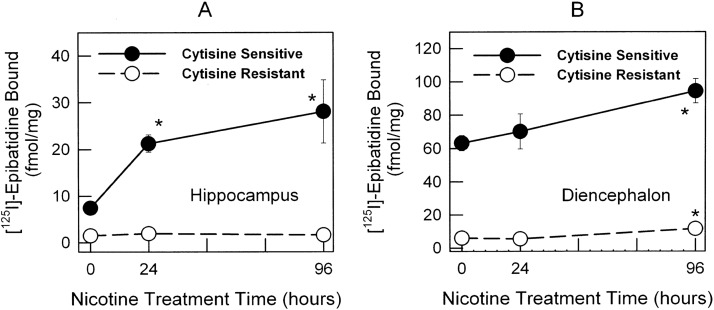
After 24 h of 1 μM nicotine treatment, up-regulation of the cytisine-sensitive component (α4β2*-nAChR), but not the cytisine-resistant [125I]epibatidine binding component (non-α4β2*, mixed receptor population including α3β4*-nAChR), in hippocampus was observed (Fig. 2A). In contrast, diencephalon cultures did not show a change in binding after a 24-h treatment (Fig. 2B). However, significant increases in cytisine-sensitive [125I]epibatidine binding were observed for both hippocampus (Fig. 2A) and diencephalon (Fig. 2B) after a 96-h treatment with 1 μM nicotine. No change was observed for the cytisine-resistant component of [125I]epibatidine binding in hippocampus at any time point (Fig. 2A). However, the cytisine-resistant [125I]epibatidine binding component showed a significant increase in diencephalon neurons after 96 h of treatment with nicotine (Fig. 2B).
Fig. 2.
Time course of the up-regulation of both cytisine-sensitive and cytisine-resistant components of [125I]epibatidine binding in hippocampus and diencephalon cultures. Binding was measured in total membranes prepared from hippocampus and diencephalon cultures at 0, 24, and 96 h for untreated neurons and neurons treated with 1 μM nicotine. Nonspecific binding was determined by using 100 μM cytisine. Results are expressed as specific [125I]epibatidine binding ± S.E.M. in fmol/mg protein. The effects of nicotine treatment on cytisine-sensitive (●) and cytisine-resistant (○) components in [125I]epibatidine binding for hippocampus cultures (A) and diencephalon cultures (B) are shown. Significant differences from control untreated neurons were calculated by using one-way ANOVA, followed by a Student-Newman-Keuls post hoc analysis. *, p < 0.05.
nAChR Alkylation.
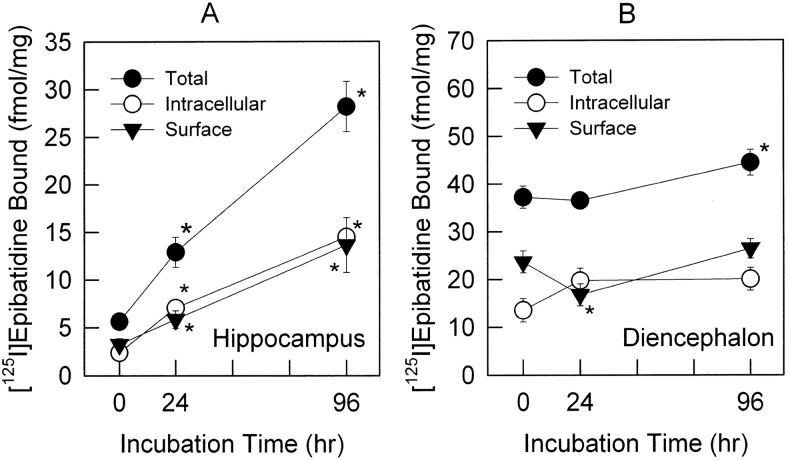
One method to determine the distribution of the receptors in the plasma membrane versus intracellular membranes makes use of an alkylation technique reported previously for nicotinic receptors in chromaffin cells (Free et al., 2005). Neurons (12–14 days in culture) were treated with 1 μM nicotine for 24 and 96 h. After washing, samples were then treated with 1 mM DTT to reduce disulfide bonds and reacted with either 0 or 100 μM BrACh and 1 mM DTNB to reoxidize unreacted sulfhydryls. Neurons treated with DTT and DTNB, but not BrACh, provided a measurement of total [125I]epibatidine binding. Samples treated with DTT, BrACh, and DTNB provided a measurement of the density of [125I]epibatidine binding sites at both surface membranes (binding eliminated by BrACh treatment) and intracellular membranes (binding retained after BrACh treatment). Total specific [125I]epibatidine binding in control hippocampus neurons (binding after DTT and DTNB treatment) was 6.3 ± 0.8 fmol/mg (Fig. 3A). After treatment with 1 μM nicotine for 24 and 96 h total specific [125I]epibatidine binding in hippocampus was significantly higher than that of controls: 12.9* ± 1.6 and 27.7* ± 2.2 fmol/mg, respectively (Fig. 3A). The percentage of total [125I]epibatidine binding sites expressed at the cell surface versus that in the intracellular pool was not significantly changed by treatment with nicotine in hippocampus [surface receptors: 57.8 ± 13.8% (0 h), 45.4 ± 9.0% (24 h), and 48.5 ± 11.2% (96 h)] (Fig. 3A). Total specific [125I]epibatidine binding in control diencephalon neurons was 38.8 ± 2.8 fmol/mg protein (Fig. 3B). After treatment with 1 μM nicotine for 24 and 96 h [125I]epibatidine binding site density was 37.1 ± 2.3 and 46.4* ± 3.3 fmol/mg, respectively (Fig. 3B). Statistically significant up-regulation was observed for diencephalon neurons treated for 96 h, but not 24 h. No differences in the percentage of surface receptors versus total receptors were noted on diencephalon cells after treatment with 1 μM nicotine for 96 h compared with untreated cells. However, there seemed to be a modest decrease in surface receptors as a percentage of total receptors after 24 h of nicotine treatment [surface receptors: 63.6 ± 6.0% (0 h), 45.9 ± 6.7% (24 h) and 59.5 ± 5.2% (96 h)] (Fig. 3B).
Fig. 3.
Determination of [125I]epibatidine binding to intracellular and surface membranes by alkylation of surface receptors. Primary neurons cultured from hippocampus and diencephalon were treated with 1 μM nicotine for 0, 24, or 96 h before the alkylation reaction as described under Materials and Methods. After alkylation, total membranes were prepared from the neurons, and total and intracellular [125I]epibatidine binding was measured. Surface binding was obtained by subtraction of the intracellular binding from the average of total binding. Total (●), intracellular (○), and surface (▾) binding sites are shown for both hippocampus (A) and diencephalon (B) cultures. Data represent mean ± S.E.M. (n = 13, five independent experiments for 0 and 96 h; n = 9, four independent experiments for 24-h treatment). Statistical differences were determined by Kruskal-Wallis one-way ANOVA on ranks, post hoc Dunn's method. *, p < 0.05.
In Situ [125I]Epibatidine Binding.
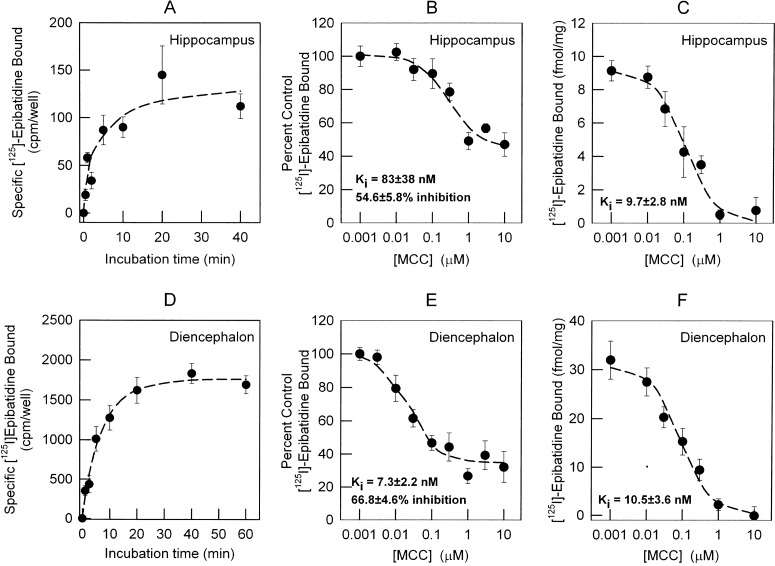
An additional method, with which to measure the cellular distribution of surface and intracellular [125I]epibatidine binding sites, is the use of differential inhibition by MCC to inhibit surface binding. The principle behind the use of MCC as a selective inhibitor of surface receptors is its relative high hydrophilicity and resultant slow permeability across cell membranes as reflected in a lower partition coefficient compared with cytisine, nicotine, or epibatidine (logP = 0, 0.2, 1.2, and 2.2 for MCC, cytisine, nicotine, and epibatidine, respectively; PubChem project website, http://pubchem.ncbi.nlm.nih.gov). MCC displays relatively high affinity for α4β2*-nAChRs (Boksa and Quirion, 1987), allowing its use in relatively low concentrations. Preliminary studies were conducted to establish the appropriate conditions for selective inhibition of surface binding sites by MCC. Because MCC may slowly penetrate cell membranes and subsequently inhibit intracellular sites, the time course for [125I]epibatidine binding was determined to establish the minimum time required to reach equilibrium. For this purpose, an [125I]epibatidine association binding was done in intact neurons in culture at 22°C. The association had two components. The fast component reached equilibrium in less than 1 min, making accurate calculation of rate constants unreliable. Estimate of the rate constant for the slow component of [125I]epibatidine (200 pM) binding was possible. Values for kslow = 0.158 ± 0.09 and 0.147 ± 0.018 min−1 with t1/2 values of 4.4 and 4.7 min were determined for hippocampus (Fig. 4A) and diencephalon (Fig. 4D), respectively. Consequently, equilibrium binding was attained by using 20-min incubation. The 20-min incubation time was used for all subsequent experiments.
Fig. 4.
Measurement of the time course for [125I]epibatidine binding and comparison of MCC inhibition of [125I]epibatidine binding in whole cells and cell membranes. A and D, the time courses for the binding of [125I]epibatidine to intact neuronal cultures from hippocampus (A) and diencephalon (D) were measured by incubating the cells with 200 pM [125I]epibatidine for the times indicated. Neurons (12–14 days) were seeded in 48-well plates and assayed for [125I]epibatidine binding in the same culture dish at room temperature (22°C). Nonspecific binding was determined by including 100 μM nicotine in the binding reaction. The inhibition of [125I]epibatidine binding by MCC in intact neurons was measured by adding the indicated concentrations of MCC (0.001–10 μM) to the incubation medium. B and E, IC50 values and residual binding for both hippocampus (B) and diencephalon (E) neuronal cultures were calculated as described under Materials and Methods. C and F, IC50 values for MCC in total membrane preparations from both hippocampus (C) and diencephalon (F) were calculated as described under Materials and Methods. Apparent Ki values were calculated by using the Cheng-Prussof equation (apparent KD for [125I]epibatidine binding in intact cells: 73 pM for hippocampus and 111 pM for diencephalon; apparent KD for membrane preparations: 25 pM). Data for the association kinetics represent the mean ± S.E.M. for four separate determinations. Data for binding in situ represent nine replicates from two independent experiments and are presented as percentage of specific binding in the absence of competitor. Binding in total membranes represents four replicates from two independent experiments and is presented as total specific binding normalized by protein concentration. All curves are least-squares fits of the data as described under Materials and Methods.
After establishing the appropriate incubation time, the pharmacology of MCC interaction with [125I]epibatidine binding sites was investigated by using the 20-min incubation time. Inhibition of [125I]epibatidine binding by MCC was measured in hippocampus and diencephalon cultures to obtain Ki values (calculated by using a one-site inhibition curve fit with a residual population resistant to inhibition). The Ki values calculated for inhibition of [125I]epibatidine binding to intact cells were 83 ± 38 and 7.3 ± 2.2 nM for hippocampus (Fig. 4B) and diencephalon (Fig. 4E), respectively. High concentrations of MCC did not totally inhibit [125I]epibatidine binding (Fig. 4, B and E). The residual binding calculated from the inhibition curves was 45.4 ± 5.9 and 33.2 ± 3.6% of the total for hippocampus and diencephalon, respectively. This component of specific [125I]epibatidine binding resistant to inhibition by MCC represents intracellular receptors (Whiteaker et al., 1998). MCC inhibition curves were also determined for [125I]epibatidine binding sites in cell membranes prepared from neurons in culture. Ki values of 9.7 ± 2.8 and 10.5 ± 3.6 nM were measured for hippocampus (Fig. 4C) and diencephalon (Fig. 4F), respectively. Complete inhibition of [125I]epibatidine binding to cell membranes by MCC was observed in both hippocampus (Fig. 4C) and diencephalon (Fig. 4F).
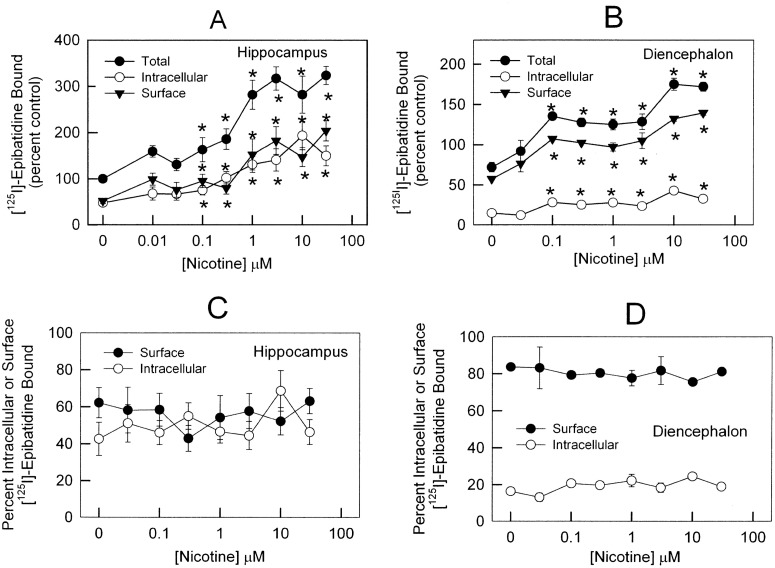
The preliminary characterization of the in situ binding establishes that using a 20-min incubation with [125I]epibatidine and measuring inhibition of the binding by 1 μM MCC to inhibit surface binding sites is appropriate for examining the effects of chronic nicotine exposure on the distribution of [125I]epibatidine binding sites. Primary cultures from hippocampus and diencephalon were exposed to one of eight concentrations of nicotine for 96 h. After washing to remove nicotine from the medium, cells were incubated with 200 pM [125I]epibatidine for 20 min in the presence of 0 μM MCC (total binding sites) or 1 μM MCC (intracellular binding sites). Total [125I]epibatidine binding was increased in a dose-dependent manner, with significant increases noted after treatment with 0.1 μM and higher concentrations of nicotine in both hippocampus (Fig. 5A) and diencephalon (Fig. 5B). Maximal binding after chronic treatment with 10 μM nicotine in hippocampus was 280 ± 40% of control (Fig. 5A) and in diencephalon was 175 ± 8% of control (Fig. 5B). The density of both surface and intracellular [125I]epibatidine binding increased after chronic nicotine treatment as well. No differences in the percentage of surface receptors in either hippocampus (Fig. 5C) or diencephalon (Fig. 5D) were noted for any nicotine treatment dose. However, consistent with the results presented above, the percentage of surface receptors in diencephalon (80%) (Fig. 5D) is higher than the percentage of surface receptors in hippocampus (50%) (Fig. 5C).
Fig. 5.
Distribution of nAChRs after chronic nicotine treatment by in situ MCC inhibition of [125I]epibatidine binding. A and B, hippocampus (A) and diencephalon (B) neurons were treated for 4 days with 0.03, 0.1, 0.3, 1, 3, 10, and 30 μM nicotine for 96 h. Samples were incubated with 200 pM [125I]epibatidine for 20 min at 22°C. Intracellular binding was determined by adding 1 μM MCC to inhibit [125I]epibatidine binding to the surface receptors. Surface binding was calculated by subtracting intracellular binding from total binding in absence of 1 μM MCC. Total (●), intracellular (○) and surface (▾) [125I]epibatidine binding are presented as the percentage of total binding to untreated samples for hippocampus (A) and diencephalon (B) cultures. C and D, surface (●) and intracellular (○) [125I]epibatidine binding, as a percentage of the total binding after treatment with each of the indicated nicotine concentrations used for chronic treatment, for hippocampus (C) and diencephalon (D) is shown. Data represent specific cpm as percentage of control neurons. *, statistical differences (p < 0.05) from control cells for total, intracellular, and surface binding obtained by one-way ANOVA, post hoc Student-Newman-Keuls.
nAChR Function after Chronic Nicotine Treatment.
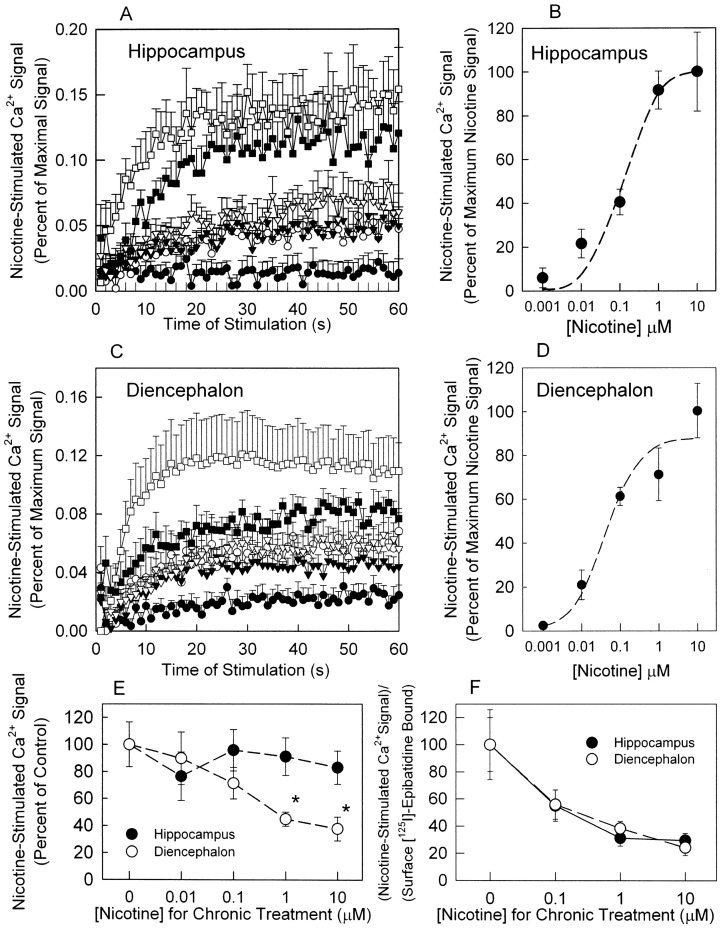
The experiments described above demonstrate that chronic nicotine treatment increased the number of surface nAChRs measured with [125I]epibatidine binding. To investigate the effects of chronic nicotine treatment on nAChR function, nicotine-stimulated Ca2+ influx was measured. Ca2+ influx has been used to measure nAChR function in several systems, including primary neuronal cultures (for review see Shen and Yakel, 2009). Initially, nicotine dose-response curves were generated for control hippocampus and diencephalon neurons (Fig. 6, A–D). The effect of acute nicotine exposure on intracellular Ca2+ in hippocampus is shown in Fig. 6A. An EC50 value of 182.7 ± 1.7 was calculated (Fig. 6B). The effect of acute nicotine exposure on intracellular Ca2+ in diencephalon is shown in Fig. 6C. An EC50 value of 84.0 ± 5.1 nM was calculated (Fig. 6D). Hill coefficients were 1.02 ± 0.52 and 0.58 ± 0.66 in hippocampus and diencephalon, respectively. A 10 μM nicotine concentration was chosen to acutely stimulate control cells and cells that had been chronically treated with nicotine (0.01, 0.1, 1, and 10 μM nicotine for 96 h). Chronic nicotine treatment had no significant effect on the increase in intracellular Ca2+ in hippocampal neurons elicited by acute nicotine stimulation (Fig. 6E). In contrast, the increase in intracellular Ca2+ for diencephalon neurons elicited by acute nicotine stimulation was reduced approximately 60% after chronic treatment with 1 or 10 μM nicotine (Fig. 6E).
Fig. 6.
Intracellular Ca2+ determination in hippocampus and diencephalon neurons. A and C, time course of nicotine-induced Ca2+ influx in untreated hippocampus (A) and diencephalon (C) neurons cultured for 12 days was measured by using these nicotine concentrations: 0 μM (●), 0.001 μM (○), 0.01 μM (▾), 0.1 μM (▿), 1 μM (■), and 10 μM (□). Each point represents the mean ± S.E.M. for four separate experiments. B and D, concentration-response curves were calculated by using the area under the curve for each nicotine concentration, as shown in A and C, for hippocampus (B) and diencephalon (D) and using nonlinear least-squares to fit the curves as described under Materials and Methods. E, subsequently, neurons were chronically treated with 0.01, 0.1, 1, and 10 μM nicotine for 4 days and then assayed for Ca2+ influx stimulated with 10 μM nicotine. Results are presented as the percentage of the response measured for samples treated with 0 μM nicotine. F, the ratio of nicotine-stimulated increases in intracellular Ca2+ normalized by the percentage of surface binding after chronic nicotine treatment (data from E) divided by the amount of surface [125I]epibatidine binding measured after the same nicotine treatment (shown in Fig. 5, A and B). Data represented as mean ± S.E.M. of three independent experiments (n = 3–4 each). Significant statistical differences were found by Kruskal-Wallis ANOVA test, post hoc Dunn's method. *, p < 0.05.
The functional activity of nAChR in response to chronic nicotine exposure in hippocampus and diencephalon was estimated by dividing the functional responses (Fig. 6E) by surface binding (Fig. 5, A and B). The estimated relative function per unit surface of the [125I]epibatidine binding site decreased after chronic nicotine treatment by virtually the same extent for both hippocampus and diencephalon cells (Fig. 6F).
Discussion
Chronic nicotine treatment increases the number of nAChR binding sites in rodent and human brain (Marks et al., 1983, 2011; Schwartz and Kellar, 1983; Benwell et al., 1988; Pauly et al., 1991; Breese et al., 1997; Perry et al., 1999; Sparks and Pauly, 1999; Nguyen et al., 2003), as well as in cells expressing nAChRs (Peng et al., 1994, 1997; Bencherif et al., 1995; Warpman et al., 1998; Whiteaker et al., 1998; Gentry et al., 2003), including primary neuronal cultures (Bencherif et al., 1995; Dávila-García et al., 1999; Nashmi et al., 2003; Lomazzo et al., 2011; Govind et al., 2012). Here, we demonstrate in primary neuronal cultures from hippocampus and diencephalon that chronic nicotine exposure elicits saturable, concentration-dependent up-regulation of high-affinity [125I]epibatidine binding sites without changing the ratio of surface to intracellular receptors. However, the increase in plasma membrane receptors is not accompanied by an increase in function measured as nicotine-induced Ca2+ influx, corroborating data obtained in chronically nicotine-treated mice (Marks et al., 1993, 2004) and cell lines expressing α4β2*-nAChRs (Peng et al., 1994; Gopalakrishnan et al., 1996; Fenster et al., 1999; Kuryatov et al., 2000), indicating that on average the surface receptors after chronic nicotine treatment are not fully functional.
Neuronal Cultures Resemble Adult Brain.
The expression and regulation of nAChRs in primary neuronal cultures resemble that observed for adult brain.
Basal levels of high-affinity [125I]epibatidine binding sites in hippocampus were relatively low respective to adult tissue, but diencephalon displayed a similar density as observed in the thalamus of adult mice (Marks et al., 2004). Likewise, reflecting the expression in adult rat brain, primary rat hippocampus and cortex neuronal cultures have a lower density of nAChR binding sites than subcortical tissue (Dávila-García et al., 1999).
Nicotine-induced nAChR up-regulation in rodents differs among brain regions: cortex or hippocampus show an approximately 2-fold increase in ligand binding, whereas thalamus has much less up-regulation (Marks et al., 1983, 2004, 2011; Flores et al., 1992; Sanderson et al., 1993; Sparks and Pauly, 1999; Nguyen et al., 2003). Likewise, less up-regulation was observed for primary striatal cultures than for primary cortical cultures (Lomazzo et al., 2011), a difference that is also observed after treatment in vivo. We also observed differences in the extent of nicotine-induced up-regulation for mouse neuronal cultures, finding 2.5- to 3.5-fold increases in total [125I]epibatidine binding in hippocampus but a modest 0.4-fold increase in diencephalon cultures after chronic exposure to 1 μM nicotine. Part of this regional difference could arise from the different developmental stages of these two brain regions.
In addition to the increase in α4β2*-nAChRs (measured as cytisine-sensitive [125I]epibatidine binding), a significant increase in cytisine-resistant [125I]epibatidine binding sites was noted in diencephalon cells after 96 h of treatment with 1 μM nicotine. However, little change in cytisine-resistant sites (or the subset of nAChR measured under these conditions) after chronic nicotine treatment has been noted for animals (Flores et al., 1997; Dávila-García et al., 2003; Nguyen et al., 2003; Marks et al., 2004). However, small increases in cytisine-resistant [125I]epibatidine binding sites, which failed to reach statistical significance, have been noted in several mouse brain regions after treatment with high doses of nicotine that resulted in plasma concentrations around 1 μM (Marks et al., 2004). Nicotine-induced up-regulation has been observed for α3β2- or α3β4-nAChRs after treatment with nicotine concentrations higher than those attainable in vivo (Peng et al., 1997; Meyer et al., 2001; Avila et al., 2003; Xiao and Kellar, 2004). The subunit composition of the nAChR expressed in primary mouse diencephalon neurons is currently unknown. The increase in cytisine-resistant [125I]epibatidine binding in diencephalon after chronic treatment with 1 μM nicotine could result from an up-regulation of α3β2*- or α3β4*-nAChRs.
Plasma Membrane and Intracellular Distribution of [125I]Epibatidine Binding Sites and Effects of Chronic Nicotine Exposure.
nAChR up-regulation has been described in detail using animal and cellular models. However, few reports have focused on how receptors are distributed before and after chronic ligand exposure. Using two different approaches to determine surface and intracellular [125I]epibatidine binding, we demonstrate here that chronic nicotine treatment does not significantly change the percentage of high-affinity [125I]epibatidine binding sites located at the cell surface: surface receptors increase in parallel with the total receptors. Although the proportion of receptors on the cell surface differs between diencephalon (70–80%) and hippocampus (50%), the distribution of binding sites on surface and intracellular membranes remains constant after nicotine treatment. Our results are completely consistent with those of a recent study demonstrating that the distribution of heteromeric nAChRs in rat primary cultures was unchanged by chronic nicotine treatment (Lomazzo et al., 2011). It should be noted that these results were found even though there were some technical differences between the studies, including methods for measuring the surface receptors and the age of the cultures when chronic nicotine treatment began. It should also be noted, in agreement with our findings, that the ratio of surface to intracellular epibatidine binding sites in M10 cells stably transfected with chicken α4β2-nAChRs, in which most of the receptors are intracellular (approximately 85%), was unchanged by chronic nicotine treatment as measured by MCC inhibition (Whiteaker et al., 1998), a result confirming increased receptor expression as measured by binding of mAb299, an α4 nAChR subunit-selective antibody (Peng et al., 1994).
Receptor Function after Chronic Nicotine Treatment.
Despite the increase in receptors at the plasma membrane after chronic nicotine treatment, Ca2+ influx elicited by acute nicotine exposure was decreased in diencephalon and virtually unchanged in hippocampus cultures. If the nicotine-stimulated increases in intracellular Ca2+ are normalized to plasma membrane receptor densities, the effect of chronic nicotine treatment is similar for cells from both regions: that is, chronic nicotine treatment decreases function per unit surface binding site (functionality ratio) (Fig. 6F). This result indicates that the up-regulated surface receptors are on average not fully functional. This finding is totally consistent with previous reports on chronically nicotine-treated mice where both 86Rb+ efflux and [3H]dopamine release were diminished compared with control saline-treated mice (Marks et al., 1993, 2004).
The functionality of nAChRs after chronic nicotine exposure remains controversial. Decreases in functionality ratio similar to those reported here have been observed previously in mice (Marks et al., 1993, 2004) or cell lines transfected with nAChRs (Peng et al., 1994, 1997; Gopalakrishnan et al., 1996; Fenster et al., 1999; Kuryatov et al., 2000; Avila et al., 2003). This decrease could result from functional inactivation of receptors after chronic nicotine exposure (Peng et al., 1994; Kuryatov et al., 2000; Gentry et al., 2003). However, it has also been reported that chronic nicotine treatment increases total nAChR function as a consequence of increased receptor expression in cell lines (Gopalakrishnan et al., 1996; Buisson and Bertrand, 2001), rat synaptosomes (Nguyen et al., 2004), and midbrain neurons (Nashmi et al., 2007). Differences in receptor composition, the functional response being measured, the specific cell type being investigated, or the species being examined no doubt contribute to the diversity of findings.
Summary.
In conclusion, we observed significant differences between hippocampus and diencephalon neurons in culture with respect to density, distribution, and nicotine-induced up-regulation of high-affinity [125I]epibatidine binding sites. The proportion of nAChR on the cell surface of cultures from both brain regions remained unchanged after chronic nicotine treatment, indicating that the number of receptors potentially available for function increased. However, this nicotine-induced increase in nAChR on the plasma membrane was not accompanied by an increase in function measured by agonist-stimulated increase in intracellular Ca2+, suggesting that the up-regulated receptors are on average less functional than receptors that have not been chronically exposed to nicotine. The similarity in nAChR expression and the response of the nAChR to chronic nicotine treatment between mouse brain in vivo and mouse neurons in culture indicate that primary neuronal cultures are an experimental model suitable for studying the mechanism of nAChR expression and function.
Acknowledgments
We thank Dr. Jerry A. Stitzel for generously providing cell culture facilities and equipment for the calcium influx experiments.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01 DA003194, P30 DA015663].
Part of this work was presented previously: Zambrano CA, Collins AC, and Marks MJ (2009) Detection of surface [125I]epibatidine binding in primary neuronal cultures from subcortex and hippocampus of mouse embryos and the modulation by nicotine, at the Annual Meeting of the Society for Neuroscience; 2009 Oct 17–21; Chicago, IL. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- nAChR
- nicotinic acetylcholine receptor
- ANOVA
- analysis of variance
- BrACh
- 2-(2-bromoacetyloxy)-N,N,N-trimethylethanaminium bromide
- DTT
- 1,4-dithio-dl-threitol
- DTNB
- 5,5′-dithio-bis(2-nitrobenzoic acid)
- HBSS
- Hanks' balanced salt solution
- KRH
- Krebs-Ringer-HEPES
- MCC
- methylcarbachol hydrochloride.
Authorship Contributions
Participated in research design: Zambrano and Marks.
Conducted experiments: Zambrano and Salamander.
Performed data analysis: Zambrano and Marks.
Wrote or contributed to the writing of the manuscript: Zambrano, Salamander, Collins, Grady, and Marks.
References
- Avila AM, Dávila-García MI, Ascarrunz VS, Xiao Y, Kellar KJ. (2003) Differential regulation of nicotinic acetylcholine receptors in PC12 cells by nicotine and nerve growth factor. Mol Pharmacol 64:974–986 [DOI] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ. (2011) An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol 82:828–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas RJ, Lippiello PM. (1995) Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther 275:987–994 [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. (1988) Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem 50:1243–1247 [DOI] [PubMed] [Google Scholar]
- Boksa P, Quirion R. (1987) [3H]N-methyl-carbamylcholine, a new radioligand specific for nicotinic acetylcholine receptors in brain. Eur J Pharmacol 139:323–333 [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. (1997) Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 282:7–13 [PubMed] [Google Scholar]
- Buisson B, Bertrand D. (2001) Chronic exposure to nicotine up-regulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci 21:1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Vallejo YF, Green WN, Bertrand D. (2000) The unusual nature of epibatidine responses at the α4β2 nicotinic acetylcholine receptor. Neuropharmacology 39:2561–2569 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Houghtling RA, Qasba SS, Kellar KJ. (1999) Nicotinic receptor binding sites in rat primary neuronal cells in culture: characterization and their regulation by chronic nicotine. Brain Res Mol Brain Res 66:14–23 [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Kellar KJ. (2003) Chronic nicotine administration does not increase nicotinic receptors labeled by [125I]epibatidine in adrenal gland, superior cervical ganglia, pineal or retina. J Neurochem 85:1237–1246 [DOI] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. (1999) Up-regulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci 19:4804–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Dávila-García MI, Ulrich YM, Kellar KJ. (1997) Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem 69:2216–2219 [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31–37 [PubMed] [Google Scholar]
- Free RB, McKay SB, Gottlieb PD, Boyd RT, McKay DB. (2005) Expression of native α3β4* neuronal nicotinic receptors: binding and functional studies investigating turnover of surface and intracellular receptor populations. Mol Pharmacol 67:2040–2048 [DOI] [PubMed] [Google Scholar]
- Gentry CL, Wilkins LH, Jr, Lukas RJ. (2003) Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human α4β2- and α4β4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther 304:206–216 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, Donnelly-Roberts D, Arneric SP, Sullivan JP. (1996) Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine α4β2 receptor. J Pharmacol Exp Ther 276:289–297 [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. (2012) Nicotine-induced up-regulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci 32:2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415 [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Olale FA, Choi C, Lindstrom J. (2000) Acetylcholine receptor extracellular domain determines sensitivity to nicotine-induced inactivation. Eur J Pharmacol 393:11–21 [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Sears SB, Fernandes KG. (1987) Kinetics and mechanism of l-[3H]nicotine binding to putative high affinity receptor sites in rat brain. Mol Pharmacol 31:392–400 [PubMed] [Google Scholar]
- Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ. (2011) Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J Neurochem 119:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, et al. (1999) International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 51:397–401 [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. (1983) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825 [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. (1993) Down-regulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther 266:1268–1276 [PubMed] [Google Scholar]
- Marks MJ, Laverty DS, Whiteaker P, Salminen O, Grady SR, McIntosh JM, Collins AC. (2010) John Daly's compound, epibatidine, facilitates identification of nicotinic receptor subtypes. J Mol Neurosci 40:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM. (2011) Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther 337:187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. (2004) Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology 46:1141–1157 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. (1998) Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther 285:377–386 [PubMed] [Google Scholar]
- Meyer EL, Xiao Y, Kellar KJ. (2001) Agonist regulation of rat α3β4 nicotinic acetylcholine receptors stably expressed in human embryonic kidney 293 cells. Mol Pharmacol 60:568–576 [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. (2003) Assembly of α4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced up-regulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci 23:11554–11567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, et al. (2007) Chronic nicotine cell specifically up-regulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci 27:8202–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. (2003) Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther 307:1090–1097 [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. (2004) Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem 90:40–49 [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. (1991) An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther 258:1127–1136 [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. (1997) Chronic nicotine treatment up-regulates α3 and α7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol 51:776–784 [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. (1994) Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 46:523–530 [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. (1999) Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 289:1545–1552 [PubMed] [Google Scholar]
- Sanderson EM, Drasdo AL, McCrea K, Wonnacott S. (1993) Up-regulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res 617:349–352 [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216 [DOI] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. (2009) Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin 30:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. (1999) Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57BL/6 mice. Psychopharmacology (Berl) 141:145–153 [DOI] [PubMed] [Google Scholar]
- Warpman U, Friberg L, Gillespie A, Hellström-Lindahl E, Zhang X, Nordberg A. (1998) Regulation of nicotinic receptor subtypes following chronic nicotinic agonist exposure in M10 and SH-SY5Y neuroblastoma cells. J Neurochem 70:2028–2037 [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. (2000) Identification of a novel nicotinic binding site in mouse brain using [125I]epibatidine. Br J Pharmacol 131:729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S. (1998) Agonist-induced up-regulation of α4β2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol 53:950–962 [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107 [DOI] [PubMed] [Google Scholar]
- Zarei MM, Radcliffe KA, Chen D, Patrick JW, Dani JA. (1999) Distributions of nicotinic acetylcholine receptor α7 and β2 subunits on cultured hippocampal neurons. Neuroscience 88:755–764 [DOI] [PubMed] [Google Scholar]
- Zoli M, Léna C, Picciotto MR, Changeux JP. (1998) Identification of four classes of brain nicotinic receptors using β2 mutant mice. J Neurosci 18:4461–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]