Abstract
Drug-induced plasticity of excitatory synapses has been proposed to be the cellular mechanism underlying the aberrant learning associated with addiction. Exposure to various drugs of abuse causes both morphological plasticity of dendritic spines and functional plasticity of excitatory synaptic transmission. Chronic activation of μ-opioid receptors (MOR) in cultured hippocampal neurons causes two forms of synaptic plasticity: loss of dendritic spines and loss of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. With use of live imaging, patch-clamp electrophysiology, and immunocytochemistry, the present study reveals that these two forms of synaptic plasticity are mediated by separate, but interactive, intracellular signaling cascades. The inhibition of Ca2+/calmodulin-dependent protein kinase II with 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62) blocks MOR-mediated structural plasticity of dendritic spines, but not MOR-mediated cellular redistribution of GluR1 and GluR2 AMPA receptor subunits. In contrast, the inhibition of calcineurin with tacrolimus (FK506) blocks both cellular processes. These findings support the idea that drug-induced structural and functional plasticity of dendritic spines is mediated by divergent, but interactive, signaling pathways.
Introduction
Forms of synaptic plasticity such as LTP and LTD are widely studied as cellular models of learning and memory (Malenka and Bear, 2004). Extensive study has revealed that LTP and LTD cause two major forms of synaptic plasticity: functional plasticity of excitatory synaptic transmission and structural plasticity of dendritic spine morphology and number (Tada and Sheng, 2006; Greger and Esteban, 2007; Wang et al., 2007; Cingolani and Goda, 2008). Addiction has been proposed to be a pathological form of learning (Hyman et al., 2006), and exposure to various drugs of abuse has been found to cause both morphological plasticity of dendritic spines and functional plasticity of excitatory synaptic transmission (Robinson and Kolb, 2004; Bowers et al., 2010; Russo et al., 2010; Wolf, 2010).
Our previous work has demonstrated that chronic treatment with morphine causes collapse of dendritic spines and suppresses excitatory synaptic transmission in hippocampal neurons in a postsynaptic, μ-opioid receptor (MOR)-dependent manner (Liao et al., 2005, 2007a,b). Although dendritic spine plasticity and synaptic AMPA receptor plasticity have been shown to be highly correlated in a number of studies (McKinney et al., 1999; Matsuzaki et al., 2004; Richards et al., 2005; Okamoto et al., 2009), other studies have found that changes in spine morphology and synaptic AMPA receptors can be differentially mediated (Sdrulla and Linden, 2007; Wang et al., 2007). Until the present study, it has not been known whether these two cellular processes can be differentially mediated in drug-induced plasticity of excitatory synapses.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a serine/threonine kinase that is widely known for its critical roles in synaptic plasticity and learning and memory (Silva et al., 1992; Lledo et al., 1995; Okamoto et al., 2009). Long-term morphine treatment decreases the phosphorylation activity of CaMKII in vivo (Lou et al., 1999) and in vitro (Zheng et al., 2010). Inhibition of CaMKII in the hippocampus blocks the development of morphine tolerance and dependence (Fan et al., 1999; Lu et al., 2000). Calcineurin is a phosphatase that has recently been reported to be necessary for morphine-induced internalization of the GluR1 AMPA receptor subunit (Kam et al., 2010). Overexpression of calcineurin in vivo has been shown to impair morphine reinforcement (Biala et al., 2005). Despite extensive previous studies supporting the role of both signaling proteins in morphine addiction, their role in morphine-induced plasticity of dendritic spines has not yet been fully revealed.
Here we report that divergent pathways mediate morphine-induced spine loss and the loss of synaptic AMPA receptors. The inhibition of CaMKII at its autophosphorylation site, Thr286, blocks the effect of morphine on spine morphology but not the effect of morphine on AMPA receptors. In contrast, the inhibition of calcineurin phosphatase activity blocks both morphine-induced spine loss and the loss of synaptic AMPA receptors. These results together provide the novel finding that divergent pathways mediate the structural and functional plasticity of excitatory synapses by morphine, shedding new light on the cellular mechanisms that may underlie opiate-induced behavioral changes.
Materials and Methods
High-Density Neuronal Cultures and Neuronal Transfection.
A 25-mm glass polylysine-coated coverslip (thickness 0.08 mm) was glued to the bottom of a 35-mm culture dish with a 22-mm hole using silicone sealant as described previously (Wiens et al., 2005). Dissociated neuronal cultures from rat hippocampus at postnatal days 1 to 2 were prepared as described previously (Liao et al., 2001). Neurons were plated onto prepared 35-mm culture dishes at a density of 106 cells/dish. The age of cultured neurons was counted from the day of plating as 1 day in vitro (DIV). All experiments were performed on neurons from at least three independent cultures. Neurons at 7 to 10 DIV were transfected with appropriate plasmids using the standard calcium phosphate precipitation method as described previously (Wiens et al., 2005). After transfection, neurons were put back into a tissue culture incubator (37°C, 5% CO2) and allowed to mature and develop until 3 weeks in vitro, a time at which neurons express high numbers of dendritic spines with mature morphologies. CaMKII wild-type (WT) and CaMKII dominant-negative constructs (generous gifts from Dr. Richard Huganir, Johns Hopkins University, Baltimore, MD) were tagged with GFP (referred to as GFP) on the N terminus and expressed in the pRK5 vector and driven by a cytomegalovirus (CMV) promoter (Clontech, Mountain View, CA). Rac1N17 (dominant-negative; referred to as Rac−), or Rac1V12 (constitutively active; referred to as Rac+) were tagged with GFP and expressed in the pRK5 vector and driven by a CMV promoter. The Rac1 constructs have been characterized and used previously (Li et al., 2002; Wiens et al., 2005). The GFP and DsRed constructs (Clontech) were also expressed in the pRK5 vector and driven by a CMV promoter. Concentrations used were 10 μM morphine, 10 μM naloxone, 10 μM d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), 10 μM 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62) (Tokumitsu et al., 1990), 1 μM tacrolimus (FK506) (Lieberman and Mody, 1994; Kam et al., 2010), and 30 μM N,N,N,-trimethyl-5-[(tricyclo]3.3.1.13,7[dec-1-ylmethyl) amino]-1-pentanaminiumbromide hydrobromide (IEM-1460) (Fortin et al., 2010). Drugs were added to the culture media at 21 DIV and remained for the length of the experiments.
Low-Density Neuronal Cultures.
To detect the distribution of endogenous synaptic proteins with high resolution, low-density neuronal cultures were prepared as described previously with some modifications (Hoover et al., 2010). Dissociated neuronal cultures from rat hippocampus at postnatal days 1 to 2 were plated onto 12-well culture plates at a density of 5 to 10 × 104 cells/well. Each well contained a polylysine-coated 12-mm glass coverslip on the bottom. To maintain the low-density cultures for a long time (up to 1 month), the above 12-mm coverslips with low-density cultured neurons were transferred to 60-mm dishes (four coverslips per dish; the coverslips faced up) that contained high-density neuronal cultures after 1 week in vitro. In previous studies, dishes with a glial feed layer were often used to support low-density cultures. We have found that high-density neuronal cultures are far better supporters than pure glial cells.
Electrophysiology.
Miniature excitatory postsynaptic currents (mEPSCs) were recorded from cultured dissociated rat hippocampal neurons at 21 to 25 DIV with a glass pipette (resistance of ∼5 MΩ) at holding potentials of −55 mV and filtered at 1 kHz as described previously (Liao et al., 2005). Input and series resistances were checked before and after the recording of mEPSCs, which lasted 5 to 20 min. There were no significant difference in the series resistances and input resistance among various groups of experiments. One recording sweep lasting 200 ms was sampled for every 1 s. Neurons were bathed in artificial cerebrospinal fluid at room temperature (25°C) with 100 μM 2-amino-5-phosphonovalerate (an N-methyl-d-aspartate receptor antagonist), 1 μM tetrodotoxin (a sodium channel blocker), and 100 μM picrotoxin (GABAA receptor antagonist), gassed with 95% O2-5% CO2. The artificial cerebrospinal fluid contained 119 mM NaCl, 2.5 mM KCl, 5.0 mM CaCl2, 2.5 mM MgCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4, and 11 mM glucose. The internal solution in the patch pipette contained 100 mM cesium gluconate, 0.2 mM EGTA, 0.5 mM MgCl2, 2 mM ATP, 0.3 mM GTP, and 40 mM HEPES (pH 7.2 with CsOH). All mEPSCs were analyzed with the Mini Analysis program designed by Synaptosoft (Decatur, GA). The detection criterion for mEPSCs was set as the peak amplitude (3 pA). Each mEPSC event was visually inspected, and only events with a distinctly fast-rising phase and a slow-decaying phase were accepted. The frequency and amplitude of all accepted mEPSCs were directly read out using the analysis function in the Mini Analysis program. The averaged parameters from each neuron were treated as single samples in any further statistical analyses.
Time-Lapse Live Imaging Method.
To label dendrites, high-density neurons at 7 to 10 DIV were transfected with plasmids encoding GFP, GFP-tagged molecules, and/or DsRed. The 35-mm culture dishes fit tightly in a homemade holding chamber on a fixed platform above an inverted Nikon microscope sitting on an X-Y translation stage (Thorlabs, Newton, NJ). A 60× oil lens was used for all imaging experiments. Original images were 157.3 μm wide (x-axis) and 117.5 μm tall (y-axis). The z-axis was composed of 15 images, taken at 0.5-μm intervals. The location of any neuron of interest was recorded by the reading of the X-Y translation stage. The culture dish was immediately put back into a tissue culture incubator after each observation. Neurons could be found again in the next observation using the X-Y translation stage (accuracy, 4 μm).
Immunocytochemistry in Fixed Tissues.
Cultured neurons were fixed and permeabilized successively with 4% paraformaldehyde, 100% methanol, and 0.2% Triton X-100 (Hoover et al., 2010). For all immunocytochemistry primary and secondary antibodies, a dilution factor of 1:50 in 10% donkey serum in phosphate-buffered saline was used. Commercial antibodies against PSD-95 were used as a postsynaptic marker [rabbit polyclonal (Invitrogen, Carlsbad, CA); mouse monoclonal (Millipore Corporation, Billerica, MA)] (Liao et al., 2001). The rabbit polyclonal antibodies against the C terminus of GluR1 or Glu2/3 subunits were generous gifts from Dr. Richard Huganir (Johns Hopkins University). Antisera against MOR were produced against a synthesized 15-residue peptide (NHQLENLEAETAPLP) corresponding to amino acids 384 to 398 (Arvidsson et al., 1995). A commercial antibody against CaMKII was used (mouse monoclonal; Invitrogen). Finally, fluorescein isothiocyanate (green) or rhodamine (red)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to recognize these primary antibodies.
To estimate the amount of glutamate receptors in dendritic spines, fixed rat neurons immunoreactive for PSD-95 (mouse monoclonal; Millipore Corporation) and a GluR antibody (GluR1 or GluR2/3) were photographed and processed with MetaMorph software (Molecular Devices, Sunnyvale, CA). as described previously (Hoover et al., 2010). Then, immunoreactive clusters of PSD-95 were autoselected using MetaMorph software, and the location of these clusters was transferred to images displaying glutamate receptor immunoreactivity on the same neuron. PSD-95 immunoreactivity was used to identify dendritic spines. A cursor was placed in the center of the glutamate receptor clusters in dendritic spines to estimate glutamate receptor immunoreactivity as fluorescent pixel intensity in the spines (value Y1). Another cursor was placed in an adjacent dendritic shaft to measure glutamate receptor fluorescent pixel intensity (value Y2), and the ratio of glutamate receptor immunoreactive fluorescence intensity in spines/dendrites (Y1/Y2) was plotted on the y-axis.
Western Blots.
Western blot experiments were performed as described previously (Wiens et al., 2005). In brief, cultured neurons were lysed in modified radioimmunoprecipitation assay buffer on ice and then harvested with a cell scraper. The same amount of protein was loaded in each lane. To determine CaMKII phosphorylation activity, cell lysates were run on the Western blot and then stained with a phospho-CaMKII antibody and a CaMKII antibody (GenScript, Piscataway, NJ). To determine Rac1 activity, a Rac1 activation kit (Millipore Corporation) was used. Cell lysates were immunoprecipitated with Rac/cdc42 Assay Reagent (PAK-1 PBD, agarose) to isolate active Rac1. Immunoprecipitated lysates and control lysates were run on a gel and then stained with a Rac1 antibody (mouse monoclonal; Millipore Corporation). Rac1 proteins in total cell lysates were loaded as controls. Rac1 activation was quantified with ImageQuant TL (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and measured as a proportion of active Rac1 to total Rac1 (Li et al., 2002).
Image Analysis.
Time-lapse live images from the same neuron at 21 DIV were taken before and at various time points after drug treatments as described previously (Liao et al., 2005). All digital images were analyzed with the MetaMorph Imaging System (Universal Imaging Corporation). Unless stated otherwise, all images of live neurons were taken as stacks and were averaged into one image before further analysis. In addition to simple averaging, stacks of images were also processed by deconvolution of nearest planes using MetaMorph. A stack of deconvolved images was further averaged into one single image. A dendritic protrusion with an expanded head that was 50% wider than its neck was defined as a spine. The number of spines from a dendrite was manually counted and normalized per 100 μm of dendritic length; only dendrites with 50 μm or more of analyzable dendritic shaft were counted. The number of protrusions from a dendrite was manually counted, including both spines and nonspine protrusions. Student's t tests were used for comparison between parameters from two groups, whereas ANOVA tests were used for comparison between parameters from multiple groups (n = number of neurons; p < 0.05, significant). All data are reported as mean ± S.E.
Results
Inhibition of Both CaMKII and Calcineurin Prevents Morphine-Induced Spine Collapse.
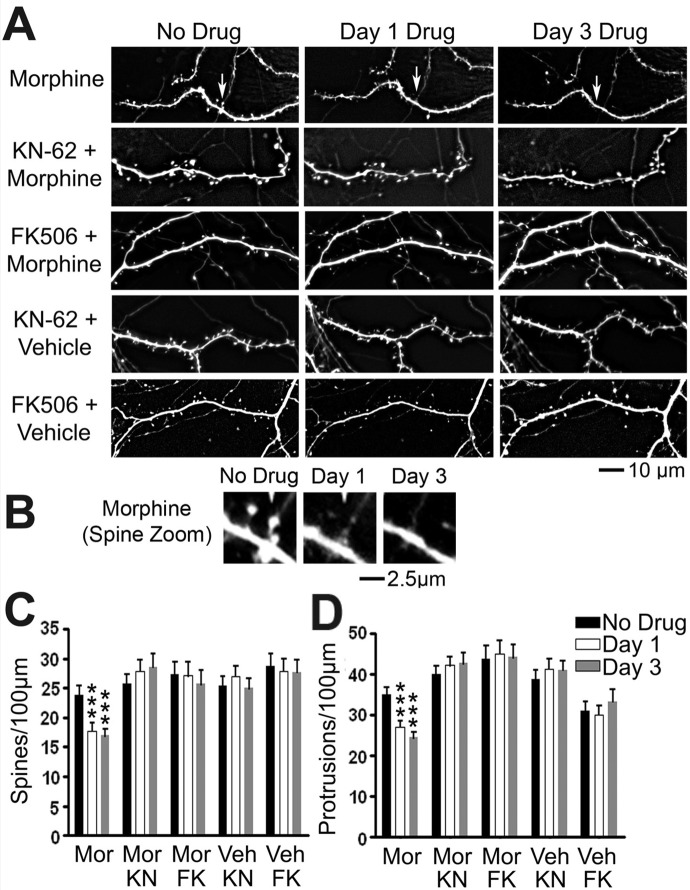
Our previous studies have demonstrated that chronic postsynaptic activation of MORs leads to two forms of plasticity of dendritic spines: loss of preexisting spines and loss of synaptic AMPA receptors (Liao et al., 2005, 2007a,b; Kam et al., 2010). Here, we used our time-lapse live imaging system to clarify the signaling pathway that mediates morphine-induced spine loss (Fig. 1). MOR activity postsynaptically modulates the structural plasticity of dendritic spines (Liao et al., 2005, 2007a). CaMKII and calcineurin are known to play an important role in structural plasticity of dendritic spines during LTP and LTD (Silva et al., 1992; Matsuzaki et al., 2004). However, their roles in drug-induced morphological changes in spines remain unknown. To test their roles in MOR-mediated spine plasticity, we blocked CaMKII kinase activity with KN-62 (Tokumitsu et al., 1990) and calcineurin phosphatase activity with FK506 (Lieberman and Mody, 1994). Cultured rat hippocampal neurons at 21 DIV were treated by adding vehicle, morphine (10 μM), morphine / KN-62 (10 μM), morphine plus FK506 (1 μM), KN-62 alone, or FK506 alone to the culture media for the length of the experiment (Fig. 1A). In past studies, we have found that vehicle treatment alone has no significant effect on dendritic spine density (Liao et al., 2005). A dendritic protrusion with a head 50% wider than its neck is defined as a dendritic spine. Morphine treatment significantly decreased both spine and total protrusion density (number/100-μm length of dendrites) 1 to 3 days after treatment (Fig. 1, B and C; n = 8–10 neurons/group, p < 0.01; repeated-measures one-way ANOVA). Treatment with either KN-62 or FK506 alone had no significant effects on the density of dendritic spines (Fig. 1B) or protrusions (Fig. 1C). The presence of KN-62 or FK506 prevented the changes in both spine and protrusion density caused by morphine (Fig. 1, B and C; before versus day 1 morphine and no drug versus day 3 morphine, p < 0.001; n = 10 neurons/group). We believe that the application of KN-62 prevents the decrease in CaMKII activity that is caused by morphine treatment (Zheng et al., 2010) and that FK506 prevents increases in calcineurin activity caused by morphine treatment (Kam et al., 2010). These results indicate that the activation of both CaMKII, and calcineurin is necessary for morphine-induced changes in spine density.
Fig. 1.
Inhibition of both CaMKII and calcineurin prevents morphine-induced spine collapse. A, representative live images of GFP-labeled cultured hippocampal neurons first taken at 21 DIV and then imaged again 1 and 3 days after drug treatment. Scale bar, 10 μm. B, zoomed image of a collapsing spine from the morphine-treated lane of A. Scale bar, 2.5 μm. C, quantification of spine density per 100 μm of dendrite length in neurons described in A. Morphine (Mor) treatment causes a significant decrease in spine density that is blocked by cotreatment with KN-62 (KN) or FK506 (FK). D, quantification of protrusion density of neurons described in A. Morphine treatment causes a significant decrease in protrusion density that is blocked by cotreatment with KN-62 or FK506. Repeated-measures two-way ANOVA; Bonferroni post-test: ***, p < 0.001; n = 8 to 10 neurons/group. Veh, vehicle.
Expression of Dominant-Negative Mutant CaMKII Prevents Morphine-Induced Spine Collapse.
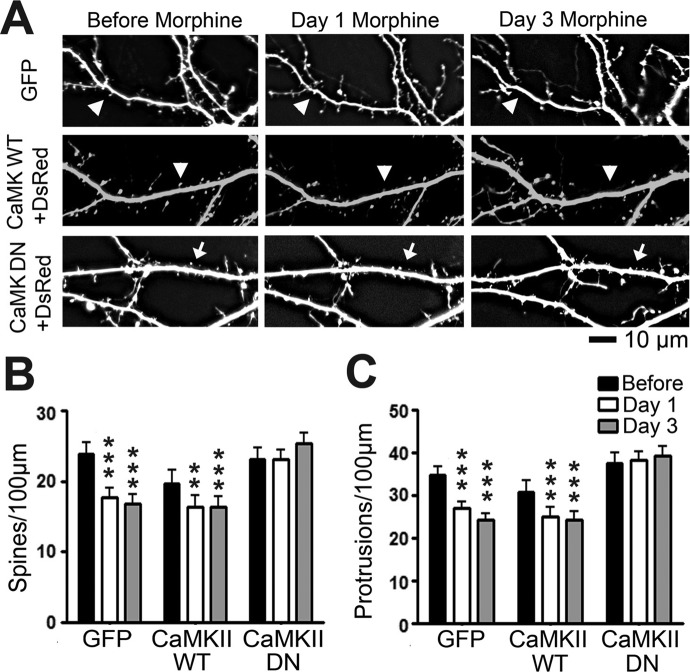
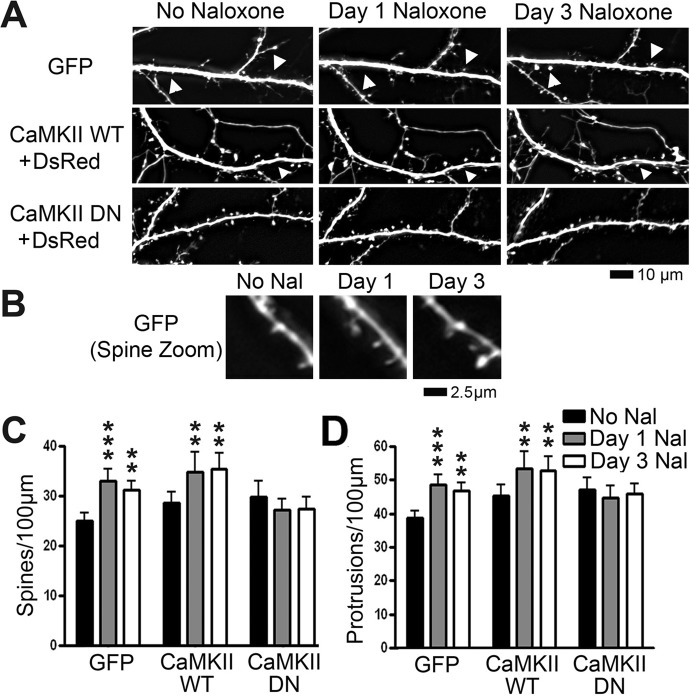
To further explore the necessity of CaMKII for MOR-induced structural plasticity, we enlisted the use of a dominant-negative CaMKII construct with a point mutation at autophosphorylation site Thr286 (Giese et al., 1998) (a generous gift from Dr. Richard Huganir, John Hopkins University). Mice expressing CaMKII with this mutation show disruptions in spatial learning and hippocampal CA1 LTP (Giese et al., 1998). At 7 DIV, we transfected cultured hippocampal neurons with a plasmid encoding GFP alone, two plasmids encoding GFP-tagged wild-type CaMKII and DsRed or two plasmids encoding GFP-tagged dominant-negative CaMKII and DsRed. Whereas the GFP-tagged CaMKII constructs are appropriate for examining the cellular distribution of CaMKII, DsRed is more appropriate for visualizing the morphology of dendrites. Neurons were then treated with vehicle, morphine, or naloxone (10 μM) at 21 DIV. Naloxone is a nonspecific MOR antagonist that we have shown causes an increase in spine density (Liao et al., 2005). We imaged the neurons before drug exposure, and then 1 and 3 days after drug exposure. There was no significant effect of morphine on the density of dendritic spines in neurons that had been transfected with dominant-negative CaMKII (Fig. 2, A–C). In contrast, morphine caused a significant decrease in both spine and total protrusion density in neurons expressing GFP or wild-type CaMKII after 1 and 3 days of morphine treatment (Fig. 2, A–C). Naloxone significantly increased the density of dendritic spines and protrusions in these neurons at 1 and 3 DIV (Fig. 3, A–C). Naloxone did not cause a significant change in spine or protrusion density in neurons transfected with dominant-negative CaMKII (Fig. 3, B and C; repeated-measures two-way ANOVA, interaction p < 0.01; Bonferroni post-test used for differences between individual groups; n = 8–10 neurons/group). These results further support the fact that CaMKII kinase activity is required for opioid-induced structural plasticity.
Fig. 2.
Expression of dominant-negative (DN) mutant CaMKII prevents morphine-induced spine collapse. A, representative live images of cultured hippocampal neurons first taken at 21 DIV and then imaged again 1 and 3 days after drug treatment. Neurons were transfected with plasmids encoding either GFP alone, DsRed and GFP-tagged CaMKII WT, or DsRed and CaMKII DN tagged with GFP at 7 DIV. Row 1 shows GFP; rows 2 and 3 show DsRed. Arrows denote spine collapse. Scale bar, 10 μm. B, quantification of spine density in A. Morphine treatment causes a significant decrease in spine density. The effect persists when neurons are transfected with CaMKII WT but is blocked when neurons are transfected with CaMKII DN. C, quantification of protrusion density in A. Morphine treatment causes a significant decrease in protrusion density. The effect persists when neurons are transfected with CaMKII WT but is blocked when neurons are transfected with CaMKII DN. Repeated-measures two-way ANOVA; Bonferroni post-test: **, p < 0.01; ***, p < 0.001; n = 8 to 10 neurons/group.
Fig. 3.
Expression of dominant-negative (DN) mutant CaMKII prevents naloxone-induced increases in spine density. A, representative live images of cultured hippocampal neurons first taken at 21 DIV and then imaged again 1 and 3 days after drug treatment. Neurons were transfected with plasmids encoding either GFP alone, DsRed and GFP-tagged CaMKII WT, or DsRed and CaMKII DN tagged with GFP at 7 DIV. Row 1 shows GFP; rows 2 and 3 show DsRed. Arrowheads indicate growth of spines. Scale bar, 10 μm. B, zoomed image of a new spine from the GFP-transfected, naloxone (Nal)-treated lane of A. Scale bar, 2.5 μm. C, quantification of spine density in A. Naloxone treatment causes a significant increase in spine density. The effect persists when neurons are transfected with CaMKII WT but is blocked when neurons are transfected with CaMKII DN. D, quantification of protrusion density in A. Naloxone treatment causes a significant increase in protrusion density. The effect persists when neurons are transfected with CaMKII WT but is blocked when neurons are transfected with CaMKII DN. Repeated-measures two-way ANOVA; Bonferroni post-test: **, p < 0.01; ***, p < 0.001; n = 8 to 10 neurons/group.
Morphine Causes Translocalization from Dendritic Spines and Dephosphorylation of CaMKII.
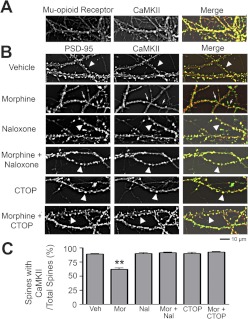
Translocation of CaMKII to or from dendritic spines has been reported to play a role in activity-dependent structural and functional plasticity of excitatory synapses (Shen and Meyer, 1999; Shen et al., 2000; Matsuzaki et al., 2004; Okamoto et al., 2004). Therefore, it is possible that activation of MOR might also suppress CaMKII activity by suppressing the clustering of this kinase in dendritic spines. To test this possibility, we first used immunocytochemistry to determine whether MOR and CaMKII are colocalized in dendritic spines. Low-density cultured hippocampal neurons at 21 DIV were costained with antibodies against MOR and CaMKII using a protocol as described previously (Hoover et al., 2010) (Fig. 4A). We found that both proteins were highly expressed in dendrites and are largely colocalized in dendritic spines (>90%). Given that postsynaptic signaling of CaMKII can be highly localized to the dendritic spine compartment (Okamoto et al., 2009), this finding supports the hypothesis that MOR modulates structural plasticity through effects on postsynaptic CaMKII signaling in the dendritic spine.
Fig. 4.
Morphine causes CaMKII translocalization from dendritic spines. A, neurons stained with anti-MOR (green in the overlay) and anti-CaMKII (red in the overlay) antibodies. MOR and CaMKII colocalize in >90% of dendritic spines. B, representative images of neurons stained with anti-PSD-95 (green in overlay) and anti-CaMKII (red in overlay) antibodies. Neurons were treated with drug at 21 DIV and then fixed at 24 DIV. Arrowheads indicate colocalization of PSD-95 and CaMKII; arrows indicate dendritic spines where CaMKII is not found. Scale bar, 10 μm. C, quantification of proportion of dendritic spines (as indicated by PSD-95) that contain CaMKII. Morphine causes the translocation of CaMKII from dendritic spines. This effect is blocked by MOR antagonists. One-way ANOVA; Bonferroni post-test: **, p < 0.01; n = 9 neurons/group. Veh, vehicle; Mor, morphine, Nal, naloxone.
To further test this possibility, low-density cultures of hippocampal neurons at 21 DIV were treated with MOR agonists and antagonists and costained with antibodies against PSD-95 (a postsynaptic marker) and CaMKII after fixation and permeabilization (Fig. 4B). Neurons were treated with vehicle, morphine, naloxone, morphine plus naloxone, CTOP (10 μM), or morphine plus CTOP for 3 days. Morphine caused a significant decrease in clustering of CaMKII in dendritic spines, as measured by the ratio of CaMKII-expressing spines to PSD-95-expressing spines (Fig. 4C; one-way ANOVA, p < 0.0001; Bonferroni post-test, p < 0.001; n = 9 neurons/group). The morphine-induced translocation of CaMKII from dendritic spines was blocked by either naloxone or CTOP, a MOR-selective antagonist, supporting the proposed mechanism that MOR activation by morphine suppresses synaptic CaMKII activity by either promoting the translocation of CaMKII from the postsynaptic compartment or inhibiting synaptic recruitment of the kinase.
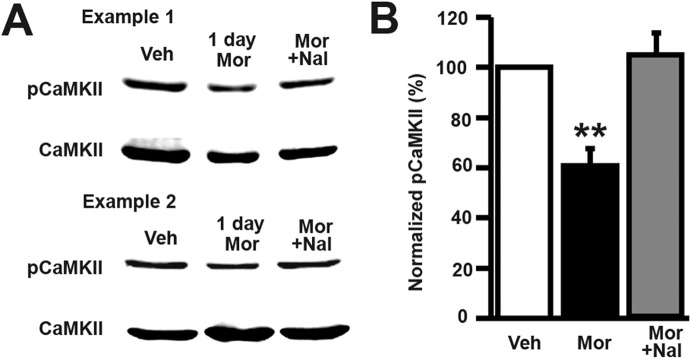
The phosphorylation of the CaMKII autophosphorylation site, Thr286, is known to activate the kinase and antibodies against this phosphorylation site are commonly used to measure CaMKII activity (Giese et al., 1998). To access the effect of morphine on CaMKII phosphorylation we treated cultured hippocampal neurons with vehicle, morphine, or morphine plus naloxone for 1 day and then made lysates of the cells. We ran the lysates on a Western blot and then probed for phospho-CaMKII (Thr286) and total CaMKII. We found that morphine significantly decreases phosphorylation of CaMKII and that this decrease is blocked by naloxone cotreatment with morphine (Fig. 5; one-way ANOVA, p < 0.05; Bonferroni post-test, p < 0.01; n = 4 cultures/group). These data add to previously reported data showing that morphine causes biphasic changes in CaMKII activity: acute morphine treatment (45 min) causes an increase in CaMKII phosphorylation (Lou et al., 1999), whereas chronic morphine treatment for 3 days causes a decrease in CaMKII phosphorylation after 3 days of morphine treatment (Zheng et al., 2010). Our results extend these previous findings showing that the decreasing phase of the biphasic effect of morphine on CaMKII activity occurs as early as 1 day into morphine treatment and can be blocked by the MOR antagonist naloxone. These results support our proposed model in which chronic morphine causes spine loss via a CaMKII-dependent signaling pathway.
Fig. 5.
Morphine exposure in vitro causes a decrease in phosphorylated CaMKII after 1 day of treatment. A, in the two representative samples, the top rows are Western blots of total cell lysates probed with a phospho (p)-CaMKII antibody (Thr286); the bottom rows are the same lysates that have been probed with a total CaMKII antibody. B, quantification of the blots shown in A. Relative optical density of phosphorylated CaMKII over total CaMKII was normalized by the nontreated control. Morphine decreases phospho-CaMKII, and this effect is blocked by naloxone. One-way ANOVA, Bonferroni post-test, **, p < 0.01; n = 4 sets of lysates. Veh, vehicle; Mor, morphine, Nal, naloxone.
Rac1 Is Necessary for Morphine-Induced Spine Loss.
CaMKII has been shown to modulate Rac1 activity through the guanine-nucleotide exchange factor kalirin-7 (Xie et al., 2007; Penzes and Jones, 2008). Rac1 is a GTPase that has been demonstrated to regulate the structure and function of excitatory synapses (Tashiro et al., 2000; Wiens et al., 2005). Therefore, we hypothesized that chronic activation of MORs causes loss of preexisting spines by inhibiting the CaMKII-Rac1 signaling pathway.
To test this possibility, we used a Rac1 activation kit (Upstate Biotechnology) to detect MOR-mediated changes in Rac1 activity (Li et al., 2002). High-density cultured rat hippocampal neurons at 21 DIV were treated with no drug, morphine, morphine plus CTOP, or CTOP alone. To determine the amount of active Rac1 proteins, cell lysates were immunoprecipitated with PAK1, a downstream effector of Rac1, and detected using an anti-Rac1 antibody in Western blots (Fig. 6A). The same antibody was used to detect total Rac1 proteins in inputs of total cell lysates in the control lanes (Fig. 6A). Compared with the untreated control group, the amount of active Rac1 proteins was significantly decreased by morphine, whereas CTOP blocked the effect of morphine (Fig. 5B; one-way ANOVA, Bonferroni post-test; n = 4 sets of lysates), supporting our hypothesis that CaMKII-Rac1 inhibition mediates the effects of chronic activation of MORs on dendritic spines.
Fig. 6.
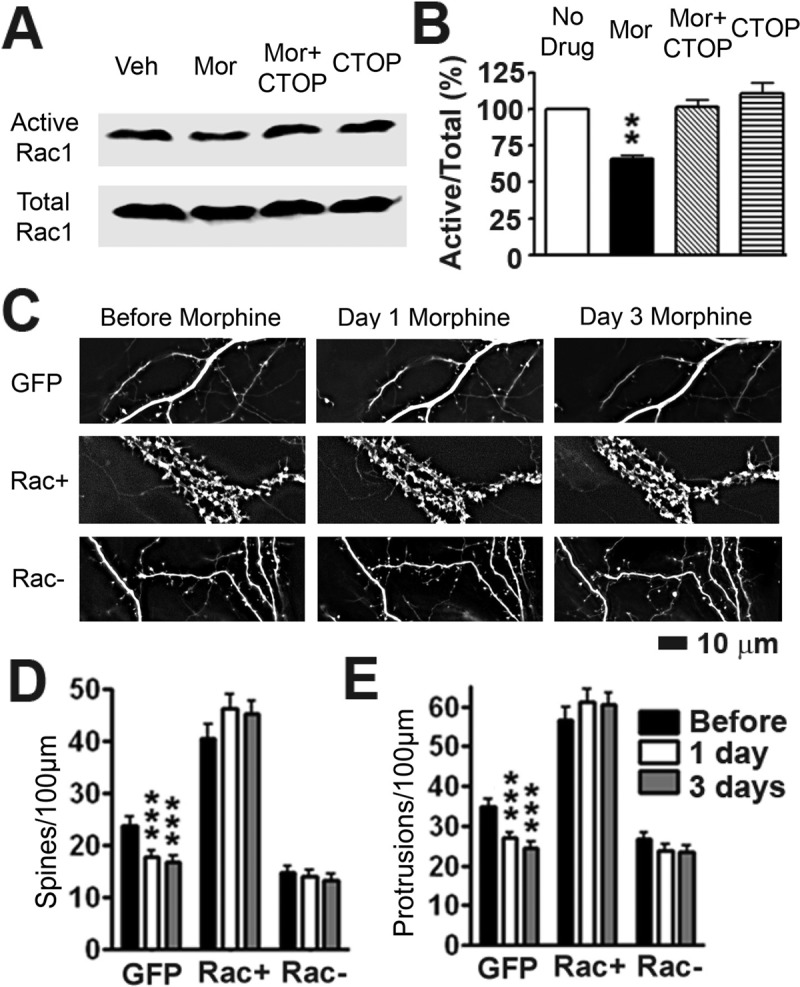
Rac1 is necessary for morphine-induced spine loss. A, the top row shows active Rac1 (see Materials and Methods). The bottom row shows input Rac1 proteins in total cell lysates. B, quantification of the blots shown in A. Relative optical density of active Rac1 over total Rac1 was normalized by the nontreated control. Morphine decreases Rac1 activity, which is blocked by CTOP (one-way ANOVA, Bonferroni post-test, p < 0.01; n = 4 sets of lysates). C, representative live images of cultured hippocampal neurons first taken at 21 DIV and then imaged again 1 and 3 days after drug treatment. Neurons were transfected with either GFP, DsRed and GFP-tagged Rac+ or DsRed and GFP-tagged Rac- at 7 DIV. GFP images are displayed. For Rac+ and Rac− groups, DsRed images were used for quantification. Arrows indicate collapse of spines. Scale bar, 10 μm. D, quantification of spine density in C. Morphine treatment causes a significant decrease in spine density that is blocked when neurons are transfected with Rac1− or Rac+. E, quantification of protrusion density in C. Morphine treatment causes a significant decrease in protrusion density that is blocked when neurons are transfected with Rac1− or Rac+. Repeated-measures two-way ANOVA, main effect of transfection, p < 0.0001, interaction p < 0.0001; Bonferroni post-test: **, p < 0.01; ***, p < 0.001; n = 8 to 10 neurons/group. Veh, vehicle; Mor, morphine.
To further test this hypothesis, we used our time-lapse live imaging system to determine how up- or down-regulation of Rac1 activity would affect the effects of morphine on dendritic spines (Fig. 6C). Neurons expressing GFP, GFP-tagged constitutively active Rac1 plus DsRed, and GFP-tagged dominant-negative Rac1 plus DsRed were imaged before and 1 day and 3 days after morphine treatment (Fig. 6). The expression of Rac− caused a significant decrease in spine density compared with the control; morphine caused no further collapse of dendritic spines. Transfection of Rac+ alone caused a significant increase in spine density, clamping spine density at a high level and preventing morphine-induced spine loss (Fig. 6, C–E; repeated-measures two-way ANOVA, main effect of transfection, p < 0.0001, interaction p < 0.0001; Bonferroni post-test used for differences between individual groups; n = 8–10). These results demonstrate that MOR-mediated structural plasticity of dendritic spines requires changes in Rac1 activity, shedding light on the downstream mechanisms in morphine-induced spine loss.
Calcineurin Inhibition, but Not CaMKII Inhibition, Blocks Morphine-Induced Decrease in the Amplitude of AMPAR-Mediated mEPSC Responses.
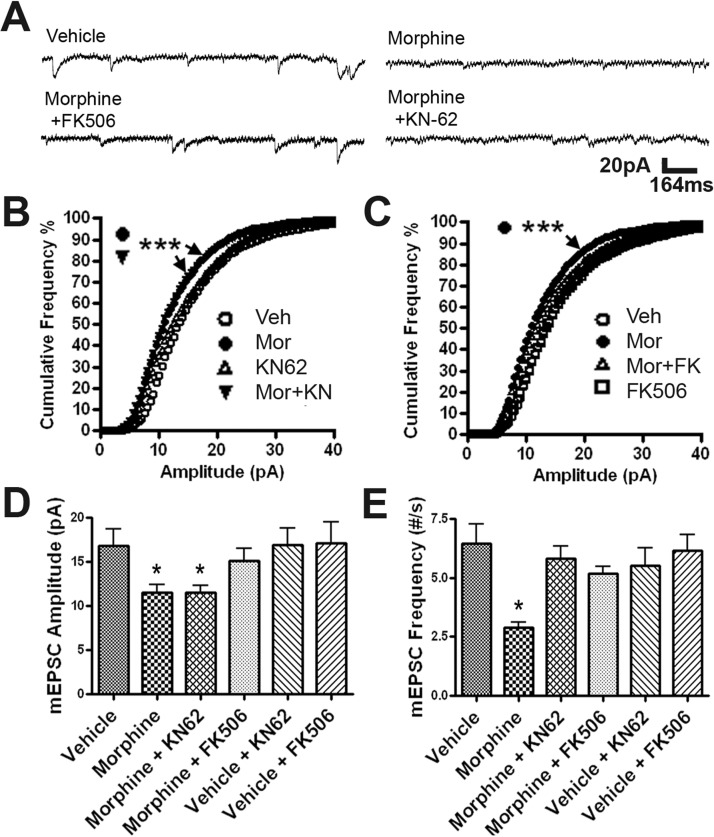
As shown in Fig. 1, CaMKII and calcineurin play similar roles in MOR-mediated structural plasticity of dendritic spines. We were surprised that our electrophysiological studies revealed that these two signaling proteins play different roles in functional plasticity of dendritic spines (Fig. 7). To elucidate the roles of calcineurin and CaMKII in morphine-induced functional plasticity, we used a whole-cell voltage-clamp technique to measure mEPSCs in mature cultured hippocampal neurons (21–25 DIV) (Fig. 7). Neurons at 19 to 21 DIV were treated with no drug, morphine, KN-62, KN-62 plus morphine, FK506, or FK506 plus morphine for at least 3 days in a manner similar to that for the morphological experiments summarized in Fig. 1. Treatment with KN-62 or FK506 alone had no significant effect on mEPSC amplitude or frequency (Fig. 7, D and E). Consistent with our previous studies (Liao et al., 2005, 2007a), morphine significantly decreased the amplitude and frequency of mEPSCs in comparison with neurons treated with vehicle (Fig. 7, D and E). The mEPSC amplitude is believed to be determined by the strength of the postsynaptic response caused by the release of only one synaptic vesicle (Del Castillo and Katz, 1954). Of interest, the effect of morphine on mEPSC amplitude persisted in the presence of KN-62 (Fig. 7B; Kolmogorov-Smirnov test, p < 0.0001; n = 10) but was blocked by FK506 (Fig. 7C), indicating that CaMKII and calcineurin might play different roles in modulating the trafficking of AMPA receptors into or out of dendritic spines. Consistent with analyses in Fig. 7, B and C, average mEPSC amplitude was significantly decreased by morphine treatment, and this effect was blocked by FK506 but not by KN-62 (Fig. 7D; one-way ANOVA; n = 10 in each group, compared with untreated control). In contrast, the presence of either KN-62 or FK506 blocks the effect of morphine on the frequency of mEPSCs (Fig. 7E; one-way ANOVA; n = 10 in each group, compared with untreated control). A decrease in the frequency of mEPSCs can result from either a decrease in releasing probability or number of synapses (subsequently decreasing number of releasing sites) (Del Castillo and Katz, 1954; Liao et al., 2007a). Therefore, combined with our morphological analyses (Figs. 1 and 2), our electrophysiological data indicate that both CaMKII inhibition and calcineurin activation reduce the number of functional synapses by causing collapse of dendritic spines.
Fig. 7.
Calcineurin inhibition, but not CaMKII inhibition, blocks the morphine-induced decrease in the amplitude of AMPA receptor-mediated mEPSC responses. A, representative traces of mEPSC recordings in neurons with four different treatments. Scale bar, x-axis, 164 ms; y-axis, 20 pA. B, cumulative frequency graph of mEPSC amplitude of neurons treated with vehicle (Veh), morphine (Mor), KN-62, and morphine plus KN-62 (Mor+KN). Neurons treated with morphine and morphine plus KN-62 have significantly more small amplitude mEPSCs than neurons treated with vehicle. Kolmogorov-Smirnov test between vehicle and morphine, between vehicle and morphine plus KN-62, p < 0.0001. C, cumulative frequency graph of mEPSC amplitude of neurons treated with vehicle, morphine, morphine plus FK506, and vehicle plus FK506. Neurons treated with morphine were found to be significantly different from vehicle. Kolmogorov-Smirnov test between vehicle and morphine, p < 0.0001. D, average mEPSC amplitudes of each group. The mEPSC amplitude of neurons treated with morphine or morphine plus KN-62 was significantly decreased compared with that for vehicle. E, Average mEPSC frequencies of each group. The mEPSC frequency of neurons treated with morphine was significantly decreased compared with that for vehicle. One-way ANOVA, Bonferroni post-test: *, p < 0.05; ***, p < 0.001; n = 10 neurons/group.
Calcineurin Inhibition, but Not CaMKII Inhibition, Protects against Morphine-Induced Loss of AMPA Receptors in Dendritic Spines.
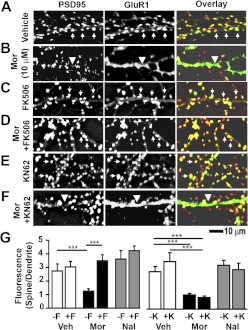
The morphine-induced reduction in mEPSC amplitude may result from loss of AMPA receptors in dendritic spines through endocytosis (Liao et al., 2005; Kam et al., 2010). To determine the roles of calcineurin and CaMKII in morphine-induced loss of AMPA receptors in dendritic spines, we examined the morphine-induced cellular redistribution of GluR1 and GluR2 subunits of AMPA receptors in the presence of either FK506 or KN-62 (Figs. 8 and 9). Low-density rat hippocampal neurons were cultured at 21 DIV, treated with vehicle, morphine, or naloxone for 3 days, and stained with anti-PSD-95 and anti-GluR1 antibodies after fixation and permeabilization (Fig. 8. A–F). We found that FK506, but not KN-62, blocked morphine-induced loss of GluR1 clustering (Fig. 8G; one-way ANOVA, Bonferroni post-test; n = 8 neurons/group). This indicates that calcineurin, but not CaMKII, is necessary for morphine-induced removal of GluR1 from dendritic spines.
Fig. 8.
Calcineurin inhibition, but not CaMKII inhibition, protects against morphine (Mor)-induced removal of GluR1 AMPA receptor subunits from the synapse. A–F, representative images of neurons stained with anti-PSD-95 (red in overlay) and anti-GluR1 (green in overlay) antibodies. Arrows indicate colocalization of PSD-95 and GluR1; arrowheads indicate dendrites where GluR1 is found in the dendritic shaft. Scale bar, 10 μm. G, quantification of the GluR1 clustering ratio (spine/dendrite). Morphine causes the cellular redistribution of GluR1; this effect is blocked by FK506 (F) but not by KN-62 (K). One-way ANOVA, Bonferroni post-test: ***, p < 0.001; n = 8 neurons/group. Veh, vehicle; Mor, morphine, Nal, naloxone.
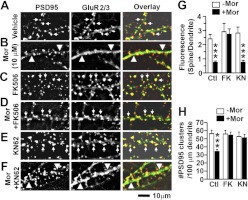
Fig. 9.
Calcineurin inhibition, but not CaMKII inhibition, protects against morphine (Mor)-induced removal of GluR2 AMPA receptor subunits from the synapse. A–F, representative images of neurons stained with anti-PSD-95 (red in overlay) and anti-GluR2 (green in overlay) antibodies. Arrows indicate colocalization of PSD-95 and GluR2; arrowheads indicate dendrites where GluR2 is found in the dendritic shaft. Scale bar, 10 μm. G, quantification of the GluR2 florescence ratio (spine/dendrite). Morphine causes the cellular redistribution of GluR2; this effect is blocked by FK506 (FK) but not by KN-62 (KN). One-way ANOVA, Bonferroni post-test. H, quantification of spine density by counting the number of PSD-95 clusters per 100 μm of dendrite length. Both FK506 and KN-62 prevent morphine-induced spine loss. One-way ANOVA, Bonferroni post-test. ***, p < 0.001; n = 8 neurons/group. Ctl, control.
To determine whether calcineurin and CaMKII also play different roles in MOR-mediated changes in GluR2 clustering in dendritic spines, cultured neurons were treated with vehicle, morphine, morphine plus FK506, or morphine plus KN-62 for 3 days and then stained with anti-PSD-95 and anti-GluR2 antibodies (Fig. 9, A–F). Similar to our GluR1 results, FK506, but not KN-62, blocked morphine-induced loss of GluR2 clustering (Fig. 9G; one-way ANOVA, Bonferroni post-test; n = 8 neurons/group). This indicates that calcineurin, but not CaMKII, is necessary for morphine-induced removal of GluR2 from dendritic spines. Consistent with our live imaging data from high-density cultures (Fig. 1), FK506 and KN-62 block the morphine-induced decrease in the number of PSD-95 clusters in low-density cultures (Fig. 9H; one-way ANOVA, Bonferroni post-test; n = 8 neurons/group), further confirming that these two molecules play similar roles in MOR-mediated structural plasticity of dendritic spines.
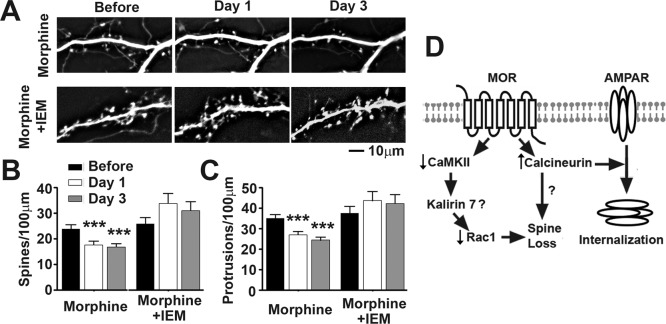
Given that the calcineurin and CaMKII are Ca2+/calmodulin-activated proteins, it is possible that the binding of morphine to MOR leads to a change in Ca2+ dynamics. Although it is not clear how morphine causes changes in intracellular Ca2+, it is probably not due to an influx through N-methyl-d-aspartate channels (Kam et al., 2010). The presence of Ca2+-permeable (CP) AMPA glutamate receptors (GluR2-lacking) at synapses has been shown to be highly plastic (Liu and Zukin, 2007; Fortin et al., 2010). CP-AMPA receptors could play a role in the effects of morphine on spine density. To test this, we transfected neurons with GFP and imaged them before drug treatment and 1 day and 3 days after drug treatment. One group of neurons received morphine treatment, whereas the other received morphine plus IEM-1460 (30 μM), an inhibitor of CP-AMPA receptors. We found that cotreatment of IEM-1460 with morphine prevents morphine from causing a significant decrease in both spine and protrusion density (Fig. 10, repeated-measures two-way ANOVA, Bonferroni post-test; n = 10 neurons/group). This finding indicates that the binding of morphine to MOR may lead to time-dependent changes in AMPA receptor subunit composition and changes in Ca2+ dynamics that cause morphine-induced spine loss.
Fig. 10.
CP-AMPA receptor antagonist IEM-1460 (IEM) prevents morphine-induced spine collapse. A, representative live images of GFP-labeled cultured hippocampal neurons first taken at 21 DIV and then imaged again 1 and 3 days after drug treatment. Scale bar, 10 μm. B, quantification of spine density per 100 μm of dendritic length in neurons described in A. Morphine treatment causes a significant decrease in spine density that is blocked by cotreatment with IEM-1460, an inhibitor of CP-AMPA receptors. C, quantification of protrusion density of neurons described in A. Morphine treatment causes a significant decrease in protrusion density that is blocked by cotreatment with IEM-1460. Repeated-measures two-way ANOVA, Bonferroni post-test: ***, p < 0.001; n = 10 neurons/group. D, diagram illustrating the roles of CaMKII and calcineurin in morphine-induced plasticity of dendritic spines.
Discussion
Persistent structural and functional changes in dendritic spines might be the basis of the abnormal learning that accompanies addiction (Robinson and Kolb, 2004; Bowers et al., 2010; Russo et al., 2010). The present study provides evidence supporting the fact that addictive drug-induced structural and functional plasticity of excitatory synapses can be mediated through separate intracellular signaling cascades (see diagram in Fig. 10D).
Differential Roles of CaMKII and Calcineurin in Morphine-Induced Changes in Synaptic Function.
The strength of synaptic responses can be altered by changes in either quantal content (releasing probability multiplied by number of releasing sites) or quantal size (postsynaptic response per synaptic vesicle), which is often estimated by measuring the amplitude and frequency of miniature excitatory postsynaptic potential or mEPSC responses (Del Castillo and Katz, 1954). Although both calcineurin and CaMKII have been reported to play roles in AMPA receptor trafficking during activity-dependent synaptic plasticity (Silva et al., 1992; Mulkey et al., 1994; Hayashi et al., 2000; Dell'Acqua et al., 2006; Kam et al., 2010), surprisingly, only calcineurin is necessary for morphine-induced reductions in the amplitude of AMPA receptor-mediated mEPSC responses (Fig. 7). Given the assumption that the amount of neurotransmitter per vesicle is constant, the decrease in mEPSC amplitude caused by morphine probably indicates that a loss of postsynaptic AMPA receptors (Liao et al., 2001) and calcineurin, but not CaMKII, is necessary for that decrease. This result is consistent with our previous study, which showed that chronic activation of MORs induces endocytosis of AMPA receptors by calcineurin-dependent dephosphorylation of GluR1 subunits at S845 (Kam et al., 2010). In contrast, both calcineurin and CaMKII are necessary for morphine-induced reductions in the frequency of AMPA receptor-mediated mEPSC responses (Fig. 7). Given that the effect of morphine on cultured hippocampal neurons is mediated postsynaptically (Liao et al., 2005, 2007a,b), morphine-induced decreases in mEPSC frequency are probably due to dendritic spine loss (i.e., a decrease in the number of functional synapses). This finding supports our concept that calcineurin and CaMKII both participate in MOR-mediated spine loss.
Similar Roles of CaMKII and Calcineurin in Morphine-Induced Changes in Spine Density.
Consistent with our functional analyses (see above), our time-lapse live imaging experiments revealed that both calcineurin and CaMKII are necessary for morphine-induced spine loss (Figs. 1–3). CaMKII has been linked to a number of regulators of actin-binding proteins, including Rac1 and RhoA, which are involved in the structural plasticity of dendritic spines (Cingolani and Goda, 2008; Okamoto et al., 2009). One of the most widely studied regulators of spine morphogenesis is Rac1, a small GTPase. The present study provides direct evidence that the CaMKII-Rac1 signaling pathway contributes to addictive drug-induced structural plasticity of dendritic spines (Fig. 6).
Our study and previous studies of other researchers support roles of the CaMKII-Rac1 pathway in AMPA receptor trafficking during neuronal development and activity-dependent synaptic plasticity (Wiens et al., 2005; Xie et al., 2007). Surprisingly, the inhibition of CaMKII activity does not block morphine-induced changes in the amplitude of AMPA receptor-mediated mEPSC responses (Fig. 7), GluR1 redistribution (Fig. 8), or GluR2 redistribution (Fig. 9). Taken together, these results indicate that opiate-induced changes in AMPA receptor trafficking are modulated through a signaling pathway that is different from those for neuronal development and activity-dependent synaptic plasticity.
The mechanistic link between calcineurin and CaMKII in MOR-mediated structural plasticity of dendritic spines remains to be determined. One possibility is that CaMKII acts downstream from the phosphatase calcineurin because it has been previously shown that calcineurin indirectly regulates CaMKII activity via inhibitory-1 and protein phosphatase 1 (Lisman and Zhabotinsky, 2001; Colbran and Brown, 2004; Ishida et al., 2008). Protein phosphatase 1 has been shown to dephosphorylate CaMKII and dissociate CaMKII from the PSD (Strack et al., 1997; Yoshimura et al., 1999), raising the possibility of a role for calcineurin-dependent dephosphorylation of CaMKII and translocation of CaMKII from dendritic spines (Figs. 4 and 5). Another possibility is that calcineurin-dependent AMPA receptor subunit internalization may change AMPA receptor calcium dynamics and subsequently cause spine collapse via the CaMKII-Rac1 signaling pathway (see diagram in Fig. 10D).
Morphine-Induced Loss of GluR1 and GluR2 from Dendritic Spines Depends upon Calcineurin, but Not CaMKII.
The present study reveals that calcineurin, but not CaMKII, is necessary for morphine-induced decreases in the clustering of both the GluR1 and GluR2 subunits of the AMPA glutamate receptors in dendritic spines (Figs. 8 and 9), further supporting the hypothesis that the two signaling molecules play different roles in morphine-induced plasticity of excitatory synapses. Recent in vivo research has found that four injections of morphine over 48 h followed by 12 h of withdrawal cause an increase in synaptic GluR1 AMPA receptor subunits and field excitatory postsynaptic potential magnitude (Billa et al., 2010a). Our past and present findings indicate that after morphine has been constantly applied to the dish for 3 days, in vitro GluR1 and GluR2 AMPA receptor subunits both cluster less in the dendritic spine, and mEPSC amplitude is decreased (Liao et al., 2005, 2007a). The lack of a withdrawal time period in our treatment protocol may be the reason that there are differences in our groups' findings. Another explanation could be that the systematic administration of morphine leads to circuit changes that indirectly affect AMPA glutamate receptor signaling in the hippocampus.
Our previous study has shown that chronic morphine exposure increases the activity of calcineurin and that calcineurin is necessary for morphine-induced GluR1 internalization (Kam et al., 2010). Because calcineurin is a Ca2+-dependent protein, morphine might cause a change in intracellular Ca2+ to stimulate calcineurin activity. Various opioids have been shown to modulate levels of intracellular calcium and the activity of calmodulin (Nehmad et al., 1982; Smart et al., 1997). Research has indicated that mGluRs and secondary messengers phospholipase C/protein kinase C are involved in morphine-induced signal transduction and development of tolerance; therefore, morphine may affect intracellular Ca2+ levels via mGluRs (Fundytus and Coderre, 1996).
Our findings cannot determine whether the time scale of morphine-induced removal of GluR1 from dendritic spines is different from that of GluR2. GluR2-lacking AMPA receptors are Ca2+-permeable, whereas GluR2-containing AMPA receptors are not (Liu and Zukin, 2007). Drugs of abuse have been shown to alter AMPA receptor subunit composition and AMPA receptor Ca2+ permeability (Conrad et al., 2008; Mameli et al., 2009; Billa et al., 2010b; Wolf and Ferrario, 2010). Given the various roles of CP-AMPA receptors in synaptic plasticity (Liu and Zukin, 2007; Fortin et al., 2010), it is possible that morphine may affect intracellular Ca2+ levels via modulation of AMPA receptor calcium permeability. Although data in Fig. 10 support the role of CP-AMPA receptors in morphine-induced spine loss, further research must be conducted to determine how morphine exposure leads to increased calcineurin activity.
Roles of Structural and Functional Plasticity of Dendritic Spines in Hippocampal Neurons in Drug Addiction.
It has been known for more than two decades that unilateral microinjections of morphine into the rat hippocampus can produce a conditioned place preference (Corrigall and Linseman, 1988). Because of the classic hypothesis that the mesolimbic dopaminergic pathway is the main rewarding pathway (Bowers et al., 2010), the hippocampus has not been incorporated into the addiction neurocircuitry until very recently. Addiction is increasingly regarded as a pathological form of learning and memory (Jones and Bonci, 2005; Hyman et al., 2006). Therefore, the present models of drug addiction almost always incorporate memory systems including the hippocampus, prefrontal cortex, and amygdala (Koob and Volkow, 2009; Morón and Green, 2010).
Although structural and functional changes in dendritic spines during activity-dependent plasticity have been correlated in numerous studies (Matsuzaki et al., 2004; Okamoto et al., 2009), some studies have shown that the molecular mediators of functional and structural plasticity can be dissociated (Wang et al., 2007; Cingolani and Goda, 2008). The present study has extended this concept of separate but interactive signaling pathways for mediating structural and functional plasticity of excitatory synapses to addiction research.
Persistent structural and functional changes in dendritic spines caused by drugs of abuse have been proposed to mediate the aberrant learning associated with addiction (Robinson and Kolb, 2004; Hyman et al., 2006). Recent studies have attempted to determine the roles of synaptic plasticity in addictive drug-induced behavioral changes by either blocking cocaine-induced structural plasticity (Pulipparacharuvil et al., 2008; Russo et al., 2009) or cocaine-induced changes in glutamate receptor function (Moussawi et al., 2009, 2011). These behavioral studies often yield conflicting findings (Pulipparacharuvil et al., 2008; Moussawi et al., 2009, 2011; Russo et al., 2009), which may result from the complexity of neuronal circuitry or divergent intracellular mechanisms underlying structural and functional plasticity of dendritic spines caused by drugs of abuse. The present study provides direct experimental evidence that these two forms of drug-induced plasticity can be mediated by separate, but interacting, signaling pathways. The new knowledge gained from our in vitro cellular studies should help us better understand the relationship between structural and functional plasticity in addiction and promote the design of more comprehensive in vivo experiments in the future.
In conclusion, the learning and memory associated with drug-induced behavioral changes requires long-lasting changes in synaptic strength, which can be achieved through either alterations in the amount of functional AMPA receptors in dendritic spines or changes in spine morphology. The present study demonstrates that these two forms of drug-induced plasticity can be mediated through separate but interactive intracellular cascades. The new concept of separate but interactive mediators for functional and structural synaptic plasticity caused by drugs of abuse should significantly advance our understanding of the diverse cellular mechanisms underlying addictive behaviors.
Acknowledgments
We thank Paul Higgins and Susan Bushek for technical support. We thank Dr. Richard Huganir for providing antibodies and DNA constructs. We thank Dr. Daniel Miller, Dr. Benjamin Smith, and Dr. Rachel Penrod for their thoughtful comments on this manuscript.
This project was supported by the National Institutes of Health National Institute on Drug Abuse [Grants T32-DA07234, R01-DA020582, K02-DA025048, P50-DA011806, R01-DA007339, R01-DA000564, R01-DA016674]; the National Institutes of Health National Institute of General Medical Sciences [Grants 5T32GM008471, P32-GM00847]; the Michael J. Fox Foundation; and the American Health Assistance Foundation.
The authors have no conflicts of interest to disclose.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- LTP
- long-term potentiation
- LTD
- long-term depression
- MOR
- μ-opioid receptor
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- GluR
- glutamate receptor
- DIV
- day 1 in vitro
- WT
- wild-type
- GFP
- enhanced green fluorescent protein
- CMV
- cytomegalovirus
- CTOP
- d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2
- KN-62
- 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine
- FK-506
- tacrolimus
- IEM-1460
- N,N,N,-trimethyl-5-[(tricyclo]3.3.1.13,7[dec-1-ylmethyl) amino]-1-pentanaminiumbromide
- mEPSC
- miniature excitatory postsynaptic current
- PSD
- postsynaptic density
- ANOVA
- analysis of variance
- CP
- Ca2+-permeable.
Authorship Contributions
Participated in research design: Miller, Loh, Law, and Liao.
Conducted experiments: Miller, Zhang, Dummer, Cariveau, and Liao.
Performed data analysis: Miller, Zhang, Dummer, Cariveau, and Liao.
Wrote or contributed to the writing of the manuscript: Miller and Liao.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. (1995) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15:3328–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Betancur C, Mansuy IM, Giros B. (2005) The reinforcing effects of chronic d-amphetamine and morphine are impaired in a line of memory-deficient mice overexpressing calcineurin. Eur J Neurosci 21:3089–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, Morón JA. (2010a) Increased insertion of glutamate receptor 2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol 77:874–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Morón JA. (2010b) Disruption of morphine-conditioned place preference by a δ2-opioid receptor antagonist: study of μ-opioid and δ-opioid receptor expression at the synapse. Eur J Neurosci 32:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. (2010) AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron 67:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9:344–356 [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. (2004) Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 14:318–327 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Linseman MA. (1988) Conditioned place preference produced by intra-hippocampal morphine. Pharmacol. Biochem. Behav 30:787–789 [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. (1954) Quantal components of the end-plate potential. J Physiol 124:560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. (2006) Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol 85:627–633 [DOI] [PubMed] [Google Scholar]
- Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. (1999) Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol 56:39–45 [DOI] [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. (2010) Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci 30:11565–11575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Coderre TJ. (1996) Chronic inhibition of intracellular Ca2+ release or protein kinase C activation significantly reduces the development of morphine dependence. Eur J. Pharmacol 300:173–181 [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. (1998) Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science 279:870–873 [DOI] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. (2007) AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol 17:289–297 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287:2262–2267 [DOI] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, et al. (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68:1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598 [DOI] [PubMed] [Google Scholar]
- Ishida A, Sueyoshi N, Shigeri Y, Kameshita I. (2008) Negative regulation of multifunctional Ca2+/calmodulin-dependent protein kinases: physiological and pharmacological significance of protein phosphatases. Br J Pharmacol 154:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Bonci A. (2005) Synaptic plasticity and drug addiction. Curr Opin Pharmacol 5:20–25 [DOI] [PubMed] [Google Scholar]
- Kam AY, Liao D, Loh HH, Law PY. (2010) Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci 30:15304–15316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2009) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. (2002) Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem 277:15207–15214 [DOI] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Loh HH, Law PY. (2007a) Agonist-dependent postsynaptic effects of opioids on miniature excitatory postsynaptic currents in cultured hippocampal neurons. J Neurophysiol 97:1485–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. (2007b) Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH. (2005) Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA 102:1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. (2001) Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci 21:6008–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. (1994) Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature 369:235–239 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31:191–201 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134 [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. (1995) Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA 92:11175–11179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou L, Zhou T, Wang P, Pei G. (1999) Modulation of Ca2+/calmodulin-dependent protein kinase II activity by acute and chronic morphine administration in rat hippocampus: differential regulation of alpha and beta isoforms. Mol Pharmacol 55:557–563 [PubMed] [Google Scholar]
- Lu L, Zeng S, Liu D, Ceng X. (2000) Inhibition of the amygdala and hippocampal calcium/calmodulin-dependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci Lett 291:191–195 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21 [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. (2009) Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12:1036–1041 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Dürr R, Gähwiler BH, Thompson SM. (1999) Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci 2:44–49 [DOI] [PubMed] [Google Scholar]
- Morón JA, Green TA. (2010) Exploring the molecular basis of addiction: drug-induced neuroadaptations. Neuropsychopharmacology 35:337–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. (2011) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci USA 108:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369:486–488 [DOI] [PubMed] [Google Scholar]
- Nehmad R, Nadler H, Simantov R. (1982) Effects of acute and chronic morphine treatment of calmodulin activity of rat brain. Mol Pharmacol 22:389–394 [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. (2009) The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology 24:357–366 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. (2004) Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci 7:1104–1112 [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. (2008) Dendritic spine dynamics—a key role for kalirin-7. Trends Neurosci 31:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. (2008) Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59:621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Mateos JM, Hugel S, de Paola V, Caroni P, Gähwiler BH, McKinney RA. (2005) Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proc Natl Acad Sci USA 102:6166–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47:33–46 [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, et al. (2009) Nuclear factor κB signaling regulates neuronal morphology and cocaine reward. J Neurosci 29:3529–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdrulla AD, Linden DJ. (2007) Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci 10:546–548 [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. (1999) Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284:162–166 [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. (2000) Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci 3:881–886 [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. (1992) Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science 257:201–206 [DOI] [PubMed] [Google Scholar]
- Smart D, Hirst RA, Hirota K, Grandy DK, Lambert DG. (1997) The effects of recombinant rat mu-opioid receptor activation in CHO cells on phospholipase C, [Ca2+]i and adenylyl cyclase. Br J Pharmacol 120:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. (1997) Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem 68:2119–2128 [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 16:95–101 [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. (2000) Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10:927–938 [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. (1990) KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 265:4315–4320 [PubMed] [Google Scholar]
- Wang XB, Yang Y, Zhou Q. (2007) Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci 27:12419–12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens KM, Lin H, Liao D. (2005) Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci 25:10627–10636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. (2010) The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. (2010) AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev 35:185–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56:640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Sogawa Y, Yamauchi T. (1999) Protein phosphatase 1 is involved in the dissociation of Ca2+/calmodulin-dependent protein kinase II from postsynaptic densities. FEBS Lett 446:239–242 [DOI] [PubMed] [Google Scholar]
- Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY. (2010) Modulations of neuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J Neurosci 30:8102–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]