Abstract
We reported previously the formation of a glutathionyl conjugate of the active metabolite (AM) of clopidogrel and the covalent modification of a cysteinyl residue of human cytochrome P450 2B6 in a reconstituted system (Mol Pharmacol 80:839–847, 2011). In this work, we extended our studies of the metabolism of clopidogrel to human liver microsomes in the presence of four reductants, namely, GSH, l-Cys, N-acetyl-l-cysteine (NAC), and ascorbic acid. Our results demonstrated that formation of the AM was greatly affected by the reductant used and the relative amounts of the AM formed were increased in the following order: NAC (17%) < l-Cys (53%) < ascorbic acid (61%) < GSH (100%). AM-thiol conjugates were observed in the presence of NAC, l-Cys, and GSH. In the case of GSH, the formation of both the AM and the glutathionyl conjugate was dependent on the GSH concentrations, with similar Km values of ∼0.5 mM, which indicates that formation of the thiol conjugates constitutes an integral part of the bioactivation processes for clopidogrel. It was observed that the AM was slowly converted to the thiol conjugate, with a half-life of ∼10 h. Addition of dithiothreitol to the reaction mixture reversed the conversion, which resulted in a decrease in AM-thiol conjugate levels and a concomitant increase in AM levels, whereas addition of NAC led to the formation of AM-NAC and a concomitant decrease in AM-GSH levels. These results not only confirm that the AM is formed through oxidative opening of the thiolactone ring but also suggest the existence of an equilibrium between the AM, the thiol conjugates, and the reductants. These factors may affect the effective concentrations of the AM in vivo.
Introduction
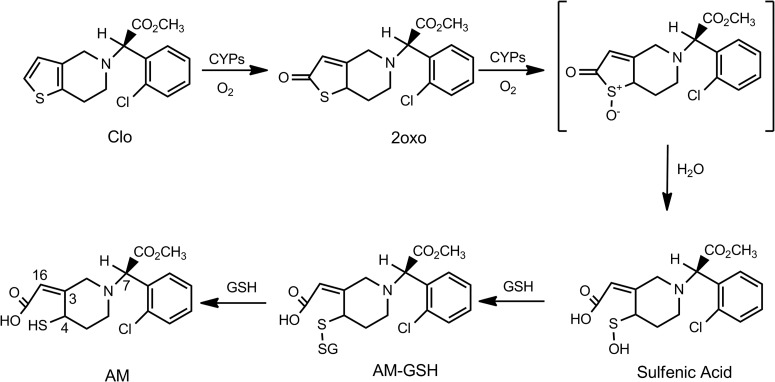
Clopidogrel (Plavix) is commonly prescribed to reduce cardiovascular event rates for patients with acute coronary syndromes, particularly those undergoing percutaneous coronary interventions, because of its antiplatelet activity. Clopidogrel prevents platelet aggregation through irreversible inhibition of the platelet P2Y12 receptor with covalent modification of the cysteinyl residues of the P2Y12 receptor (Ding et al., 2003; Savi et al., 2006; Algaier et al., 2008). It is well established that clopidogrel is bioactivated to its pharmacologically active metabolite (AM) by hepatic cytochrome P450 (P450) enzymes (Savi et al., 1992; Kazui et al., 2010). Specifically, clopidogrel is bioactivated through two sequential oxidative processes, as elegantly elucidated by Dansette et al. (2009, 2011, 2012). In the first step, clopidogrel is mono-oxygenated to yield 2-oxo-clopidogrel, which has no antiplatelet activity. In the second step, 2-oxo-clopidogrel is further oxidized to yield an unstable sulfenic acid intermediate. Reduction of the sulfenic acid by GSH leads to opening of the thiolactone ring to yield a glutathionyl conjugate, followed by a thiol-disulfide exchange to yield the AM. The reaction scheme is illustrated in Fig. 1. Recent studies by Dansette et al. (2011, 2012) demonstrated that the AM produced by P450s possesses a cis-exocyclic double bond at C3-C16, whereas hydrolysis of 2-oxo-clopidogrel by the esterase PON1 leads to formation of an endo-isomer, an inactive form. It is generally thought that the reactive thiol group of the active metabolite covalently modifies the cysteinyl residues of the platelet P2Y12 receptor, leading to prevention of platelet aggregation. It is currently not known whether the glutathionyl conjugate of clopidogrel exhibits antiplatelet activity.
Fig. 1.
Bioactivation of clopidogrel by P450s. Clo, clopidogrel; 2oxo, 2-oxo-clopidogrel. The numbers shown in the AM structure indicate the numbering of the two chiral centers (C4 and C7) and the exocyclic double bond (C3 and C16).
Although it is widely used as an antiplatelet agent, clopidogrel has shown significant interindividual variability in drug responses. Lack of response or “resistance” to clopidogrel therapy may be seen in ∼45% of the patient population (Mason et al., 2005). Many pharmacogenetic and pharmacogenomic studies have been performed in the past 5 to 6 years, in attempts to identify genetic markers for these variable drug responses. Many target genes, such as those for CYP2C19 (Hulot et al., 2006; Collet et al., 2009; Mega et al., 2009), CYP3A5 (Suh et al., 2006), P-glycoprotein (Taubert et al., 2006), the P2Y12 receptor (Fontana et al., 2003), and PON1 (Bouman et al., 2011), have been indicated. Most, but not all, of the studies on CYP2C19 polymorphisms established a correlation between the variable drug responses and a loss-of-function mutation in the CYP2C19 gene (CYP2C19*2) (Collet et al., 2009; Hulot et al., 2010; Mega et al., 2010; Simon et al., 2011). Correlations of the variable drug responses with other genetic markers have not been established. However, all of those studies failed to account for the majority of the variable drug responses. Even in the case of CYP2C19, CYP2C19*2 accounts for only ∼12% of the overall variation in the responses to clopidogrel therapy (Shuldiner et al., 2009). Therefore, other factors are most likely involved but have not yet been identified.
These factors may be associated with the bioactivation of clopidogrel. In particular, from both mechanistic and clinical perspectives, some important questions regarding the formation of the AM and the glutathionyl conjugate have not been addressed adequately. For example, what are the factors that determine the concentration of the AM? Does the AM undergo thiol-disulfide exchange? Does the glutathionyl conjugate of the AM have antiplatelet activity? Answers to these questions should provide crucial information for a full understanding of the variable drug responses to clopidogrel therapy.
We reported the formation of a glutathionyl conjugate of the AM in a reconstituted system with CYP2B6, one of the key P450 isoforms involved in the bioactivation of clopidogrel (Zhang et al., 2011). We also observed that Cys475 of CYP2B6 was covalently modified to form an AM-Cys conjugate. These observations prompted us to conduct additional studies to understand the general reactivity of the AM. In this work, we extended our previous studies to human liver microsomes (HLMs), to investigate the factors that affect the formation of the AM. Our results showed that GSH, l-Cys, and NAC were all capable of forming AM-thiol conjugates and that formation of the AM was greatly affected by the identity of the reductants used. In addition, we observed thiol-disulfide exchange reactions between the AM, the conjugates, and various reductants. These factors may affect the effective concentrations of the AM in vivo.
Materials and Methods
Chemicals.
HLMs pooled from 150 donors were purchased from BD Biosciences (San Jose, CA). Racemic 2-oxo-clopidogrel hydrochloride and (S)-(+)-clopidogrel hydrogen sulfate were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). GSH, l-Cys, NAC, dithiothreitol (DTT), ascorbic acid, NADP+, glucose-6-phosphate, and glucose-6-phosphate dehydrogenase were purchased from Sigma-Aldrich (St. Louis, MO).
Detection of AM and AM-Thiol Conjugates of 2-Oxo-clopidogrel Formed by HLMs.
To generate the AM and AM-thiol conjugates, the metabolic reactions were performed in 0.2 ml of 50 mM potassium phosphate solution (pH 7.4) containing 1 mg/ml HLMs, 80 μM 2-oxo-clopidogrel, 1 mM concentrations of each of the four reductants (GSH, NAC, l-Cys, or ascorbic acid), 0.1 M KF, 1 mM glucose-6-phosphate, and 1 mM NADP+. KF was used to inhibit the activities of esterases; hydrolysis of the methyl ester clopidogrel to the carboxylic acid metabolite is fully inhibited with 0.1 M KF, whereas the esterase activity of PON1 is only partially inhibited with this concentration (Dansette et al., 2012). The catalytic reaction was initiated with the addition of 1 unit of glucose-6-phosphate dehydrogenase, and then the mixtures were incubated at 37°C for 30 min. The reaction was terminated with the addition of 0.1 ml of 10% acetic acid in acetonitrile. For quantitative analysis, 200 pmol of clopidogrel was added to the quenched samples as the internal standard (IS). The samples were centrifuged at 16,000g for 10 min to precipitate the protein aggregates, and the supernatants were stored on ice. Aliquots of 50 μl of the supernatants were loaded into an ESI-LC/MS system for analysis of the AM and AM-thiol conjugates, as described below.
ESI-LC/MS Analysis of AM and AM-Thiol Conjugates of 2-Oxo-clopidogrel.
The AM and AM-thiol conjugates were analyzed with a LCQ Deca XP ion-trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). The IS and metabolites were separated on a reverse-phase C18 column (2 × 100 mm, 3 μm, 110 Å; Phenemonex, Torrance, CA) by using a binary mobile phase reported by Dansette et al. (2012). The mobile phase consisted of 10 mM ammonium acetate (solvent A) and an acetonitrile/methanol/water (7:2:1) mixture (solvent B). The gradient used to elute metabolites and IS was as follows: use of 40% solvent B for 4 min, a linear increase to 100% solvent B in 15 min, and maintenance at 100% solvent B for 2 min. The flow rate was 0.2 ml/min. The mass spectrometer was tuned with 1 μM clopidogrel in 50% solvent B, in the positive electrospray ionization mode. The instrumental settings for MS were as follows: heated capillary temperature, 200°C; spray voltage, +4.5 kV; sheath gas flow, 60 arbitrary units; auxiliary gas flow, 20 arbitrary units. The precursor ions were scanned at m/z 300 to 700, and MS2 spectra were obtained by using the dependent scan mode; the four most-abundant ions were fragmented through collision-induced dissociation at a 35% energy level, and the fragment ions were recorded at m/z 100 to 700.
Determination of Km and Vmax Values for Metabolism of 2-Oxo-clopidogrel by HLMs.
To evaluate quantitatively the formation of the AM and AM-thiol conjugates, we determined the kinetic parameters for the metabolism of 2-oxo-clopidogrel. The metabolic reactions were performed as described above except that 2-oxo-clopidogrel was incubated at 0, 1, 3, 5, 10, 30, or 50 μM at 37°C for 30 min. The calibration standards were prepared under the same conditions as for the samples except that the standards were quenched before the reactions were initiated. The concentrations of 2-oxo-clopidogrel remaining were quantified on the basis of the calibration curve. The Km and Vmax values were obtained by fitting the specific activity values obtained with various concentrations of 2-oxo-clopidogrel to the Michaelis-Menten equation with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA).
Because authentic standards for the AM and AM-thiol conjugates were not commercially available, we were able to determine only the relative amounts of the AM and AM-thiol conjugates, by calculating the analyte/IS area ratios. The area ratio for the AM was calculated as the ratio of the area under the curve for m/z 356.1 to that for m/z 322.1, whereas the area ratios for the AM-GSH, AM-l-Cys, and AM-NAC conjugates were calculated as the ratios of the area under the curve for m/z 661.0, 475.1, and 517.1, respectively, to that for m/z 322.1. The same approach was used to determine the Km for GSH for the metabolism of 2-oxo-clopidogrel at various concentrations of GSH (0–5 mM).
Studies of Thiol-Disulfide Exchange in HLMs.
To examine the thiol-disulfide exchange reactions, the metabolism of 2-oxo-clopidogrel by HLMs was first performed in the presence of 1 mM GSH in 50 mM potassium phosphate solution (pH 7.4). The reaction mixture (2 ml) was incubated at 37°C for 30 min in the presence of 1 mg/ml HLMs, 80 μM 2-oxo-clopidogrel, 1 mM GSH, 0.1 M KF, and the NADP+-regenerating system. The reaction was terminated by adding 0.4 ml of the reaction mixture to three separate vials containing 0.2 ml of ice-cold acetonitrile. Small volumes (∼6 μl) of DTT or NAC were added to each vial to yield final concentrations of 5 mM. An equal volume of water was added to the control sample. The samples were then incubated at room temperature for 30 min, to allow thiol exchange to occur. The samples were acidified with acetic acid and stored on ice before ESI-LC/MS analyses. In a separate experiment to measure thiol-disulfide exchange between the AM and the AM-GSH conjugate, aliquots of the reaction mixture were analyzed immediately after quenching, without preincubation at room temperature. The same samples were then analyzed under the same conditions over a period of 20 h.
Results
Metabolism of 2-Oxo-clopidogrel by HLMs in the Presence of GSH.
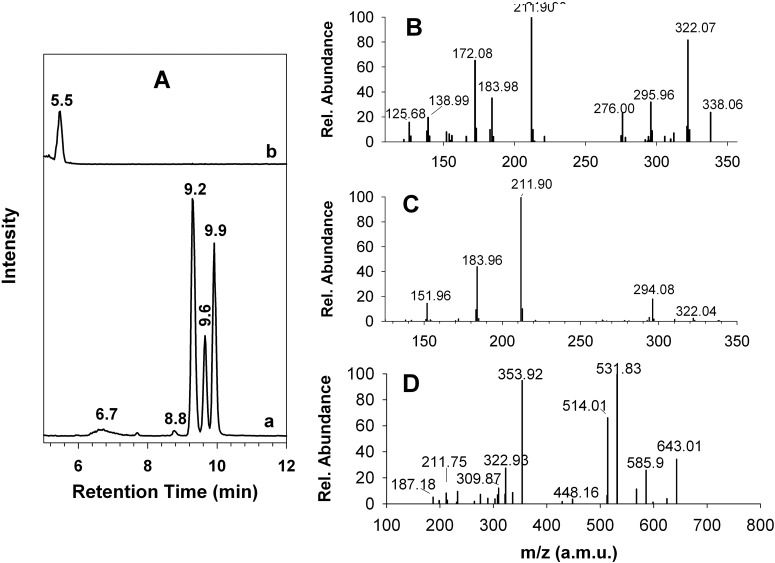
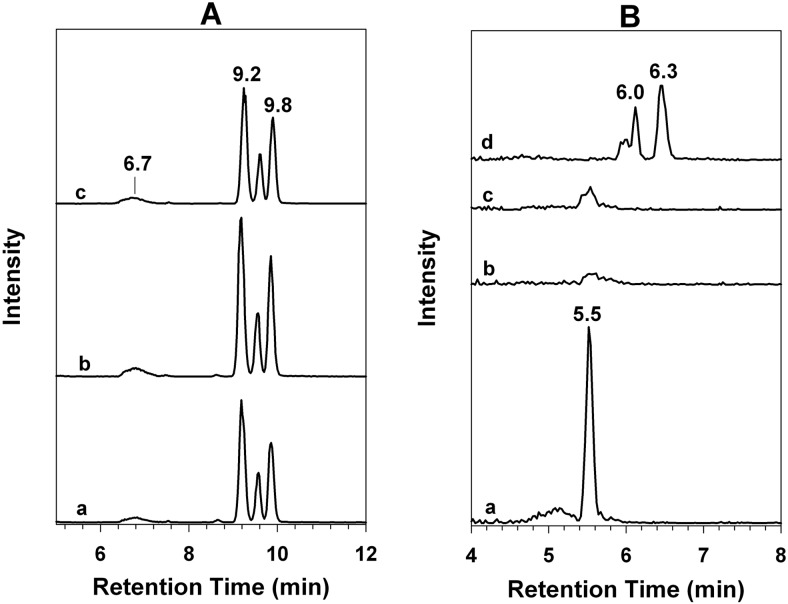
Traditionally, the metabolism of clopidogrel has been assessed in the presence of 1 to 10 mM GSH. Therefore, we first characterized this metabolic reaction in the presence of 1 mM GSH. As shown in Fig. 2A, metabolism of 2-oxo-clopidogrel by HLMs led to the formation of multiple metabolites. The extracted ion chromatogram (EIC) at m/z 356.1 (MH+ for the AM) showed the formation of metabolites M1 to M5, which eluted at 6.7, 8.8, 9.2, 9.6, and 9.9 min, respectively. All five peaks in the mass spectrum exhibited a base peak at m/z 356.1 and an isotope peak at m/z 358.1, whose intensity was ∼40% of that of the base peak (data not shown). The presence of this pair of isotope peaks indicated that M1 to M5 contained one chlorine atom, presumably originating from 2-oxo-clopidogrel. The identities of M1 to M5 could be assigned on the basis of their MS2 spectra. The MS2 spectra of M2 to M5 were very similar. A representative MS2 spectrum for M5 is shown in Fig. 2B. This spectrum showed major fragment ions at m/z 211.90, 322.07, and 172.08 and was almost identical to that of the AM, as reported (Pereillo et al., 2002; Dansette et al., 2011, 2012). The MS2 spectrum of M1 differed from that of M5 (Fig. 2C) but was consistent with that of an endo-isomer of the AM in which the double bond had migrated from the exocyclic position to the endocyclic piperdinyl ring (Dansette et al., 2011, 2012). The endo-isomer is an inactive product of 2-oxo-clopidogrel that is produced not by P450s but rather by PON1, through hydrolysis of 2-oxo-clopidogrel.
Fig. 2.
EICs and MS2 spectra of the AM and the AM-GSH conjugate produced by HLMs. The AM and the AM-GSH conjugate were generated through incubation at 37°C for 30 min in a reaction mixture containing 1 mg/ml HLMs, 80 μM 2-oxo-clopidogrel, and 1 mM GSH, as described under Materials and Methods. A, EICs at m/z 356.1 for the AM (trace a) and at m/z 661.0 for the AM-GSH conjugate (trace b). B, MS2 spectrum of the precursor ion m/z 356.1 at 9.9 min. C, MS2 spectrum of the precursor ion m/z 356.04 at 6.7 min. D, MS2 spectrum of the precursor ion m/z 661.0 at 5.5 min.
In addition to the multiple active metabolites, we observed the AM-GSH conjugate. The EIC at m/z 661.0 (equivalent to MH+ of the AM-GSH conjugate) exhibited a peak at 5.5 min (Fig. 2A, trace b). The MS2 spectrum of this peak (Fig. 2D) was in excellent agreement with findings we reported previously for the glutathionyl conjugate of the AM observed in a reconstituted system (Zhang et al., 2011). The two major MS2 peaks, at m/z 531.83 and 353.92, could be attributed to a neutral loss of 129 from the precursor ion m/z 661.0 and the AM fragment cleaved from the mixed disulfide bond of the glutathionyl conjugate, respectively. Clearly, metabolism of 2-oxo-clopidogrel by HLMs produced both the AM and the AM-GSH conjugate. When clopidogrel was substituted for 2-oxo-clopidogel as substrate, we observed similar metabolic profiles for the AM and the AM-GSH conjugate but with lower yields (data not shown).
Dependence of Metabolism of 2-Oxo-clopidogrel by HLMs on Concentrations of GSH.
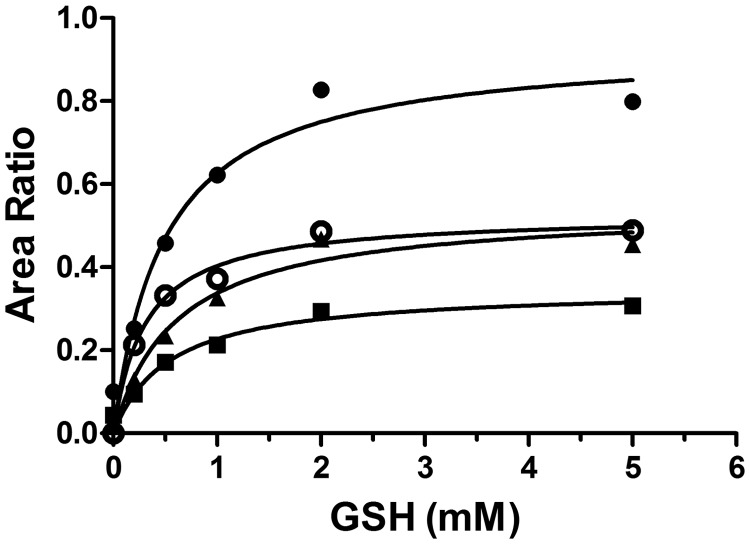
To examine the dependence of 2-oxo-clopidogrel metabolism on GSH, we determined the Km values for GSH for the formation of the AM and the AM-GSH conjugate. As shown in Fig. 3, the formation of M3 to M5 and the AM-GSH conjugate required GSH, as observed previously (Kazui et al., 2010), and exhibited saturation kinetics, with Km values of 0.3 mM for AM-GSH, 0.49 mM for M3, 0.53 mM for M4, and 0.60 mM for M5. The Km values for the formation of the AM and the AM-GSH conjugate were very similar, which indicates that formation of the glutathionyl conjugate constitutes an integral part of the bioactivation processes for 2-oxo-clopidogrel through P450-catalyzed reactions.
Fig. 3.
Dependence on GSH of the metabolism of 2-oxo-clopidogrel by HLMs. Metabolism of 2-oxo-clopidogrel was performed in 50 mM potassium phosphate buffer (pH 7.4) with 80 μM 2-oxo-clopidogrel and varying concentrations of GSH (0–5 mM) at 37°C for 30 min, and the AM and AM-GSH conjugate levels were analyzed with ESI-LC/MS, as described under Materials and Methods. The relative amounts of the AM were calculated as the ratio of the area under the curve for m/z 356.1 to that for m/z 322.0, whereas the relative amounts of the AM-GSH conjugate were calculated as the ratio of the area for m/z 661.0 to that for m/z 322.0. ●, M3 eluting at 9.2 min; ■, M4 eluting at 9.6 min; ▴, M5 eluting at 9.9 min; ○, AM-GSH eluting at 5.5 min.
Metabolism of 2-Oxo-clopidogrel by HLMs in the Presence of Alternative Reductants.
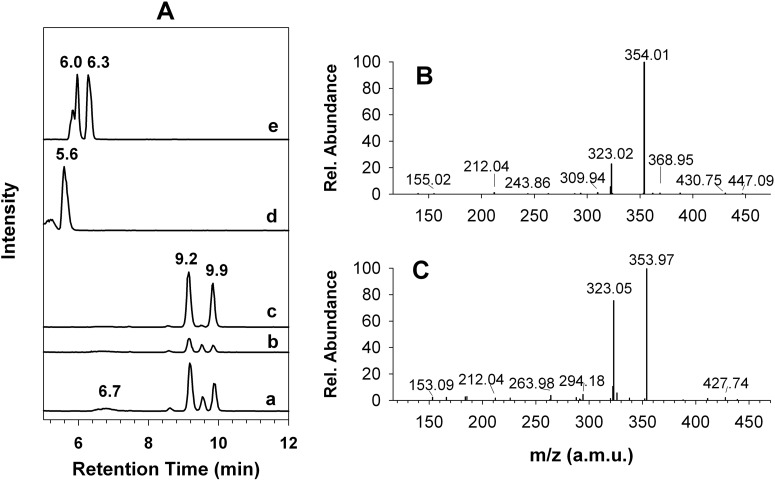
In addition to GSH, ascorbic acid, NAC, and l-Cys were used to generate the AM. This is the first report to compare their effects on the production of the AM under the same conditions as used for GSH. Metabolism of 2-oxo-clopidogrel in the presence of l-Cys yielded the AM, as observed in the presence of GSH (Fig. 4A, trace a). M2 to M5 were observed at the same retention times as noted in the presence of GSH; however, the relative amount of AM formed was 44% less than that formed in the presence of GSH. M2 to M5 were also observed in the presence of NAC (Fig. 4A, trace b); however, their amounts were significantly decreased. Compared with GSH findings, only ∼17% of the AM was produced in the presence of NAC. The relative amount of the AM generated in the presence of 1 mM ascorbic acid was approximately 61%, compared with GSH findings (Fig. 4A, trace c). The intensity of M4 was negligible (Fig. 4A, trace c). It appears that ascorbic acid showed stereoselectivity for M3 and M5.
Fig. 4.
Formation of the AM and the AM conjugates by HLMs in the presence of ascorbic acid, l-Cys, and NAC. The AM and the AM conjugates were formed through incubation at 37°C for 30 min in a reaction mixture containing 1 mg/ml HLMs, 80 μM 2-oxo-clopidogrel, and 1 mM concentrations of the reductants, as indicated under Materials and Methods. A, traces a, b, and c, EICs at m/z 356.1 in the presence of l-Cys, NAC, and ascorbic acid, respectively; traces d and e, EICs at m/z 475.1 and 517.1, respectively. B, MS2 spectrum of the AM-l-Cys conjugate at 5.6 min. C, MS2 spectrum of the AM-NAC conjugate at 6.3 min.
In addition to the AM, thiol conjugates with the AM were observed in the presence of NAC and l-Cys. The AM-l-Cys conjugate eluted at 5.6 min in the EIC of m/z 475.1 (MH+ of the AM-l-Cys conjugate) (Fig. 4A, trace d). The MS2 spectrum of the AM-l-Cys conjugate (Fig. 4B) exhibited a major fragment ion at m/z 354.01, which was attributed to the AM fragment cleaved from the mixed disulfide bond, similar to the AM-GSH conjugate shown in Fig. 2. In contrast, two major AM-NAC conjugates were observed at 6.0 and 6.3 min, with a minor conjugate at 5.8 min (Fig. 4A, trace e). The MS2 spectra of the precursor ion at m/z 517.1 (MH+ of the AM-NAC conjugates) for the two major AM-NAC conjugates were similar to that of the AM-l-Cys conjugate, with a major peak at m/z 354 (Fig. 4C).
Kinetic Parameters for Metabolism of 2-Oxo-clopidogrel by HLMs in the Presence of GSH, NAC, and l-Cys.
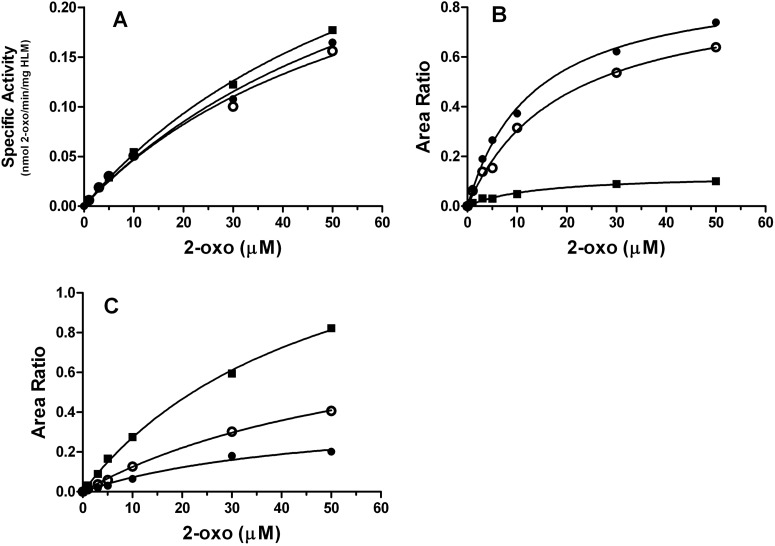
To compare quantitatively the effects of the different reductants, we determined the Km and Vmax values for the metabolism of 2-oxo-clopidogrel (Fig. 5). The rates of consumption of 2-oxo-clopidogrel with HLMs in the presence of the three reductants did not differ significantly (Fig. 5A). For example, the Km and Vmax values for the consumption of 2-oxo-clopidogrel in the presence of GSH were 75 μM and 0.4 nmol of 2-oxo per min per mg of HLMs, respectively, which yielded a catalytic efficiency (Vmax/Km) of 0.0053 (Table 1). The Km, Vmax, and catalytic efficiency values for the consumption of 2-oxo-clopidogrel in the presence of l-Cys and NAC were similar (within 11%) to those obtained with GSH. The presence of different reductants did not appear to affect the rates of consumption of 2-oxo-clopidogrel.
Fig. 5.
Determination of kinetic parameters for the metabolism of 2-oxo-clopidogrel by HLMs in the presence of GSH (●), l-Cys (○), and NAC (■). Metabolism of 2-oxo-clopidogrel (2-oxo) was performed in 50 mM potassium phosphate buffer (pH 7.4) with 1 mM reductant and varying concentrations (0–50 μM) of 2-oxo-clopidogrel at 37°C for 30 min, and the amount of 2-oxo-clopidogrel consumed and the products formed were determined with ESI-LC/MS, as described under Materials and Methods. A, kinetic profile for the consumption of 2-oxo-clopidogrel. The amount of 2-oxo-clopidogrel remaining after 30 min of incubation was quantified with ESI-LC/MS, on the basis of a calibration curve prepared with 2-oxo-clopdogrel standards in the same matrix as the samples. The net difference between substrate and 2-oxo-clopidogrel remaining yielded the amount of 2-oxo-clopidogrel consumed. B, kinetic profile for the formation of the total amount of the AM (sum of M2–M5). The relative amount of the AM was calculated as the ratio of the area for m/z 356.1 to that for m/z 322.0. C, kinetic profile for the formation of the AM-thiol conjugates. The relative amount of AM-thiol was calculated as the ratio of the area for MH+ for the AM-thiol conjugate to that for m/z 322.0. The MH+ ions for the AM-NAC and AM-l-Cys conjugates are m/z 517.1 and 475.1, respectively.
TABLE 1.
Summary of apparent Km and Vmax values for metabolism of 2-oxo-clopidogrel in the presence of GSH, NAC, and l-Cys
The metabolic reactions were performed at 37°C for 30 min, as described under Materials and Methods.
| Reductant | 2-Oxo-clopidogrel Consumption |
AM |
AM-Thiol Conjugate |
||||
|---|---|---|---|---|---|---|---|
| Km | Vmax | Vmax/Km | Km | Vmax | Km | Vmax | |
| μM | nmol · min−1 · mg HLMs−1 | μM | auc · min−1 · mg HLMs−1 | μM | auc · min−1 · mg HLMs−1 | ||
| GSH | 75 ± 8 | 0.40 ± 0.07 | 0.0053 | 13 ± 2 | 0.92 ± 0.02 | 46 ± 5 | 0.41 ± 0.05 |
| l-Cys | 64 ± 5 | 0.35 ± 0.04 | 0.0055 | 18 ± 3 | 0.88 ± 0.01 | 66 ± 7 | 0.95 ± 0.02 |
| NAC | 72 ± 4 | 0.43 ± 0.03 | 0.0059 | 14 ± 1 | 0.12 ± 0.01 | 50 ± 4 | 1.6 ± 0.09 |
auc, area under the curve.
As shown in Table 1, the Km values for 2-oxo-clopidogrel for the production of the total amount of the AM were determined to be 13 μM for GSH, 14 μM for NAC, and 18 μM for l-Cys (Fig. 5B); these values were not significantly different. However, the Vmax for the production of the AM in the presence of NAC differed greatly from values for the other two reactions. The Vmax for the formation of the AM in the presence of NAC was 7.6-fold less than that obtained in the presence of GSH and 7.3-fold less than that measured in the presence of l-Cys. Therefore, NAC seemed to decrease the formation of the AM. The Km values for the formation of the AM-thiol conjugates were also similar, with Km values in the range of 46 to 66 μM. It is difficult to compare the Vmax values for the different thiol conjugates directly, on the basis of the area ratios alone, because the MS responses of the conjugates might differ.
Thiol-Disulfide Exchange between AM, AM-Thiol Conjugates, and Thiol Reductants.
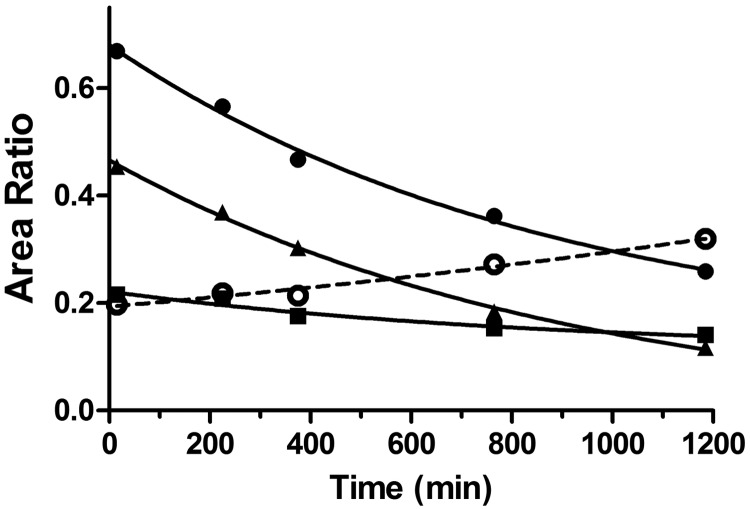
Because the reaction mixture for the metabolism of 2-oxo-clopidogrel by HLMs in the presence of GSH contained the AM, the AM-GSH conjugate, and GSH in both its reduced and oxidized forms after 30 min of incubation, one simple way to examine the thiol-disulfide exchange reaction was to monitor the time-dependent changes in the levels of the AM and the AM-GSH conjugate. As shown in Fig. 6, the relative amounts of the major AM metabolites (M3–M5) decreased slowly, with identical rate constants of 1.1 × 10−3 min−1 (t1/2 ∼ 10 h), whereas the relative amount of the AM-GSH increased slowly, with a comparable rate constant of 0.4 × 10−3 min−1. The simultaneous decrease in the amount of the AM and increase in the amount of the conjugate indicated that the AM was converted to the AM-GSH conjugate, at least partially.
Fig. 6.
Time-dependent changes in the relative amounts of the AM and the AM-GSH conjugate after termination of the metabolic reactions. The AM and the AM-GSH conjugate were formed through incubation at 37°C for 30 min in a reaction mixture containing 1 mg/ml HLMs, 80 μM 2-oxo-clopidogrel, and 1 mM GSH, as described under Materials and Methods. Aliquots of the reaction mixture were analyzed with ESI-LC/MS at designated times, and the amounts of metabolites M3 to M5 were calculated as the ratios of the areas for the metabolites to that for the IS. The area for the IS was integrated from the EIC at m/z 322.0, the areas for M3 to M5 were integrated from the EIC at m/z 356.1, and the area for AM-GSH was integrated from the EIC at m/z 661.0. ●, area ratio for M3; ■, area ratio for M4; ▴, area ratio for M5; ○, area ratio for AM-GSH.
To examine the thiol-disulfide exchange further, we added a second reductant, such as DTT or NAC, after 30 min of incubation with GSH and monitored the changes in the amounts of the AM and the AM-thiol conjugates. Addition of 5 mM DTT led to an 86% decrease in the amount of the AM-GSH conjugate, with a concomitant 40% increase in the amount of the AM (Fig. 7). This observation indicated that the disulfide bond of the AM-GSH conjugate was reduced by DTT through thiol-disulfide exchange. Addition of excess NAC led to a ∼80% decrease in the amount of the AM-GSH conjugate, without significantly affecting the amount of the AM. Addition of NAC led to formation of the AM-NAC conjugate, as evidenced by the peaks at 6.0 and 6.3 min in the EIC for m/z 517.1 (Fig. 7B, trace d). These results clearly demonstrated that thiol-disulfide exchange could occur between the AM, the conjugates, and reductants such as DTT and NAC.
Fig. 7.
Thiol-disulfide exchange between the AM and the AM-GSH conjugate in the presence of excess DTT and NAC. The AM and the AM-GSH conjugate were generated as described for Fig. 6. For thiol-disulfide exchange, DTT or NAC was added at 5 mM, as described under Materials and Methods. A, traces a, b, and c, EICs for the AM at m/z 356.1 for samples incubated with water, DTT, and NAC, respectively. B, traces a, b, and c, EICs for AM-GSH at m/z 661.0 for samples incubated with water, DTT, and NAC, respectively; trace d, same as trace c except recorded at m/z 517.1 for the AM-NAC conjugate.
Discussion
We demonstrated with HLMs that metabolism of 2-oxo-clopidogrel in the presence of four different reductants (i.e., GSH, NAC, l-Cys, and ascorbic acid) led to the formation of the active thiol metabolites. In the presence of the three thiol-containing reductants, we also observed formation of the corresponding AM-thiol conjugates (Figs. 2 and 4). The multiplicity of the active metabolite forms observed is attributable to the existence of multiple diastereomers of 2-oxo-clopidogrel. In principle, eight diastereomers would be expected as a result of the combination of two chiral centers (at C7 and C4) and one exocyclic double bond (between C3 and C16) (Fig. 1). According to Pereillo et al. (2002), the (7S,7R)-diastereomers exhibited very similar chromatographic properties and could not be resolved with conventional, reverse-phase, C18 columns. Therefore, M2 to M5 would be expected to include the two trans- and two cis-isomers of the (4S,4R)-diastereomers. The MS2 spectra of M2 to M5 were in excellent agreement with that reported for the AM of clopidogrel (Pereillo et al., 2002; Dansette et al., 2011, 2012). Quantitative analysis of the AMs of clopidogrel in human plasma, historically referred to as H1, H2, H3, and H4, showed that H3 and H4 were the major metabolites (Tuffal et al., 2011). The double bonds of H3 and H4 are in the cis-configuration (Pereillo et al., 2002; Dansette et al., 2012). Therefore, metabolites M3 and M5 are most likely the cis-isomers of the active metabolite, whereas the minor metabolites M2 and M4 are the trans-isomers.
As shown in Fig. 3, production of the major metabolites M3 to M5 was clearly dependent on the concentrations of GSH. In the absence of GSH, only negligible amounts of M3 to M5 were produced, which might be attributable to the presence of GSH or other reductants in the HLM samples. In the presence of GSH, however, formation of the active metabolites was evident and exhibited saturation kinetics, with a Km of 0.3 mM for GSH. The intracellular levels of GSH in mammalian cells are in the range of 0.5 to 10 mM (Meister and Anderson, 1983). These high levels of GSH should readily allow microsomal P450s to produce the active metabolite. Similar Km values for the formation of the AM and the AM-GSH conjugate suggest that the formation of both the AM and the AM-GSH conjugate is dependent on GSH and the formation of the conjugate is an integral part of the metabolic processes of bioactivation of clopidogrel by P450s. Our results support the two-step bioactivation mechanism proposed by Dansette et al. (2009, 2012).
Simultaneous formation of both the AM and the AM-thiol conjugate under a variety of conditions seems to indicate the existence of an equilibrium between the AM, GSH, and the AM-GSH conjugate, as illustrated in the equations R-S-OH + GSH → R-SSG + H2O and R-SSG + GSH ⇆ R-SH + GSSG, where R-S-OH, R-SSG, and R-SH represent the sulfenic acid intermediate, the AM-GSH conjugate, and the AM, respectively. It is well established that thiol-disulfide interchanges occur in a number of biochemical processes, with a range of rate constants (103-107 s−1) (Houk et al., 1987). As shown in Fig. 6, conversion of R-SH back to the R-SSG conjugate is a much slower process than formation of the active metabolite. Therefore, formation of R-SH is favored. The concentration of R-SH in this equilibrium is ultimately governed by the redox potentials of R-S-OH, R-SSG, and GSH. The use of alternative reductants with more-positive redox potentials would be expected to favor formation of the conjugate R-SSG, leading to decreases in the active metabolite levels. This is exactly what we observed in the presence of NAC and l-Cys (Fig. 4).
The standard redox potentials (E°) for GSH and l-Cys are −0.24 and −0.22 V (pH 7.0, 25°C) for the redox pairs GSH/GSSG and reduced cysteine/oxidized cysteine, respectively, as determined with the thiol-disulfide exchange reaction (Jocelyn, 1967). No experimental data could be found for the absolute redox potentials of NAC, but its redox potential was calculated to be +63 mV above the E° value for GSH/GSSG (Noszál et al., 2000). Therefore, NAC would be expected to exhibit a greater propensity to form a disulfide bond than GSH and l-Cys. Conversely, NAC would disfavor formation of the active thiol metabolite. The levels of the AM produced in the presence of GSH, l-Cys, and NAC were in the following order: GSH (100%) > l-Cys (56%) > NAC (17%). As shown in Fig. 5C, more of the AM-NAC conjugate than the AM-GSH conjugate appears to be formed. It should be noted that direct comparison of the relative amounts of the AM-NAC and AM-GSH conjugates on the basis of area ratios is difficult, because the conjugates' responses to the MS detector might differ. However, the large difference (∼4-fold) in the relative amounts of the AM-NAC and AM-GSH conjugates formed in the presence of 50 μM 2-oxo-clopidogrel provides indirect evidence that NAC has a greater propensity for forming disulfide bonds than does GSH. This is consistent with the observations that the amount of the AM produced in the presence of NAC was ∼17% of that produced in the presence of GSH, whereas the overall consumption of 2-oxo-clopidogrel remained almost constant. One plausible explanation is that the AM-NAC conjugate produced in the equation R-S-OH + GSH → R-SSG + H2O is not converted to R-SH in the equation R-SSG + GSH ⇆ R-SH + GSSG because of the higher E° value for NAC. This is also consistent with the observation that the addition of NAC led to a decrease in R-SH levels, with a concomitant increase in AM-NAC conjugate levels (Fig. 7). It should be emphasized that the concentration of the AM is largely dependent on the equation R-SSG + GSH ⇆ R-SH + GSSG, because the sulfenic acid is an unstable intermediate that likely has a high redox potential, and its reduction is not likely to be the rate-limiting step.
The metabolism of 2-oxo-clopidogrel in the presence of ascorbic acid differs from that with the other reductants in two respects. First, ascorbic acid favors formation of the cis-isomers over the trans-isomer. The cis/trans ratio in the presence of ascorbic acid is approximately 6-fold higher than that in the presence of GSH, which indicates that ascorbic acid preferentially reduces the cis-isomer of the sulfenic acid. Second, the redox potential of ascorbic acid is −0.081 V (pH 7) (Fruton, 1934), which is the highest value among the four reductants used. However, ascorbic acid is more effective than NAC in producing the active thiol metabolite. Compared with GSH, the relative amount of the active thiol metabolite is 61%, similar to results with l-Cys. This is likely attributable to direct reduction of the sulfenic acid without the aforementioned equilibrium involving thiol-disulfide exchange.
Because of its high concentrations in hepatocytes, GSH probably serves as the primary reductant for the generation of active thiol metabolites in human livers. Inevitably, the AM-GSH conjugate would be generated as well. The presence of the equilibrium indicated in the equation R-SSG + GSH ⇆ R-SH + GSSG may contribute to the variations in the concentrations of the active thiol metabolites of clopidogrel in blood plasma. This is particularly relevant considering that clopidogrel has a narrow therapeutic range; overdosing may cause severe bleeding, whereas underdosing may not achieve the desired therapeutic effect. The maximal concentration of the active isomer H4 in blood plasma is approximately ∼30 ng/ml (or 0.1 μM) after a 300-mg loading dose (Takahashi et al., 2008; Tuffal et al., 2011). This low level of active metabolite may be prone to changes as a result of factors other than P450-catalyzed reactions, such as the concentrations of GSH or other reductants that may be associated with oxidative stress or inflammation. In addition, a study by Hagihara et al. (2011) demonstrated that glutaredoxin and thioredoxin accelerated the conversion of the glutathionyl conjugate of the active metabolite of prasugrel, another thienopyridine antiplatelet agent like clopidogrel, to the active metabolite. It is unclear to what extent glutaredoxin and thioredoxin may contribute to the metabolism of clopidogrel to its active metabolite. More studies are required to investigate the factors that determine the effective concentrations of the active metabolite in vivo, so that patients may be given the correct doses for maximal efficacy.
This work was supported in part by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA20090] and the National Institutes of Health National Cancer Institute [Grant CA16954].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- AM
- active metabolite
- P450
- cytochrome P450
- DTT
- dithiothreitol
- EIC
- extracted ion chromatogram
- ESI
- electrospray ionization
- LC
- liquid chromatography
- MS
- mass spectrometry
- MS2
- tandem mass spectrometry
- HLM
- human liver microsome
- IS
- internal standard
- PON1
- paraoxonase 1
- NAC
- N-acetyl-l-cysteine.
Authorship Contributions
Participated in research design: Zhang.
Conducted experiments: Zhang.
Contributed new reagents or analytic tools: Zhang and Hollenberg.
Performed data analysis: Zhang and Hollenberg.
Wrote or contributed to the writing of the manuscript: Zhang, Lau, and Hollenberg.
References
- Algaier I, Jakubowski JA, Asai F, von Kügelgen I. (2008) Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost 6:1908–1914 [DOI] [PubMed] [Google Scholar]
- Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. (2011) Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17:110–116 [DOI] [PubMed] [Google Scholar]
- Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, et al. (2009) Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373:309–317 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Libraire J, Bertho G, Mansuy D. (2009) Metabolic oxidative cleavage of thioesters: evidence for the formation of sulfenic acid intermediates in the bioactivation of the antithrombotic prodrugs ticlopidine and clopidogrel. Chem Res Toxicol 22:369–373 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Rosi J, Bertho G, Mansuy D. (2011) Paraoxonase-1 and clopidogrel efficacy. Nat Med 17:1040–1041 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Rosi J, Bertho G, Mansuy D. (2012) Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol 25:348–356 [DOI] [PubMed] [Google Scholar]
- Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. (2003) Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood 101:3908–3914 [DOI] [PubMed] [Google Scholar]
- Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P. (2003) Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108:989–995 [DOI] [PubMed] [Google Scholar]
- Fruton JS. (1934) Oxidation-reduction potentials of ascorbic acid. J Biol Chem 105:79–85 [Google Scholar]
- Hagihara K, Kazui M, Kurihara A, Kubota K, Ikeda T. (2011) Glutaredoxin and thioredoxin can be involved in producing the pharmacologically active metabolite of a thienopyridine antiplatelet agent, prasugrel. Drug Metab Dispos 39:208–214 [DOI] [PubMed] [Google Scholar]
- Houk J, Singh R, Whitesides GM. (1987) Measurement of thiol-disulfide interchange reactions and thiol pKa values. Methods Enzymol 143:129–140 [DOI] [PubMed] [Google Scholar]
- Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. (2006) Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108:2244–2247 [DOI] [PubMed] [Google Scholar]
- Hulot JS, Collet JP, Silvain J, Pena A, Bellemain-Appaix A, Barthélémy O, Cayla G, Beygui F, Montalescot G. (2010) Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol 56:134–143 [DOI] [PubMed] [Google Scholar]
- Jocelyn PC. (1967) The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem 2:327–331 [DOI] [PubMed] [Google Scholar]
- Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 38:92–99 [DOI] [PubMed] [Google Scholar]
- Mason PJ, Jacobs AK, Freedman JE. (2005) Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol 46:986–993 [DOI] [PubMed] [Google Scholar]
- Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, et al. (2009) Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 360:354–362 [DOI] [PubMed] [Google Scholar]
- Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, et al. (2010) Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Anderson ME. (1983) Glutathione. Annu Rev Biochem 52:711–760 [DOI] [PubMed] [Google Scholar]
- Noszál B, Visky D, Kraszni M. (2000) Population, acid-base, and redox properties of N-acetylcysteine conformers. J Med Chem 43:2176–2182 [DOI] [PubMed] [Google Scholar]
- Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM, Maffrand JP, Picard C. (2002) Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 30:1288–1295 [DOI] [PubMed] [Google Scholar]
- Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, Defreyn G, Maffrand JP. (1992) Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol 44:527–532 [DOI] [PubMed] [Google Scholar]
- Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Hervé C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, et al. (2006) The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103:11069–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, et al. (2009) Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Bhatt DL, Bergougnan L, Farenc C, Pearson K, Perrin L, Vicaut E, Lacreta F, Hurbin F, Dubar M. (2011) Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharmacol Ther 90:287–295 [DOI] [PubMed] [Google Scholar]
- Suh JW, Koo BK, Zhang SY, Park KW, Cho JY, Jang IJ, Lee DS, Sohn DW, Lee MM, Kim HS. (2006) Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ 174:1715–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Pang H, Kawabata K, Farid NA, Kurihara A. (2008) Quantitative determination of clopidogrel active metabolite in human plasma by LC-MS/MS. J Pharm Biomed Anal 48:1219–1224 [DOI] [PubMed] [Google Scholar]
- Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, Kastrati A, Schömig A, Schömig E. (2006) Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther 80:486–501 [DOI] [PubMed] [Google Scholar]
- Tuffal G, Roy S, Lavisse M, Brasseur D, Schofield J, Delesque Touchard N, Savi P, Bremond N, Rouchon MC, Hurbin F, et al. (2011) An improved method for specific and quantitative determination of the clopidogrel active metabolite isomers in human plasma. Thromb Haemost 105:696–705 [DOI] [PubMed] [Google Scholar]
- Zhang H, Amunugama H, Ney S, Cooper N, Hollenberg PF. (2011) Mechanism-based inactivation of human cytochrome P450 2B6 by clopidogrel: involvement of both covalent modification of cysteinyl residue 475 and loss of heme. Mol Pharmacol 80:839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]