Abstract
Cisplatin is one of the most effective broad-spectrum anticancer drugs. Its effectiveness seems to be due to the unique properties of cisplatin, which enters cells via multiple pathways and forms multiple different DNA-platinum adducts while initiating a cellular self-defense system by activating or silencing a variety of different genes, resulting in dramatic epigenetic and/or genetic alternations. As a result, the development of cisplatin resistance in human cancer cells in vivo and in vitro by necessity stems from bewilderingly complex genetic and epigenetic changes in gene expression and alterations in protein localization. Extensive published evidence has demonstrated that pleiotropic alterations are frequently detected during development of resistance to this toxic metal compound. Changes occur in almost every mechanism supporting cell survival, including cell growth-promoting pathways, apoptosis, developmental pathways, DNA damage repair, and endocytosis. In general, dozens of genes are affected in cisplatin-resistant cells, including pathways involved in copper metabolism as well as transcription pathways that alter the cytoskeleton, change cell surface presentation of proteins, and regulate epithelial-to-mesenchymal transition. Decreased accumulation is one of the most common features resulting in cisplatin resistance. This seems to be a consequence of numerous epigenetic and genetic changes leading to the loss of cell-surface binding sites and/or transporters for cisplatin, and decreased fluid phase endocytosis.
I. Introduction: The Complexities of Cisplatin Sensitivity and Resistance
Cisplatin is a small-molecule platinum compound that was accidentally discovered to inhibit the growth of Escherichia coli and later was found to kill tumor cells as well (Rosenberg, 1973). The antitumor toxicities of platinum compounds and their clinical application in the late 1970s was a milestone in the development of successful cancer chemotherapeutic agents. Platinating compounds including cisplatin, carboplatin, and oxaliplatin are still front-line clinical therapies and constitute part of the treatment regimen for patients with many types of cancers, including head and neck, testicular, ovarian, cervical, lung, colorectal and relapsed lymphoma. The cytotoxic lesions caused by platinating agents are known as platinum-DNA adducts, which primarily form intrastrand cross-links that activate the apoptotic pathway, resulting in cell death (Siddik, 2003). Patients usually have a good initial response to cisplatin-based chemotherapy but later relapse, because the development of cisplatin resistance, either acquired or intrinsic, markedly reduces its clinical effectiveness. Tissue culture studies suggest that this resistance can result from epigenetic changes at molecular and cellular levels, including reduced accumulation of the platinum compounds by either active efflux/sequestration/secretion or impaired influx, detoxification by GSH conjugates, metallothioneins and other antioxidants, increased levels of DNA damage repair (nucleotide excision repair and mismatch repair), changes in DNA-methylation status, alterations of membrane protein trafficking as a result of defective organization and distribution of the cytoskeleton, overexpression of chaperones, up- or down-regulated expression of microRNA (miRNA1), transcription factors and small GTPases, inactivation of the apoptosis pathway, activation of the EMT pathway, and others.
Studies of the mechanism of resistance to platinum have revealed a plethora of complex resistance mechanisms. On more detailed analysis, these mechanisms seem to reflect activation of intrinsic pathways used during development or as defense against environmental toxins. The purpose of this review is to provide an overview of the mechanisms of cellular resistance to cisplatin. We discuss the relevance of these in vitro studies to cisplatin resistance in clinical cancer.
II. Basic Features
A. Pleiotropic Phenotype Associated with Cisplatin Resistance
The myriad of phenotypic changes that appear in human cisplatin-resistant (CP-r) cells have been well documented. They include cross-resistance to many structurally related or unrelated drugs, decreased accumulation of platinum in CP-r cells in association with a decline in platinum-DNA adduct levels, changes in gene expression levels involved in almost every aspect of cell survival, such as apoptosis, DNA damage-repair, chaperones, transporters, the cell cycle, protein trafficking, transcription factors, oncogenes, small GTPases, GSH and related enzymes, cytoskeletal proteins, mitochondria, etc. (Reed, 1998; Shen et al., 2004a., 2006; Kohno et al., 2005; Liang et al., 2008; Kasherman et al., 2009; Shen and Gottesman, 2012). Cells develop resistance to cisplatin and other anticancer drugs by establishing a complicated self-defense system to escape exogenous cytotoxic compounds. To survive, they activate an overall abnormal phenotype that is either defective or active/defensive by silencing or activating the expression of a variety of genes when exposed to platinum compounds.
B. Reduced Accumulation Is a Prominent Feature of Cisplatin Resistance
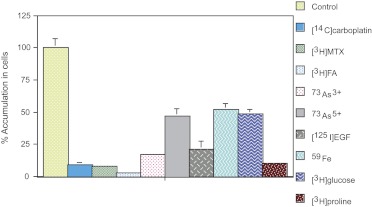
One of the most prominent characteristics of cellular resistance to cisplatin is the reduced accumulation of the compound. As a consequence of reduced uptake or retention, the formation of platinum-DNA adducts is correspondingly decreased, reducing cytotoxicity, resulting in more resistance to the platinum compound. In our human hepatoma cisplatin-resistant 7404-CP20 cells, significant reductions in platinum-DNA adduct formation (9-fold) and ribosomal RNA gene-specific interstrand cross-link formation (12-fold) were found, but the removal rates of the total platinum-DNA adducts and gene-specific interstrand cross-links were similar to those in their parental 7404 cells (Johnson et al., 1996). Using radiolabeled compounds, we found that accumulation of carboplatin, sodium arsenite, sodium arsenate, methotrexate, folic acid, epidermal growth factor, iron, glucose, and proline were significantly reduced in the human cisplatin-resistant cells as shown in Fig. 1 (Shen et al., 2004b). We also discovered that the uptake of [14C]carboplatin in 7404 parental cells is time-, temperature-, and energy-dependent and that the rate of uptake is reduced in human hepatoma cisplatin-resistant 7404-CP20 cells. The efflux of [14C]carboplatin in cisplatin-resistant cells was comparable to efflux in the parental cisplatin-sensitive cells. There was little effect of temperature (between 37°C and 4°C) on efflux in cisplatin-resistant cells, indicating that passive diffusion of the compound may not be primarily responsible for uptake. These results also suggest that impaired uptake may play an important role, at least in part, in the reduced accumulation of cisplatin and other compounds. Reduced expression of the multidrug resistance-associated transporters MRP1 and MRP2, and aquaporins AQP2 and AQP9 (Shen et al., 1998; Hall et al., 2008) and mislocalization of the folate-binding protein (FBP) and MRP1 (Shen et al., 1998; Liang et al., 2003), were found to be associated with resistance, leading to the hypothesis that a defective membrane transporter/binding protein/carrier for cisplatin might exist. It is noteworthy that there was significantly reduced expression of FBP in CP-r cells, decreased uptake of radiolabeled folic acid, and an association with cross-resistance to methotrexate, which is a derivative antifolate product, suggesting a more general defect in transporter-mediated uptake.
Fig. 1.
Reduced uptake of various radiolabeled compounds was detected in KB-CP20 cells, compared with parental CP-s KB-3-1 cells. [Reprinted from Shen DW, Su A, Liang XJ, Pai-Panandiker A, and Gottesman MM (2004) Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer 91:270–276.]
But what is the mechanism for decreased accumulation of platinum in CP-r cells? A reasonable explanation is that this occurs either through decreased influx or increased efflux. Decreased influx could result from defective transporters or channels, as described above, or functional/structural changes in organelles or membrane potential. Active efflux could result from increased export, secretion, or exocytosis of the platinum compounds.
III. Membrane Transporters and Cisplatin Resistance
A. ATP-Binding Cassette Transporters
The first multidrug resistance transporter to be identified, ABCB1, an ATP-binding cassette transporter known for years as P-glycoprotein or MDR1, functions as a pump to efflux a variety of structurally unrelated anticancer drugs, leading to cellular resistance to related substrates but not to cisplatin (Gottesman et al., 2002; Gottesman, 2002; Gillet and Gottesman, 2010). Investigators searched intensively to find transporters of platinum compounds analogous to MDR1, and several have been found, including the efflux ATPases (MRPs, ATP7A/B), and the solute carrier importers CTR1, the SLCs, AQP2, and AQP9. The multidrug resistance-associated protein MRP1 (ABCC1) and other MRPs are now known to play important roles in detoxification and chemoprotection by transporting a wide range of compounds, especially secondary metabolite conjugates of lipophilic substances with glutathione, glucuronate, and sulfate. Although they modulate the pharmacokinetics of many drugs, no direct correlation with cisplatin resistance has been found in vitro or in patients (Grant et al., 1993; Hamaguchi et al., 1993; Zelcer et al., 2001; Filipits et al., 2007). However, a recent study has shown that increased expression and altered N-linked glycosylation of MRP1 and MRP4 in an oxaliplatin-selected ovarian tumor cell line was associated with resistance to oxaliplatin and cisplatin, with markedly reduced accumulation of platinum drugs (Beretta et al., 2010). The observed N-glycosylation defect of oxaliplatin-resistant cells was linked to reduced levels of N-acetylglucosamine-1-phosphotransferase and mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetyl-glucosaminyltransferase. It should be noted that cisplatin-resistant cells are not commonly cross-resistant to oxaliplatin, whereas oxaliplatin-resistant cells usually are cross-resistant to cisplatin.
B. Role of Copper Transporter 1 in Cisplatin Uptake
More recently, it has been reported that the copper transporter CTR1, which is a major influx transporter, plays an important role in mediating uptake of platinum compounds. Ishida et al. (2002) demonstrated that uptake of cisplatin is mediated by the copper transporter Ctr1 in yeast and mammals. It has been further confirmed in human cells that cisplatin triggers rapid degradation of the copper membrane transporter CTR1, with diminished influx of cisplatin, resulting in resistance to the drug (Lin et al., 2002; Holzer et al., 2006). Genetic knockout of CTR1 results in cellular resistance to cisplatin in vivo. Cells with increased CTR1 expression exhibit increased platinum accumulation and, in most instances, increased sensitivity to cisplatin. The role of CTR1 in cisplatin-resistant cells has been reviewed by Howell et al. (2010).
C. Transmembrane Protein 205, a Putative Membrane Transporter, Confers Cisplatin Resistance
As mentioned above, active efflux/sequestration/secretion may also play important roles in decreased accumulation of cisplatin in human CP-r cells. In a recent report, we identified a membrane protein named TMEM205 that was associated with cellular resistance to cisplatin (Shen et al., 2010). TMEM205 has four transmembrane domains and is predicted, on the basis of its nucleotide sequences, to be a secretion-related protein. Its expression is increased in our CP-r cell lines, as demonstrated by immunoblotting, confocal examination, and immunoelectron microscopy. Stable transfection of the TMEM205 gene confers approximately 2.5-fold resistance to cisplatin. Uptake assays with Alexa Fluor-cisplatin showed reduced accumulation in CP-r KB-CP.3 and KB-CP.5 cells and in TMEM205-transfected cells. Analysis of TMEM205 expression profiles in normal human tissues indicates a differential expression pattern with higher expression levels in the liver, pancreas, and adrenal glands. These results indicate that overexpression of TMEM205 in CP-r cells may play a role in cellular resistance to platinum and would also be valuable as a biomarker or target in cancer chemotherapy.
D. The Glucose Transporter 1
One important consequence of selection of KB cells in cisplatin is reduced expression and internalization of Glut1 (glucose transporter 1), normally located on the cell surface. This reduces glucose uptake, resulting in induction of Sirt1 in cisplatin-resistant cells, causing cisplatin resistance (Liang et al., 2008). The mitochondrial phenotype associated with up-regulation of Sirt1 by glucose starvation or Sirt1 transfection decreases apoptosis and makes cells more resistant to cisplatin. Therefore, although Glut1 is not proposed to directly transport cisplatin, the mislocalization of the transporter exacerbates the cisplatin resistance phenotype.
IV. Endocytosis and Cisplatin Resistance
A. Defective Endocytic Uptake in Cisplatin-Resistant Cells
Reduced endocytosis is another way for cells to decrease uptake of cisplatin normally achieved via cellular fluid-phase endocytosis. Chauhan et al. (2003) reported that the uptake of horseradish peroxidase and Texas Red dextran was decreased severalfold in KB-CP-r cells, indicating a general defect in fluid-phase endocytosis. Treatment of KB cells by bafilomycin A1, a known inhibitor of the vacuolar proton pump, mimicked the phenotype seen in KB-CP-r cells with reduced uptake of horseradish peroxidase and [14C]carboplatin, suggesting that a defective endocytic uptake of cisplatin may be involved in cellular resistance to the compound. Liang et al. (2006) further tracked lipids in the endocytic recycling compartment (ERC) and found that the distribution of the ERC is altered in early-stage cisplatin-resistant KB-CP.5 cells, compared with parental KB-3-1 cells. The altered distribution of the ERC in KB-CP.5 cells was found to be related to the amount and distribution of stable detyrosinated microtubules (Glu-α-tubulin). In addition, cells with a dispersed ERC under nonpermissive conditions were more resistant to cisplatin, indicating that this resistance might be due, in part, to reduced uptake of cisplatin resulting from an endocytic defect.
A recent study using metallofullerene nanoparticles to circumvent tumor resistance to cisplatin has demonstrated that pretreatment of the human prostate cisplatin-resistant PC-3-luc cells with [Gd@C82(OH)22]n enhanced intracellular accumulation of cisplatin and formation of cisplatin-DNA adducts by restoring defective endocytosis (Liang et al., 2010). Therefore, a defective endocytic pathway may constitute a cellular defense mechanism that protects the cell against toxic compounds from the exogenous environment by reducing influx of the compounds.
B. Endocytosis and Copper Transporter 2
Recently, Blair et al. (2011) also reported that CTR2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Their research indicates that CTR2 regulates the transport of cisplatin in part by controlling the rate of macropinocytosis via activation of the Rho gene family.
V. Countering Cisplatin Toxicity: Heat Shock Proteins, GTPases, Ribosomal Proteins
A. Heat Shock Proteins
Heat shock proteins are stress-inducible molecular chaperones that are abundantly expressed in multiple compartments of the cell, modulating protein homeostasis to protect against oxidant-induced DNA damage and apoptosis. The 10-, 27-, 60-, and 70-kDa heat-shock proteins and others have been linked to stress response in protein folding and unfolding in drug resistance (Mandic et al., 2003; Shan et al., 2003; Zhao and Houry, 2005). Cisplatin also induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling (Mandic et al., 2003). Because cisplatin is a DNA-damaging agent and an inducer of apoptosis, it is reasonable to expect changes in HSPs in the development of cellular resistance to the drug. In an earlier report (Shen et al., 1995), we found that HSP60 was significantly expressed in both human cervical and liver carcinoma CP-r cells using two-dimensional gel electrophoreses and amino acid micro-sequencing. It was further demonstrated that overexpression of HSP10 renders KB-3-1 cells somewhat more resistant to cisplatin. A cDNA for HSP10 was identified by functional cloning from a retroviral cDNA library of cDNAs (Shen et al., 2006) as seen in Table 1. Up-regulation of HSP27, HSP70, HSP72, GRP78, and HSP90 have been reported to be involved in CP-r ovarian cancer (Arts et al., 1999; Liu et al., 2002), breast cancer (Vargas-Roig et al., 1998), colon cancer (Belfi et al., 1999), cervical HeLa cells (Huang et al., 2000), and laryngeal carcinoma cells (Brozovic et al., 2001). However, overexpression of ERp29, which up-regulates Hsp27 in breast cancer cells, increases resistance to doxorubicin, but not cisplatin or paclitaxel (Zhang and Putti, 2010). Application of the HSP inhibitor 5-(2,4-dihydroxy-5-isopropylphenyl)-4-(4-morpholin-4-ylmethylphenyl)isoxazole-3-carboxylic acid ethylamide (NVP-AUY922) was shown to be effective at reducing A2780 tumor growth in vivo (Nagengast et al., 2010). Tumor necrosis factor-associated protein 1 has been reported to protect cells from oxidative stress and apoptosis (Montesano Gesualdi et al., 2007). HSP75 was recently identified as a mitochondrial chaperone; it is overexpressed in cisplatin-resistant ovarian tumors and ovarian carcinoma cell lines (Landriscina et al., 2010). Because heat-shock proteins are stress response-related proteins, increased expression of heat-shock proteins in CP-r cells also suggests a cellular defense reaction in cooperation with other genes to mediate resistance, although the precise function of these proteins in this regard remains to be further elucidated. Cowen and colleagues (Cowen and Lindquist, 2005; Cowen et al., 2009) also demonstrated that HSP90 plays an important role in the drug resistance of yeast and fungus and could be a target for clinical therapy of yeast- and fungus-induced diseases.
TABLE 1.
Genes identified as involved in mediating cisplatin resistance by gene transfer or siRNA inhibition
All genes confer cisplatin resistance, except G-catenin and CTR1, which confer sensitivity to cisplatin
| Gene | Regulation | Method | Reference |
|---|---|---|---|
| TMEM205 | Up | Gene transfer | Shen et al., 2010 |
| RAB8 | Up | Gene transfer | Shen and Gottesman, 2012 |
| GCF2/LRRFIP1a | Up | Gene transfer/siRNA | Shen et al., 2012 |
| PCAF (p300/CBP-associated factor) | Up | Gene transfer | Hirano et al., 2010 |
| G-catenin | Down | Gene transfer | Liang et al., 2004 |
| Nrf2 | Up | siRNA | Cho et al., 2008 |
| HSP10, 27, 60 70, 90 | Up | Gene transfer/gene expression | Mandic et al., 2003; Zhao and Houry, 2005; Shen et al., 2006 |
| RPL36 | Up | Gene transfer | Shen et al., 2006 |
| SIRT1 | Up | Gene transfer/siRNA | Liang et al., 2008 |
| CTR1 | Down | Gene knockout | Howell et al., 2010 |
| ATP7A/B | Up | Gene transfer | Owatari et al., 2007; Safaei et al., 2008 |
| Tip60 | Up | Knockdown | Miyamoto et al., 2008 |
| TWIST, Snail | Up | siRNA | Zhuo et al., 2008a,b |
GCF2/LRRFIP1 is shown to be upregulated in CP-r cells, repressing the expression of the microfilament regulator RhoA, disrupting trafficking of transporters to the cell surface, and conferring resistance to cisplatin.
B. Small GTPases
The low molecular weight GTP-binding proteins of the Rho family, including RhoA, RhoB, RhoC, and Rac, all belong to the Ras tumor suppressor gene family. Fritz et al. (1995) found that RhoB was DNA damage-inducible and involved in the early steps of signal transduction upon genotoxic stress. Reduced expression of small GTPases such as Rab5, Rac1, and RhoA was detected in our human cisplatin-resistant cells in association with decreased accumulation of radiolabeled compounds, as listed in Table 2 (Shen et al., 2004a). The Rho family has been recently recognized to be involved in the formation of the cytoskeleton and in protein trafficking and endocytosis (Shim et al., 2010; Shen et al., 2012), suggesting that a regulated defensive system is expressed in CP-r cells to control the influx of cytotoxic chemicals. On the other hand, Rab8 was found to be significantly increased in CP-r cells (Table 1). It colocalizes with TMEM205 in late endocytic vesicles and increases TMEM205-mediated cisplatin resistance (Table 1) (Shen and Gottesman, 2012). It was demonstrated that H-Ras enhances DNA repair through the Ras/phosphatidylinositol 3-kinase/Rac1 pathway in NIH3T3 cells (Cho et al., 2002) and up-regulates ERCC1, one of the key enzymes involved in nucleotide excision repair, to protect against platinum-based anticancer agents (Youn et al., 2004). In addition, inhibition of Ras may enhance the cisplatin sensitivity of human glioblastoma (Messina et al., 2004). Blair et al. (2011) reported that the small GTPases Rac1 and CDC42 are associated with regulation of uptake of cisplatin. Our recent studies (Shen et al., 2012) demonstrate that knockdown of the small GTPase RhoA by GCF2, a transcription repressor, results in increased resistance to cisplatin (Table 1) (see detailed discussion in section VII). Therefore, the small GTPase family may also play important roles in mediating cellular resistance to drugs through either activation of the DNA-damage repair pathway or silencing genes related to the cytoskeleton and protein trafficking, thus reducing endocytic uptake of the platinum compound.
TABLE 2.
Changes in gene expression in CP-r cells determined by reverse transcription-polymerase chain reaction, immunoblotting, immunofluorescence, and functional cloning
| Type of protein | Expression | Gene | Reference |
|---|---|---|---|
| Membrane proteins | Reduced | MRP1, MRP2 | Liang et al., 2003; Shen et al., 2000 |
| MRP4, MRP5 | D.-W. Shen and M. M. Gottesman, unpublished data | ||
| FBP | Liang et al., 2003; Shen et al., 2004a | ||
| Small GTPases | Reduced | Rab5, Rac1, RhoA | Shen et al., 2004b |
| Transcription/regulation | Reduced | Histone H1, Histone H3 | D.-W. Shen and M. M. Gottesman, unpublished data |
| Cytoskeleton | Reduced | β-actin, F-actin, α-tubulin, β-tubulin, Dyn2, filamin 90 and 250, keratin 5/8 | Liang et al., 2008; Shen et al., 2000); D.-W. Shen and M. M. Gottesman, unpublished data |
| Endocytic/exocytic | Reduced | ERC, STX6 | D.-W. Shen and M. M. Gottesman, unpublished data |
| Tumor suppressor | Reduced | Maspin | A. Pai-Panandiker and M. M. Gottesman, unpublished data |
| Membrane proteins | Increased | Caveolin, Ror1 (transmembrane TMEM205 receptor) | Shen et al., 2010; D.-W. Shen and M. M. Gottesman, unpublished data |
| Small GTPases | Increased | Rab8 | Shen and Gottesman, 2011 |
| Transcription/Regulation | Increased | SIRT1 | Liang et al., 2008 |
| GCF2 | Shen et al., 2012 | ||
| Chaperones | Increased | HSP60 | Shen et al., 1995 |
| HSP10 | Shen et al., 2006 | ||
| Ribosomal proteins | Increased | RPL36, RPL36a, RPL27 | Shen et al., 2006 |
| Enzymes | Increased | ALDH3 [Aldehyde dehydrogenase III], ACADM (acyl-CoA dehydrogenase, C4 to C12, straight chain | D.-W. Shen and M. M. Gottesman, unpublished data |
C. Ribosomal Proteins
The ribosome plays an essential role in the structural assembly of proteins. When cells encounter environmental stress, ribosomes help to coodinate the expression of stress-response genes (Holcik and Sonenberg, 2005). The ribosomal protein L36 was found to confer cisplatin resistance in human carcinoma cells (Shen et al., 2006). This was discovered by functional cloning from a retroviral cDNA library made from KB-CP.5 cells, which are at an early stage of resistance to cisplatin, and suggests that ribosomal proteins play roles in the mediation of cisplatin resistance. In human breast cancer MCF-7 cells, reduced expression of the ribosomal protein P0 was found by proteomic analysis (Smith et al., 2007). It has been found that degradation of the ribosomal protein L37 leads to cell cycle arrest in an L11- and p53-dependent manner after exposure to cisplatin, suggesting that cisplatin-induced DNA damage can initiate ribosomal stress. Moreover, ectopic L37 overexpression can attenuate the DNA damage response mediated by p53 (Llanos and Serrano, 2010). These data imply that there is a mechanistic link between platinum-induced DNA damage and the ribosomal stress response pathway.
VI. Epigenetic Changes in Cisplatin Resistance: Chromatin, DNA-Methylation, Histones
A. Chromatin-Mediated Cisplatin Resistance
Since the early 1990s, investigators have noted that changes in chromatin structure may be involved in the chemoresistance of human CP-r cells (de Jong et al., 1993; Kato et al., 2000). Sharma et al. (2010) reported that drug tolerance is associated with heterogeneity within a cancer cell population, and a small subpopulation of reversibly “drug-tolerant” cells can exhibit increased drug resistance, including to cisplatin, maintaining viability via engagement of IGF-1 receptor signaling and an altered chromatin state that requires the histone demethylase RBP2/KDM5A/Jarid1A. These findings suggest that cancer cell populations employ a dynamic survival strategy in which individual cells transiently assume a reversibly drug-tolerant state to protect the population from eradication by potentially lethal exposures. This phenotype can be reversed by treatment with insulin-like growth factor-1 receptor inhibitors or chromatin-modifying agents, potentially yielding a therapeutic opportunity. Piwil2, a member of the PIWI/Argonaute gene family, is a key factor in regulating chromatin modification, playing important roles in stem cell self-renewal, RNA silencing, and translational regulation in various organisms. Wang et al. (2011) demonstrated that cisplatin could induce chromatin relaxation in Mili-wild type mouse embryonic fibroblasts, but not in Mili-knockout mouse embryonic fibroblasts. They found that overexpression of Piwil2 in some cancers could lead to cellular cisplatin resistance, possibly as a result of enhanced chromatin condensation, affecting normal DNA repair. This is an interesting field that warrants further exploration.
B. DNA Methylation
DNA methylation is an epigenetic phenomenon that plays a critical role in many cellular processes, including silencing of certain genes. Aberrant promoter hypermethylation and consequent gene silencing are epigenetic hallmarks of multidrug resistance. It has been suggested that aberrant DNA methylation can affect the sensitivity of cancers to antineoplastic agents by altering the expression of genes critical to drug response. DNA hypermethylation may play a major role in generating drug-resistant phenotypes by inactivating genes that are required for cytotoxicity (Chang et al., 2010). Epigenetic profiling is useful in identifying molecular mediators for cancer drug sensitivity (pharmacoepigenomics). In a previous report (Shen et al., 2004a), we demonstrated that significantly reduced accumulation of methotrexate and other compounds in human cisplatin-resistant hepatoma and epidermal carcinoma cells was associated with a silencing of the folate-binding gene (FBP) (Table 2). We found that the silenced FBP gene in the CP-r cells could be re-activated by a DNA demethylation agent, 2-deoxy-5-aza-cytidine (DAC), indicating that hypermethylation occurred in the CP-r cells. The uptake of radiolabeled [14C]carboplatin, folic acid, and methotrexate was increased in an early stage CP-r cell line (KB-CP1) after treatment with DAC in association with increased expression of FBP, suggesting that hypermethylation may contribute to cellular resistance to carboplatin, methotrexate, and other compounds. However, cells highly resistant to cisplatin (KB-CP20 and 7404-CP20) did not respond to DAC.
A similar feature was also detected in male germ cell tumors; cisplatin treatment induces de novo promoter hypermethylation in vivo. Acquired cisplatin resistance in vitro alters the expression of specific genes, but the highly resistant cells fail to reactivate gene expression after treatment with demethylating and histone deacetylase inhibiting agents (Koul et al., 2004). These results may imply that DNA methylation plays an important role in the early development of a drug-resistant phenotype by inactivating genes that are required for cytotoxicity.
Epigenetic profiling provides information valuable for identifying molecular mediators of cisplatin resistance. It has been found that several hundred genes were down-regulated in two pairs of cisplatin-resistant cell lines and could be reactivated by the DNA methyltransferase inhibitor DAC. Among them, 14 genes were hypermethylated in resistant cell lines, as confirmed by bisulfite sequencing (Chang et al., 2010). The authors concluded that DNA methylation is a frequent event in cells that are continuously exposed to cisplatin and that methylation-induced gene silencing may play a role in the development of resistance to cytotoxic chemotherapeutic drugs. Wermann et al. (2010) also found global DNA methylation in fetal human germ cells and germ cell tumors in association with cisplatin resistance using a high-throughput methylation screen for changes in the methylation sites of 14,000 genes.
When examining the DNA methylation of 32 promoter-associated CpG islands in human cancer cell lines from the National Cancer Institute (NCI) drug-screening panel (NCI-60 panel), it was found that the frequency of aberrant hypermethylation of these islands ranged from 2 to 81% of the NCI-60 cancer cell lines. Among them, hypermethylation of the p53 homolog p73 and associated gene silencing was strongly correlated with sensitivity to alkylating agents, including cisplatin (Shen et al., 2007). Using small interfering RNA (siRNA) to down-regulate p73 expression in multiple cell lines substantially increased sensitivity to the alkylating agents, indicating that epigenetic silencing of p73 directly modulates drug sensitivity. After analysis of the microarray data on 23 human cancer cell lines, 36 non–small-cell lung cancer specimens, and 3 matching sets of CP-r and CP-s cells, Ibanez de Caceres et al. (2010) reported specific silencing by promoter hypermethylation of insulin-like growth factor-binding protein-3 in CP-r cells. They found a strong correlation between methylation status and cisplatin response in tumor specimens: a loss of insulin-like growth factor-binding protein expression, mediated by promoter-hypermethylation, resulted in a reduction of tumor cell sensitivity to cisplatin in non–small-cell lung cancer.
C. Histone Modification
Histone modification has recently been shown to be important for maintaining chromatin structure and function. Histone acetylation has a critical regulatory role in both transcription and DNA repair. Miyamoto et al. (2008) reported that expression of histone acetyltransferase genes are associated with cisplatin resistance. Overexpression of the acetyl-transferase Tip60 was observed in cisplatin-resistant human lung cancer cells. Knockdown of Tip60 expression rendered cells sensitive to cisplatin in association with the down-regulation of several DNA repair genes. A combined inhibition of DNA methylation and histone acetylation was found to enhance gene re-expression and drug sensitivity (Steele et al., 2009). Treatment of the cisplatin-resistant human ovarian A2780/cp70 cells with DAC, an inhibitor of DNA methylation, and belinostat, an inhibitor of histone deacetylase, resulted in a marked increase in expression of epigenetically silenced MLH1 and MAGE-A1 both in vitro and in vivo, and enhanced sensitivity to cisplatin compared with DAC alone. We recently found that histone deacetylases, which remove acetyl groups from histones, allowing for chromatin expansion and genetic transcription, were significantly overexpressed in our human cervical CP-r cells, especially the group histone deacetylases 1, 3, and 4 (D.-W. Shen and M. M. Gottesman, unpublished data). As suggested by data noted above, studies on DNA methylation and histone acetylation should provide a broad molecular basis for understanding the mechanisms of cisplatin resistance.
VII. Transcription Factors Affecting Cisplatin Sensitivity
A. Transcription Factors Play a Major Role in Cisplatin Resistance
During the last decade, the importance of transcription factors in the acquisition of cellular resistance to cisplatin has become more apparent. These factors include the Y-box binding protein-1, CCAAT-binding transcription factor 2, activating transcription factor 4, zinc-finger factor 143, the nuclear transcription factor-κB, the microphthalmia-associated transcription factor, the forkhead transcription factor O, and mitochondrial transcription factor A (Torigoe et al., 2005; Lee et al., 2009; Lei and Quelle, 2009; Li et al., 2009; Keenen et al., 2010). Hirano et al. (2010) reported that p300/CBP-associated factor (PCAF) was overexpressed in human prostate and epidermal cisplatin-resistant P/CDP6 and HeLa/CP4 cells. PCAF-overexpressing cells also showed enhanced expression of E2F1 and conferred cellular resistance to chemotherapeutic agents. PCAF is a transcription cofactor with intrinsic histone acetyltransferase activity. It induces cell-cycle arrest and/or apoptosis by regulating the function of the tumor suppressor p53 and the apoptosis mediators p73 and Bax. E2F1 is a transcription factor that is acetylated and stabilized by PCAF in response to DNA damage, suggesting that PCAF-mediated overexpression of E2F1 may play an important role in regulating cell viability or apoptosis resistance in cisplatin-resistant cells. Suppression of PCAF expression reduced Y-box binding protein-1 expression in KK47 human urothelial cancer cells, rendering cells sensitive to cisplatin and doxorubicin (Shiota et al., 2010).
B. Nuclear Factor (Erythroid-Derived 2)-Like 2
The cap‘n’collar transcription factor Nrf2 regulates the cellular defense response of genes encoding drug-metabolizing enzymes and drug transporters, as well as enzymes involved in the glutathione, thioredoxin, and peroxiredoxin antioxidant pathways. It was first found that the levels of Nrf2 expression in Nrf2-deficient murine embryonic cells and human ovarian cancer SK-OV cisplatin-resistant cells correlated with the extent of resistance to cisplatin. Loss of Nrf2 or inhibition by siRNA resulted in increased cell death, cytotoxicity, and apoptosis in response to cisplatin treatment compared with control cells (Cho et al., 2008). Ohta et al. (2008) further reported that loss of Keap1 function activated Nrf2, causing cells to become more resistant to cisplatin. It was found that Nrf2 mediated intrinsic resistance to environmental stressors, such as isothiocyanates, epoxides, peroxides, hydroquinones and quinones, NaAsO2, and various mutagens, including cisplatin (Higgins and Hayes, 2011). Ren et al. (2011) reported that brusatol, an inhibitor of the Nrf2 pathway, sensitized a broad spectrum of cancer cells and A549 xenografts to cisplatin and other chemotherapeutic drugs by inhibiting the Nrf2-mediated defense mechanism. It has been reported that inhibitors of transcription factors, such as 3-(4-methylphenylsulfonyl)-2-propenenitrile (Bay11-7082) and sulfasalazine, could induce apoptosis and enhance sensitivity to the chemotherapeutic agents cisplatin and fluorouracil (Li et al., 2009). These inhibitors may have potential for future clinical application.
C. GC-Binding Factor 2
In our own studies, we found that LRRFIP1 (also known as GCF2), a negative transcription factor, is significantly overexpressed in human CP-r cells (Tables 1 and 2), revealing a novel complex regulatory pathway downstream from GCF2 involving RhoA, microfilaments, and internalization of membrane transport proteins, as shown in Fig. 1 (Shen et al., 2012). Increased expression in GCF2-transfected KB-3-1 cells results in reduced RhoA expression, disruption of actin-filamin dynamics, mislocalization of the membrane protein MRP1, and reduction in cisplatin accumulation, leading to 3-fold resistance to cisplatin. An siRNA targeted to GCF2 suppressed the expression of GCF2 in CP-r cells, increased RhoA expression, restored the fine structure of actin microfilaments, and reversed the resistance of single-step selected CP-r cells. The siRNA also caused the membrane protein MRP1 to relocate to the cell surface. These data demonstrate that GCF2 plays crucial roles in the regulation of membrane protein trafficking by reducing levels of the small GTPase RhoA, which regulates the actin/filamin network, causing reduced accumulation of cisplatin and drug resistance. Application of inhibitors of transcription factors, such as siRNA (Shen et al., 2012), Bay11-7082, sulfasalazine (Li et al., 2009), and brusatol (Ren et al., 2011) may restore the normal phenotype of cellular response by enhancing sensitivity to chemotherapeutic agents and provide new strategies for a combined regimen in cancer treatment to prevent the acquisition of drug resistance.
VIII. Epithelial to Mesenchymal Transition, Wingless Gene, and Protein Kinase B Differentiation Pathways
In the past several years, the epithelial to mesenchymal transition (EMT) and the Wnt (wingless gene) pathways have drawn increasing interest in the areas of developmental biology, cell differentiation, cell cycle, stem cells, cancer progression and treatment, and drug resistance (Lee et al., 2006; Baum et al., 2008; Kalluri and Weinberg, 2009; Thiery et al., 2009; Wellner et al., 2009; Raimondi et al., 2010; Roussos et al., 2010; Singh and Settleman, 2010).
A. Epithelial to Mesenchymal Transition
Arumugam et al. (2009) found an inverse correlation between levels of E-cadherin and Zeb-1 and resistance to cisplatin, gemcitabine, and fluorouracil, suggesting that the EMT pathway may contribute to drug resistance in pancreatic cancer CP-r cells. Depletion of the zinc finger transcription factors TWIST and Snail, which are inducers of the EMT pathway, has been reported to sensitize A549 human lung cancer cells and head and neck squamous cell carcinoma cells to cisplatin (Zhuo et al., 2008a,b; Hsu et al., 2010). A study in our laboratory showed that overexpression of Snail 1 was found in human cervical and hepatoma CP-r cells (Table 2),but only in the cells highly resistant to cisplatin, not in early-stage, low-level CP-r cells, indicating that alteration of Snail expression may occur in later stages of the development of cisplatin resistance to facilitate cell survival under higher platinum selective pressure. E-cadherin (CDH1), another marker of the EMT, was also overexpressed in KB-CP20 cervical carcinoma cells, which are highly resistant to cisplatin, but not in low-level CP-r cells and not in hepatoma CP-r cells, suggesting that CDH1 overexpression is not a common characteristic of CP-r cells and may not play an initial role in the development of cellular resistance to cisplatin (D.-W. Shen and M. M. Gottesman, unpublished data). SFRP5, a member of the secreted frizzled-related protein (SFRP) family, has been shown to be important in regulating the EMT/Wnt signaling pathway. Overexpression of SFRP5 inhibited EMT signaling. The restoration of SFRP5 down-regulated AKT2 and sensitized ovarian cancer cells to chemotherapeutic agents, suggesting that epigenetic silencing of SFRP5 leads to activation of the Wnt pathway and contributes to ovarian cancer progression and chemoresistance through TWIST-mediated EMT and AKT2 signaling (Su et al., 2010). These effects are consistent with the poor response to platinum-based chemotherapy in patients with methylation of SFRP5 (Su et al., 2010). Sayan et al. (2009)) found that Smad interacting-protein 1 [SIP1 (ZEB2)], a zinc-finger E-box-binding family member, protects cells from DNA damage-induced apoptosis. They observed that the antiapoptotic effect of SIP1 was independent of either cell cycle arrest or loss of cell-cell adhesion and was associated with reduced phosphorylation of ATM/ATR targets.
B. Wingless Gene
The network proteins in the Wnt signaling pathway have been known to play important roles in gene regulation, cell proliferation, and apoptosis. Shou et al. (2002) found that overexpression of the human Dkk-1 gene, a transcriptional target of the p53 tumor suppressor, encodes a powerful inhibitor of the Wnt signaling pathway and could sensitize brain tumor cells to the DNA-damaging agents cisplatin and N,N′-bis(2-chloroethyl)-N-nitrosourea, but not to vincristine, which is an inhibitor of microtubules, showing a specific effect of human Dkk-1 on induction of cellular apoptosis. This observation was further confirmed in head and neck cancer Cal27 cells, where DKK1 negatively regulated cellular resistance to cisplatin (Gosepath et al., 2008). Overexpression of β-catenin, a Wnt marker, and OCT-4, a stem cell marker, were recently detected in A549 CP-r cells (Tang et al., 2010; Teng et al., 2010). Knocking down β-catenin by siRNA down-regulated the Wnt target gene cyclin D1 and resulted in decreased drug resistance, indicating that Wnt signaling may play a role in cisplatin resistance. EMX2, a human homolog of the Drosophila melanogaster empty spiracles gene, is a homeodomain-containing transcription factor. The function of EMX2 has been linked to the WNT signaling pathway. Okamoto et al. (2010) reported that EMX2 was dramatically down-regulated in lung cancer tissue samples and that this down-regulation was associated with methylation of the EMX2 promoter. Restoration of EMX2 expression in lung cancer cells lacking endogenous EMX2 expression suppressed cell proliferation and invasive phenotypes, inhibited canonical WNT signaling, and sensitized lung cancer cells to the treatment of the cytotoxic drug cisplatin. Uematsu et al. (2007) found that targeting the Wnt signaling pathway with siRNA for the gene Disheveled could enhance cisplatin cytotoxicity to mesothelioma cells. Dvl2, a member of Dishevelled (Dsh) family of proteins that act directly downstream of Frizzled receptors, has been known to be involved in canonical and noncanonical Wnt signaling pathways, playing essential roles in cellular differentiation and cell polarity. Our data demonstrate that Dvl2 was significantly expressed in human cervical KB-CP-r cells (Table 2). Increased levels of expression were correlated with levels of resistance to cisplatin.
C. Protein Kinase B
The protein kinase B/Akt signaling pathway plays essential roles in tumor growth, transformation, cell survival, and induced apoptosis and mediates drug sensitivity (Chakraborty et al., 2010; Liu et al., 2010; Tan et al., 2010; Ou et al., 2011). Findings indicate that PDK1 regulates vascular remodeling and promotes EMT in cardiac development (Feng et al., 2010) and that physical association of PDK1 with AKT1 is sufficient for pathway activation independent of membrane localization and phosphatidylinositol 3 kinase (Ding et al., 2010). Our recent data demonstrate that PDK1 was significantly expressed in human cervical KB-CP-r cells, and the increased levels were correlated with levels of resistance to cisplatin.
IX. MicroRNA Involvement
miRNAs are noncoding RNA post-transcriptional gene regulators of ∼20 nucleotides in length that function by targeting mRNA for degradation or sequestering. miRNAs have crucial roles in diverse biological processes, such as phenotypic stabilization, mediating the stress response, apoptosis, proliferation, and maintaining translational thresholds (Bushati and Cohen, 2007; Kosik, 2010; Leung and Sharp, 2010), essentially all areas affected when cells undergo the pleiotropic phenotypic changes that cause cisplatin-induced multidrug resistance. It is no surprise given their function that alterations in miRNA expression have also been shown to be involved in tumor growth and response to chemotherapy (Croce, 2009).
Several miRNAs have been shown to sensitize cisplatin-resistant cell lines to cisplatin and other drugs by regulating cell apoptosis. Yang et al. (2008a) reported miRNA expression profiling in human ovarian cancer and found that miR-214 induces cell survival and cisplatin resistance by targeting PTEN. In gastric and lung cell lines, the miRNA cluster miR-200bc/429 was shown to promote apoptosis by targeting B-cell lymphoma 2 (Bcl2) and X-linked inhibitor of apoptosis protein (XIAP), which sensitized resistant lines to vincristine as well as cisplatin (Zhu et al., 2012a). Zhu et al. (2012b) have also shown that miR-497 can target Bcl-2 and sensitize cells to cisplatin. miR-141 was found to be the most highly expressed miRNA in CP-r cell lines and was expressed ectopically in the CP-s cell lines, directly targeting the 3′-untranslated region of Yes-associated protein 1 (YAP1), which is known to have an important role in apoptosis induced by DNA-damaging agents. Thus, down-regulated YAP1 expression by overexpression of miRNA-141 renders cells more resistant to DNA-damaging drugs (Imanaka et al., 2011). Ye et al. (2011) reported that overexpression of miR-376c blocked cisplatin-induced cell death, whereas siRNA anti-miR-376c enhanced the effect of cisplatin. The effects of miR-376c were partially compensated by the overexpression of activin receptor-like kinase 7 (ALK7), which is a target of miR-376c. In addition, miRNA has been shown to play a role when cisplatin induces EMT, resulting in cells that have increased motility, invasiveness, and chemotherapeutic resistance. Specifically, miR-200b and miR-15b were down-regulated in EMT cells compared with tongue squamous cell carcinoma parental cells. Mimics for miR-200b and miR-15b were able to reverse EMT behavior and resulted in cells becoming sensitive to cisplatin (Sun et al., 2012). These investigations provided valuable insight and the identification of miRNA involved in mechanisms common among multidrug-resistant cultured cell lines. However, because miRNAs are reproducibly accurate identifiers of cell type (Kosik, 2010) and involved in cellular stress response (Leung and Sharp, 2010), we would expect variations between cell lines depending on tissue origin and culture techniques.
To explore possible roles of miRNA in the development of cancer in patients, many researchers look for correlations between miRNA expression and outcome, or use miRNAs as biomarkers (Croce, 2009). However, little research has been done that combines clinical expression data and cell culture with regard to cisplatin-induced multidrug resistance. Hamano et al. (2011) evaluated clinical samples and observed that overexpression of miR-200c and miR-21 and underexpression of miR-145 correlated significantly with shortened overall patient survival. The authors followed the correlative study with in vitro assays showing significantly increased miR-200c expression in esophageal cancer CP-r cells compared with their parent cells (∼1.7-fold). Anti-miR-200c-transfected cells were more sensitive to cisplatin. Studies indicated that miR-200c-induced chemoresistance may be mediated through activation of the Akt signaling pathway.
Researchers continue to document expression changes in miRNAs associated with resistance to cisplatin as well as to identify targets for miRNA. As shown in Table 3, changes in miRNAs are diverse, mostly because of the variety of cells employed. However, these studies do have similarities. The target mRNA of the miRNAs involved in cisplatin resistance are related to drug tolerance and cell survival pathways and show that although miRNA expression is phenotype specific, the mechanisms regulated by miRNA show similarities among cell lines. Table 3 summarizes the recent findings on changes in miRNAs associated with cisplatin resistance. Further exploration of miRNA target mRNA should provide valuable insights concerning the dysregulation of miRNA in CP-r cells. In particular, research is needed to identify miRNA targeting mRNA in the numerous mechanisms involved in the pleiotropic phenotypic changes seen in multidrug-resistant cells outside of apoptotic pathways.
TABLE 3.
Changes in miRNA associated with chemosensitivity to cisplatin
| miRNA | Cells | Targets | References |
|---|---|---|---|
| miR-7 | MCF-7 | MRP1 | Pogribny et al., 2010 |
| miR-141 | ESCC | YAP1 | Imanaka et al., 2011 |
| miR-148a | SCC, EAC | Unknown | Hummel et al., 2011 |
| miR-181b | A549 | BCL2 | Zhu et al., 2010 |
| miR-181d | UMSCC-1, SQ20B | Unknown | Dai et al., 2011 |
| miR-196 | A549 | Unknown | Tang et al., 2010 |
| miR-200c | TE8-R | Akt | Hamano et al., 2011 |
| miR-345 | MCF-7 | MRP1 | Pogribny et al., 2010 |
| miR-497 | A549 | BCL2 | Zhu et al., 2011b |
| miR-130a | A2780CIS | M-CSF | Sorrentino et al., 2008 |
| Let-7i | 2008, SKOV3, MCF7 | Unknown | Yang et al., 2008b |
| miR-214 | HIOSE | PTEN/Akt | Yang et al., 2008a |
| miR-372/373 | TGCT | NEO1/LATS2 | Duale et al., 2007 |
| miR-98 | SCC-4 | HMGA2 | Hebert et al., 2007 |
KB, human cervical epidermal carcinoma cells; ESCC, human esophageal squamous cell carcinoma; A549, human lung cancer cells; TEB-R, human esophageal squamous cell carcinoma, CP-r; UMSCC-1 & SQ20B, human head and neck squamous cell carcinoma; MCF7, human breast adenocarcinoma cells; SKOV3 & 2008, human ovarian cancer cells; HIOSE, human immortalized ovarian surface epithelial cells; TGCT, testicular germ cell tumor cells; SCC-4, human oral squamous cell carcinoma (SCC) cells.
X. DNA Repair and BRCA Mutant Genes in Cisplatin Resistance
It has been known since the mid-1980s that cisplatin is mutagenic. Burnouf et al. (1987) found that cisplatin efficiently induces mutations in E. coli. More than 90% of the mutations are single-base-pair substitutions occurring at potential sites of cisplatin adducts (ApG and GpG) The paradigm for the mechanism of action of cisplatin points to platinum binding to nuclear DNA as the critical pathway for cell killing, established in a review by Roberts and Thomson (1979). In recent times, attention has also been paid to this assumption and possible alternative cellular targets (including mitochondrial DNA) (Gibson, 2009).
The cellular response to platinum-DNA damage is complex and involves recognition and repair steps. Depending on the nature of the platinum-DNA lesion, one of two primary repair pathways that have been defined will be involved: mismatch repair and nucleotide excision repair (NER) (Martin et al., 2008). Examples exist of increased expression of genes associated with both NER and mismatch repair pathways in cisplatin-resistant cells and consequent increases in repair activity. For example, excision repair cross-complementation group 1 (ERCC1) protein plays a critical role in NER and has been up-regulated in resistant ovarian cancer cell lines (Ferry et al., 2000), and a survival benefit has been found for patients with low ERCC1 (i.e., low NER capacity) in patients with metastatic lung cancer (Olaussen et al., 2006).
The homologous recombination pathways associated with BRCA1/2 have emerged as playing an important role in platinum sensitivity. Discovering that the tumor suppressors BRCA1 and BRCA2 are associated with resistance to cisplatin was a milestone. Recent reports indicate loss of the wild-type allele through somatic alterations in breast and ovarian cancer (Wang and Figg, 2008). BRCA1/2 play important roles in homologous recombination-mediated repair of DNA double-strand breaks. Because of this, BRCA1/2-deficient cancers often have a better response to DNA cross-linking agents such as platinum analogs, and to poly (ADP-ribose) polymerase (PARP) inhibitors. Over time, however, the majority of these BRCA1/2-deficient cancers become resistant, and patients die from refractory disease. Recent studies have demonstrated that acquired resistance to platinum analogs or PARP inhibitors in tumors carrying frame-shift BRCA1/2 mutations come from restored BRCA1/2 expression and recovery of homologous recombination function as a result of secondary intragenic mutations that correct the open reading frames of mutated BRCA1/2. Swisher et al. (2008) reported that although ovarian carcinomas with mutated BRCA1 or BRCA2 are sensitive to platinum compounds, such carcinomas eventually develop platinum resistance, which may be mediated by secondary intragenic mutations in BRCA2 that restore the wild-type function. Sakai et al. (2008, 2009) demonstrated secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated ovarian cancers, confirming that acquired resistance to cisplatin was due to secondary intragenic mutations in BRCA2 that restore the wild-type BRCA2 reading frame. Edwards et al. (2008) also reported that most of the deletions in BRCA2 were associated with small tracts of homology and possibly arose from error-prone repair caused by BRCA2 deficiency. They note that similar ORF-restoring mutations are present in carboplatin-resistant ovarian tumors from c.6174delT mutation carriers. It is noteworthy that the resistance regained as a result of restoration of wild-type BRCA2 can be overcome by treatment with 6-thioguanine (6TG), a PARP inhibitor, which induces DNA double-strand breaks that are repaired by homologous recombination, resulting in cell death (Issaeva et al., 2010). This is because homologous recombination is reactivated in PARP inhibitor-resistant BRCA2-defective cells, but it is not fully restored for the repair of 6TG-induced lesions. It is likely that several recombinogenic lesions could be formed after 6TG treatment. It has been noted that mutations in BRCA1 and BRCA2 are present throughout the world, and therefore it would be useful to develop a form of genetic testing for BRCA1/2 mutations and targeted therapies for those with such mutations (Narod, 2010).
XI. Conclusions
In nature, intrinsic and acquired drug resistance occurs in all living organisms, from bacteria to human cancer cells. Resistance is a natural cellular self-defense mechanism developed by evolution to protect cells from toxic natural products and other environmental stressors. During the development of cisplatin resistance in human cancer cells in vivo and in vitro, numerous epigenetic and/or genetic changes can occur, probably reflecting activation of many different pathways that protect cells against environmental toxins. Because cisplatin has many different routes of cell entry and multiple cellular targets (the commonly accepted mechanism of cell killing is via platinum-DNA binding), resistance to this platinum compound is very complex, requiring multiple pathways, with profound changes at both the molecular and cellular levels concerning cell survival, apoptosis, developmental pathways, endocytosis, DNA methylation, gene activation/silencing, and mutation mediated by multiple transcription factors and miRNAs.
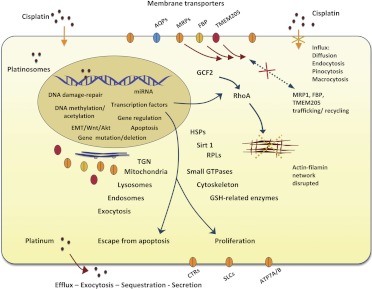
Figure 2 outlines potential pathways that can mediate cisplatin resistance, showing that decreased influx of cisplatin could result from blocked endocytosis, pinocytosis, macrocytosis, or mislocalization of membrane transporters or mediators. One potential mediator of decreases in these multiple uptake mechanisms is the transcriptional repressor GCF2. Increased levels of GCF2 silence the small GTPase RhoA, which disrupts the cytoskeletal network, resulting in defective membrane protein trafficking. Increased efflux could be mediated by elevated expression of the membrane transporters TMEM205, ATP7A/B, MRPs, etc., although the exact mechanism remains to be further investigated. Other mechanisms also play important roles in mediation of cisplatin resistance, such as the DNA-damage repair pathway and the apoptosis pathway. DNA methylation, including hyper- or demethylation and histone acetylation or deacetylation, could activate or silence gene expression via miRNA or transcription factors, triggering cellular defense systems to protect cells from cytotoxic agents such as anticancer compounds. In addition, enzymes related to binding platinum-containing compounds, to detoxification, and stress response chaperones may also be involved in initial protection mechanisms. Finally, because many of the genes able to confer CP-r affect cell differentiation and cell growth, it should not be surprising that different cell types express different resistance mechanisms.
Fig. 2.
Schematic illustration of cellular self-defense systems that mediate cisplatin resistance. Cisplatin binds to DNA, triggers the activation or silencing of a number of gene regulatory pathways, such as those related to DNA-damage repair, DNA methylation, histone acetylation, miRNA, EMT, Wnt, transcription factors, and apoptosis, and also inducing gene mutation or deletion. Mislocalization of membrane proteins, such as MRP1, FBP, and TMEM205, largely results from the up-regulation of the transcription factor GCF2, which silences small GTPase rhoA expression, interrupting assembly and or organization of the cytoskeletal actin/filamin network. This in turn results in internalization of several membrane proteins in the intracellular cytoplasm, with decreased influx and accumulation of cisplatin in the CP-r cells. This could also be due to a defective influx route (i.e., reduced endocytosis) or to other putative proteins that are needed for cisplatin uptake. HSPs, Sirt1, ribosomal proteins (RPLs), and GSH-related enzymes may play roles in regulating cellular response and detoxification of the compound. These cell self-defense mechanisms in CP-r cells serve to allow survival and growth of cancer cells exposed to cisplatin.
As with other areas of drug resistance, a great deal of understanding of resistance at the cellular level has brought about little improvement in efficacy against intractable malignancies. The reasons are manifold. One of the major challenges in understanding cisplatin resistance is that a broad range of individual resistance mechanisms has been identified (according to PubMed, more than 6000 papers match the search term “cisplatin resistance”). There is conflicting evidence for most of these mechanisms about whether any one mechanism may or may not be observed in cisplatin-resistant cell lines. For example, the literature on the uptake role of human organic cation transporters is inconsistent. Rather than weakening the relevance of these mechanisms, it reinforces a limitation that must be acknowledged—individual resistance pathways are probably activated on a tissue-specific basis. Clearly there is a large difference in the response of testicular cancer compared with glioblastoma.
In fact, testicular cancers may be the exception to the rule in their seemingly singular solution to cisplatin resistance. The exquisite sensitivity of testicular cancer has been shown to be due to wild-type p53 hypersensitivity (Gutekunst et al., 2011). Despite the high cure rate, some germ cell tumors do develop resistance, and this seems to be due primarily to high levels of cytoplasmic p21 (Koster and de Jong, 2010). In general, p21 is a downstream mediator of cell death but cytosolic localization of p21 protects cells from Fas-mediated apoptosis.
It would be instructive (but cumbersome) to be able to compare whether 5 to 10 resistance mechanisms are up-regulated in cisplatin-resistant cell lines generated under the same conditions as cell lines from a range of solid-tumor origins. Complicating interpretation relating to resistance mechanisms, even individual labs use cell lines with varying levels of resistance to cisplatin, and it is unclear whether low- and high-grade resistance carry the same mechanism(s) of action. No comprehensive study of this has been undertaken, and it is likely that highly resistant cells are not physiologically relevant.
A multitude of reports demonstrate that the sole contribution of an individual gene product to cells is sufficient to confer resistance to cisplatin. Yet it is also known that hundreds of changes exist in any individual cisplatin-resistant cell line. Clearly hundreds of gene changes are not additive, because there is an upper limit to cisplatin resistance. Although it has not been clearly demonstrated, it seems that the pleiotropy introduces multiple redundant pathways, collectively and individually conferring adequate resistance to protect cancer cells through the pathways described in this review. It seems to follow, then, that tackling the individual effectors (such as transporters or glutathione expression) shown in Fig. 2 will not resolve the resistant phenotype. The challenge is in identifying the core underlying regulators of the global changes effected in cisplatin-resistant cells, to inhibit the development of resistance.
Two facts argue that the primary alterations in cisplatin-resistant cells affect regulatory pathways: 1) the finding of multiple changes in many pathways in cells selected in a single-step in toxic concentrations of cisplatin, and 2) the ability to replicate some of the complexity of alterations in cisplatin-resistant cells by transduction of specific regulatory proteins and miRNAs. Of the variety of regulatory mechanisms altered in cisplatin-resistant cells, those under control of DNA methylation and acetylation, transcription factors, and/or miRNAs seem most likely to be the primary basis for cisplatin resistance.
There is certainly a focus in the medicinal chemistry field on the development of new platinum-based agents. Reports of new platinum complexes are often accompanied by testing against cisplatin-resistant cell lines. Based on current knowledge of the plethora of platinum-resistance mechanisms, it seems unlikely that the answer to cisplatin resistance is another platinum compound. Resistance that has developed to a given mode of action will demonstrate cross-resistance to other compounds that act in the same way, and sensitivity to compounds with differing modes of action is expected. Carboplatin, oxaliplatin, and cisplatin do have different activity profiles, but the search for small molecules insensitive to known cisplatin resistance mechanisms probably lies with small molecules disrupting pathways described in this review, such as DNA methylation and DNA repair and those that elicit collateral sensitivity in cisplatin-resistant cells.
Virtually all of the mechanisms and pathways discussed in this review were derived from studies in our laboratory and in many other laboratories using cultured human cancer cells of many different origins. Although it is clear that in most cases these cultured cells have basal patterns of expression of genes associated with drug resistance that differ significantly from patterns of gene expression seen in clinical cancers (Gillet et al., 2011), these studies on acquired resistance to cisplatin explore the range of potential options available to cells to escape the toxicity of cisplatin. There are virtually no studies that examine the resistance mechanisms that we describe in this review before and after development of cisplatin resistance in clinical cancers, so their relative contribution to clinical resistance, and the variety of mechanisms from individual to individual, is unknown. Improved chemotherapy of human cancers requires that such studies be performed and that more relevant in vitro systems be developed to explore strategies to overcome or circumvent these resistance mechanisms.
Given the above challenges, it seems that the “elephant in the room” is the question of which of the cisplatin-resistant mechanisms are important and which are amenable to direct pharmacological intervention? The two commonly recurring observations in cisplatin-resistant cells are reduced cellular accumulation of cisplatin and increased nuclear repair pathways. In both cases, small molecule intervention is possible. For example, Ishida et al. (2002) showed that in some cultured cancer cells, copper chelators increase copper transporter levels and cisplatin uptake. Interfering with nuclear repair may be more challenging to healthy tissue dealing with cisplatin, but a sound understanding of critical repair pathways may allow for intervention. As small interfering RNAs move toward clinical application, the opportunity to target specific genes at the site (for example the intraperitoneal cavity for ovarian cancer) may offer a complementary way forward on a patient-by-patient basis.
Acknowledgments
This research was funded by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute. We thank George Leiman for editorial assistance.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Shen, Pouliot, Hall, and Gottesman.
This article is available online at http://pharmrev.aspetjournals.org.
- 6TG
- 6-thioguanine
- ABC
- ATP-binding cassette
- AQP
- aquaporin
- Bay11-7082
- 3-(4-methylphenylsulfonyl)-2-propenenitrile
- Bcl2
- B-cell lymphoma 2
- BRCA
- breast cancer
- CP-r
- cisplatin-resistant
- CP-s
- cisplatin-sensitive
- CTR
- copper transporter
- DAC
- 2-deoxy-5-aza-cytidine
- Dkk-1
- Dickkopf-related protein 1
- EMT
- epithelial-mesenchymal transition
- EMX2
- empty spiracles homolog 2
- ERC
- endocytic recycling compartment
- FBP
- folate-binding protein
- GCF2
- GC-binding factor 2
- HSP
- heat shock protein
- MDR
- multidrug resistance
- miRNA
- microRNA
- MRP
- multidrug resistance-associated protein
- NCI
- National Cancer Institute
- NER
- nucleotide excision repair
- Nrf2
- nuclear factor (erythroid-derived 2)-like 2
- NVP-AUY922
- 5-(2,4-dihydroxy-5-isopropylphenyl)-4-(4-morpholin-4-ylmethylphenyl)isoxazole-3-carboxylic acid ethylamide
- OCT
- organic cation transporter
- PARP
- poly(ADP-ribose) polymerase
- PCAF
- p300/CBP-associated factor
- PDK1
- 3-phosphoinositide-dependent protein kinase-1
- SFRP
- secreted frizzled-related protein
- siRNA
- small interfering RNA
- SLC
- solute carrier
- TMEM
- transmembrane
- Wnt
- wingless gene.
References
- Arts HJ, Hollema H, Lemstra W, Willemse PH, De Vries EG, Kampinga HH, Van der Zee AG. (1999) Heat-shock-protein-27 (hsp27) expression in ovarian carcinoma: relation in response to chemotherapy and prognosis. Int J Cancer 84:234–238 [DOI] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. (2009) Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 69:5820–5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP. (2008) Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 19:294–308 [DOI] [PubMed] [Google Scholar]
- Belfi CA, Chatterjee S, Gosky DM, Berger SJ, Berger NA. (1999) Increased sensitivity of human colon cancer cells to DNA cross-linking agents after GRP78 up-regulation. Biochem Biophys Res Commun 257:361–368 [DOI] [PubMed] [Google Scholar]
- Beretta GL, Benedetti V, Cossa G, Assaraf YG, Bram E, Gatti L, Corna E, Carenini N, Colangelo D, Howell SB, et al. (2010) Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem Pharmacol 79:1108–1117 [DOI] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Adams PL, Abada PB, Pesce CE, Safaei R, Howell SB. (2011) Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol Pharmacol 79:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovic A, Simaga S, Osmak M. (2001) Induction of heat shock protein 70 in drug-resistant cells by anticancer drugs and hyperthermia. Neoplasma 48:99–103 [PubMed] [Google Scholar]
- Burnouf D, Duane M, Fuchs RP. (1987) Spectrum of cisplatin-induced mutations in Escherichia coli. Proc Natl Acad Sci USA 84:3758–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205 [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, et al. (2010) Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143:897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Monitto CL, Demokan S, Kim MS, Chang SS, Zhong X, Califano JA, Sidransky D. (2010) Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res 70:2870–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SS, Liang XJ, Su AW, Pai-Panandiker A, Shen DW, Hanover JA, Gottesman MM. (2003) Reduced endocytosis and altered lysosome function in cisplatin-resistant cell lines. Br J Cancer 88:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Jeong HG, Lee JS, Woo ER, Hyun JW, Chung MH, You HJ. (2002) Oncogenic H-Ras enhances DNA repair through the Ras/phosphatidylinositol 3-kinase/Rac1 pathway in NIH3T3 cells. Evidence for association with reactive oxygen species. J Biol Chem 277:19358–19366 [DOI] [PubMed] [Google Scholar]
- Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. (2008) Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett 260:96–108 [DOI] [PubMed] [Google Scholar]
- Cowen LE, Lindquist S. (2005) Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189 [DOI] [PubMed] [Google Scholar]
- Cowen LE, Singh SD, Köhler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al. (2009) Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci USA 106:2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. (2009) Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Xie CH, Neis JP, Fan CY, Vural E, Spring PM. (2011) MicroRNA expression profiles of head and neck squamous cell carcinoma with docetaxel-induced multidrug resistance. Head Neck 33:786–791 [DOI] [PubMed] [Google Scholar]
- de Jong S, Timmer-Bosscha H, de Vries EG, Mulder NH. (1993) Effect of novobiocin on cisplatin cytotoxicity and DNA interstrand cross-link formation in a cisplatin-resistant, small-cell lung carcinoma cell line. Int J Cancer 53:110–117 [DOI] [PubMed] [Google Scholar]
- Ding Z, Liang J, Li J, Lu Y, Ariyaratna V, Lu Z, Davies MA, Westwick JK, Mills GB. (2010) Physical association of PDK1 with AKT1 is sufficient for pathway activation independent of membrane localization and phosphatidylinositol 3 kinase. PLoS One 5:e9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duale N, Lindeman B, Komada M, Olsen AK, Andreassen A, Soderlund EJ, Brunborg G. (2007) Molecular portrait of cisplatin induced response in human testis cancer cell lines based on gene expression profiles. Mol Cancer 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. (2008) Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451:1111–1115 [DOI] [PubMed] [Google Scholar]
- Feng Q, Di R, Tao F, Chang Z, Lu S, Fan W, Shan C, Li X, Yang Z. (2010) PDK1 regulates vascular remodeling and promotes epithelial-mesenchymal transition in cardiac development. Mol Cell Biol 30:3711–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry KV, Hamilton TC, Johnson SW. (2000) Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol 60:1305–1313 [DOI] [PubMed] [Google Scholar]
- Filipits M, Haddad V, Schmid K, Huynh A, Dunant A, André F, Brambilla E, Stahel R, Pignon JP, Soria JC, et al. (2007) Multidrug resistance proteins do not predict benefit of adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: International Adjuvant Lung Cancer Trial Biologic Program. Clin Cancer Res 13:3892–3898 [DOI] [PubMed] [Google Scholar]
- Fritz G, Kaina B, Aktories K. (1995) The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem 270:25172–25177 [DOI] [PubMed] [Google Scholar]
- Gibson D. (2009) The mechanism of action of platinum anticancer agents–what do we really know about it? Dalton Trans (48):10681–10689 [DOI] [PubMed] [Google Scholar]
- Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al. (2011) Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci USA 108:18708–18713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet JP, Gottesman MM. (2010) Mechanisms of multidrug resistance in cancer. Methods Mol Biol 596:47–76 [DOI] [PubMed] [Google Scholar]
- Gosepath EM, Eckstein N, Hamacher A, Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD, Kassack MU. (2008) Acquired cisplatin resistance in the head-neck cancer cell line Cal27 is associated with decreased DKK1 expression and can partially be reversed by overexpression of DKK1. Int J Cancer 123:2013–2019 [DOI] [PubMed] [Google Scholar]
- Gottesman MM. (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627 [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58 [DOI] [PubMed] [Google Scholar]
- Grant SC, Kris MG, Gralla RJ, Clark RA, Tyson LB. (1993) Dose-ranging evaluation of the substituted benzamide dazopride when used as an antiemetic in patients receiving anticancer chemotherapy. Cancer Chemother Pharmacol 31:442–444 [DOI] [PubMed] [Google Scholar]
- Gutekunst M, Oren M, Weilbacher A, Dengler MA, Markwardt C, Thomale J, Aulitzky WE, van der Kuip H. (2011) p53 hypersensitivity is the predominant mechanism of the unique responsiveness of testicular germ cell tumor (TGCT) cells to cisplatin. PLoS One 6:e19198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. (2008) The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol 48:495–535 [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Godwin AK, Yakushiji M, O'Dwyer PJ, Ozols RF, Hamilton TC. (1993) Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res 53:5225–5232 [PubMed] [Google Scholar]
- Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, et al. (2011) Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res 17:3029–3038 [DOI] [PubMed] [Google Scholar]
- Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. (2007) High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins LG, Hayes JD. (2011) The cap‘n’collar transcription factor Nrf2 mediates both intrinsic resistance to environmental stressors and an adaptive response elicited by chemopreventive agents that determines susceptibility to electrophilic xenobiotics. Chem Biol Interact 192:37–45 [DOI] [PubMed] [Google Scholar]
- Hirano G, Izumi H, Kidani A, Yasuniwa Y, Han B, Kusaba H, Akashi K, Kuwano M, Kohno K. (2010) Enhanced expression of PCAF endows apoptosis resistance in cisplatin-resistant cells. Mol Cancer Res 8:864–872 [DOI] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N. (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6:318–327 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. (2006) Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol 70:1390–1394 [DOI] [PubMed] [Google Scholar]
- Howell SB, Safaei R, Larson CA, Sailor MJ. (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, Chang CC, Tzeng CH, Wu KJ, Kao JY, et al. (2010) Regulation of excision repair cross-complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res 16:4561–4571 [DOI] [PubMed] [Google Scholar]
- Huang TG, Ip SM, Yeung WS, Ngan HY. (2000) Changes in p21WAF1, pRb, Mdm-2, Bax and Bcl-2 expression in cervical cancer cell lines transfected with a p53 expressing adenovirus. Eur J Cancer 36:249–256 [DOI] [PubMed] [Google Scholar]
- Hummel R, Watson DI, Smith C, Kist J, Michael MZ, Haier J, Hussey DJ. (2011) Mir-148a improves response to chemotherapy in sensitive and resistant oesophageal adenocarcinoma and squamous cell carcinoma cells. J Gastrointest Surg 15:429–438 [DOI] [PubMed] [Google Scholar]
- Ibanez de Caceres I, Cortes-Sempere M, Moratilla C, Machado-Pinilla R, Rodriguez-Fanjul V, Manguán-García C, Cejas P, López-Ríos F, Paz-Ares L, de CastroCarpeño J, et al. (2010) IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 29:1681–1690 [DOI] [PubMed] [Google Scholar]
- Imanaka Y, Tsuchiya S, Sato F, Shimada Y, Shimizu K, Tsujimoto G. (2011) MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J Hum Genet 56:270–276 [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99:14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaeva N, Thomas HD, Djureinovic T, Djurenovic T, Jaspers JE, Stoimenov I, Kyle S, Pedley N, Gottipati P, Zur R, et al. (2010) 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res 70:6268–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Shen D, Pastan I, Gottesman MM, Hamilton TC. (1996) Cross-resistance, cisplatin accumulation, and platinum-DNA adduct formation and removal in cisplatin-sensitive and -resistant human hepatoma cell lines. Exp Cell Res 226:133–139 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasherman Y, Sturup S, Gibson D. (2009) Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. J Med Chem 52:4319–4328 [DOI] [PubMed] [Google Scholar]
- Kato K, Nomoto M, Izumi H, Ise T, Nakano S, Niho Y, Kohno K. (2000) Structure and functional analysis of the human STAT3 gene promoter: alteration of chromatin structure as a possible mechanism for the upregulation in cisplatin-resistant cells. Biochim Biophys Acta 1493:91–100 [DOI] [PubMed] [Google Scholar]
- Keenen B, Qi H, Saladi SV, Yeung M, de la Serna IL. (2010) Heterogeneous SWI/SNF chromatin remodeling complexes promote expression of microphthalmia-associated transcription factor target genes in melanoma. Oncogene 29:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Uchiumi T, Niina I, Wakasugi T, Igarashi T, Momii Y, Yoshida T, Matsuo K, Miyamoto N, Izumi H. (2005) Transcription factors and drug resistance. Eur J Cancer 41:2577–2586 [DOI] [PubMed] [Google Scholar]
- Kosik KS. (2010) MicroRNAs and cellular phenotypy. Cell 143:21–26 [DOI] [PubMed] [Google Scholar]
- Koster R, de Jong S. (2010) Lessons learned from testicular cancer: identification of cytoplasmic p21 as an Achilles' heel of cisplatin resistance. Cell Cycle 9:4776–4777 [DOI] [PubMed] [Google Scholar]
- Koul S, McKiernan JM, Narayan G, Houldsworth J, Bacik J, Dobrzynski DL, Assaad AM, Mansukhani M, Reuter VE, Bosl GJ, et al. (2004) Role of promoter hypermethylation in Cisplatin treatment response of male germ cell tumors. Mol Cancer 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landriscina M, Laudiero G, Maddalena F, Amoroso MR, Piscazzi A, Cozzolino F, Monti M, Garbi C, Fersini A, Pucci P, et al. (2010) Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res 70:6577–6586 [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Cho JS, Yuk DY, Moon DC, Jung JK, Yoo HS, Lee YM, Han SB, Oh KW, Hong JT. (2009) Obovatol enhances docetaxel-induced prostate and colon cancer cell death through inactivation of nuclear transcription factor-kappaB. J Pharmacol Sci 111:124–136 [DOI] [PubMed] [Google Scholar]
- Lei H, Quelle FW. (2009) FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res 7:1294–1303 [DOI] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. (2010) MicroRNA functions in stress responses. Mol Cell 40:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Li YY, Tsao SW, Cheung AL. (2009) Targeting NF-kappaB signaling pathway suppresses tumor growth, angiogenesis, and metastasis of human esophageal cancer. Mol Cancer Ther 8:2635–2644 [DOI] [PubMed] [Google Scholar]
- Liang XJ, Finkel T, Shen DW, Yin JJ, Aszalos A, Gottesman MM. (2008) SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol Cancer Res 6:1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XJ, Meng H, Wang Y, He H, Meng J, Lu J, Wang PC, Zhao Y, Gao X, Sun B, et al. (2010) Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc Natl Acad Sci USA 107:7449–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XJ, Mukherjee S, Shen DW, Maxfield FR, Gottesman MM. (2006) Endocytic recycling compartments altered in cisplatin-resistant cancer cells. Cancer Res 66:2346–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XJ, Shen DW, Garfield S, Gottesman MM. (2003) Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res 63:5909–5916 [PubMed] [Google Scholar]
- Liang XJ, Shen DW, Gottesman MM. (2004) Down-regulation and altered localization of gamma-catenin in cisplatin-resistant adenocarcinoma cells. Mol Pharmacol 65:1217–1224 [DOI] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. (2002) The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62:1154–1159 [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Birnbaum MJ, Stoffers DA. (2010) Three-amino-acid-loop-extension homeodomain factor Meis3 regulates cell survival via PDK1. Proc Natl Acad Sci USA 107:20494–20499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JR, Opipari AW, Tan L, Jiang Y, Zhang Y, Tang H, Nuñez G. (2002) Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res 62:924–931 [PubMed] [Google Scholar]
- Llanos S, Serrano M. (2010) Depletion of ribosomal protein L37 occurs in response to DNA damage and activates p53 through the L11/MDM2 pathway. Cell Cycle 9:4005–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. (2003) Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem 278:9100–9106 [DOI] [PubMed] [Google Scholar]
- Martin LP, Hamilton TC, Schilder RJ. (2008) Platinum resistance: the role of DNA repair pathways. Clin Cancer Res 14:1291–1295 [DOI] [PubMed] [Google Scholar]
- Messina S, Leonetti C, De Gregorio G, Affatigato V, Ragona G, Frati L, Zupi G, Santoni A, Porcellini A. (2004) Ras inhibition amplifies cisplatin sensitivity of human glioblastoma. Biochem Biophys Res Commun 320:493–500 [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Izumi H, Noguchi T, Nakajima Y, Ohmiya Y, Shiota M, Kidani A, Tawara A, Kohno K. (2008) Tip60 is regulated by circadian transcription factor clock and is involved in cisplatin resistance. J Biol Chem 283:18218–18226 [DOI] [PubMed] [Google Scholar]
- Montesano Gesualdi N, Chirico G, Pirozzi G, Costantino E, Landriscina M, Esposito F. (2007) Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress 10:342–350 [DOI] [PubMed] [Google Scholar]
- Nagengast WB, de Korte MA, Oude Munnink TH, Timmer-Bosscha H, den Dunnen WF, Hollema H, de Jong JR, Jensen MR, Quadt C, Garcia-Echeverria C, et al. (2010) 89Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl Med 51:761–767 [DOI] [PubMed] [Google Scholar]
- Narod SA. (2010) BRCA mutations in the management of breast cancer: the state of the art. Nat Rev Clin Oncol 7:702–707 [DOI] [PubMed] [Google Scholar]
- Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, et al. (2008) Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res 68:1303–1309 [DOI] [PubMed] [Google Scholar]
- Okamoto J, Hirata T, Chen Z, Zhou HM, Mikami I, Li H, Yagui-Beltran A, Beltran A, Johansson M, Coussens LM, et al. (2010) EMX2 is epigenetically silenced and suppresses growth in human lung cancer. Oncogene 29:5969–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, et al. (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355:983–991 [DOI] [PubMed] [Google Scholar]
- Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, Brekken R, Wurz R, Tasker A, Polverino T, et al. (2011) TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell 41:458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owatari S, Akune S, Komatsu M, Ikeda R, Firth SD, Che XF, Yamamoto M, Tsujikawa K, Kitazono M, Ishizawa T, et al. (2007) Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res 67:4860–4868 [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. (2010) Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer 127:1785–1794 [DOI] [PubMed] [Google Scholar]
- Raimondi C, Gianni W, Cortesi E, Gazzaniga P. (2010) Cancer stem cells and epithelial-mesenchymal transition: revisiting minimal residual disease. Curr Cancer Drug Targets 10:496–508 [DOI] [PubMed] [Google Scholar]
- Reed E. (1998) Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev 24:331–344 [DOI] [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA 108:1433–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JJ, Thomson AJ. (1979) The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol 22:71–133 [DOI] [PubMed] [Google Scholar]
- Rosenberg B. (1973) Platinum coordination complexes in cancer chemotherapy. Naturwissenschaften 60:399–406 [DOI] [PubMed] [Google Scholar]
- Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. (2010) AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res 70:7360–7364 [DOI] [PubMed] [Google Scholar]
- Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB. (2008) Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol 73:461–468 [DOI] [PubMed] [Google Scholar]
- Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, Wurz K, Higgins J, Villegas E, Taniguchi T. (2009) Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res 69:6381–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ, Couch FJ, et al. (2008) Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, Edwards R, Mayer NJ, Qazi H, Goyal S, et al. (2009) SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci USA 106:14884–14889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. (2003) Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol 35:1135–1143 [DOI] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, et al. (2010) A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Pastan I, Gottesman MM. (1998) Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res 58:268–275 [PubMed] [Google Scholar]
- Shen DW, Akiyama S, Schoenlein P, Pastan I, Gottesman MM. (1995) Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br J Cancer 71:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Goldenberg S, Pastan I, Gottesman MM. (2000) Decreased accumulation of [14C]carboplatin in human cisplatin-resistant cells results from reduced energy-dependent uptake. J Cell Physiol 183:108–116 [DOI] [PubMed] [Google Scholar]
- Shen DW, Gottesman MM. (2012) RAB8 enhances TMEM205-mediated cisplatin resistance. Pharm Res 29:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Liang XJ, Gawinowicz MA, Gottesman MM. (2004a) Identification of cytoskeletal [14C]carboplatin-binding proteins reveals reduced expression and disorganization of actin and filamin in cisplatin-resistant cell lines. Mol Pharmacol 66:789–793 [DOI] [PubMed] [Google Scholar]
- Shen DW, Liang XJ, Suzuki T, Gottesman MM. (2006) Identification by functional cloning from a retroviral cDNA library of cDNAs for ribosomal protein L36 and the 10-kDa heat shock protein that confer cisplatin resistance. Mol Pharmacol 69:1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Ma J, Okabe M, Zhang G, Xia D, Gottesman MM. (2010) Elevated expression of TMEM205, a hypothetical membrane protein, is associated with cisplatin resistance. J Cell Physiol 225:822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Pouliot L, Gillet JP, Ma W, Johnson A, Hall MD, Gottesman MM. (2012) The transcription factor GCF2 is an upstream repressor of the small GTPAse RhoA, regulating membrane protein trafficking, sensitivity to doxorubicin and resistance to cisplatin. Mol Pharm http://dx.doi.org/10.1021/mp300153z [DOI] [PMC free article] [PubMed]
- Shen DW, Su A, Liang XJ, Pai-Panandiker A, Gottesman MM. (2004b) Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer 91:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Kondo Y, Ahmed S, Boumber Y, Konishi K, Guo Y, Chen X, Vilaythong JN, Issa JP. (2007) Drug sensitivity prediction by CpG island methylation profile in the NCI-60 cancer cell line panel. Cancer Res 67:11335–11343 [DOI] [PubMed] [Google Scholar]
- Shim J, Lee SM, Lee MS, Yoon J, Kweon HS, Kim YJ. (2010) Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol Cell Biol 30:1421–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Yokomizo A, Tada Y, Uchiumi T, Inokuchi J, Tatsugami K, Kuroiwa K, Yamamoto K, Seki N, Naito S. (2010) P300/CBP-associated factor regulates Y-box binding protein-1 expression and promotes cancer cell growth, cancer invasion and drug resistance. Cancer Sci 101:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. (2002) Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 21:878–889 [DOI] [PubMed] [Google Scholar]
- Siddik ZH. (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279 [DOI] [PubMed] [Google Scholar]
- Singh A, Settleman J. (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Welham KJ, Watson MB, Drew PJ, Lind MJ, Cawkwell L. (2007) The proteomic analysis of cisplatin resistance in breast cancer cells. Oncol Res 16:497–506 [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. (2008) Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol 111:478–486 [DOI] [PubMed] [Google Scholar]
- Steele N, Finn P, Brown R, Plumb JA. (2009) Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer 100:758–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HY, Lai HC, Lin YW, Liu CY, Chen CK, Chou YC, Lin SP, Lin WC, Lee HY, Yu MH. (2010) Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer 127:555–567 [DOI] [PubMed] [Google Scholar]
- Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M, Zhang W, Chen W, Pan C, Liu Q, et al. (2012) MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene 31:432–445 [DOI] [PubMed] [Google Scholar]
- Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. (2008) Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res 68:2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Lee PL, Li Z, Jiang X, Lim YC, Hooi SC, Yu Q. (2010) B55β-associated PP2A complex controls PDK1-directed myc signaling and modulates rapamycin sensitivity in colorectal cancer. Cancer Cell 18:459–471 [DOI] [PubMed] [Google Scholar]
- Tang N, Zhang J, Du Y. (2010) [Curcumin promoted the apoptosis of cisplain-resistant human lung carcinoma cells A549/DDP through down-regulating miR-186*]. Zhongguo Fei Ai Za Zhi 13:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Wang X, Wang Y, Ma D. (2010) Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun 392:373–379 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890 [DOI] [PubMed] [Google Scholar]
- Torigoe T, Izumi H, Ishiguchi H, Yoshida Y, Tanabe M, Yoshida T, Igarashi T, Niina I, Wakasugi T, Imaizumi T, et al. (2005) Cisplatin resistance and transcription factors. Curr Med Chem Anticancer Agents 5:15–27 [DOI] [PubMed] [Google Scholar]
- Uematsu K, Seki N, Seto T, Isoe C, Tsukamoto H, Mikami I, You L, He B, Xu Z, Jablons DM, et al. (2007) Targeting the Wnt signaling pathway with dishevelled and cisplatin synergistically suppresses mesothelioma cell growth. Anticancer Res 27:4239–4242 [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 79:468–475 [DOI] [PubMed] [Google Scholar]
- Wang QE, Han C, Milum K, Wani AA. (2011) Stem cell protein Piwil2 modulates chromatin modifications upon cisplatin treatment. Mutat Res 708:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Figg WD. (2008) Secondary BRCA1 and BRCA2 alterations and acquired chemoresistance. Cancer Biol Ther 7:1004–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, et al. (2009) The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 11:1487–1495 [DOI] [PubMed] [Google Scholar]
- Wermann H, Stoop H, Gillis AJ, Honecker F, van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C, Looijenga LH. (2010) Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol 221:433–442 [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, et al. (2008a) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 68:425–433 [DOI] [PubMed] [Google Scholar]
- Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, et al. (2008b) MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res 68:10307–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Fu G, Cui S, Zhao S, Bernaudo S, Bai Y, Ding Y, Zhang Y, Yang BB, Peng C. (2011) MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: implications for chemoresistance. J Cell Sci 124:359–368 [DOI] [PubMed] [Google Scholar]
- Youn CK, Kim MH, Cho HJ, Kim HB, Chang IY, Chung MH, You HJ. (2004) Oncogenic H-Ras up-regulates expression of ERCC1 to protect cells from platinum-based anticancer agents. Cancer Res 64:4849–4857 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P. (2001) Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3). J Biol Chem 276:46400–46407 [DOI] [PubMed] [Google Scholar]
- Zhang D, Putti TC. (2010) Over-expression of ERp29 attenuates doxorubicin-induced cell apoptosis through up-regulation of Hsp27 in breast cancer cells. Exp Cell Res 316:3522–3531 [DOI] [PubMed] [Google Scholar]
- Zhao R, Houry WA. (2005) Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol 83:703–710 [DOI] [PubMed] [Google Scholar]
- Zhu W, Shan X, Wang T, Shu Y, Liu P. (2010) miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer 127:2520–2529 [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. (2012a) miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol 69:723–731 [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, Shu Y, Liu P. (2012b) miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol 29:384–391 [DOI] [PubMed] [Google Scholar]
- Zhuo W, Wang Y, Zhuo X, Zhang Y, Ao X, Chen Z. (2008a) Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer 62:8–14 [DOI] [PubMed] [Google Scholar]
- Zhuo WL, Wang Y, Zhuo XL, Zhang YS, Chen ZT. (2008b) Short interfering RNA directed against TWIST, a novel zinc finger transcription factor, increases A549 cell sensitivity to cisplatin via MAPK/mitochondrial pathway. Biochem Biophys Res Commun 369:1098–1102 [DOI] [PubMed] [Google Scholar]