Abstract
Parasympathetic control of murine urinary bladder consists of contractile components mediated by both muscarinic and purinergic receptors. Using intracellular recording techniques, the purinergic component of transmission was measured as both evoked excitatory junctional potentials (EJPs) in response to electrical field stimulation and spontaneous events [spontaneous EJPs (sEJPs)]. EJPs, but not sEJPs, were abolished by the application of the Na+ channel blocker tetrodotoxin and the Ca2+ channel blocker Cd2+. Both EJPs and sEJPs were abolished by the application of the P2X1 antagonist 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt (NF279). Application of phorbol dibutyrate (PDBu) increased electrically evoked EJP amplitudes with no effect on mean sEJP amplitudes. Similar increases in EJP amplitudes were produced by PDBu in the presence of either the nonselective protein kinase inhibitor staurosporine or the specific protein kinase C (PKC) inhibitor 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X). These results suggest that PDBu increases the purinergic component of detrusor transmission through increasing neurogenic ATP release via a PKC-independent mechanism.
Introduction
The storage of urine within the urinary bladder relies on the contraction of internal and external sphincter muscles located in the neck of the urinary bladder in consort with relaxation of the detrusor muscle. Conversely, urinary bladder voiding is mediated by contraction of the detrusor muscle and relaxation of the sphincter muscles (Thompson, 2004). Neuronal control of detrusor muscle function is exerted by parasympathetic cholinergic neurons; these neurons are known to store and release both acetylcholine (ACh) and ATP together as cotransmitters from synaptic vesicles (Whittaker et al., 1972; Silinsky and Hubbard, 1973; Dowdall et al., 1974; Silinsky, 1975; Unsworth and Johnson, 1990). In most mammalian species parasympathetic neurotransmission to the detrusor smooth muscle consists of a purinergic P2X1 receptor component mediated by ATP (Vial and Evans, 2000) and a muscarinic receptor component mediated by ACh (Kennedy, 2001).
The relative contributions of muscarinic and purinergic components of neurotransmission in detrusor muscle are both species- and age-dependent. In the mouse, neurotransmission to the urinary bladder detrusor muscle consists of almost equal muscarinic and purinergic components. In contrast, in healthy young adult humans it is generally believed that neurotransmission in the detrusor muscle is mediated primarily by the muscarinic portion of transmission. However, the purinergic portion of transmission increases with both age and disease, for example, in patients suffering from detrusor overactivity (Sjögren et al., 1982; Kennedy, 2001; Yoshida et al., 2001).
Injection of botulinum toxin A (Botox) into the detrusor muscle has been found to be an effective treatment for detrusor overactivity, thus implicating the parasympathetic nerve endings as both a potential cause of overactive bladder and a target for therapeutic drug discovery. One major side effect of botulinum treatment for overactive bladder is that patients experience bladder voiding impairment (Brubaker et al., 2008; Shaban and Drake, 2008; Khan et al., 2009). From these observations it seems that understanding the mechanisms for the modulation of neurotransmission in bladder detrusor muscle may lead to therapies that could either offer advantages over Botox treatment or provide mitigation for the voiding impairment induced by Botox treatment through enhancing neurotransmitter release at that portion of nerve terminals unaffected by Botox.
We know of no previous studies that have exploited the temporal and quantal resolution that might be achieved through the application of electrophysiological techniques to the study of prejunctional modulation of nerve-evoked neurotransmission purinergic component of evoked transmitter release in murine detrusor muscle.
Phorbol esters are known to cause rapid increases in evoked neurotransmitter release at a wide range of loci, at both central and peripheral nerve endings either through protein kinase C (PKC)-dependent pathways (Wardell and Cunnane, 1994) or PKC-independent pathways, which are generally thought to be mediated by Munc13 (Betz et al., 1998; Searl and Silinsky, 1998; Rhee et al., 2002; Silinsky and Searl, 2003). Munc13 is a nerve terminal protein containing the C1 phorbol binding domain that through interaction with syntaxin, a critical member of the secretory apparatus, promotes transmitter release either by increases in the numbers of vesicles available for release (Searl and Silinsky, 2008; Chang et al., 2010) or effects on the probability of release (Basu et al., 2007). At a number of synapses both PKC-dependent and PKC-independent pathways have been identified as mechanisms by which phorbol esters promote neurotransmitter release (Wierda et al., 2007; Lou et al., 2008).
In addition to the PKC-dependent postjunctional effects of phorbol esters on bladder smooth muscle contraction (Wang et al., 2012), phorbol esters have been found to exert prejunctional effects. Thus, phorbol esters were found to increase [14C]ACh overflow in the rat urinary bladder through a M1 muscarinic receptor-dependent PKC pathway, with the effects of phorbol esters inhibited by both PKC inhibitors and atropine (Somogyi et al., 1997). In contrast to these findings, contraction studies in murine detrusor muscle have found that the application of phorbol esters selectively increases the purinergic component of neurotransmitter release through a PKC-dependent effect on P/Q-type Ca2+ channels (Liu and Lin-Shiau, 2000).
The principal aims of this study were 3-fold: 1) to test the feasibility and utility of applying electrophysiological techniques for the measurement of quantal neurotransmitter release in the murine detrusor muscle, 2) to determine the effects of the application of a phorbol ester on the electrophysiological correlate of purinergic transmission in the detrusor muscle, and 3) to determine whether the acute effects of phorbol dibutyrate on neurotransmitter release in the murine detrusor are mediated through PKC-dependent or PKC-independent mechanisms.
Materials and Methods
General.
The methods used for anesthesia and exsanguinations are in accordance with guidelines laid down by our institutional animal welfare committee and the National Institutes of Health (Institute of Laboratory Animal Resources, 1996). Specifically, mice (B6129F2J, 20–30 g in weight), were anesthetized with 5% isoflurane for 3 to 5 min, until unresponsive to touch, and this was followed by cervical dislocation and exsanguination. The urinary bladder was removed and placed in physiological solution containing 137 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 30 mM HEPES, 24 mM NaH2CO3, 1 mM NaH2PO4, and 11 mM dextrose, pH 7.2 to 7.4 gassed with O2, in a Sylgard (silicone elastomer; Dow Corning, Midland, MI)-lined chamber (volume, approximately 4 ml). The bladder was dissected along the ventral wall with a longitudinal incision, from the bladder neck (posterior) to the top of the dome (anterior), and the bladder was opened and pinned flat, interior surface uppermost. The urothelium and suburothelium were removed with careful dissection. The detrusor muscle was then repined, outside surface uppermost, and muscle cuts were made along the craniocaudal axis to isolate muscle contractions. Silver wire electrodes were placed on the surface of the muscle sheet. The chamber was mounted on the heated stage (Dagan, Minneapolis, MN) of an inverted microscope (Nikon, Tokyo, Japan) and maintained at 36 to 37°C by superfusion with warmed physiological saline solution at a constant flow rate (12 ml · min−1). Unless otherwise stated, experiments were performed in the combined presence of 10 to 15 μM nifedipine to block the postjunctional L-type Ca2+ channels, thus revealing the underlying excitatory junctional potential, 100 nM atropine to block muscarinic-mediated contractions of the muscle, and 300 μM hexamethonium to block ganglionic nicotinic receptors. Electrically evoked responses were elicited by using field stimulation of the preparation (0.5-ms duration at a frequency of 0.1 Hz), with the bath electrode located to minimize the stimulus artifact. Intracellular recordings were made from the smooth muscle cells with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 60–100 MΩ) with a high input impedance amplifier (Axoclamp-2A; Molecular Devices, Sunnyvale, CA). Signals were filtered (5-kHz low pass), amplified, and digitized at 1 kHz (Digidata 1200 A/D converter; Molecular Devices). Signals were recorded directly to the computer by using winEDR software and analyzed by using winEDR and winWCP programs (Professor J. Dempster, Strathclyde University, Glasgow, UK). Data were aggregated by using Microsoft (Redmond, WA) Excel, Corel (Mountain View, CA) Quattro Pro, and Sigma Plot and Sigma Stat software packages (SPSS Inc., Chicago, IL). All drugs were from Sigma-Aldrich (St. Louis, MO) except 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt (NF279) was from Tocris Bioscience (Ellisville, MO), and tetrodotoxin (TTX) was a gift from Dr. W Marszalec (Dagan, Minneapolis, MN).
Because of the nonlinear summation of EJP, increases in EJP amplitude are likely to be underestimated. We therefore also provide estimates of EJP and sEJP amplitudes corrected according to the equation of Stevens (1976), corrected to a membrane potential of 50 mV; namely, v′ = 50.In [E/(E − v)], where E is the resting membrane potential; v is the measured (s)EJP amplitude; and v′ is the corrected (s)EJP amplitude, assuming a reversal potential for the EJP conductance of O mV (Evans et al., 1996). The accuracy of this correction depends on the capacitance and resistance properties of the detrusor smooth muscle electrical syncytium.
Statistical Methods.
Comparisons were made by either parametric [Student's paired t test or analysis of variance (ANOVA)] or nonparametric (Wilcoxon signed rank test) statistics (Glantz, 1992). For the purpose of discussion of the results, differences between groups were considered significant when p < 0.05. Unless otherwise stated, n represents the number of single experiments carried out at single intracellular impalements on individual preparations. Data are presented as means ± 1 S.E.M.
Results
General Observations.
In the absence of any blocking drugs, detrusor muscle preparations contracted both spontaneously and in response to electrical field stimulation of the tissue. Both spontaneous and evoked action potentials could be recorded from detrusor muscle preparations, but these events were usually accompanied by contractions and loss of the intracellular muscle impalement. Coapplication of the L-type Ca2+ channel blocker nifedipine (10–15 μM) and the muscarinic antagonist atropine (100 nM) resulted in a rapid abolition of spontaneous contractile activity and a slower abolition of electrically evoked contractions in all preparations. In the combined presence of the ganglionic blocker hexamethonium, atropine, and nifedipine, membrane potentials were quiescent.
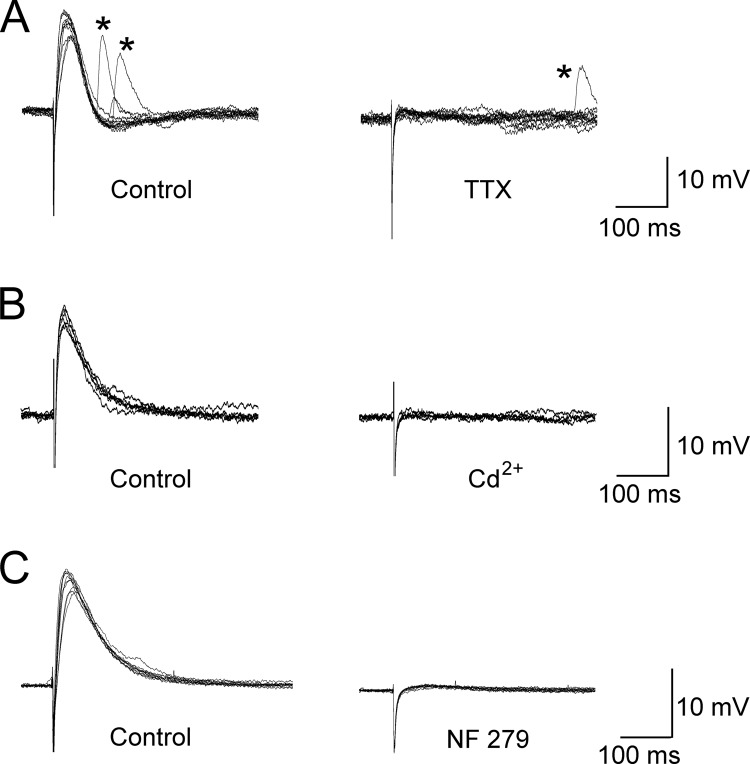
Under conditions in which all cholinergic activity and muscle contractions were blocked, intracellular membrane potentials ranged from −32 to −58 mV with an average potential of −42.7 ± 1.7 mV (n = 41). Electrically evoked EJPs could be readily recorded in response to field stimulation of the preparation (Fig. 1) as well as sEJPs (Fig. 1A). The neurogenic origin of these electrically evoked EJPs was tested with the Na+ channel blocker TTX. As shown in Fig. 1A, application of 2 μM TTX resulted in a rapid abolition of the evoked response (n = 6), whereas sEJPs persisted in the presence of TTX. The effect of TTX on EJPs was reversible on wash. As shown in Fig. 1B, application of the Ca2+ channel blocker Cd2+ (200 μM) resulted in rapid abolition of the electrically evoked response (n = 5). The purinergic nature of the EJPs was tested by the applications of the P2X1 competitive antagonist NF279 (10–20 μM) and the desensitizing P2X agonist α,β-methylene ATP. Application of NF279 caused a gradual reduction and eventual abolition of both EJPs and sEJPs (n = 6; see Fig. 1C) with no effect on membrane potentials. In contrast, application of α,β-methylene ATP caused an initial immediate depolarization (10–15 mV) followed by a slow-developing (5–15 min) repolarization that was accompanied by an abolition of both evoked EJPs and sEJPs (n = 5; data not shown). Taken in combination, these results confirm both the purinergic and neurogenic origin of the evoked EJPs recorded from the detrusor muscle preparation and further suggest that these responses may be measured with a quantal-level resolution with these techniques.
Fig. 1.
Properties of electrically evoked EJPs recorded from mouse detrusor muscle. A, the effect of 2 μM TTX on electrically evoked responses recorded from detrusor muscle. sEJPs (indicated by *) were still present after abolition of the evoked response by TTX. B, the effect of the inorganic voltage-dependent Ca2+ channel blocker Cd2+ (200 μM) on electrically evoked responses. Cd2+ produced a rapid abolition of the evoked response, indicating the calcium dependence of the evoked EJP. C, the effect of the P2X1 receptor antagonist NF279 (15 μM) on electrically evoked responses. Application of NF279 abolishes the evoked response, indicating the purinergic nature of these responses.
The Effects of Phorbol Dibutyrate on EJPs Recorded from the Murine Detrusor Muscle.
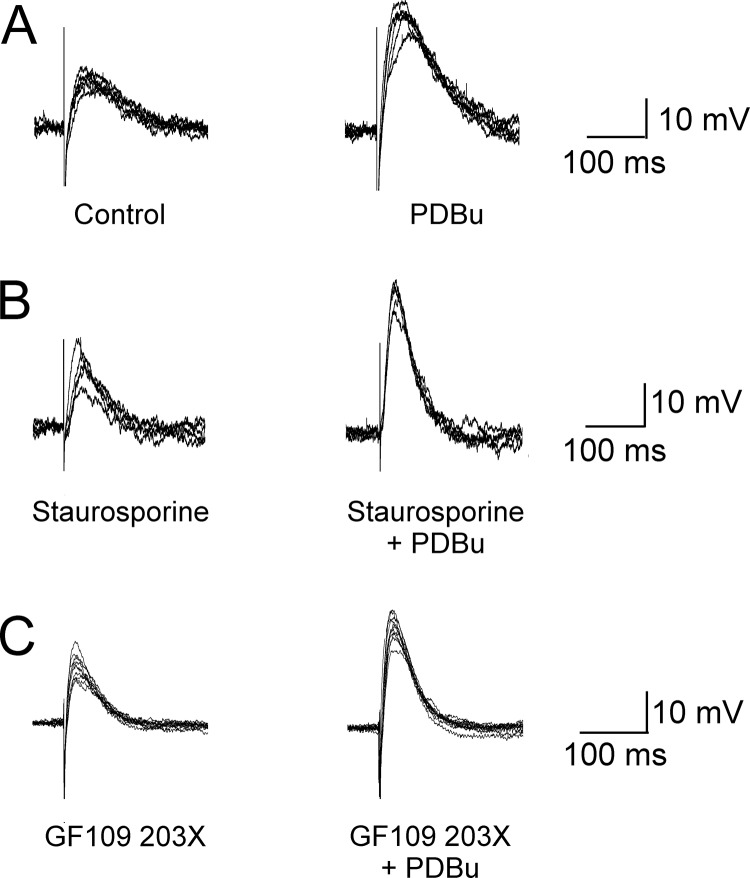
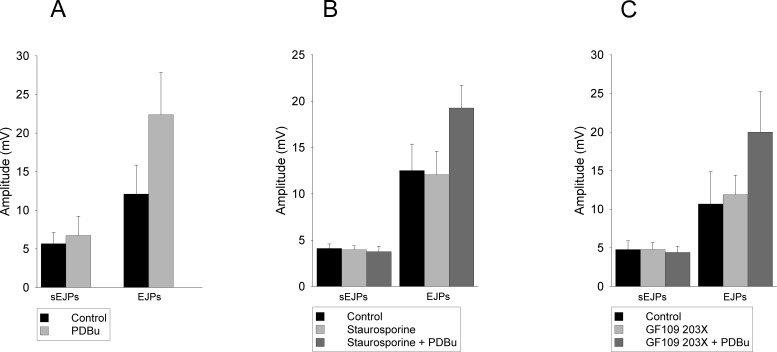
Application of 100 nM phorbol dibutyrate caused a rapid (3- to 8-min onset) augmentation of the electrically evoked EJPs (Figs. 2A and 3A). The mean EJP amplitude was 12.1 ± 3.7 mV in control versus 22.4 ± 5.5 mV in PDBu (p = 0.018; n = 6; Student's paired t test). In addition, as shown in Fig. 3A, the amplitudes of sEJPs were unaffected. Specifically, the mean sEJP amplitude was 5.7 ± 1.5 mV in control versus 6.7 ± 2.5 mV in PDBu. (After application of Stevens' correction for nonlinear summation, EJP amplitudes were 15.5 ± 5.8 mV in control versus 35.3 ± 11.7 mV in PDBu; sEJP amplitudes were 6.11 ± 1.7 mV in control versus 7.47 ± 3.0 in PDBu.)
Fig. 2.
The effect of PKC inhibitors on PDBu-mediated increases in electrically evoked EJP amplitudes. A, superimposed evoked responses before and after the application of 100 nM PDBu on evoked EJPs. B, the effects of 100 nM PDBu on evoked EJPs in the presence of 1 μM staurosporine. C, the effects of 100 nM PDBu on evoked EJPs in the presence of 10 μM GF109203X. As shown in B and C, the PDBu-evoked increase of EJP amplitude was also accompanied by an increased rate of repolarization of the membrane potential. It seems likely that this increased rate of repolarization results from the activation of K+ channels and may serve to limit the measured increase in EJP amplitude in these experiments. In support of this suggestion, it has been reported that both BK and SK K+Ca channels act to limit bladder smooth muscle responses to nerve stimulation in mouse (Herrera et al., 2005; Werner et al., 2007) and human urinary bladder in vitro (Darblade et al., 2006).
Fig. 3.
The effects of PDBu and PKC inhibitors on EJP and sEJP amplitudes. A, after application of PDBu EJP amplitudes were increased to 197 ± 16% of control, and sEJP amplitudes were 110% ± 10% of control (n = 6 experiments). B, application of PDBu in the presence of staurosporine resulted in an increase in EJP amplitude to 177 ± 16% of the amplitude measured in staurosporine alone. The sEJP amplitudes were 102 ± 7.5% of the amplitudes measured in staurosporine alone (n = 6 experiments). C, after application of PDBu in the presence of GF109203X EJP amplitudes were increased to 170 ± 14% of the amplitudes measured in GF109203X alone, and sEJP amplitudes were 101 ± 11% of the amplitudes measured in GF109203X alone (n = 5 experiments).
Because both PKC-dependent and PKC-independent pathways have been implicated in the effects of phorbol esters on evoked neurotransmitter output, we investigated the abilities of two PKC inhibitors to inhibit the PDBu-mediated increase in ATP release. For this study, we chose to use both staurosporine, a nonselective kinase inhibitor, and the more selective PKC inhibitor 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X) (10 μM), both of which act on the C3 domain (ATP binding site) of PKC (Newton, 1995). As shown in Fig. 3, B and C, these agents had no detectable effect on the evoked response themselves or the increases in evoked response resulting from the application of 100 nM phorbol dibutyrate (Figs. 2, B and C and 3, B and C). Mean EJP amplitudes were 12.5 ± 3.8 mV in control versus 12.1± 2.5 mV in staurosporine versus 19.3 ± 2.5 mV in staurosporine + PDBu (p = 0.031, Student's paired t test; p < 0.05, Dunnet's method, repeated-measures ANOVA; n = 6). Mean sEJP amplitudes were 4.3 ± 0.5 mV in control versus 4.9 ± 0.4 mV in staurosporine versus 5.0 ± 0.6 mV in staurosporine + PDBu. (Applying Stevens' correction for nonlinear summation, EJP amplitudes were 16.3 ± 4.1 mV in control, 14.6 ± 2.9 mV in staurosporine, and 26.2 ± 2.9 mV in staurosporine and PDBu. sEJP amplitudes were 5.2 ± 0.1 mV in control, 5.5 ± 0.5 mV in staurosporine, and 5.5 ± 0.25 mV in staurosporine and PDBu.) For GF109203X experiments mean EJP amplitudes were 10.7 ± 4.2 mV in control versus 11.9 ± 3.4 mV in GF109203X versus 20.0 ± 5.3 mV in GF109203X + PDBu (p = 0.036; n = 5; p < 0.05, determined by Dunnet's method, repeated-measures ANOVA). Mean sEJP amplitudes were 4.8 ± 1.2 mV in control versus 4.9 ± 0.9 mV in GF109203X versus 4.4 ± 0.8 mV in GF109203X + PDBu (n = 5). (Applying Stevens' correction for nonlinear summation, EJP amplitudes were 14.8 ± 6.8 mV in control, 16.6 ± 5.8 mV in GF109203X, and 37.3 ± 13.8 mV in GF109203X and PDBu. sEJP amplitudes were 5.4 ± 1.5 mV in control, 5.4 ± 1.3 mV in GF109203X, and 5.0 ± 1.2 mV in GF109203X and PDBu.) Comparing EJP amplitudes recorded in PDBu alone with those in staurosporine + PDBu and GF109203X + PDBu there was no significant difference in ANOVA: PDBu alone versus staurosporine + PDBu, p = 0.39; Wilcoxon signed rank test; PDBu alone versus EJP amplitudes recorded in GF109203X + PDBu, p = 0.5; Wilcoxon signed rank test.
Discussion
Historically, the first recordings of purinergic EJPs were made by Burnstock and Holman (1960) from guinea-pig vas deferens. It was later found that responses to locally applied ATP both mimicked the nerve-evoked EJP, and both the EJP and responses to ATP were abolished by α,β-methylene ATP in this preparation (Sneddon and Burnstock, 1984). Recordings of electrically evoked EJPs from smooth muscle cells of the rabbit urinary bladder detrusor muscle (using both microelectrode and sucrose gap methods) were first published by Creed et al., (1983). Later work demonstrated that the atropine-resistant EJPs recorded in the bladders of rabbits, guinea-pigs, and pigs were inhibited by desensitization of the ATP receptor with α,β-methylene ATP (Hoyle and Burnstock, 1985; Hashitani and Suzuki, 1995; Fujii, 1988; Brading and Mostwin, 1989; Bramich and Brading, 1996). In other studies, high concentrations of the purinergic antagonist suramin were found to reduce the amplitude of EJPs recorded by the sucrose gap method in rabbit and guinea-pig bladder (Creed et al., 1994). The purinergic component of detrusor muscle neurotransmission was further supported by experiments with whole-cell patch-clamped smooth muscle cells isolated from guinea-pig bladder in which concentration jump applications of ATP closely mimicked the time course of response predicted from recordings of the EJP (Inoue and Brading, 1990).
Further detailed analysis of intracellularly recorded EJPs recorded in the guinea-pig bladder (Bramich and Brading, 1996; Hashitani et al., 2000) found that electrically evoked EJPs varied greatly in amplitude and time course, even when recorded from cells at similar distances from the stimulating electrodes. As the stimulation strength was reduced, the amplitude of EJPs was decreased in two or three discreet steps, suggesting that these EJPs are composed of more than one conductance and the variation in EJP amplitude may be related to the low degree of coupling between smooth muscle cells in and between muscle bundles. Meng et al. (2008) and Young et al. (2008) found that in murine detrusor muscle sEJPs led to the production of spontaneous L-type action potentials.
As reported here, in addition to the sEJPs previously recorded from the murine detrusor muscle, electrical field stimulation of the detrusor muscle in the presence of nifedipine results in the production of EJPs. These electrically evoked responses (but not the sEJPs) are reversibly blocked by TTX, indicating the dependence of the evoked EJPs on neuronal Na+ channels. In addition, the evoked EJPs (but not the sEJPs) are abolished by Cd2+, indicating the dependence of the evoked events on Ca2+ channels. Finally, both the evoked EJPs and sEJPs are reversibly blocked by the P2X1 antagonist NF279, indicating the dependence of these events on P2X1 receptor gated channels.
Initial reports on the effects of phorbol esters on neurotransmitter release often assumed that these effects were mediated through actions on protein kinase C. However, the discovery of homologous C1 binding domains in proteins other than PKC, such as Munc13, led to a reevaluation of the mechanisms underlying the modulatory effects of phorbols on neurotransmitter release at a variety of synapses (Silinsky and Searl, 2003). The relative magnitude of the putative roles of the PKC- and non-PKC-dependent actions of phorbol esters in enhancing neurotransmitter release seems to differ from synapse to synapse. Thus at the frog neuromuscular junction PKC inhibitors failed to demonstrate any role of PKC in the acute affects of phorbol esters (Searl and Silinsky, 1998). In the experiments presented here, application of the phorbol ester PDBu resulted in a significant increase in mean EJP amplitudes with no measurable effect on the amplitudes of sEJPs, thus indicating that the enhancement of the evoked response by PDBu is mediated through increases in the number of quanta released in response to nerve stimulation. Preapplication of either 1 μM staurosporine (which has been found to produce complete inhibition of PKC activity in isolated neuronal preparations at a concentration of 200 nM; Considine et al., 1992) or 10 μM GF109203X, (which has a reported IC50 of 10 nM; Gordge and Ryves, 1994) failed to have any effect on the increases in transmitter release in response to the acute application of phorbol esters. This suggests that in the detrusor muscle enhancement of the purinergic component of neurotransmission by phorbol esters is mediated by a non-PKC-dependent process, i.e., Munc-13.
Previously, on the basis of radioactively labeled acetylcholine overflow experiments, the enhancement of detrusor neurotransmission by phorbol esters was attributed to the prejunctional actions of PKC (Somogyi et al., 1997). There are several notable points of difference between our study and those previous reports that might in part account for the difference. First, the effects previously reported pertained to the cholinergic component of neurotransmission that may be under different prejunctional control than the purinergic component of transmission in detrusor muscle (e.g., Waterman, 1996 but see Lawrence et al., 2010). In addition, the PKC-dependent effect found previously was mediated through an action on L-type Ca2+ channels. One shortcoming of our approach is the necessary presence of the L-type Ca2+ channel blocker nifedipine throughout, which may mask any prejunctional effect that is exerted through the enhancement of L-type Ca2+ channel function. In addition, the previous report used both lower concentrations of phorbol ester applied over a much longer time course than we were regularly able to achieve with electrophysiological techniques. Nevertheless, despite these limitations, our results demonstrate a clear prejunctional enhancement of electrically evoked neuromuscular transmission by a phorbol ester that is mediated through a PKC-independent pathway.
In conclusion, the application of electrophysiological recording techniques to the detrusor muscle of the mouse allows the study of neuromodulation with a degree of resolution not easily attainable with other techniques. In addition, application of the phorbol ester PDBu results in a PKC-independent increase in neurotransmitter release as determined by the amplitudes of electrically evoked responses. It is likely that the effects of PDBu are mediated through the activation of Munc13, a presynaptic protein that contains a phorbol ester binding domain and is thought to enhance release through the promotion of synaptic vesicle priming (Betz et al., 1998; Rhee et al., 2002; Silinsky and Searl, 2003).
Acknowledgments
We thank Dr. Jeffrey Glassroth for his support and encouragement during his all too brief time as Interim Dean at the Northwestern University Feinberg School of Medicine; Dr. Kevin McVary (Department of Urology, Northwestern University Feinberg School of Medicine for his collegiality, valuable insight, and general encouragement of our endeavors; and Dr. W. Marszalec (Department of Molecular Pharmacology and Biological Chemistry, Northwestern University Feinberg School of Medicine) for the PS-12 Dagan Corporation power supply.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA016513]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS12782]; and Northwestern Memorial Hospital.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- ACh
- acetylcholine
- PDBu
- phorbol dibutyrate
- EJP
- excitatory junctional potential
- sEJP
- spontaneous EJP
- TTX
- tetrodotoxin
- PKC
- protein kinase C
- Botox
- botulinum toxin A
- ANOVA
- analysis of variance
- NF279
- 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt
- GF109203X
- 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide.
Authorship Contributions
Participated in research design: Searl and Silinsky.
Conducted experiments: Searl.
Performed data analysis: Searl.
Wrote or contributed to the writing of the manuscript: Searl and Silinsky.
References
- Basu J, Betz A, Brose N, Rosenmund C. (2007) Munc13–1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J Neurosci 27:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. (1998) Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21:123–136 [DOI] [PubMed] [Google Scholar]
- Brading AF, Mostwin JL. (1989) Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br J Pharmacol 98:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramich NJ, Brading AF. (1996) Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol 492:185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker L, Richter HE, Visco A, Mahajan S, Nygaard I, Braun TM, Barber MD, Menefee S, Schaffer J, Weber AM, et al. (2008) Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 180:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Holman ME. (1960) Autonomic nerve-smooth muscle transmission. Nature 187:951–952 [DOI] [PubMed] [Google Scholar]
- Chang CY, Jiang X, Moulder KL, Mennerick S. (2010) Rapid activation of dormant presynaptic terminals by phorbol esters. J Neurosci 30:10048–10060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sherwin JR, Simpson LL. (1992) Role of protein kinase C in short-term transmission at the mammalian neuromuscular junction. J Pharmacol Exp Ther 263:1269–1274 [PubMed] [Google Scholar]
- Creed KE, Callahan SM, Ito Y. (1994) Excitatory neurotransmission in the mammalian bladder and the effects of suramin. Br J Urol 74:736–743 [DOI] [PubMed] [Google Scholar]
- Creed KE, Ishikawa S, Ito Y. (1983) Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338:149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. (2006) Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68:442–448 [DOI] [PubMed] [Google Scholar]
- Dowdall MJ, Boyne AF, Whittaker VP. (1974) Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J 140:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. (1996) Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol 497:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. (1988) Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol 404:39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SA. (1992) Primer of Biostatistics, McGraw Hill Inc., New York [Google Scholar]
- Gordge PC, Ryves WJ. (1994) Inhibitors of protein kinase C. Cell Signal 6:871–882 [DOI] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GD. (2000) Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524:565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. (1995) Electrical and mechanical responses produced by nerve stimulation in detrusor smooth muscle of the guinea-pig. Eur J Pharmacol 284:177–183 [DOI] [PubMed] [Google Scholar]
- Herrera GM, Etherton B, Nausch B, Nelson MT. (2005) Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289:R402–R409 [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Burnstock G. (1985) Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene-ATP. Eur J Pharmacol 114:239–240 [DOI] [PubMed] [Google Scholar]
- Inoue R, Brading AF. (1990) The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol 100:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kennedy C. (2001) The role of purines in the peripheral nervous system, in Purinergic and Pyrimidinergic Signalling 1 (Abbracchio M, Williams M. eds) pp 296–297, Springer, New York [Google Scholar]
- Khan S, Kessler TM, Apostolidis A, Kalsi V, Panicker J, Roosen A, Gonzales G, Haslam C, Elneil S, Fowler CJ, et al. (2009) What a patient with refractory idiopathic detrusor overactivity should know about botulinum neurotoxin type A injection. J Urol 181:1773–1778 [DOI] [PubMed] [Google Scholar]
- Lawrence GW, Aoki KR, Dolly JO. (2010) Excitatory cholinergic and purinergic signaling in bladder are equally susceptible to botulinum neurotoxin A consistent with co-release of transmitters from efferent fibers. J Pharmacol Exp Ther 334:1080–1086 [DOI] [PubMed] [Google Scholar]
- Liu SH, Lin-Shiau SY. (2000) Protein kinase C regulates purinergic component of neurogenic contractions in mouse bladder. J Urol 164:1764–1767 [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. (2008) Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci 28:8257–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng E, Young JS, Brading AF. (2008) Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27:79–87 [DOI] [PubMed] [Google Scholar]
- Newton AC. (1995) Protein kinase C: structure, function, and regulation. J Biol Chem 270:28495–28498 [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Südhof TC, Takahashi M, Rosenmund C, et al. (2002) β-Phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108:121–133 [DOI] [PubMed] [Google Scholar]
- Searl TJ, Silinsky EM. (1998) Increases in acetylcholine release produced by phorbol esters are not mediated by protein kinase C at motor nerve endings. J Pharmacol Exp Ther 285:247–251 [PubMed] [Google Scholar]
- Searl TJ, Silinsky EM. (2008) Mechanisms of neuromodulation as dissected using Sr2+ at motor nerve endings. J Neurophysiol 99:2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban AM, Drake MJ. (2008) Botulinum toxin treatment for overactive bladder: risk of urinary retention. Curr Urol Rep 9:445–451 [DOI] [PubMed] [Google Scholar]
- Silinsky EM. (1975) On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol 247:145–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Hubbard JI. (1973) Release of ATP from rat motor nerve terminals. Nature 243:404–405 [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Searl TJ. (2003) Phorbol esters and neurotransmitter release: more than just protein kinase C? Br J Pharmacol 138:1191–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren C, Andersson KE, Husted S, Mattiasson A, Moller-Madsen B. (1982) Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol 128:1368–1371 [DOI] [PubMed] [Google Scholar]
- Sneddon P, Burnstock G. (1984) Inhibition of excitatory junction potentials in guinea-pig vas deferens by α,β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol 100:85–90 [DOI] [PubMed] [Google Scholar]
- Somogyi GT, Zernova GV, Tanowitz M, de Groat WC. (1997) Role of L- and N-type Ca2+ channels in muscarinic receptor-mediated facilitation of ACh and noradrenaline release in the rat urinary bladder. J Physiol 499:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. (1976) A comment on Martin's relation. Biophys J 16:891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. (2004). Geriatric urologic disorders, in Applied Therapeutics: The Clinical Use of Drugs (Koda-Kimble MA. ed), 8th ed, pp 101–124, Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- Unsworth CD, Johnson RG. (1990) Acetylcholine and ATP are coreleased from the electromotor nerve terminals of Narcine brasiliensis by an exocytotic mechanism. Proc Natl Acad Sci U S A 87:553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Evans RJ. (2000) P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Kendig DM, Trappanese DM, Smolock EM, Moreland RS. (2012) Phorbol 12,13-dibutyrate-induced, protein kinase C-mediated contraction of rabbit bladder smooth muscle. Front Pharmacol 2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell CF, Cunnane TC. (1994) Biochemical machinery involved in the release of ATP from sympathetic nerve terminals. Br J Pharmacol 111:975–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA. (1996) Multiple subtypes of voltage-gated calcium channel mediate transmitter release from parasympathetic neurons in the mouse bladder. J Neurosci 16:4155–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. (2007) Frequency encoding of cholinergic and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292:R616–R624 [DOI] [PubMed] [Google Scholar]
- Whittaker VP, Essman WB, Dowe GH. (1972) The isolation of pure cholinergic synaptic vesicles from the electric organs of elasmobranch fish of the family Torpedinidae. Biochem J 128:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. (2007) Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron 54:275–290 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, Murakami S, Kawabe K, Ueda S. (2001) Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol 36:99–109 [DOI] [PubMed] [Google Scholar]
- Young JS, Meng E, Cunnane TC, Brain KL. (2008) Spontaneous purinergic neurotransmission in the mouse urinary bladder. J Physiol 586:5743–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]