Abstract
A significant challenge that most therapeutic agents face is their inability to be delivered effectively. Nanotechnology offers a solution to allow for safe, high-dose, specific delivery of pharmaceuticals to the target tissue. Nanoparticles composed of biodegradable polymers can be designed and engineered with various layers of complexity to achieve drug targeting that was unimaginable years ago by offering multiple mechanisms to encapsulate and strategically deliver drugs, proteins, nucleic acids, or vaccines while improving their therapeutic index. Targeting of nanoparticles to diseased tissue and cells assumes two strategies: physical and chemical targeting. Physical targeting is a strategy enabled by nanoparticle fabrication techniques. It includes using size, shape, charge, and stiffness among other parameters to influence tissue accumulation, adhesion, and cell uptake. New methods to measure size, shape, and polydispersity will enable this field to grow and more thorough comparisons to be made. Physical targeting can be more economically viable when certain fabrication techniques are used. Chemical targeting can employ molecular recognition units to decorate the surface of particles or molecular units responsive to diseased environments or remote stimuli. In this review, we describe sophisticated nanoparticles designed for tissue-specific chemical targeting that use conjugation chemistry to attach targeting moieties. Furthermore, we describe chemical targeting using stimuli responsive nanoparticles that can respond to changes in pH, heat, and light.
I. Introduction
Current drug therapies have advanced significantly over the past few decades, and we find ourselves in a time of a medical revolution in our collective aim to treat each disease. Although dozens of new drugs appear each year, almost all of them continue to be wearing two hats: a pharmaceutical drug and, at certain concentrations, a toxic substance. Our ability to tip the balance toward the beneficial side of the equation to broaden the therapeutic window has largely been dependent on improved delivery methods to prevent nondiseased tissue from being affected. In addition to drug safety, pharmaceutical agents quickly get cleared or metabolized into a different, sometimes toxic side product and thus minimizing their therapeutic activity and duration. Furthermore, advances in genomics have allowed us to take major leaps toward personalized medicine and create novel tools for gene delivery or gene knockdown that appear to be promising. Nucleic acid-based therapies, however, are not as stable or readily taken up by cells as are small-molecule agents. Naked DNA and siRNA molecules degrade rapidly and their large size and charge make them difficult for delivery and thus requiring large amounts to be effective.
Nanotechnology has the potential to transform the pharmaceutical field by offering the ability to encapsulate and strategically deliver drugs, proteins, nucleic acids, or vaccines while improving their therapeutic index (Fig. 1). It is becoming evident that nanotechnology applied toward medicine (nanomedicine) will prove to be effective in creating new therapies but also in giving old therapies new life. Encapsulating pharmaceuticals in nano- or microparticles offers a solution to multiple problems in medicine underscored by the relatively new boom in interests from chemist and biologists. Nanoparticles can be made from various materials including lipids (Buse and El-Aneed, 2010); inorganic materials such as gold, carbon, and iron oxide (Huang et al., 2011); proteins (Maham et al., 2009); and polymeric systems (Zhang et al., 2008). Lipids, which are widely used and well characterized in carrier systems, are especially advantageous for targeting, because their dynamic nature allows clustering of peptides or other ligands, enhancing the affinity of the interaction with target cells (Poon et al., 2011). However, this same dynamic nature also makes them less stable than other carriers. Inorganic materials provide the advantage of stability; but again, this strength is also a disadvantage, especially in the case of gold, as their retention in the body could limit clinical applications.
Fig 1.
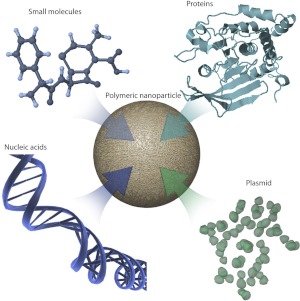
Types of therapeutic payloads that can be encapsulated into polymeric nanoparticles. Small polymeric delivery systems in the nano and micro range can be used to deliver diverse therapeutics, including small molecules, proteins, nucleic acids, and diagnostic agents. The small size of these delivery vehicles allows them to circulate the body and reach various target regions. Polymeric particles protect the therapeutic payload from degradation, increase clearance, and reduce unwanted side effects.
Biodegradable polymeric nanoparticle systems offer many advantages for biocompatibility, superior controlled release, size control, and low toxicity. The ability of polymers to degrade into safe small components that can be cleared by the body is almost as important as the ability to formulate the particles. Biodegradable polymeric nanoparticles can be formulated in a variety of ways and modified to easily encapsulate, embed within, or bind drugs to the exterior of nanoparticles. The most commonly used polymers include poly(lactide-coglycolide) (PLGA1), poly(lactic acid), poly(ε-caprolactone), chitosan, and poly(alkylcyanoacrylates) (Kumari et al., 2010). In this review, we will highlight the various strategies of delivering therapeutic payloads using polymeric nano- and microparticles that target sites of disease via physical and chemical approaches. We also showcase some studies that are paving the road to improved therapeutic delivery. Biodistribution and degradation kinetics is also an important consideration when designing polymeric particles.
II. Physical Targeting
A. Size
Improved circulation time of pharmacological agents is an important feature when designing better therapeutics. Nanotechnology quickly became an attractive system for the delivery of molecules to prevent immediate clearance from the kidneys. For example, a study using different sizes of quantum dots indicates that only particles with a diameter of 5.5 nm resulted in rapid and efficient urinary excretion (Choi et al., 2007). Delivery vehicles can range from 10 nm to several micrometers in diameter, and what size is best remains an ongoing debate. To no surprise, there is not one particular size for all applications and several studies have demonstrated opposing results using nanoparticles or microparticles. In addition, the methods used to determine the size of particles differs between different research groups. Some groups measure the diameter calculated from volume (volume-weighted), whereas others consider the diameter measured using scanning electron microscopy (number-weighted) to be a better option. Furthermore, reported particle sizes may be misleading due to a high polydispersity of size caused by imperfect methods of preparation. The polydispersity can affect the apparent number and volume diameter and, more importantly, can result in microparticle formulation procedures that produce microparticles in combination with some nanoparticles and vice versa. In addition, failure to provide the instruments used to make measurements with the correct inputs or inappropriate dilutions can produce inaccurate results. Therefore extreme caution must be taken when preparing particles to ensure polydispersity is minimized and measured properly.
For immunology applications, the size of particles can affect the distribution and its intended goals. The two most common targets include the lymph node and dendritic cells. In Table 1, we highlight and summarize several key studies for immunology applications comparing the size used in those experiments and include the actual reported particle size rather than categorizing them simply as “nano” or “micro.” For example, it was shown that smaller particles preferably target dendritic cells in lymph nodes, whereas larger particles are better for dendritic cells in the periphery where they transport larger particles from the site of injection to the lymph node (Reddy et al., 2006; Manolova et al., 2008). Whether particles are delivered to local or systemic lymph nodes may be less important than whether they activate dendritic cells and generate complete, long-lasting protection. Therefore, additional studies will need to be done to address what nanoparticle size is best for specific immunological outcomes.
TABLE 1.
Size comparisons for physical delivery in immunology applications
| Particle Sizes | Polymer | Delivery Method | Measured Immune Response | Efficient Size | Reference |
|---|---|---|---|---|---|
| 110 nm, 800–900 nm | PLGA (RG 503) | I.P., I.M., I.N./I.M. | IgG1/IgG2 level | No difference | Wendorf et al., 2008 |
| 1–10 μm, >10 μm | PLGA | S.C. | IgG | 1–10 μm | Eldridge et al., 1991 |
| 1.5 μm, 72.6 μm | PLGA | S.C. | IgG | 1.5 μm | O'Hagan et al., 1993 |
| 200 nm, 500 nm, 1 μm | PLGA (RG 506) | S.C., P.O., I.N. | IgG2a/IgG1 | No difference | Gutierro et al., 2002 |
| <500nm, 2 μm, >7 μm | PLGA (RG 505) | I.P. | T-cell activation | <500 nm | Nixon et al., 1996 |
| 200–600 nm, 1.5–4.7 μm | PLGA (RG 505) | Parenteral | T-cell activation | 200–600 nm primed the Th2 response, whereas 1.5–4.7 μm induced the Th1 response | Conway et al., 2001 |
| 7.5, 15.7, 40.4, 50.0 μm | PLLA | I.P., S.C. | IgG | I.P., 7.5 μm; S.C., no difference | Nakaoka et al., 1996 |
| 1, 4, 7, 15, 21 μm | PDLLA | P.O. | IgG | 4 μm | Tabata et al., 1996 |
| 4, 7, 26 μm | PDLLA | P.O. | IgA | 7 μm | Tabata et al., 1996 |
| <2, 2–8, 10–70, 50–150 μm | PDLLA | I.M. | IgG | 2–8 μm | Katare et al., 2005 |
| 200 nm, 1.5 μm | PEG-PLA | I.N. | IgG and IgA | No difference | Vila et al., 2004 |
| 100 nm, 500 nm, >1 μm | SB(43)-PVAL-g-PLGA | P.O., I.N. | IgG and IgA | P.O., 100 nm; I.N., 100 and 500 nm | Jung et al., 2001 |
| 0.4, 1, 3 μm | Chitosan | I.N. | IgG and IgA | IgG production, no difference; IgA production, 0.4 and 1 μm | Nagamoto et al., 2004 |
| 35 nm, 3.5 μm | pH-sensitive hydrogel | S.C. | T-cell activation | No difference | Cohen et al., 2009 |
I.N., intranasal; PLLA, poly(l-lactic acid); PDLLA, poly(dl-lactic acid); SB(43)-PVAL-g-PLGA, sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-coglycolide).
For cancer applications, many research groups exploit the enhanced permeability and retention effect (EPR) of cancerous tissues. The EPR is due to the unique vasculature environment of cancer tissue characterized by extensive and defective vasculature and impaired lymphatic drainage. One of the size safety criteria for intravenous micro- or nanoparticles is that it should not produce vascular occlusion and should not be able to circulate and reach the target area without producing any harmful effects (Wong et al., 2008). But a size limit for particles intended to accumulate in the tumor area by EPR is related to the permeability of the tumor. Several studies have measured the pore cutoff size of different subcutaneous tumors, and it appears that the majority of tumor's cutoff size ranges between 380 to 780 nm in diameter (Hobbs et al., 1998; Hashizume et al., 2000). It is not surprising that there is a relatively large range for a cutoff size because most individual tumors will have distinct vasculature with exceptionally larger or smaller pore cutoff sizes according to the type, location, and cause of the tumors. For example, cranially grown or hormone-dependent tumors were found to have relatively smaller cutoff sizes (Hobbs et al., 1998). To address the particle size question in tumor targeting, different studies were made to determine the optimum size that can meet the safety, circulation t1/2, EPR, and uptake criteria. In Table 2, we highlight several studies using physical targeting for tumors. The studies listed in Table 2 have different compositions and sizes and thus cannot be directly compared because they also measure different responses and use different models.
TABLE 2.
Size comparisons for physical delivery in cancer and other applications
| Particle Sizes | Polymer | Delivery Method | Measured Response | Efficient size | Reference |
|---|---|---|---|---|---|
| 315 nm, 1 μm, 10 μm | PLGA | Intratumoral | Decrease in tumor volume | 1 and 10 μm | Chakravarthi et al., 2010 |
| Cubic particles with side length of 2, 3, or 5 μm | Cationic cross-linked PEG hydrogel | Incubated with HeLa cells for 4 h | Internalization | Cubic particles with side length of 2 μm | Gratton et al., 2008b |
| Cylindrical particles of D = 0.5 or 1 μm and H = 1 μm | Cationic cross-linked PEG hydrogel | Incubated with HeLa cells for 4 h | Internalization | No difference | Gratton et al., 2008b |
| Cylindrical particles of D = 150 nm and H = 450 nm (AR = 3) or D = 100 nm and H = 300 nm (AR = 3) | Cationic cross-linked PEG hydrogel | Incubated with HeLa cells for 4 h | Internalization extent and rate | Same extent; cylindrical particles of D = 150 nm and H = 450 nm internalize faster | Gratton et al., 2008b |
| 0.7, 1, 2.5, 3 μm | Silica beads | I.V. | Accumulation in heart, tumor, kidney and brain | Increased accumulation with decreased diameter; N.B., also affected by injected dose | Decuzzi et al., 2010 |
D, diameter; H, height; AR, aspect ratio.
Physical targeting to tumor regions may not be sufficient if the drug particles cannot reach the inner tumor mass. Because tumors have a more dense extracellular matrix than normal tissue (Jain, 1990), large nanoparticles cannot always efficiently penetrate the tumor parenchyma. Delivery vehicles can be designed to release the therapeutic drug within exposed cancer cells or at the tumor region where the drug can then penetrate surrounding tissue. Most drugs can penetrate within a tumor mass once it reaches the tumor site, but many drugs become resistant and thus need to be included within subcarriers that can infiltrate deeper into the tumor mass and enter all cancer cells. Smaller particles, those around 20 nm or smaller, can diffuse into deep tumor mass more readily (Pluen et al., 2001; McKee et al., 2006; Popović et al., 2010). Wong et al. (2011) combined the capability of EPR targeting using large nanoparticles with the ability for deep tumor mass penetration using small nanoparticles. In this study, 100-nm nanoparticles reached the tumor site by circulation and EPR effect followed by degradation and release of 10-nm nanoparticles that were able to better penetrate into the cancer tissue (Wong et al., 2011).
B. Shape
Architectural design of biomaterial structures must be investigated in detail to fully control and understand the biological properties attributed to nanoparticles. The shape of particles is an important consideration and can have an influence on the rate of cellular uptake and body distribution. The shape of particles can be spherical, disk-like, rod-like, and flexible in shape among others (Fig. 2) (Petros and DeSimone, 2010). However, the contributions of shape have been difficult to assess because of the ease of making spherical particles and the challenge of designing other shaped particles.
Fig 2.
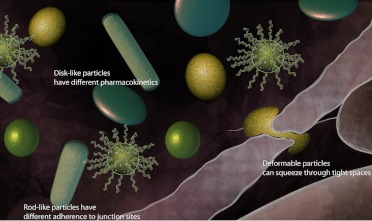
Shape effects. A particle's shape can influence the rate of cellular uptake and body distribution. Particles can be designed to have increased distribution (spherical or disk-like), adherence to junction sites (rod-like), improved uptake by cells (softness), or improved penetration into various gaps (deformable particles).
1. Effect of Shape on Uptake.
One study used a unique system to systematically control and compare the shape of nanoparticles. The system relied on a lithographic fabrication method called “particle replication in nonwetting templates” and was used to prepare cationic, nonspherical, cross-linked PEG particles (Gratton et al., 2008b). They showed that cylindrical particles are internalized by HeLa cells more efficiently than cube-shaped particles. Moreover, different cylindrical particles showed comparable extents of internalization after 4 h regardless of the aspect ratio or size. The internalization kinetics of particles having a diameter of 150 nm and height of 450 nm was significantly higher than smaller or less elongated ones. They concluded that rod-shaped particles of specific sizes could be a good choice to use as a delivery carrier. This is also in agreement with Huang et al. (2010) who showed that rod-shaped mesoporous silica nanoparticles have better uptake rates and extents compared with spherical and short rod particles in human melanoma cells. However, they report that rod-shaped particles have a negative effect on cell viability and other cellular functions.
A different study looking at gold nanoparticles demonstrated contradictory results. Chithrani et al. (2006) demonstrated that spherical nanoparticles showed better uptake by HeLa cells. Champion and Mitragotri (2006) explained the variation in uptake as a function of different shapes by using geometrically anisotropic polystyrene beads of five different shapes in addition to spherical particles. Phagocytosis by alveolar macrophages showed that the “local particle shape at the point of initial contact” affected initiation of phagocytosis; shallow curvature at this point triggers phagocytosis most effectively. Thus, particles whose shape throughout their surface is a shallow curve (e.g., spheres) are taken up efficiently, whereas those with such a shape only at certain parts of their surfaces (e.g., ellipsoids) are not. Furthermore, this study found that completion was controlled by the volume of particles, irrespective of shape. Although at optimum orientation (small angle relative to the cell surface), macrophages (radius 7.5 ± 2.5 μm) showed the ability to phagocyte particles as large as themselves, even small particles (0.2% of the macrophage volume) were not taken up if they were in unfavorable orientation. This phenomena was used to design worm-like particles that have minimum size normalized curvature to inhibit endocytosis (Champion and Mitragotri, 2009).
2. Effect of Shape on Flow.
Changing a particle's shape can modulate the distribution of particles throughout the circulatory system. For example, intravenous injection of particles will result in varying distribution and circulation when comparing spherical and nonspherical particles (Decuzzi et al., 2010). Discoidal particles seem to accumulate less in the liver but can reach the lungs and other organs. A more comprehensive study compared the flow dynamics of different-sized spherical, elliptical, circular, or rod-shaped particles. In this study, they use 1-, 3-, or 6-μm particles of different shapes and placed them through synthetic microvascular networks to evaluate and compare their flow and adherence properties (Doshi et al., 2010). The rod-shaped particles appear to have higher adhesion properties. The effect of the particle's shape is more significant when comparing larger particles, where lower adhesion of spherical particles occurs at the channel junctions. The different adhesion and flow properties can be used to improve targeting. For example, rod-shaped particles may be considered when targeting particles to the endothelium junctions because they have better adhesion than round particles.
The effect of shape on the in vivo circulation time was shown by Geng et al. (2007). They presented a copolymer with a hydrophilic chain of PEG and an inert or biodegradable hydrophobic chain. These copolymers can assemble into cylindrically shaped micelles known as filomicelles that showed longer circulation time compared with their spherical alternatives after injection into animals.
3. Red Blood Cell Simulation.
Small natural objects such as red blood cells can be used as inspiration for the shape of particles. Several groups have studied red blood cells (RBCs) as a model microstructured vesicle that is biconcave, 8 μm in diameter, and able to deform and pass through small blood vessels and sinusoidal pores in the spleen. Can particles be designed to imitate these properties? Various studies have attempted to simulate RBCs' unique deformability by using polymeric microparticles. Shape was concluded to be one of the main factors that contributes to deformability of particles and determines their ultimate biological properties. Haghgooie et al. synthesized PEG hydrogel microparticles into four different shapes (disks, rings, crosses, and S shapes) to study their flow behavior in microfluidic channels (Haghgooie et al., 2010). Although all four shapes affected the flexibility of the microparticles, S-shaped ones were reported to be the highest in flexibility, whereas disks were the least. No biological experiments were done to test the consequences of these differences in flexibility.
RBCs were also mimicked using low-density cross-linked hydrogel disks prepared using the particle replication in nonwetting templates method. Pharmacokinetics and distribution of these particles were studied after intravenous injection in mice and demonstrated that these RBC-like particles had a much longer circulation half-life (Merkel et al., 2011). In another study, poly(4-styrenesulfonate) and hemoglobin were assembled layer by layer to produce RBC-shaped particles (Doshi et al., 2009). These particles were described as able to pass through small channels carrying oxygen, drugs, or imaging agents.
C. Particle Stiffness
The rigidity of particles has a clear effect on distribution, as mentioned previously with soft and flexible particles that mimic RBCs (section II.B.3), but it can also affect cellular uptake. The differences in rigidity can be studied by changing the cross-linking density to alter the rigidity of hydrogel particles. Only a few studies have been done to show that different types of cells have different preferences for particle uptake. For example, HeLa cells can take up soft particles better than harder ones (You and Auguste, 2009), whereas macrophages prefer hard ones (Beningo and Wang, 2002). Because of the different applications and different cellular targets, more investigation is warranted to compare the same particles in different cell lines. Some studies looking at particle size as the variable may also be indirectly looking at rigidity, which can also influence the results. This is a critical challenge of these studies, which persists in all studies on the biological consequences of particle physiochemical properties. More mechanisms that can allow the evaluation of each variable separately are needed.
D. Charge
The net charge of particles can affect the circulation half-life, tissue retention, and/or cell entry capabilities and should be well understood to improve the delivery of particles. Several groups have assessed the charge presented on the particle's surface to assess cellular uptake. Most studies are in agreement with the observation that there is better uptake of positively charged particles in most cell lines with some exceptions for certain macrophages (Gratton et al., 2008a) and some stem cells (Lorenz et al., 2006; Chung et al., 2007). Better uptake of cationic particles may be due to the electrostatic interaction with the cell membrane. On the other hand, in vivo distribution of 150-nm particles having a ζ-potential of ∼−15 mV showed more efficient accumulation at the tumor site compared with more negative or positive particles or bigger particles (He et al., 2010). Because cationic particles can improve their uptake but cause undesired interaction with any cell type (Dellian et al., 2000), negatively charged particles with longer circulation half-lives may be designed to switch to positively charged ones at the site of action (Sankaranarayanan et al., 2010).
E. Polyethylene Glycolation
Opsonization occurs when foreign organisms or particles are covered with proteins that function in directing phagocytic cells toward them. The net charge carried by particles or their hydrophobic character can cause undesirable interaction with opsonin proteins (Chonn et al., 1991; Müller et al., 1992; Norman et al., 1992). Once particles are opsonized, macrophages in the reticuloendothelial system initiate phagocytosis and cause immediate removal from the bloodstream before delivering the therapeutic cargo. Addition of poly(ethylene glycol) (PEG) to the particle's surface, known as PEGylation, can be used to cover any undesired charge or surface properties and effectively prolong their circulation time to achieve their intended application (Gref et al., 1994; van Etten et al., 1995; Mainardes et al., 2009; Shan et al., 2009) (Fig. 3). PEGylation of particles must have the right surface coverage to function as a good stealth system. Low surface coverage forms loose mushroom-like structures and results in open spaces between the PEG chains to allow opsonin proteins to interact with the particles. Dense surface coverage will form a brush-like configuration when presented on the surface of the particles and can cause particles to lose their flexibility, decreasing its steric hindrance properties (Storm et al., 1995; Owens and Peppas, 2006). To reach the optimal PEGylation density, polymer molecular weight, length, branching, and particle formulation procedures are variables that can affect the final results and must be empirically determined for each system (Gref et al., 1994; Photos et al., 2003; Prencipe et al., 2009; Shan et al., 2009). Although PEGylation can give particles stealth power for longer circulation times, it has some drawbacks. If the particles are required to be taken up by cells, PEGylation can be a factor that decreases their uptake (Ehrenberg et al., 2009; Mainardes et al., 2009). To overcome this hurdle, several groups have designed particles to have the PEG chain removed when it reaches the target area (Boomer et al., 2003; Romberg et al., 2008; Hatakeyama et al., 2009). These advanced particles have long circulation times to reach the target tissue where they shed the PEG chains, thus exposing charged particles that promote cellular uptake.
Fig 3.
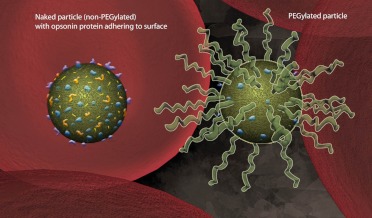
PEG provides stealth. Particles can be PEGylated to cover surface properties and neutralize the net charge to effectively reduce binding by opsonin proteins and eventual clearance by macrophages. This strategy can prolong the circulation time of particles in the body.
III. Chemical Targeting
To improve delivery and reduce unwanted side effects, the nanotechnology field is beginning to evolve and take advantage of biological, chemical, and physical properties of tissues and individual cells to improve targeting. We will loosely define this field as chemical targeting. Chemical targeting of nanoparticles involves multiple advanced strategies that may include materials that are responsive to pH, reactive oxygen species, heat, proteases, external physical stimuli (light, heat, magnetic field) or targeting through specific binding and cell-penetrating peptides. The multiple strategies can be applied independently or combined to improve therapeutic delivery.
A. Ligands and Antibodies
To achieve higher specificity, nanoparticles can be conjugated with targeting moieties on the surface that bind specifically to receptors or other molecular structures on the cell's surface (Fig. 4). In Table 3, we list a few examples of chemical targeting via antibodies or ligands that improve delivery.
Fig 4.
Cell-specific targeting using antibody-conjugated particles. Antibodies that bind specifically to receptors or other structures on the cell's outer membrane can be used for chemical targeting and improve therapeutic delivery. EGFR is a common target receptor that is overexpressed in cancer cells and used as a way to deliver therapeutics to those cells.
TABLE 3.
Chemical targeting using ligands or antibodies
| System | Target | Ligand/Antibody | Reference |
|---|---|---|---|
| PLGA | EGFR | Anti-EGFR antibody | Acharya et al., 2009 |
| PEG-coated chitosan | TfR; receptor-mediated transport across the BBB | Anti-OX26 antibody | Aktaş et al., 2005 |
| PLGA-PEG | PSMA on prostate cancer cells | Anti-PSMA ligand (DCL) | Sanna et al., 2011 |
| PLGA | αvβ3 Receptor overexpressed on cancerous and endothelial cells | Arg-Gly-Asp (RGD) sequence | Wang et al., 2011 |
| Amphiphilic block copolymers (PEG-PBLA) | Cancer cells overexpressing FBPs | Folate | Bae et al., 2007; De et al., 2008 |
| Acid-degradable polymer | DEC-205-expressing DCs | Anti-DEC-205 antibody | Kwon et al., 2005; Bandyopadhyay et al., 2011 |
| PLGA-PEG | C-type lectin receptor DC-SIGN on DCs | Anti-hD1 antibody | Cruz et al., 2010 |
| PLGA | Mammalian CD8 T cells | Anti-CD8 antibody | Bicho et al., 2010 |
| Polymers based on HPMA | Hepatic ASGPR | Galactosamine | Seymour et al., 2002 |
TfR, transferrin receptor; PSMA, prostate-specific membrane antigen; FBP, folate-binding protein; HPMA, N-(2-hydroxypropyl)methacrylamide; ASGPR, asialoglycoprotein receptor.
Advances in antibody production and their accepted therapeutic value, underscored by numerous U.S. Food and Drug Administration-approved antibody therapeutics, make these biological molecules excellent candidates for nanoparticle targeting. Antibodies are also easily conjugated to multiple types of particles, with PLGA being the preferred polymeric system due to its Food and Drug Administration validation. Nanoparticles can be adsorbed or conjugated to antibodies by using a variety of methods, including biotin-streptavidin, thiolation, or cross-linking agents such as bis(sulfosuccinimidyl) suberate. Because antibodies are “polar” in their binding activity, it is also important to consider orientation of the binding versus random binding. This problem can be addressed by taking advantage of the sugar molecule normally found in the Fc region of antibodies or bioengineered active groups. Regardless of orientation, it seems that a significant amount of active binding sites must be present after antibody conjugation to prove useful. The ability to have active targeting sites accessible and not bound to the particle depends on conjugation techniques.
1. Blood-Brain Barrier.
Antibody conjugated nanoparticles have been employed to overcome the blood-brain barrier (BBB) when targeting the brain. The BBB results from the selectivity of the tight junctions between endothelial cells in central nervous system vessels that restricts the passage of microscopic objects, hydrophobic molecules, and most proteins. Overcoming the difficulty of targeted delivery of therapeutic agents to the brain presents a major challenge to treatment of most brain disorders. Aktaş et al. (2005) demonstrated the potential of targeting chitosan nanoparticles to the brain using a mouse monoclonal antibody, OX26, against the rat transferrin receptor. The transferrin receptor has been reported to be in high abundance at the brain microvascular endothelium (Chen et al., 1998). Their goal was the inhibition of caspase-3-like protease activity using encapsulated N-benzyloxycarbonyl-DEVD-fluoromethyl ketone peptide because of its therapeutic significance in the treatment of stroke and related neurological disorders. Their strategy involved the use of biotin-labeled PEG to coat chitosan nanoparticles and streptavidin-labeled antibodies for the conjugation. The resulting “smart” cationic particles are thought to interact with the negative charges of the brain endothelium and allow for targeted-induced transport across the BBB. Indeed, they show that OX26-conjugated nanoparticles can penetrate into the brain whereas the OX26-free NPs cannot (Aktaş et al., 2005).
2. Targeting Cancer.
Nanoparticle targeting with antibodies or ligands has been greatly applied in cancer and immunological diseases. For instance, several strategies take advantage of popular cell surface targets that are overexpressed by cancer cells. The most common targets include the epidermal growth factor receptor (EGFR), vascular endothelial growth factor, folate receptor, the transferrin receptor, and several glycoproteins, among others. EGFR, a receptor highly expressed in breast cancer, has been used with PLGA nanoparticles containing rapamycin that were surface-conjugated with an EGFR antibody (Acharya et al., 2009). In vitro analysis shows that the antibody-conjugated particles offer cancer cell growth control greater than that of nanoparticles without targeting or just rapamycin by itself (Acharya et al., 2009). Although in vitro results usually do not reflect what happens in vivo, current proof-of-concept experiments support the notion that targeting will help improve therapeutic delivery.
Antibody-conjugated nanoparticles can also be used for the targeted delivery of nucleic acids. The use of siRNA as a therapeutic has shown a lot of promise, but the main obstacle is preventing it from degrading upon delivery and improved targeting. CALAA-01, a nanoparticle formulation to deliver siRNA in tumor cells, is the first nanoparticle of its type to enter human clinical trials (Davis, 2009). The nanoparticle consists of a cyclodextrin-containing polymer and PEG. Similar to the strategy used to target and enter the BBB, human transferrin was used as a targeting ligand for binding to transferrin receptors that are typically up-regulated on cancer cells. Developments resulting from CALAA-01 clinical trials will help pave the way when considering the design, safety, and efficacy of targeted siRNA delivery systems (Eifler and Thaxton, 2011).
3. Targeting for Vaccines.
Several groups have also leveraged the power of conjugated nano- and microparticles for improved delivery and activation of antigen-presenting cells. Dendritic cells are thought to be the most potent antigen-presenting cells for the stimulation of naive T cells but are difficult to target because of their low numbers compared with macrophages. For vaccines, an ideal target molecule has been DEC 205 because of its specificity in dendritic cells (DCs) and its ability to provide receptor-mediated endocytosis (Kwon et al., 2005; Cruz et al., 2010; Bandyopadhyay et al., 2011). In vivo results from vaccinations using nanoparticles with DEC-205 targeting have shown mixed results but are nevertheless encouraging. Kwon et al. (2005) demonstrated that there is improved targeting of nanoparticles using DEC 205 in the draining inguinal lymph nodes and show a higher percentage of T cells expressing IFN-γ, but no targeting was observed in the mesenteric or popliteal lymph nodes. Cross-linking densities must also be considered when constructing conjugated nanoparticles as this may affect DC targeting and activation (Bandyopadhyay et al., 2011).
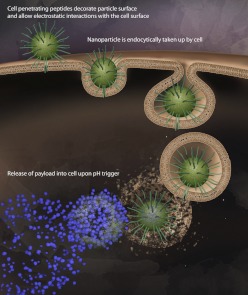
B. Cell-Penetrating Peptides
Physical and antigen-based chemical targeting facilitates the localization of particles to the target region; however, in applications where cell internalization is critical to therapeutic outcome, physical and even some chemical targeting approaches fall short. Although some forms of specific targeting enhance uptake by promoting receptor-mediated endocytosis, others do not. To improve cellular entry and delivery of drugs within cells, researchers have taken a page from the playbook of viruses with some considerable success over the past 20 years (Brasseur and Divita, 2010). Cell-penetrating peptides (CPPs) are peptides that are traditionally derived from viral structures that are employed to assist particles to enter cells (Deshayes et al., 2005) (Fig. 5). The most commonly employed peptide sequence is the arginine rich TAT peptide derived from HIV that has been successfully used with nanoparticles to improve cellular entry (Frankel and Pabo, 1988; Ziegler et al., 2005; Torchilin, 2008). Enhanced CPPs derived from TAT are usually arginine-rich peptides and have been engineered to be severalfold more efficient at cellular entry (Wender et al., 2000). More than 30 peptides have been identified with varying composition and properties and are usually classified as cationic, amphipathic with a large fraction of basic residues, or hydrophobic (Fischer et al., 2005).
Fig 5.
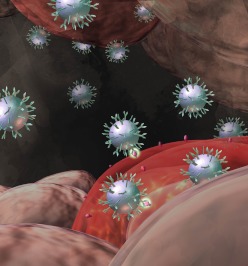
CPPs. CPP-conjugated nanoparticles can be employed to increase entry into multiple types of cells, including nonphagocytic cells. Combining CPPs with particles composed of pH-responsive polymers allows efficient entry into cells followed by cargo release in response to acidic conditions of the endosome.
Cellular studies using CPP-conjugated nanoparticles have demonstrated the capabilities of this approach to efficiently target nanoparticles to nonphagocytic cells. In one example, a polymeric micelle that has a hydrophobic core made of poly(l-lactic acid) and a hydrophilic shell consisting of PEG conjugated to TAT was used to effectively improve cellular uptake of nanoparticles. Using microscopy and flow cytometry analysis, they demonstrate that TAT attached to the micelles helped translocate the nanoparticles into the cells and near the nucleus, suitable for drugs targeting transcription factors (Sethuraman and Bae, 2007). A similar study used the 9-mer arginine peptide conjugated to acid-degradable polymeric nanoparticles and demonstrated that this delivery vehicle is effective at promoting particle uptake in nonphagocytic epithelial cells (Cohen et al., 2008). Beyond in vitro models, the use of CPP-conjugated nanoparticles is still in its infancy but will continue to be part of combined targeting approaches as a result of the attractive feature of the use of CPPs to remove the constraint of molecules that are intrinsically incapable of cellular uptake. Furthermore, CPPs alone may only allow improved cell entry but may still limit nanoparticles in their ability to target specific tissues, because pharmacokinetic studies of CPPs attached to DOPA seem to enter into all the major organs (Sarko et al., 2010).
C. Stimuli-Responsive Nanoparticles
Using biological cues to creatively target pharmacological agents is always a good method to improve specificity and minimize side effects. However, this approach is not always available or significant given our current knowledge of biological pathways and available technology. An alternative method is to combine bioresponsive materials with internal or external physical stimuli. These stimuli may include polymeric nanoparticles responsive to changes in pH, reactive oxygen species, temperature, and light, among others (Table 4).
TABLE 4.
Examples of stimuli responsive strategies
| Response to | Target | Examples |
|---|---|---|
| pH | Acidic compartments found at pathological tissues (tumor sites) or within cells in the endosomes. | Gao et al., 2005; Bae et al., 2007; Cohen et al., 2008, 2009; Lee et al., 2008 |
| Thermal | Heat applied external or from physiologically changes | De et al., 2008; Rahimi et al., 2008; Peng et al., 2011 |
| Light | Externally applied light | Suzuki and Tanaka, 1990; Goodwin et al., 2005; Jiang et al., 2006; Fomina et al., 2010 |
| Redox | Regions where reactive oxidative and reductive species are abundant (inflammation and tumor sites) | Napoli et al., 2004; Rehor et al., 2005; Reddy et al., 2006; Wilson et al., 2010; Mahmoud et al., 2011 |
| Enzyme | Regions of high protease expression such as cancerous tissue | Olson et al., 2009; Andresen et al., 2010; Whitney et al., 2010; van Duijnhoven et al., 2011; Wong et al., 2011 |
1. Protease-Activated Systems.
Tumor expansion followed by metastasis requires a breakdown of extracellular matrix by proteases. The most commonly studied proteases are matrix metalloproteases 2 and 9 (MMP-2 and MMP-9), because they are highly expressed in the tumor microenvironment and promote angiogenesis for the tumor (Turpeenniemi-Hujanen, 2005). These proteases usually cleave a conserved peptide sequence. Delivery systems have been developed to take advantage of this specific protease activity and implement a short cleavable sequence to target release of cargo or activate an additional targeting module such as a cell-penetrating peptide (Olson et al., 2009). A more elaborate, multistage nanoparticle system used physical targeting via the EPR effect to target leaky vesicles followed by protease-dependent release of smaller nanoparticles (Wong et al., 2011). The smaller (10 nm) nanoparticles were then able to penetrate deep in the tumor, which is required for delivery into the tumor's dense collagen matrix.
Using only MMP-2 or MMP-9 for protease targeting may not be suitable for all tissues or tumors. In fact, a biodistribution study to analyze tumor targeting of MMP activity revealed that metalloproteinase targeting is not as efficient as previously believed and is most likely caused by cleavage in the vascular compartment rather than tumor-specific cleavage (van Duijnhoven et al., 2011). To improve protease targeting of activatable cell-penetrating peptides, Whitney et al. (2010) developed a novel screen using phage display to identify unique tumor-specific proteases. They identified a peptide sequence that is cleaved not by metalloproteinases but by plasmin and elastases. Both of these enzymes were demonstrated to be highly overexpressed by tumors in mice. These advancements will continue to add specificity and improve the way nanoparticle systems can be targeted.
2. pH.
Nanoparticles that are triggered by a decrease in pH have been an attractive system for improved delivery of encapsulated therapies. pH-responsive systems can be applied for external (tumor sites) and internal (endosomes) cellular release (Shen et al., 2008). The extracellular matrix of tumor sites has a relatively low pH (pH ∼6.5) due to the cell's high metabolic activity and limited oxygen availability. Cancer cells respond to this microenvironment by undergoing anaerobic glycolysis resulting in higher production of lactic acid. Several studies have successfully developed and demonstrated the potential of using this particular pathological stimuli to deliver encapsulated drugs (Gao et al., 2010). For example, delivery of doxorubicin encapsulated in a poly(l-histidine)/PEG-based polymeric system seems to improve the circulation half-life and increases the local drug concentration in low-pH tumor sites (Gao et al., 2005).
Nanoparticles can also be triggered to release drugs once they are taken up by cells via endocytosis (You and Auguste, 2009). As nanoparticles are taken up by cells, they progress through the endocytic pathway and eventually to the lysosome. The early endosome begins to acidify within minutes and progressively becomes more acidic as it moves toward the lysosomes where the pH can be as low as 4. The pH difference within endosomes is an attractive system because particles can be maintained in stable conditions until they enter the targeted cells. In addition, changes in ionic strength causes a proton sponge effect in certain cationic buffering polymers that can allow the endosome to release encapsulated material. Delivery of drugs using pH-responsive nanoparticles via the endosome and into the cytoplasm is very effective and in some cases seems also to improve drug resistance (Lee et al., 2005).
Delivery of nucleic acid therapeutics for gene or siRNA delivery seems to be very promising when delivered using pH-responsive materials. DNA or siRNA delivery is very inefficient when delivered by itself as a result of degradation from nucleases and the inability for these large negatively charged molecules to cross the membrane. However, even if nucleic acids cross the membrane and enter through the endosome, they can become degraded in the lysosome if they are not released into the cytoplasm. Convertine et al. (2010) used diblock copolymer nanoparticles, incorporating poly(propylacrylic acid) to induce a hydrophobic switch upon low pH, for the delivery of siRNA and demonstrate in vitro a significant knockdown of GAPDH mRNA levels using low siRNA concentrations. Delivery of siRNA using pH responsive nanoparticles is still in its early stages but is already creating a buzz in this growing field (Keller, 2009; Ozpolat et al., 2010; Shim and Kwon, 2010).
A combination of pH-responsive nanoparticles with other targeting strategies can also help to further target and enhance the therapeutic outcome. Combining cell-penetrating peptides with pH-responsive nanoparticles has been successfully used to efficiently enter and then release drug content into nonphagocytic cells (Cohen et al., 2008). One group combined three strategies for improved delivery: PEGylation, pH response, and cell-penetrating peptides. They used stealth nanoparticles designed to take advantage of pH changes to shed their PEG molecules, which resulted in exposure of cell-penetrating peptides to enter cells (Lee et al., 2008).
3. Temperature.
Thermally responsive polymeric nanoparticles can be used to take advantage of local temperature differences or via external heat application. Nanoparticles that contain temperature-sensitive polymers can shrink or expand when triggered by heat. The balance between the hydrophobic and hydrophilic segments determines the sensitivity of heat. Temperature-responsive polymeric systems have been successfully applied for controlled release of drugs or proteins from gels; however, only a few groups have shown success in control release of drugs from particles in the physiological temperature range. Particles made with poly(N-isopropylacrylamide) polymers are the type most commonly used for thermoresponsive systems (Zhou et al., 2007; Zhao et al., 2011). It has been successfully used to encapsulate doxorubicin in poly(N-isopropylacrylamide-coacrylamide-coallylamine) nanoparticles, where it can be triggered to release its content at a temperature of 41°C, just above body temperature (Rahimi et al., 2008). Recent in vivo studies are also beginning to show some promising results to treat cancer using thermosensitive nanoparticles based on poly(N-isopropylacrylamide-co-((2-dimethylamino)ethylmethacrylate) copolymers. For instance, the chemotherapeutic drug SN-38 can be delivered using thermosensitive nanoparticles to efficiently suppress colon tumor growth when combined with hyperthermia (Peng et al., 2011). Poly(N-isopropylacrylamide)-based systems are limited by the polymer's toxicity, immunogenicity, and short blood circulation time, but these could be overcome by PEGylation or other creative designs to shield the thermosensitive portion of the nanoparticle (Zhao et al., 2011). This field of thermoresponsive nanoparticles is still in the emerging stage but will likely be useful for therapeutic applications requiring localized controlled release.
4. Light.
Light-activated release of pharmacological agents is one of the most promising controlled delivery systems for specific targeting that has begun to emerge with exciting possibilities. Having spatial and timed control release of a drug complex is highly desired when treating localized diseases such as cancer. One can imagine a system where release of a drug only occurs when a physician remotely shines light or a laser to release the drug locally, leaving all other tissue, including skin, intact (Fig. 6).
Fig 6.
Light-responsive nanoparticles. Emerging delivery systems can be designed to degrade and release their therapeutic cargo upon light activation using UV, visible, or near-infrared light. The goal of this technology is to precisely control the time and location of particle degradation within a person's body and thereby minimize side effects.
Light-activated particles are created using optically active substances such as functional dyes, metals, and light-sensitive polymers that are capable of degrading or releasing their cargo (Bédard et al., 2010). Light-responsive polymers have been designed to respond to UV, visible light, and near-infrared (NIR) light. Particle encapsulation and UV-triggered release of drug compounds can be developed using amphiphilic block compolymers composed of poly(ethylene oxide) and poly (2-nitrobenzyl methacrylate) (Jiang et al., 2006). UV light has the highest power and can break polymeric bonds more easily but can also damage surrounding tissue and skin. Particles that are responsive to visible light instead of UV offer a less harmful alternative. Light-responsive systems can incorporate a chromophore that can absorb light, which is then dissipated locally as heat, ultimately altering the swelling of the particle (Suzuki and Tanaka, 1990). These systems have limitations, however, because most visible light cannot penetrate well into tissue, and the reactions are slow when converting light into thermal energy.
NIR light between 750 and 1000 nm has been shown to penetrate the skin more deeply with minimal risk to surrounding tissue or skin. This optical window presents an opportunity to develop novel stimuli-responsive systems to control delivery of pharmaceuticals in a harmless yet effective manner. Advances in this field have demonstrated promising results. For example, a micellar composition of PEG and 2-diazo-1,2-naphthoquinone were combined to form NIR light-triggered particles that can release their cargo when irradiated with a laser emitting at 795 nm (Goodwin et al., 2005). Polymers with even more sensitivity have been developed using self-immolative monomers that can potentially sense a single triggering event to degrade the entire particle (Fomina et al., 2010). In vitro studies show that these polymeric nanoparticles can be used to encapsulate small molecules and can be released rapidly, followed by degradation, making them suitable for biomedical applications. Although the field of NIR light-responsive particles is relatively new, it seems to be one the most promising systems to control drug delivery.
D. Coopting Cell Migration
A few groups have attempted the innovative strategy of targeting nanoparticles by co-opting the ability of certain cell types to migrate to particular tissues. Although only one of these involved polymeric particles (Cheng et al., 2010), the strategy used with liposomes could also be applied. Cheng et al. (2010) demonstrated that bone marrow-derived mesenchymal stem cells can carry commercial polystyrene nanospheres, attached via biotin-avidin affinity, to tumor microspheres in vitro. Alternatively, nanoparticles can be covalently attached, for example by decoration with maleimide moieties, which react with cell-surface thiols (Stephan et al., 2010). The latter study demonstrated that cell-attached liposomes can release their contents; however, whether this approach increases efficiency of nanoparticle delivery to the target tissue remains to be determined. The molecules released in that case were cytokines intended to enhance expansion of the T cells to which they were attached. The goal of enhanced delivery could be a challenge because cells may internalize the attached particles before reaching the target. In any case, translating this strategy into a clinically relevant system would require creative strategies to avoid inducing immune responses.
IV. Conclusion
Targeting strategies can be divided into two categories: physical and chemical targeting. Physical targeting involves physical parameters and, with certain fabrication techniques, offers a commercially viable method of efficiently delivering therapeutic particles to diseased tissue and cells. Fabrication techniques are necessarily varied and thus produce various degrees of polydispersity, thereby affecting conclusions on the effects of these parameters on biological interactions in vivo and in vitro. Exacerbating these issues is the fact that measurements and data analysis of size and polydispersity are not straightforward and can produce measurements that do not accurately describe the nature of these small particles. Both of these limits, fabrication and characterization of nanoparticles, will be overcome eventually. Nevertheless, particle size has proven to be important to targeting cancer and is a much more economically viable method than chemical targeting. Larger particles take advantage of the EPR effect and thus accumulate in the tumor site. However, larger particles are unable to penetrate into tumors the way smaller particles can. Therefore, structures with smart and dynamic size properties seem to hold promise in circumventing this seemingly no-win situation. For immunological applications, smaller particles better target dendritic cells in lymph nodes, whereas larger particles better target DCs in the periphery.
In investigating affects of shape, the limits have been fabrication techniques; new techniques developed have enabled recent insights on shape effects. There is still much to do in this area within the limits of the available fabrication techniques.
Recently the stiffness of a particle was shown to be useful in targeting uptake by certain cell types. For example, HeLa cells are better able to engulf soft particles whereas macrophages are better able to engulf harder particles. This could be the reason why some studies show particles that are softer (because of a low cross-linking density) and circulate for longer (possibly because of reduced macrophage uptake).
Chemical targeting can be split into two categories: ex vivo and in vivo strategies. The most well studied in vivo strategies involve molecular recognition. However, these are generally limited by the tendency of targeting moieties to enhance delivery to unwanted organs (i.e., spleen and liver) even more than they enhance delivery to intended tissues. For example, modification of PEG-PLGA particles with tumor antigen-binding aptamer increases accumulation in tumors from 0.5 to 1.2%, but simultaneously increases accumulation in liver from 25 to 35% (Gu et al., 2008). An alternative approach to specific targeting involves responsive systems that can recognize a diseased biochemical environment. Ex vivo conditions include light-activated systems. In terms of in vivo chemical-targeting efforts, antibody conjugation is a good strategy when significant amounts of active binding sites are present after conjugation. The field of optically and thermally responsive nanoparticles is still in its infancy but will likely be useful for therapeutic applications requiring localized release.
Nanotechnology has been improving and will eventually be used to treat multiple diseases that have few or no current effective therapy. The number of researchers with diverse training in this field is growing and will help lead to novel developments in chemistry, engineering, and biomedical applications. Interdisciplinary collaborations in this field will continue to revolutionize how we deliver drugs, plasmids, siRNA, proteins, and diagnostic agents. We look forward to the promise of polymeric systems, as they will continue to improve and offer new ways to control release, improve targeting, and limit toxicity.
Acknowledgments
This work was supported by a University of California San Diego Institutional Research and Academic Career Development Award Fellowship through the National Institutes of Health National Institute of General Medical Sciences [Grant GM06852] (to J.M.); the National Institutes of Health Director's New Innovator Award [Grant 1-DP2-OD006499-01]; and King Abdulaziz City for Science and Technology.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Morachis, Mahmoud, and Almutairi.
This article is available online at http://pharmrev.aspetjournals.org.
- BBB
- blood-brain barrier
- CPP
- cell-penetrating peptide
- DC
- dendritic cell
- EGFR
- epidermal growth factor receptor
- EPR
- enhanced permeability and retention
- MMP
- matrix metalloprotease
- NIR
- near-infrared
- PEG
- poly(ethylene glycol)
- PEGylation
- polyethylene glycolation
- PLGA
- poly(lactide-coglycolide)
- RBC
- red blood cell
- siRNA
- small interfering RNA
- SN-38
- 7-ethyl-10-hydroxycamptothecin.
References
- Acharya S, Dilnawaz F, Sahoo SK. (2009) Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 30:5737–5750 [DOI] [PubMed] [Google Scholar]
- Aktaş Y, Yemisci M, Andrieux K, Gürsoy RN, Alonso MJ, Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Riguera R, Sargon MF, et al. (2005) Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug Chem 16:1503–1511 [DOI] [PubMed] [Google Scholar]
- Andresen TL, Thompson DH, Kaasgaard T. (2010) Enzyme-triggered nanomedicine: drug release strategies in cancer therapy. Mol Membr Biol 27:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Nishiyama N, Kataoka K. (2007) In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug Chem 18:1131–1139 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. (2011) The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials 32:3094–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard MF, De Geest BG, Skirtach AG, Möhwald H, Sukhorukov GB. (2010) Polymeric microcapsules with light responsive properties for encapsulation and release. Adv Colloid Interface Sci 158:2–14 [DOI] [PubMed] [Google Scholar]
- Beningo KA, Wang YL. (2002) Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 115:849–856 [DOI] [PubMed] [Google Scholar]
- Bicho A, Peça IN, Roque AC, Cardoso MM. (2010) Anti-CD8 conjugated nanoparticles to target mammalian cells expressing CD8. Int J Pharm 399:80–86 [DOI] [PubMed] [Google Scholar]
- Boomer JA, Inerowicz HD, Zhang ZY, Bergstrand N, Edwards K, Kim JM, Thompson DH. (2003) Acid-triggered release from sterically stabilized fusogenic liposomes via a hydrolytic DePEGylation strategy. Langmuir 19:6408–6415 [Google Scholar]
- Brasseur R, Divita G. (2010) Happy birthday cell penetrating peptides: already 20 years. Biochim Biophys Acta 1798:2177–2181 [DOI] [PubMed] [Google Scholar]
- Buse J, El-Aneed A. (2010) Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomedicine 5:1237–1260 [DOI] [PubMed] [Google Scholar]
- Chakravarthi SS, De S, Miller DW, Robinson DH. (2010) Comparison of anti-tumor efficacy of paclitaxel delivered in nano- and microparticles. Int J Pharm 383:37–44 [DOI] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. (2006) Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 103:4930–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. (2009) Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 26:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. (1998) Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 18:4914–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Kastrup CJ, Ramanathan R, Siegwart DJ, Ma M, Bogatyrev SR, Xu Q, Whitehead KA, Langer R, Anderson DG. (2010) Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano 4:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithrani BD, Ghazani AA, Chan WC. (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668 [DOI] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. (2007) Renal clearance of quantum dots. Nat Biotechnol 25:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonn A, Cullis PR, Devine DV. (1991) The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. The Journal of Immunology 146:4234–4241 [PubMed] [Google Scholar]
- Chung TH, Wu SH, Yao M, Lu CW, Lin YS, Hung Y, Mou CY, Chen YC, Huang DM. (2007) The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3–L1 cells and human mesenchymal stem cells. Biomaterials 28:2959–2966 [DOI] [PubMed] [Google Scholar]
- Cohen JA, Beaudette TT, Tseng WW, Bachelder EM, Mende I, Engleman EG, Fréchet JM. (2009) T-cell activation by antigen-loaded pH-sensitive hydrogel particles in vivo: the effect of particle size. Bioconjug Chem 20:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Almutairi A, Cohen JA, Bernstein M, Brody SL, Schuster DP, Fréchet JM. (2008) Enhanced cell penetration of acid-degradable particles functionalized with cell-penetrating peptides. Bioconjug Chem 19:876–881 [DOI] [PubMed] [Google Scholar]
- Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. (2010) pH-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules 11:2904–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Madrigal-Estebas L, McClean S, Brayden DJ, Mills KH. (2001) Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine 19:1940–1950 [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, Torensma R, Figdor CG. (2010) Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J Control Release 144:118–126 [DOI] [PubMed] [Google Scholar]
- Davis ME. (2009) The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 6:659–668 [DOI] [PubMed] [Google Scholar]
- De P, Gondi SR, Sumerlin BS. (2008) Folate-conjugated thermoresponsive block copolymers: highly efficient conjugation and solution self-assembly. Biomacromolecules 9:1064–1070 [DOI] [PubMed] [Google Scholar]
- Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. (2010) Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release 141:320–327 [DOI] [PubMed] [Google Scholar]
- Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. (2000) Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer 82:1513–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. (2005) Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci 62:1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. (2010) Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Control Release 146:196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S. (2009) Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci USA 106:21495–21499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg MS, Friedman AE, Finkelstein JN, Oberdörster G, McGrath JL. (2009) The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 30:603–610 [DOI] [PubMed] [Google Scholar]
- Eifler AC, Thaxton CS. (2011) Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol Biol 726:325–338 [DOI] [PubMed] [Google Scholar]
- Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. (1991) Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun 59:2978–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. (2005) Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem 6:2126–2142 [DOI] [PubMed] [Google Scholar]
- Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. (2010) UV and near-IR triggered release from polymeric nanoparticles. J Am Chem Soc 132:9540–9542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193 [DOI] [PubMed] [Google Scholar]
- Gao W, Chan JM, Farokhzad OC. (2010) pH-Responsive nanoparticles for drug delivery. Mol Pharm 7:1913–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Lee DH, Kim DI, Bae YH. (2005) Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J Drug Target 13:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. (2007) Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AP, Mynar JL, Ma Y, Fleming GR, Fréchet JM. (2005) Synthetic micelle sensitive to IR light via a two-photon process. J Am Chem Soc 127:9952–9953 [DOI] [PubMed] [Google Scholar]
- Gratton SE, Napier ME, Ropp PA, Tian S, DeSimone JM. (2008a) Microfabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm Res 25:2845–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. (2008b) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. (1994) Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603 [DOI] [PubMed] [Google Scholar]
- Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. (2008) Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA 105:2586–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierro I, Hernández RM, Igartua M, Gascón AR, Pedraz JL. (2002) Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine 21:67–77 [DOI] [PubMed] [Google Scholar]
- Haghgooie R, Toner M, Doyle PS. (2010) Squishy non-spherical hydrogel microparticles. Macromol Rapid Commun 31:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. (2000) Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 156:1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, Harashima H. (2009) A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release 139:127–132 [DOI] [PubMed] [Google Scholar]
- He C, Hu Y, Yin L, Tang C, Yin C. (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31:3657–3666 [DOI] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 95:4607–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Barua S, Sharma G, Dey SK, Rege K. (2011) Inorganic nanoparticles for cancer imaging and therapy. J Control Release 155:344–357 [DOI] [PubMed] [Google Scholar]
- Huang X, Teng X, Chen D, Tang F, He J. (2010) The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 31:438–448 [DOI] [PubMed] [Google Scholar]
- Jain RK. (1990) Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev 9:253–266 [DOI] [PubMed] [Google Scholar]
- Jiang J, Tong X, Morris D, Zhao Y. (2006) Toward photocontrolled release using light-dissociable block copolymer micelles. Macromolecules 39:4633–4640 [Google Scholar]
- Jung T, Kamm W, Breitenbach A, Hungerer KD, Hundt E, Kissel T. (2001) Tetanus toroid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): evaluation of antibody response after oral and nasal application in mice. Pharm Res 18:352–360 [DOI] [PubMed] [Google Scholar]
- Katare YK, Muthukumaran T, Panda AK. (2005) Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int J Pharm 301:149–160 [DOI] [PubMed] [Google Scholar]
- Keller M. (2009) Nanomedicinal delivery approaches for therapeutic siRNA. Int J Pharm 379:210–211 [DOI] [PubMed] [Google Scholar]
- Kumari A, Yadav SK, Yadav SC. (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75:1–18 [DOI] [PubMed] [Google Scholar]
- Kwon YJ, James E, Shastri N, Fréchet JM. (2005) In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci USA 102:18264–18268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. (2008) Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J Control Release 129:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. (2005) Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release 103:405–418 [DOI] [PubMed] [Google Scholar]
- Lorenz MR, Holzapfel V, Musyanovych A, Nothelfer K, Walther P, Frank H, Landfester K, Schrezenmeier H, Mailänder V. (2006) Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials 27:2820–2828 [DOI] [PubMed] [Google Scholar]
- Maham A, Tang Z, Wu H, Wang J, Lin Y. (2009) Protein-based nanomedicine platforms for drug delivery. Small 5:1706–1721 [DOI] [PubMed] [Google Scholar]
- Mahmoud EA, Sankaranarayanan J, Morachis JM, Kim G, Almutairi A. (2011) Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconjug Chem 22:1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardes RM, Gremião MP, Brunetti IL, da Fonseca LM, Khalil NM. (2009) Zidovudine-loaded PLA and PLA-PEG blend nanoparticles: influence of polymer type on phagocytic uptake by polymorphonuclear cells. J Pharm Sci 98:257–267 [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. (2008) Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 38:1404–1413 [DOI] [PubMed] [Google Scholar]
- McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. (2006) Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res 66:2509–2513 [DOI] [PubMed] [Google Scholar]
- Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, et al. (2011) Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci USA 108:586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto T, Hattori Y, Takayama K, Maitani Y. (2004) Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm Res 21:671–674 [DOI] [PubMed] [Google Scholar]
- Nakaoka R, Inoue Y, Tabata Y, Ikada Y. (1996) Size effect on the antibody production induced by biodegradable microspheres containing antigen. Vaccine 14:1251–1256 [DOI] [PubMed] [Google Scholar]
- Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA. (2004) Oxidation-responsive polymeric vesicles. Nat Mater 3:183–189 [DOI] [PubMed] [Google Scholar]
- Nixon DF, Hioe C, Chen PD, Bian Z, Kuebler P, Li ML, Qiu H, Li XM, Singh M, Richardson J, et al. (1996) Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine 14:1523–1530 [DOI] [PubMed] [Google Scholar]
- Norman ME, Williams P, Illum L. (1992) Human serum albumin as a probe for surface conditioning (opsonization) of block copolymer-coated microspheres. Biomaterials 13:841–849 [DOI] [PubMed] [Google Scholar]
- O'Hagan DT, Jeffery H, Davis SS. (1993) Long-term antibody responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine 11:965–969 [DOI] [PubMed] [Google Scholar]
- Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. (2009) In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 1:382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DE, 3rd, Peppas NA. (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307:93–102 [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Sood AK, Lopez-Berestein G. (2010) Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med 267:44–53 [DOI] [PubMed] [Google Scholar]
- Peng CL, Tsai HM, Yang SJ, Luo TY, Lin CF, Lin WJ, Shieh MJ. (2011) Development of thermosensitive poly(n-isopropylacrylamide-co-((2-dimethylamino) ethyl methacrylate))-based nanoparticles for controlled drug release. Nanotechnology 22:265608. [DOI] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9:615–627 [DOI] [PubMed] [Google Scholar]
- Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. (2003) Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release 90:323–334 [DOI] [PubMed] [Google Scholar]
- Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, Brown EB, Izumi Y, Campbell RB, Berk DA, et al. (2001) Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci USA 98:4628–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon Z, Lee JA, Huang S, Prevost RJ, Hammond PT. (2011) Highly stable, ligand-clustered “patchy” micelle nanocarriers for systemic tumor targeting. Nanomedicine 7:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popović Z, Liu W, Chauhan VP, Lee J, Wong C, Greytak AB, Insin N, Nocera DG, Fukumura D, Jain RK, et al. (2010) A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed Engl 49:8649–8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai H. (2009) PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J Am Chem Soc 131:4783–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi M, Kilaru S, Sleiman GE, Saleh A, Rudkevich D, Nguyen K. (2008) Synthesis and characterization of thermo-sensitive nanoparticles for drug delivery applications. J Biomed Nanotechnol 4:482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. (2006) In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release 112:26–34 [DOI] [PubMed] [Google Scholar]
- Rehor A, Hubbell JA, Tirelli N. (2005) Oxidation-sensitive polymeric nanoparticles. Langmuir 21:411–417 [DOI] [PubMed] [Google Scholar]
- Romberg B, Hennink WE, Storm G. (2008) Sheddable coatings for long-circulating nanoparticles. Pharm Res 25:55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan J, Mahmoud EA, Kim G, Morachis JM, Almutairi A. (2010) Multiresponse strategies to modulate burst degradation and release from nanoparticles. ACS Nano 4:5930–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M. (2011) Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem 54:1321–1332 [DOI] [PubMed] [Google Scholar]
- Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, Altmann A, Haberkorn U, Mier W. (2010) The pharmacokinetics of cell-penetrating peptides. Mol Pharm 7:2224–2231 [DOI] [PubMed] [Google Scholar]
- Sethuraman VA, Bae YH. (2007) TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release 118:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ, et al. (2002) Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol 20:1668–1676 [DOI] [PubMed] [Google Scholar]
- Shan X, Liu C, Yuan Y, Xu F, Tao X, Sheng Y, Zhou H. (2009) In vitro macrophage uptake and in vivo biodistribution of long-circulation nanoparticles with poly(ethylene-glycol)-modified PLA (BAB type) triblock copolymer. Colloids Surf B Biointerfaces 72:303–311 [DOI] [PubMed] [Google Scholar]
- Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ. (2008) pH-responsive nanoparticles for cancer drug delivery. Methods Mol Biol 437:183–216 [DOI] [PubMed] [Google Scholar]
- Shim MS, Kwon YJ. (2010) Efficient and targeted delivery of siRNA in vivo. FEBS J 277:4814–4827 [DOI] [PubMed] [Google Scholar]
- Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. (2010) Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16:1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm G, Belliot SO, Daemen T, Lasic DD. (1995) Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev 17:31–48 [Google Scholar]
- Suzuki A, Tanaka T. (1990) Phase transition in polymer gels induced by visible light (Letter). Nature 346:345–347 [Google Scholar]
- Tabata Y, Inoue Y, Ikada Y. (1996) Size effect on systemic and mucosal immune responses induced by oral administration of biodegradable microspheres. Vaccine 14:1677–1685 [DOI] [PubMed] [Google Scholar]
- Torchilin VP. (2008) Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers 90:604–610 [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T. (2005) Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 87:287–297 [DOI] [PubMed] [Google Scholar]
- van Duijnhoven SM, Robillard MS, Nicolay K, Grüll H. (2011) Tumor targeting of MMP-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J Nucl Med 52:279–286 [DOI] [PubMed] [Google Scholar]
- van Etten EW, ten Kate MT, Stearne LE, Bakker-Woudenberg IA. (1995) Amphotericin B liposomes with prolonged circulation in blood: in vitro antifungal activity, toxicity, and efficacy in systemic candidiasis in leukopenic mice. Antimicrob Agents Chemother 39:1954–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila A, Sánchez A, Evora C, Soriano I, Vila Jato JL, Alonso MJ. (2004) PEG-PLA nanoparticles as carriers for nasal vaccine delivery. J Aerosol Med 17:174–185 [DOI] [PubMed] [Google Scholar]
- Wang Z, Chui WK, Ho PC. (2011) Nanoparticulate delivery system targeted to tumor neovasculature for combined anticancer and antiangiogenesis therapy. Pharm Res 28:585–596 [DOI] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A 97:13003–13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendorf J, Chesko J, Kazzaz J, Ugozzoli M, Vajdy M, O'Hagan D, Singh M. (2008) A comparison of anionic nanoparticles and microparticles as vaccine delivery systems. Hum Vaccin 4:44–49 [DOI] [PubMed] [Google Scholar]
- Whitney M, Crisp JL, Olson ES, Aguilera TA, Gross LA, Ellies LG, Tsien RY. (2010) Parallel in vivo and in vitro selection using phage display identifies protease-dependent tumor-targeting peptides. J Biol Chem 285:22532–22541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. (2010) Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 9:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. (2011) Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA 108:2426–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Brugger A, Khare A, Chaubal M, Papadopoulos P, Rabinow B, Kipp J, Ning J. (2008) Suspensions for intravenous (IV) injection: a review of development, preclinical and clinical aspects. Adv Drug Deliv Rev 60:939–954 [DOI] [PubMed] [Google Scholar]
- You JO, Auguste DT. (2009) Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett 9:4467–4473 [DOI] [PubMed] [Google Scholar]
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769 [DOI] [PubMed] [Google Scholar]
- Zhao C, He P, Xiao C, Gao X, Zhuang X, Chen X. (2011) Synthesis of temperature and pH-responsive crosslinked micelles from polypeptide-based graft copolymer. J Colloid Interface Sci 359:436–442 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang K, Song Q, Liu S. (2007) Thermo-induced formation of unimolecular and multimolecular micelles from novel double hydrophilic multiblock copolymers of N,N-dimethylacrylamide and N-isopropylacrylamide. Langmuir 23:13076–13084 [DOI] [PubMed] [Google Scholar]
- Ziegler A, Nervi P, Dürrenberger M, Seelig J. (2005) The cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry 44:138–148 [DOI] [PubMed] [Google Scholar]