Abstract
A male, neutered cat was presented for lethargy, reluctance to walk, and mammary enlargement after recent treatment with megestrol acetate. Mammary fibroadenomatous hyperplasia was diagnosed on the basis of history, clinical signs, and histopathological findings. Pathogenesis, clinical signs, and treatment options for mammary fibroadenomatous hyperplasia attributed to megestrol acetate treatment are discussed.
A 1.5-year-old, neutered male, domestic shorthair was presented to the Strathmore Veterinary Clinic, Strathmore, Alberta for a pruritic, hemorrhagic dermatopathy of the chin region. A tentative diagnosis of a superficial pyoderma was made and the cat was discharged with the following therapies: topical cleansing; oral orbifloxacin (Orbax; Schering-Plough, Pointe Claire, Quebec), 2.2 mg/kg bodyweight (BW), q24h for 14 d; and oral prednisone (generic), 1 mg/kg BW, q24h for 4 d.
The cat was presented to the clinic again, approximately 1 mo later. The lesion on the chin remained pruritic, and 2 additional similar lesions were on the ventral aspect of the neck and mandible. At this time, the owners declined to have the skin biopsied. Eosinophil-granuloma complex was suspected and methylprednisolone acetate (Depo-Medrol; Upjohn, Mississauga, Ontario), 4 mg/kg BW, SC, was administered. A hypoallergenic food trial was commenced.
The cat was presented to the clinic again, 38 d after initial presentation, with continuing pruritus. The therapeutic regime was modified at this time to include oral megestrol acetate (Ovaban; Schering-Plough), 1 mg/kg BW, q24h for 5 d, and then 0.5 mg/kg BW, PO twice weekly for 21 d. This prescription was refilled 33 d after initiation of the oral megestrol acetate treatment, so that the cat received 1 mg/kg BW, PO, q24h for 2 d, followed by 0.5 mg/kg BW, PO, q24h for 16 d.
A recheck appointment was scheduled for 7 d after the prescription had been completed, that is, 58 d after initiation of megestrol acetate treatment. The cat, at this time, demonstrated acute mammary enlargement and reluctance to walk. Findings on physical examination were unremarkable, except for the chin and mammary glands. All 6 mammary glands were asymmetrically enlarged, with diameters ranging from 1.5 cm to 5 cm. The most severely affected gland was the right inguinal, which was hyperemic, warm, and edematous near the teat. Thoracic radiographs, taken to screen for possible pulmonary metastases, were within normal limits. A complete blood cell (CBC) count and chemistry panel were performed on a blood analysis machine, (IDEXX VetLab; IDEXX Laboratories, Westbrook, Maine USA), and the results were within normal limits. A urinalysis was not performed.
Differential diagnoses included mammary adenocarcinoma or carcinoma, mammary adenoma, or mammary sarcoma. Due to the recent history of progestin administration and the involvement of more than 1 gland, mammary hyperplasia was the most likely diagnosis.
A simple mastectomy of the right inguinal mammary gland and an excisional biopsy of the right inguinal lymph node were performed under general anesthesia. These tissues were submitted to the Western College of Veterinary Medicine for histological examination. The final histological diagnosis of the right inguinal gland was mammary fibroadenomatous hyperplasia (Figure 1). The right inguinal gland also had neutrophilic infiltrates beneath the ulcerated area, suggesting a superficial mastitis. The right inguinal lymph node had no histological lesions.
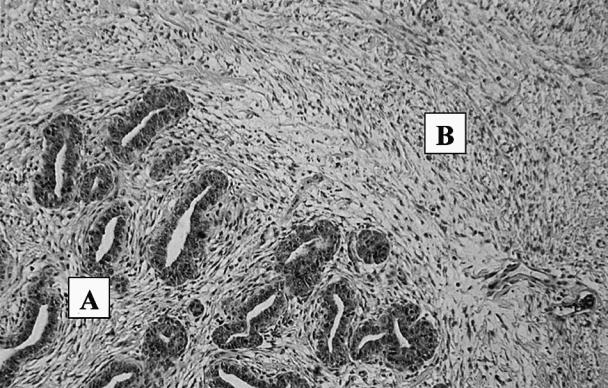
Figure. 1. Mammary fibroadenomatour hyperplasia in a 1 and 1.5 year old male neutered cat after treatment with standard dosages of megestrol acetate (progesterone analogue). (A) Mammary adenomatous hyperplasia of lobular ducts. (B) Fibrous hyperplasia of connective tissue.
Recheck examinations were performed 10 d and 15 d after surgery, that is, 68 d and 73 d after initiation of megestrol acetate treatment. Abdominal and thoracic mammary glands had regressed slightly, but the left inguinal mammary gland had enlarged slightly.
The cat was presented to the clinic 7 d later for inappetance (80 d after initiation of megestrol acetate treatment). On physical examination, the cat was mildly febrile (temperature 39.9°C; normal 38.5°C to 39.5°C) and estimated to be 5% dyhydrated. A small, ulcerated skin lesion, similar to previous lesions, was noted on the ventral chin. The left inguinal mammary gland was ulcerated, swollen, warm, painful, and asymmetrically enlarged to 4 cm in diameter. A CBC count showed a mild leukocytosis (18.9 × 109/L; normal, 6.0 to 16.9 × 109/L) and mild neutrophilia (15.5 × 109/L; normal, 2.8 to 10.5 × 109/L). A presumptive diagnosis of superficial mastitis (complicating the underlying mammary hyperplasia) was made. The cat received trimethoprim-sulfadiazine (Tribrissen; Schering Plough), 35 mg/kg BW, SC, q24h for 3 d, meloxicam (Metacam Injectable; Boehringer Ingelheim Vetmedica, Burlington, Ontario), 0.02 mg/kg BW, SC, q24h, and lactated Ringer's solution, 60 to 120 mL, SC, q24 h for the next 3 d. During this time, the cat's temperature returned to normal (38.5°C). The therapeutic plan was to return the cat to the owners once the cat was eating sufficiently on his own, to continue antibiotic treatment for 10 d, and to biopsy the skin lesions after the antibiotic course had been completed.
The owners did not wish to pursue further diagnostic tests. When the cat's skin lesions returned, they elected for euthanasia. A necropsy was not performed.
Feline mammary fibroadenomatous hyperplasia, also known as feline mammary hypertrophy or fibroepithelial hyperplasia, is a nonneoplastic, progesterone-induced condition (1). Typical signalments include young cycling cats, pregnant or pseudopregnant female cats, or older neutered male or female cats given exogenous progestins (2). Cats present with mammary enlargement, involving one or more glands. The enlargement is due to rapid proliferation of mammary duct epithelium and stroma. Hyperplastic mammary tissue may undergo spontaneous regression; may require ovariohysterectomy, if secondary, to elevated estrogen levels; or may regress once progesterone levels decline. Mammary hyperplasia must be distinguished by microscopic examination from mammary neoplasia, as the latter has a poor prognosis. Mammary neoplasms in the cat often grow rapidly, are firm and ulcerated, and metastasize early to local lymph nodes and lungs (3).
Megestrol acetate is a progestagen. In veterinary medicine, it has been used to treat pseudopregnancy in dogs; to suppress or delay estrus in cats and dogs; to treat behavior-related conditions in cats; to treat feline eosinophilic and proliferative keratopathies; and, less commonly, as an appetite stimulant (4,5). However, it has not been approved for use in the cat. Side effects noted in cats are as follows: iatrogenic adrenocortical suppression, transient diabetes mellitus, hepatotoxicity, cystic endometrial hyperplasia, and mammary hypertrophy or neoplasia (6). There is evidence that megestrol acetate has a glucocorticoid-like activity. Its use may result in suppression of the adrenocortical axis for 2 to 4 wk after treatment, 5 mg/kg BW/cat, q24h, 14d, and in hyperinsulinemia (7). Therefore, discontinuing megestrol acetate therapy abruptly is not recommended (7).
Fibroadenomatous mammary hyperplasia is a relatively uncommon sequela to the administration of megestrol acetate (8). This therapy was found to be clearly associated with mammary fibroepithelial hyperplasia in predominantly older (average age 8.1 y) neutered male or female cats (2). In the case reported here, the affected cat was 1.5 y old; however, the youngest cat reported in another study was 11 mo old (5). Secondary mastitis, ulceration, or both may be observed with this condition, as was confirmed in this case on histopathologic examination.
Maximal mammary development seems to require a synergism between pituitary hormones and ovarian steroids (2). Mammary duct epithelium and stromal proliferation (benign) is thought to be progesterone dependent (8). Steroid receptors within mammary tissue recognize progesterone and estrogen, and through these receptors hormonal action is medicated.
Immunohistochemistry techniques have been used to identify and distinguish estrogen and progesterone hormone receptors. In cases that were studies, progesterone receptors were identified in tissues of all cases of mammary hypertrophy, while estrogen receptors were recognized in only 50% of tissues (1). It is hypothesized that “the interaction of progesterone and synthetic progestins with progesterone receptors stimulates the local production of growth hormone, which would act on a potential autocrine/paracrine stimulatory loop to induce proliferation of mammary epithelial and stromal cells” (1).
In this case, one could speculate that the exogenous methylprednisolone acetate given 3 d before initiation of megestrol acetate may have allowed synergistic action between glucocorticoids and progestins, encouraging maximal mammary development. Further studies showing the interaction between these 2 hormones may be indicated.
Typically, the treatment for mammary hyperplasia in cats was ovariohysterectomy (if intact), mastectomy, or cessation of progestin therapy and allowing time for mammary regression. More recently, a progesterone-antagonist has been used successfully to treat hyperplasia in intact or neutered cats by eliminating the source of progestin (5). Seven cats affected with mammary hyperplasia were treated with aglepristone (Alizine; Aventis, Strasbourg, France), 10 mg/kg BW, SC, q24h for 4 to 5 d. Five days after the initial treatment, reduction in the size of the mammary glands, as well as change in consistency of affected glands (from rigid to soft), was observed. Corresponding biopsies taken 7 d after the first treatment showed collapse of the glandular duct lumens and reduction in the number of epithelial cells. It was reported to take 3 to 4 wk for complete involution of the glands. One of the 7 cats studied had recurrence of mammary hyperplasia 13 d after initial reduction in mammary gland size (16 d after initial treatment with aglepristone). No side effects were observed in any of the 7 cats treated with aglepristone.
In this case, progesterone blood levels were not measured, and tests for progesterone receptors on fixed mammary tissue were not performed. Therefore, the diagnosis of mammary hyperplasia due to megestrol acetate therapy is presumptive. However, the “apparent influence of a progestagen, whether present as exogenous therapy in the male or female or as endogenous steroid of ovarian origin, has been demonstrated directly and indirectly in cats with mammary hypertrophy” (6). Aglepristone may have been a viable treatment option for this cat (if commercially available) to reduce the size of all affected mammary glands.
It should be noted that the dose of megestrol acetate used was within the standard dosing regimen (never greater than 0.5 to 1 mg/kg BW, q24h) (10). However, the dose or duration of therapy with exogenous progestins cannot be used as a predictor for mammary hyperplasia (8). Veterinarians should be aware of the potential, yet uncommon, side effects discussed above. CVJ
Footnotes
Acknowledgments
The author thanks Drs. P. Dowling, K. Mealey, M. Johnson, B. Fransson, and E. O'Toole for their advice. CVJ
Dr. MacDougall's current address is Western Veterinary Specialist Centre, 1635–17th Avenue Southwest, Calgary, Alberta T2T 0E5.
Address all correspondence to Dr. MacDougall.
Reprints will not be available from the author.
References
- 1.de las Mulas JM, Millan Y, Bautista MJ, Perez J, Carrasco L. Oestrogen and progesterone receptors in feline fibroadenomatous change: An immunohistochemical study. Res Vet Pathol 2000; 68:15–21. [DOI] [PubMed]
- 2.Hayden DW, Barnes DM, Johnson KH. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: A retrospective study. Vet Pathol 1989;26:104–113. [DOI] [PubMed]
- 3.Fossum TW, Hedlund CS, Hulse DA, et al. Small Animal Surgery, 1st ed., St. Louis: Mosby-Year Book, 1997:539–544.
- 4.Adams R, ed. Veterinary Pharmacology and Therapeutics, 7th ed., Iowa: Iowa State Univ Pr. 1995:594–595,1084,1111.
- 5.Wehrend A, Hospes R, Gruber AD. Treatment of feline mammary fibroadenomatous hyperplasia with a progesterone-antagonist. Vet Rec 2001;148:346–347. [DOI] [PubMed]
- 6.Hayden DW, Johnston SD, Kiang DT, Barnes DM. Feline mammary hypertrophy/fibroadenoma comples: clinical and hormonal aspects. Am J Vet Res 1981;42:1699–1703. [PubMed]
- 7.Church DB, Watson AD, Emslie DR, Middleton DJ, Tan K, Wong D. Effects of proligestone and megestrol on plasma adrenocorticotrophic hormone, insulin and insulin-like growth factor-1 concentration in cats. Res Vet Sci 1994;56:175–178. [DOI] [PubMed]
- 8.Hayden DW, Johnson KH. Feline mammary hypertrophy-fibroadenoma complex. In: Kirk RW, ed. Current Veterinary Therapy IX, Small Animal Practice. Philadelphia: WB Saunders, 1986:477–480.
- 9.Chisholm H. Massive mammary enlargement in a cat. Can Vet J 1993;34:315. [PMC free article] [PubMed]
- 10.Plumb DC. Veterinary Drug Handbook, 3rd ed. White Bear Lake: Pharma Vet Publ, 1999:398–401.


