Abstract
The literature on post-infectious irritable bowel syndrome (IBS) is reviewed with special emphasis on recent new data. Further accounts of this phenomenon continue to be reported following a range of infections including giardiasis as well as viral and bacterial gastroenteritis. Risk factors such as severity of initial illness, female gender together with adverse psychological factors have been confirmed. Recent evidence of a genetic predisposition needs replication. Animal studies suggest activation of mast cells and inflammation driven impairment of serotonin transporter may be important, which are findings supported by some recent human studies in IBS with diarrhoea. Experimentally induced inflammation leads to damage and remodelling of enteric nerves. Similar changes have been reported in IBS patients with increase in nerves expressing transient receptor potential cation channel V1. While changes in microbiota are very likely this area has yet to be explored using modern techniques. Since the prognosis is for slow improvement, treatments should currently target the key symptoms of diarrhoea and abdominal pain. Future therapies aimed at correcting underlying mechanisms including immune activation and serotonin excess are currently being explored and may provide better treatments in the future.
Keywords: Genetics, Infection, Irritable bowel syndrome, Serotonin
Introduction
Approximately 6%-17% of patients with irritable bowel syndrome (IBS) believe their symptoms began following a bout of gastroenteritis.1 One of the first accounts of this phenomenon was by Chaudhary and Truelove2 who described a series of patients with the IBS and its association with psychological problems. A subgroup who had developed IBS following a bout of gastroenteritis had a more a favourable prognosis than those without an infectious precipitant.2 More recent studies have used a more rigorous definition of post-infectious IBS (PI-IBS), namely new IBS developing after an episode of acute infectious gastroenteritis3 which is characterised by an acute illness with ≥ 2 of the following clinical features: fever, vomiting, diarrhea and a positive stool culture.4 Although a positive stool culture is desirable this is often not available, as many cases occur while travelling when the patient may not have ready access to a doctor. One of the commonest causes of PI-IBS in the United Kingdom (UK) is Camplylobacter jejuni enteritis. A survey of 840 cases of C. jejuni enteritis reported 103 cases who had developed PI-IBS meeting Rome I criteria, 63% of whom were classified as diarrhea predominant.3 The original description by Chaudhary and Truelove2 suggested that PI-IBS had less psychiatric illness than IBS patients with no infectious precipitant,2 a feature confirmed in a series of outpatients with IBS.4
Epidemiology
The incidence of infective gastroenteritis in the UK is 19/1,000/year resulting in a high burden of consultation to primary care. However less than 1% of episodes of gastrointestinal infections in the community are reported to national surveillance systems so national statistics grossly underestimate the true incidence.5 A more recent large community survey in the UK involving over 6,800 participants showed that the overall rate of infective diarrhea was 274 cases/1,000 persons/year. Viral gastroenteritis was the commonest cause, norovirus being the most frequent organism isolated, with an incidence of 47/1,000 persons/year. The commonest bacteria was Campylobacter spp. with incidence rate of 11/1,000 persons/year which gave an estimate of > 500,000 cases in the UK in 2009.6 Other common bacterial intestinal infections were Salmonella spp. and Escherichia coli. It is worth noting that given the marked underreporting of infectious gastroenteritis the true incidence of PI-IBS may well be greater than is currently believed.
Epidemiological studies suggest that enteric infection is one of the most important risk factors for developing IBS, equal to anxiety and greater than depression, sleep disorders, smoking, body mass index and alcohol excess.7 The proportion of patients developing IBS following intestinal infections varies in different series from as low as 3.7%8 to as high as 36%.9 The highest figure was reported from the Walkerton outbreak, when the municipal water supply was simultaneously contaminated by both C. jejuni and E. coli O147. The commonest bacteria causing PI-IBS in the UK are C. jejuni, Salmonella enteritidis and Shigella flexneri. The IBS symptoms which develop are often regarded by the patient as a continuation of the initial illness, most meeting the Rome criteria for IBS with diarrhea (IBS-D) or IBS with mixed bowel habit.10-12 The similarities between PI-IBS and these other subtypes of IBS suggest that studying PI-IBS patients may provide insight into the pathophysiology of all IBS. Thus PI-IBS has been studied by many authors,9-15 possibly because the clearly defined onset makes it easier to define what is the cause and what the effect, something which could often be difficult in other subtypes of IBS.
Risk Factors
Genetic
IBS shows a familial tendency and an early study16 on monozygotic and dizygotic twins in Australia reported a heritability of 57% but later studies have suggested a weaker effect.17,18 Thus although the large twin study in Virginia18 showed concordance for IBS was greater in monozygotic twins (17%) than dizygotic twins (8%), it also showed the importance of social conditioning, since having a mother with IBS was a stronger predictor than having a dizygotic twin with IBS. Studies of single nucleotide polymorphisms (SNPs) also support a genetic influence. A greater proportion of IBS patients compared to control (46% vs 26%) are heterozygous for the -308 (G/A) SNP which a high producer of TNF-α.19 Possession of both the high producer TNF-α SNP-308 A and low producer of IL-10-1082 A allele was also more prevalent in IBS patients (9%) versus controls (3%). More recently the G allele of SNP rs4263839 in the TNFSF15 gene, which has been associated with Crohn's disease, has been demonstrated to increase the risk of IBS in a cohort of 1992 IBS patients from both Sweden and the USA (OR, 1.37).20 A closely related SNP in the TNFSF15 gene has also been shown to increase the risk of IBS-D but not IBS with constipation (IBS-C).21 The same study also found an increased prevalence of the TNF-α SNP rs1800629 genotype GA compared with GG in PI-IBS, confirming findings in an earlier study of PI-IBS patients.19 Further SNPs associated with PI-IBS have recently been reported from the Walkerton outbreak in 200722 which identified 3 gene regions where SNPs increased the risk of PI-IBS, namely Cadherin 1, IL-6 and Toll-like receptor 9. Cadherin 1, coding for E-cadherin, is a tight junction glycoprotein which influences intestinal barrier function.23 Toll-like receptor-9 is the receptor for unmethylated CpG dinucleotides which are a marker of bacterial DNA and represent the pathway whereby bacteria activate innate immunity, while IL-6 is an inflammatory cytokine activated by infection. One limitation of this study is that the significance of these associations did not withstand corrections for multiple testing possibly owing to small sample size (228 PI-IBS and 581 controls) so these associations need to be reproduced in a separate cohort.
Physical and Psychosocial
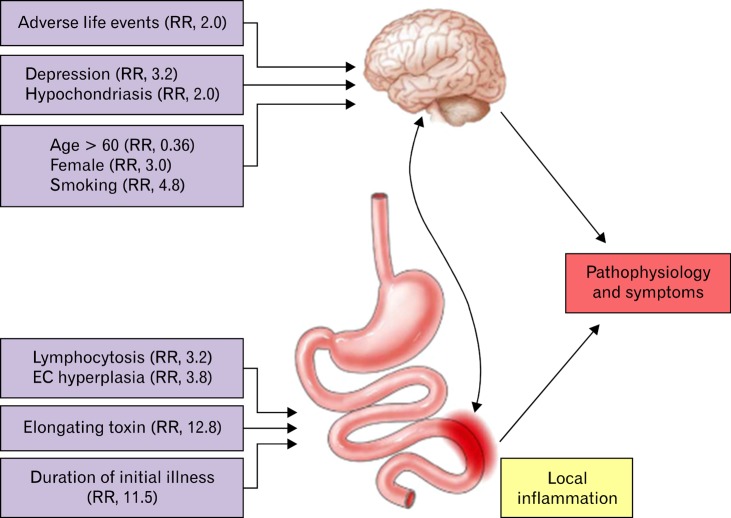
Both central psychological factors and local gut injury influence the risk of developing PI-IBS (Figure).24 Several studies confirm that high stress and anxiety levels,25 hypochondriasis (relative risk [RR], 2.0), adverse life events in the preceding 3 months (RR, 2.0)14 and depression (RR, 3.2),26 all increase the risk of developing PI-IBS. Mucosal factors are also significant and each 1 standard deviation rise in T lymphocyte and enterochromaffin (EC) cell numbers increases the risk of PI-IBS by 3.2- and 3.8-fold, respectively. Other host factors include age and smoking. Being older than 60 years protects against PI-IBS (RR, 0.36), possibly because of declining immune response with ageing, while smoking increases the risk 4.8-fold, through an as yet unclear mechanism.24 The underlying mechanisms whereby these psychological factors increase the risk of PI-IBS are unclear. Animal studies show corticotrophin releasing factor (CRF) is an important mediator of the stress response, acting via the hypothalamic-pituitary-adrenal axis in animal models of PI-IBS.27,28 CRF acting via CRF1 receptors mediates the stimulation of colonic motility by various stressors29,30 and CRF and stress enhance abdominal pain via the central CRF pathway and activation of mast cells in rats.31 CRF may also be proinflamatory, stimulating lymphocyte proliferation by increasing IL-2 receptor expression and enhancing production of both IL-1 and IL-232 as well as enhancing cytokine release (TNF-α, IL-1 and IL-6) in response to endotoxin.33 Stress also aggravates chemically induced colitis.34 Thus by enhancing the local inflammatory response to infection, stress may increase the risk of PI-IBS. This has been examined in more detail recently using an animal model of PI-IBS. Rats infected with Citrobacter rodentium who were subsequently exposed to chronic water avoidance stress showed increased level of corticosterone and epinephrine levels and an increase peripheral nociceptive signalling from rectal distension, assessed both by recordings from afferent nerves, as well as visceromotor responses in intact animals. They also found increased tissue proteases in homogenates of the colon of C. rodentium-infected mice, most likely from activated mast cells, which induced hyperexcitability in colonic dorsal root ganglia cells.35 Human studies have also shown activation of mast cells induced by immersion of hand into cold water which causes pain and sympathetic activation with raised pulse and blood pressure. This lead to the release of mast cell products such as histamine and tryptase into the lumen of the small bowel36 providing a possible mechanism for how stress could increase human small bowel secretion and motility, leading to the accelerated transit which is a characteristic of IBS-D. The same group have also shown evidence of increased numbers of mast cells in anxious IBS-D patients associated with increased release of tryptase from jejunal biopsies, a finding recently replicated by Foley et al.37
Figure.
Host and bacterial factors influencing risk of developing post infectious irritable bowel syndrome. Reproduced from Spiller and Garsed24 with kind permission of the editor of Gastroenterology. RR, relative risk; EC, enterochromaffin.
Bacterial Factors
Bacterial toxins play important part in development of intestinal inflammation during infection. C. jejuni infection is one of the commonest causes of infectious gastroenteritis, leading to PI-IBS in 9%-13% of cases.26,38 Patients infected with a strain that secret toxin which causes elongation of human epithelial (HEp-2) cells have an increased risk of developing a long term change of bowel habit with a RR of 12.8 (Figure).38 Other bacterial pathogens that cause marked gut inflammation and have been associated with PI-IBS include enterotoxigenic E. coli,39 the shiga-toxin producing E. coli O147 especially when combined with C. jejuni,22 Shigella spp.11 and S. enteritidis.40 A surrogate marker for bacterial toxicity is the duration of initial illness; initial episodes lasting more than 21 days had a RR of 11.37 (95% CI, 2.2-5.8) for PI-IBS10 compared to illness lasting less than a week. Vomiting as part of the initial illness may be protective,10 perhaps because it reduces the infecting dose.
Mechanism
Inflammation
There is limited data on the morphological and functional changes in human gastrointestinal mucosa during an outbreak of gastroenteritis. Enteric bacterial pathogens adhere to the intestinal epithelium and deliver enterotoxins or cytotoxins which may cause marked secretion or inflammation and enterocyte damage associated with symptoms of diarrhea.41,42 During the acute phase of gastroenteritis, macroscopic changes may mimic features of inflammatory bowel disease. Macroscopic features seen during colonoscopy include erythema, oedema, haemorrhagic spots, friable mucosae, ulcers and mucopus.43-45 A marked increase in gut permeability is commonly seen, possibly reflecting damage to the enterocytes.10 During the acute phase of viral gastroenteritis, there is reduction in villous surface area and villous height along with increased intraepithelial lymphocytes and gut permeability. However this rapidly resolves with little inflammatory damage,46 which may explain why PI-IBS after viral gastroenteritis is short-lived.47 Other studies following protozoan infection such as giardiasis, which is associated with PI-IBS, have confirmed inflammatory changes including villous shortening and increase of plasma cells and leucocytes in the lamina propria.48 Detailed prospective studies in men are difficult to perform so studies on animal models have been used to assess the pathophysiology of PI-IBS. Mice infected with Trichinella spiralis show transient acute mucosal inflammation with increase in mast cells and T lymphocytes which resolves as the worm migrates from the gut to encyst in muscle tissue. However although acute inflammation resolves and the mucosa heals there is persistent T lymphocyte-dependent EC cell hyperplasia,49-51 increased expression of tachykinins,52 prolonged motility dysfunction and visceral hypersensitivity that lasts for many weeks following clearance of the infection.51,53-56
Several studies have shown similar evidence of low grade "immune activation" in IBS patients.12,14,57,58 A prospective study using serial rectal biopsies on patients following C. jejuni gastroenteritis has shown raised serotonin (5-hydroxytryptamine, 5-HT)-containing EC cells, intra-epithelial lymphocytes (IELs; CD8) and T lymphocytes (CD3, CD4 and CD8) which could last up to 4 years.12,26 Increased EC cells are an independent predictor of developing PI-IBS25,26 with each standard deviation rise in EC counts being associated with a RR of 3.8. Immune activation with increased level of CD3, CD4 and CD8 are also found in unselected IBS patients.59 This "immune activation" is subtle compared with changes seen in inflammatory bowel disease or microscopic colitis and does not include neutrophil infiltration.58,59
Serotonin
EC cells are an important subgroup of enteroendocrine cells which act as "taste buds" of the intestine, responding to luminal pressure and contents including nutrients and bacterial products, transducing these to neural responses by secreting peptides and amines which activate enteric nerves. EC cell granules contain 5-HT which activates enteric reflexes via the 5-HT1p, 5-HT3, 5-HT4 and 5-HT7 receptors to stimulate secretion and propulsion of the gut.60,61 5-HT plays an important role in the motility and secretion of the gut, particularly in response to toxins such as cholera toxin which causes profuse intestinal secretion.62 Mice infected with Trichuris muris develop T-cell mediated immune activation in the gut leading to an increase in EC cells and 5-HT content.49-51 EC hyperplasia is associated with increased serotonin release, which can lead to increase motility and secretion, features which may explain diarrheal symptoms in IBS-D patients.63,64 As discussed above mice infected with T. spiralis, which showed similarities with PI-IBS in human, exhibit hyperplasia of EC in most sections of the bowel (except colon) and reduced serotonin transporter (SERT).49-51 5-HT's action is terminated by degradation by intracellular enzymes, monamino-oxidases. Being a polar molecule, 5-HT is excluded from cells and requires SERT to transport it across the cell membrane before it can be degraded. It follows that reduction in SERT leads to impaired intracellular uptake and degradation and hence increased availability of serotonin within the mucosa.56,65 This may be relevant to IBS since patients with IBS-D show similar findings including increased IELs and mast cells and reduced SERT mRNA in duodenal biopsies.37 The raised IELs are inversely correlated with level of SERT messenger RNA, in keeping with experimental data showing inflammatory cytokines, possibly from lymphocytes and macrophages, reduced SERT mRNA.66 Impairment of SERT function will increase mucosal 5-HT availability, stimulating secretion and motility which may contribute to the diarrheal symptom in IBS-D.37,63 This concept is supported by the clinical effectiveness of 5-HT3 receptor antagonists which are amongst the most potent treatments for IBS-D.67
Mast Cells
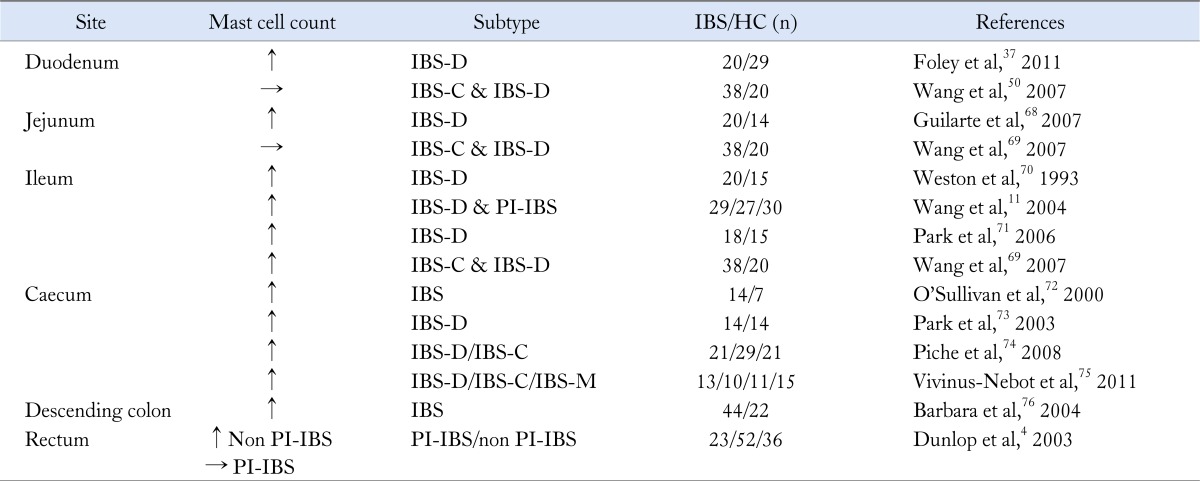
As shown in the Table increased mucosa mast cells have been found in many series of both PI-IBS11 and IBS-D patients.57,68 Mast cells products can activate enteric nerves within the lamina propria which may be relevant to IBS symptoms since the number of mast cells < 5 µm from an enteric nerve correlates with the severity of visceral pain in IBS.76,77 Increased amounts of mast cell mediators such as tryptase and histamine are found in supernatants from IBS patients' mucosal biopsies and have been shown to activate human enteric afferent nerves.78-80 The release of these mediators from mast cells may contribute to visceral hypersensitivity by activating enteric afferents.76,80 One study showed an increase in mast cells in the terminal ileum, ascending colon and rectum of IBS patients compared to healthy volunteers and higher mast cell counts in those with rectal hypersensitivity.71 Interestingly after infection, regardless of symptoms, the numbers of mast cell in close proximity to enteric nerves were increased, presumably as part of the remodeling of the mucosa after the initial injury when regenerating nerves sought out their targets.81
Table.
Evidence of Mast Cell Abnormalities in Irritable Bowel Syndrome
IBS, irritable bowel syndrome; HC, healthy controls; IBS-D, IBS with diarrhea; IBS-C, IBS with constipation; PI-IBS, post-infectious IBS; IBS-M, mixed IBS.
Cytokines
IL-1β is a key pro-inflammatory marker which is released by activated macrophages and stimulates lymphocyte proliferation. IL-1β mRNA has been shown to be increased in the terminal ileum and recto-sigmoid colon of PI-IBS patients11 and in rectal biopsies in patients who had PI-IBS, both during the acute infection and after 3 months.82 It is more convenient to assess immune activation in peripheral blood mononuclear cells (PBMCs) which have been shown to release more proinflammatory cytokines (TNF-α, IL-1β and IL-6) in response to lipopolysaccharide in patients with IBS-D but not IBS-C.23 This study had only 5 out of 20 IBS-D patients with PI-IBS who appeared to exhibit particularly high cytokine levels but this difference was not significant, though the study was obviously underpowered to test this. The origin of this increased reactivity of PBMCs is unclear since psychological stress may also lead to immune activation and increased IL-1β in PBMCs.83 IL-6, which is also increased by infection, has been shown to be increased in both IBS and depressed patients, showing the complex interaction between psychological and infectious factors.84 More recently a group from Sweden has reported increased % of PBMCs from unselected IBS patients which were CD69+, Integrin β7+ and HLRD+, indicative of T-cell activation and gut homing respectively85 though this report did not indicate if any were PI-IBS.
Changes in Enteric Nerves
The enteric nervous system plays an important role during enteric infections by orchestrating secretory action and activating motor patterns to expel the pathogens.86 T. spiralis infected mice show higher levels of substance P in the myenteric plexus52 associated with visceral hypersensitivity, muscle wall thickening and greater sensitivity to cholinergic stimuli. A more severe model of gut inflammation, the trinitrobenzenesulphonic acid colitis, shows that inflammation in the gut causes extensive remodelling of enteric nerves. This leads to changes in the neurochemical coding with increased expression of tachykinins, vasoactive polypeptide, galanin and pituitary adenylate cyclase activating polypeptide within the enteric nervous system.87,88 Similar studies in patients with symptomatic diverticular disease, who often have had prior episodes of acute diverticulitis, showed that the symptomatic patients had increased expression of substance P.89 This supports the idea that the pain in PI-IBS could be due to changes in the enteric nerves, whose slow rate of repair might explain why symptoms often remain for many years after the initial infection.
Following shigella enteritis, neuron specific enolase (paneuronal marker) and substance P were both increased in the terminal ileum and rectosigmoid tissue in PI-IBS. However this was also seen in non-infectious IBS patients suggesting there is more than one mechanism mediating these changes.11 Animal studies showed that colonic mucosal injury induced by acetic acid was followed by increased expression of the transient receptor potential vallinoid 1 (TRPV1) receptor which caused increased visceral hypersensitivity in this model.90 More recently human studies using rectal biopsies of unselected IBS patient have shown increased TRPV-1 positive neuronal fibres, whose numbers correlated with severity of abdominal pain.91 The key nerves responsible for transmitting pain to the human brain lie not in the mucosa but within the myenteric plexus which not easily obtainable in IBS patients. However in a highly selected series of 10 IBS patients with very severe symptoms, full thickness biopsy of jejunum showed increased lymphocytes and neuronal degeneration in the myenteric plexus suggesting altered enteric nerves may be important.92
Altered Microbiome
The appearance of new non-culture based techniques for profiling of the bacteria in the gastrointestinal tract has revealed an immense complexity with as many as 1,500 separate species. Each individual's intestinal microbiota depends on many factors including genetics, age, diet, antibiotics and other environmental factors including infection. There is a symbiosis between the human host and the microbiota which helps to keep the environment in the colon stable, protecting the host from pathogens via production of a range of antibacterial products including short chain fatty acids (SCFA).93 During an acute phase of bacterial enteritis, the inflammatory response inhibits the normal microbiota94 and there is reduction in SCFA and increase in the faecal pH.95 Subjects with infectious diarrhea showed decreased diversity and new strong bands which were seen using gel electrophoresis profiling indicating overgrowth of selected bacteria groups, features not seen in normal healthy controls.96 Taking a bowel preparation for screening colonoscopy in some ways simulated an acute episode of diarrhea and in some patients this appeared to alter the gut microbiota, an effect which persisted for at least 8 weeks.97
Many studies have tried to characterise the changes in intestinal faecal microbiota in IBS patients. An earlier study using traditional culture-based techniques have shown reduction of bacteria such as coliforms, Lactobacillus spp. and Bifidobacterium spp. and increased number of Enterobacteriaceae in the gut flora of IBS patients.98,99 More recently a study using DNA-based techniques showed increased numbers of Proteobacteria and Firmicutes and reduction in number of Actinobacteria and Bacteroidetes in patients with IBS-D when compared to healthy volunteers.100 Another study found changes in the composition of bacteria according to subtypes of IBS. IBS-D has lower amount of Lactobacillus spp. whereas IBS-C patients had increased amount of Veillonella spp.101 These findings vary between studies which may be due to different laboratory techniques and possibly due to the heterogeneity of IBS patients. Indeed it is unlikely that attempting to characterise all the bacteria will be helpful since it is the overall metabolic effect which is likely to determine bowel symptoms. Thus in the future it is likely that dividing into enterotypes, ie, clusters of associated bacteria will improve the link between microbiota and gut symptoms which at present are uncertain.
Prognosis
There is a slow decline in prevalence of PI-IBS in the years following initial diagnosis. A 5 year review following Salmonella spp. infection showed 7 out of 11 patients still had disturbed bowel function, but only 5 had diarrhea more than 1×/week.102 A 6 year follow-up study in PI-IBS revealed 43% of PI-IBS patients had recovered103 while a meta-analysis of PI-IBS reported steady reduction in IBS following infection, with odds ratio for those infected compared to noninfected controls of 7.6 at 3 months and 3.8 at 3 years.15 The long term follow up of the outbreak of gastroenteritis in Walkerton showed a decline in the prevalence of PI-IBS from 28% to 15.4% after 8 years.104 Risk factors for developing PI-IBS were similar to previous reports and included female gender, younger age and fever or weight loss during the acute enteric infection. A history of severe psychiatric illness that is untreated may impair recovery. These data show that the clinician can reassure the patients that the prognosis is one of improvement and the chances of developing a new condition like inflammatory bowel disease is very low.
Management
Management of PI-IBS should be tailored according to patient's symptom severity. It is important to ensure that patient has realistic expectations of treatment and recognise that although they are likely to improve, symptoms may persist for some years. Meanwhile treatment should be given according to the predominant symptoms. Patient with abdominal pain and anxiety should try mild tranquilisers like low dose tricyclics agents such as amitriptyline 10-30 mg at night, a practice supported by consensus of experts but without high quality randomised controlled trials.105 The only recent good quality study used desipramine 50 mg which is much less well tolerated, though if compliance is good it does lead to superior response rates compared to placebo.106 The symptoms of diarrhea and urgency respond well to loperamide, 2 mg with each loose stool.107,108 However this often induces bloating and distension, so many patients prefer to only use loperamide when they are travelling or on special occasions, when urgency would be very inconvenient.
Our increase in understanding of the underlying pathophysiology has opened more opportunities to target treatment to specific mechanisms that may cause PI-IBS/IBS. Given the underlying immune activation prednisolone was a logical choice but at a dose of 30 mg daily for 3 weeks proved to be ineffective in symptomatic relief of IBS despite reducing T-lymphocyte numbers in the rectal mucosa.109 A proof of concept study concluded that ketotifen, a mast cell stabiliser, decreased visceral hypersensitivity and reduced IBS symptoms although there was poor correlation with mast cell activation assessed in biopsies.110 More recently, mesalazine, an anti-inflammatory agent, has been used. Some pilot studies have shown reduction in mast cell numbers and improvement of overall well-being, abdominal discomfort and altered gut microbiota111,112 but the studies were underpowered (n = 20 and n = 12, respectively). Another uncontrolled study using mesalazine in PI-IBS and IBS patients with predominantly diarrhea concluded that there was improvement in stool consistency, frequency and abdominal discomfort.113 However given the high placebo response in IBS, a large double blind and placebo controlled study using mesalazine for treatment of IBS is now needed to convincingly confirm or refute these findings.
Given the data on the relationship between immune activation and increased availability of 5-HT in IBS-D, a 5-HT antagonist would be a logical treatment for PI-IBS. A 5-HT3 antagonist, alosetron, was highly effective for unselected IBS-D patients with beneficial effect on abdominal pain and overall well being.114 Unfortunately owing to its side effect of severe constipation and ischaemic colitis, this drug was initially withdrawn and is now only available under very restricted conditions. Other 5-HT3 antagonists include ramosetron and ondansetron. Ramosetron is a potent 5-HT3 antagonist, used at the very low dose of 5 µg daily, not available in Europe or America but available in Japan and Korea where it is approved for both males and females. Ischemic colitis has not been reported and the pivotal randomised controlled trial demonstrated 47% response rate compared to 27% on placebo, giving a number needed to treat of 5, suggesting it is one of the most potent IBS treatments for IBS-D.115 Ondansetron, a 5-HT3 antagonist may be another potential treatment of IBS-D patients. It has been widely used as an anti-emetic for over 2 decades with an excellent safety profile and no reports of ischemic colitis. Our recent randomised placebo controlled trial showed a responder rate, defined as a 50% reduction in days per week with loose stool of 70% on ondansetron versus 33% on placebo. This gives a number needed to treat of 2.7 making it one of the most potent drugs for IBS-D. The mode dose was very low at 4 mg on alternate days with a low incidence of constipation (9%), which virtually always responded to dose reduction.116 An alternative approach is to inhibit serotonin synthesis and a tryptophan hydroxylase 1 inhibitor has been shown recently to benefit IBS-D.117
Conclusion
PI-IBS developing after viral, bacterial and protozoan infections continues to be reported. These recent studies have confirmed the importance of the previously established risk factors, namely severity of initial illness, female gender and adverse psychological factors. Recent evidence suggests a genetic predisposition involving excessive cytokine response. Mucosal immune activation involving mast cells and lymphocytes with secondary impairment of serotonin transporter have been demonstrated in IBS-D. While the prognosis is for slow improvement, treatments should currently target the key symptoms of diarrhea and abdominal pain. Loperamide is effective for diarrhea while tricyclic antidepressants benefit pain. Future disease modifying therapies aimed at correcting underlying mechanisms including immune activation and serotonin excess are currently being explored and may provide better treatments in the future.
Acknowledgements
RS and CL acknowledge the support of the NIHR Biomedical Research Unit in Gastrointestinal Diseases at the Nottingham University Hospitals NHS Trust and Nottingham University.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Longstreth GF, Hawkey CJ, Mayer EA, et al. Characteristics of patients with irritable bowel syndrome recruited from three sources: implications for clinical trials. Aliment Pharmacol Ther. 2001;15:959–964. doi: 10.1046/j.1365-2036.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary NA, Truelove SC. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962;31:307–322. [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler JG, Sethi D, Cowden JM, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam CC, Rodrigues LC, Viviani L, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruigómez A, García Rodríguez LA, Panés J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–469. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Borgaonkar MR, Ford DC, Marshall JK, Churchill E, Collins SM. The incidence of irritable bowel syndrome among community subjects with previous acute enteric infection. Dig Dis Sci. 2006;51:1026–1032. doi: 10.1007/s10620-006-9348-1. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JK, Thabane M, Garg AX, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 14.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol. 2005;100:1340–1344. doi: 10.1111/j.1572-0241.2005.41700.x. [DOI] [PubMed] [Google Scholar]
- 18.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 19.van der Veek PP, van den Berg M, de Kroon YE, Verspaget HW, Masclee AA. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–2516. doi: 10.1111/j.1572-0241.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 20.Zucchelli M, Camilleri M, Andreasson AN, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;60:1671–1677. doi: 10.1136/gut.2011.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiller R, Swan C, Campbell E, et al. Identifying and testing candidate genes underlying the inflammatory basis of irritable bowel syndrome. Gut. 2011;60(suppl 1):A164. [Google Scholar]
- 22.Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502–1513. doi: 10.1053/j.gastro.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 25.Spence MJ, Moss-Morris R. The cognitive behavioural model of irritable bowel syndrome: a prospective investigation of patients with gastroenteritis. Gut. 2007;56:1066–1071. doi: 10.1136/gut.2006.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(suppl 7):33–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Gué M, Del Rio-Lacheze C, Eutamene H, Théodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 32.Singh VK, Leu SJ. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett. 1990;120:151–154. doi: 10.1016/0304-3940(90)90025-5. [DOI] [PubMed] [Google Scholar]
- 33.Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gué M, Bonbonne C, Fioramonti J, et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol. 1997;272(1 Pt 1):G84–G91. doi: 10.1152/ajpgi.1997.272.1.G84. [DOI] [PubMed] [Google Scholar]
- 35.Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108.e5. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 37.Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–1443.e1. doi: 10.1053/j.gastro.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 38.Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis. 2001;184:606–609. doi: 10.1086/322845. [DOI] [PubMed] [Google Scholar]
- 39.Okhuysen PC, Jiang ZD, Carlin L, Forbes C, DuPont HL. Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in North American travelers to Mexico. Am J Gastroenterol. 2004;99:1774–1778. doi: 10.1111/j.1572-0241.2004.30435.x. [DOI] [PubMed] [Google Scholar]
- 40.Mearin F, Pérez-Oliveras M, Perelló A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Guerrant RL, Steiner TS, Lima AA, Bobak DA. How intestinal bacteria cause disease. J Infect Dis. 1999;179(suppl 2):S331–S337. doi: 10.1086/513845. [DOI] [PubMed] [Google Scholar]
- 42.Ina K, Kusugami K, Ohta M. Bacterial hemorrhagic enterocolitis. J Gastroenterol. 2003;38:111–120. doi: 10.1007/s005350300019. [DOI] [PubMed] [Google Scholar]
- 43.Speelman P, Kabir I, Islam M. Distribution and spread of colonic lesions in shigellosis: a colonoscopic study. J Infect Dis. 1984;150:899–903. doi: 10.1093/infdis/150.6.899. [DOI] [PubMed] [Google Scholar]
- 44.Khuroo MS, Mahajan R, Zargar SA, et al. The colon in shigellosis: serial colonoscopic appearances in Shigella dysenteriae I. Endoscopy. 1990;22:35–38. doi: 10.1055/s-2007-1012784. [DOI] [PubMed] [Google Scholar]
- 45.Tedesco FJ, Hardin RD, Harper RN, Edwards BH. Infectious colitis endoscopically simulating inflammatory bowel disease: a prospective evaluation. Gastrointest Endosc. 1983;29:195–197. doi: 10.1016/s0016-5107(83)72583-6. [DOI] [PubMed] [Google Scholar]
- 46.Troeger H, Loddenkemper C, Schneider T, et al. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070–1077. doi: 10.1136/gut.2008.160150. [DOI] [PubMed] [Google Scholar]
- 47.Marshall JK, Thabane M, Borgaonkar MR, James C. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol. 2007;5:457–460. doi: 10.1016/j.cgh.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Hanevik K, Dizdar V, Langeland N, Hausken T. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 2009;9:27. doi: 10.1186/1471-230X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motomura Y, Ghia JE, Wang H, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–481. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Steeds J, Motomura Y, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 52.Swain MG, Agro A, Blennerhassett P, Stanisz A, Collins SM. Increased levels of substance P in the myenteric plexus of Trichinella-infected rats. Gastroenterology. 1992;102:1913–1919. doi: 10.1016/0016-5085(92)90313-n. [DOI] [PubMed] [Google Scholar]
- 53.Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224–1232. doi: 10.1053/gast.1997.v113.pm9322517. [DOI] [PubMed] [Google Scholar]
- 54.Barbara G, De Giorgio R, Deng Y, Vallance B, Blennerhassett P, Collins SM. Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology. 2001;120:1729–1736. doi: 10.1053/gast.2001.24847. [DOI] [PubMed] [Google Scholar]
- 55.Bercik P, Wang L, Verdú EF, et al. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179–187. doi: 10.1053/j.gastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Keating C, Beyak M, Foley S, et al. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 2008;586(Pt 18):4517–4530. doi: 10.1113/jphysiol.2008.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 58.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 59.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 60.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19(suppl 2):25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 61.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Bearcroft CP, André EA, Farthing MJ. In vivo effects of the 5-HT3 antagonist alosetron on basal and cholera toxin-induced secretion in the human jejunum: a segmental perfusion study. Aliment Pharmacol Ther. 1997;11:1109–1114. doi: 10.1046/j.1365-2036.1997.d01-1389.x. [DOI] [PubMed] [Google Scholar]
- 63.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55–60. [PubMed] [Google Scholar]
- 65.Coleman NS, Foley S, Dunlop SP, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 66.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 67.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang SH, Dong L, Luo JY, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weston AP, Biddle WL, Bhatia PS, Miner PB., Jr Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- 71.Park JH, Rhee PL, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21(1 Pt 1):71–78. doi: 10.1111/j.1440-1746.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 72.O'Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 73.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piche T, Saint-Paul MC, Dainese R, et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468–473. doi: 10.1136/gut.2007.127068. [DOI] [PubMed] [Google Scholar]
- 75.Vivinus-Nébot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107:75–81. doi: 10.1038/ajg.2011.315. [DOI] [PubMed] [Google Scholar]
- 76.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 77.Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 79.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 81.Mearin F, Perelló A, Balboa A, et al. Pathogenic mechanisms of postinfectious functional gastrointestinal disorders: results 3 years after gastroenteritis. Scand J Gastroenterol. 2009;44:1173–1185. doi: 10.1080/00365520903171276. [DOI] [PubMed] [Google Scholar]
- 82.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brydon L, Edwards S, Jia H, et al. Psychological stress activates interleukin-1beta gene expression in human mononuclear cells. Brain Behav Immun. 2005;19:540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 85.Ohman L, Isaksson S, Lindmark AC, et al. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–1212. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 86.Spiller RC. Role of nerves in enteric infection. Gut. 2002;51:759–762. doi: 10.1136/gut.51.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simpson J, Sundler F, Humes DJ, et al. Prolonged elevation of galanin and tachykinin expression in mucosal and myenteric enteric nerves in trinitrobenzene sulphonic acid colitis. Neurogastroenterol Motil. 2008;20:392–406. doi: 10.1111/j.1365-2982.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 88.Miampamba M, Sharkey KA. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil. 1998;10:315–329. doi: 10.1046/j.1365-2982.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- 89.Simpson J, Sundler F, Humes DJ, Jenkins D, Scholefield JH, Spiller RC. Post inflammatory damage to the enteric nervous system in diverticular disease and its relationship to symptoms. Neurogastroenterol Motil. 2009;21:847–847.e58. doi: 10.1111/j.1365-2982.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 90.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 91.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 93.Balamurugan R, Janardhan HP, George S, Chittaranjan SP, Ramakrishna BS. Bacterial succession in the colon during childhood and adolescence: molecular studies in a southern Indian village. Am J Clin Nutr. 2008;88:1643–1647. doi: 10.3945/ajcn.2008.26511. [DOI] [PubMed] [Google Scholar]
- 94.Isolauri E, Salminen S, Ouwehand AC. Microbial-gut interactions in health and disease. Probiotics. Best Pract Res Clin Gastroenterol. 2004;18:299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Fujita K, Kaku M, Yanagase Y, et al. Physicochemical characteristics and flora of diarrhoeal and recovery faeces in children with acute gastro-enteritis in Kenya. Ann Trop Paediatr. 1990;10:339–345. doi: 10.1080/02724936.1990.11747455. [DOI] [PubMed] [Google Scholar]
- 96.Mai V, Braden CR, Heckendorf J, Pironis B, Hirshon JM. Monitoring of stool microbiota in subjects with diarrhea indicates distortions in composition. J Clin Microbiol. 2006;44:4550–4552. doi: 10.1128/JCM.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mai V, Greenwald B, Morris JG, Jr, Raufman JP, Stine OC. Effect of bowel preparation and colonoscopy on post-procedure intestinal microbiota composition. Gut. 2006;55:1822–1823. doi: 10.1136/gut.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 99.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krogius-Kurikka L, Lyra A, Malinen E, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malinen E, Rinttilä T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 102.McKendrick MW. Post Salmonella irritable bowel syndrome - 5 year review. J Infect. 1996;32:170–171. doi: 10.1016/s0163-4453(96)91715-6. [DOI] [PubMed] [Google Scholar]
- 103.Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413. doi: 10.1136/gut.51.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 105.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 107.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol. 1996;31:463–468. doi: 10.3109/00365529609006766. [DOI] [PubMed] [Google Scholar]
- 108.Camilleri M. Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clin Pharmacol Ther. 2012;91:44–59. doi: 10.1038/clpt.2011.261. [DOI] [PubMed] [Google Scholar]
- 109.Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77–84. doi: 10.1046/j.1365-2036.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 110.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 111.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther. 2009;30:245–252. doi: 10.1111/j.1365-2036.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- 112.Andrews CN, Griffiths TA, Kaufman J, Vergnolle N, Surette MG, Rioux KP. Mesalazine (5-aminosalicylic acid) alters faecal bacterial profiles, but not mucosal proteolytic activity in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2011;34:374–383. doi: 10.1111/j.1365-2036.2011.04732.x. [DOI] [PubMed] [Google Scholar]
- 113.Bafutto M, Almeida JR, Leite NV, Oliveira EC, Gabriel-Neto S, Rezende-Filho J. Treatment of postinfectious irritable bowel syndrome and noninfective irritable bowel syndrome with mesalazine. Arq Gastroenterol. 2011;48:36–40. doi: 10.1590/s0004-28032011000100008. [DOI] [PubMed] [Google Scholar]
- 114.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 115.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202–1211. doi: 10.1080/00365520802240255. [DOI] [PubMed] [Google Scholar]
- 116.Garsed K, Hastings M, Marciani L, et al. Ondansetron is an effective treatment for patients with diarrhoea predominant irritable bowel syndrome. Gut. 2011;60:A40. [Google Scholar]
- 117.Brown PM, Drossman DA, Wood AJ, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]