Abstract
Continent ileostomy can be defined as a surgical procedure that facilitates planned intermittent evacuation of a bowel reservoir through an ileostomy. It was devised by Nils Kock in 1969. Subsequently, continent ileostomy (or Kock pouch) became a viable alternative in the management of patients who had traditionally required an end ileostomy. Kock pouch appeared to provide substantial physical and psychosocial benefits over a conventional ileostomy. The procedure became popular until ileal pouch anal anastomosis (IPAA) was introduced in 1980. Despite its benefits, continent ileostomy had many short term complications including intubation problems, ileus, anastomotic leaks, peritonitis and valve problems. Operative mortalities have also been reported in the literature. Most of these problems have been eliminated with increasing experience; however, valve-related problems remain as an “Achilles’ heel” of the technique. Many modifications have been introduced to prevent this problem. Some patients have had their pouch removed because of complications mainly related to valve dysfunction. Although revision rates can be high, most of the patients who retain their reservoirs are satisfied with regard to their health status and quality of life. Today, this procedure is still appropriate for selected patients for whom pouch surgery is not possible or for patients who have failed IPAA. Both the patient and their physician must be highly motivated to accept the risk of failure and the subsequent need for revisional operations.
Keywords: Continent ileostomy, Kock pouch, Ileal reservoir, Surgical technique
INTRODUCTION
Continent ileostomy (CI) allows planned evacuation of the small bowel through a reservoir equipped with a nipple valve. The operation was first reported by Kock in 1969[1]. Subsequently, CI (or Kock pouch) became a viable alternative to conventional end ileostomy. This new procedure was met with initial skepticism since most patients did well with a Brooke type end ileostomy[2]. However, some complained that an external appliance was inconvenient, some experienced complications from the conventional ileostomy, while others sought alternative procedures because of sexual and social reasons. The procedure became popular until ileal pouch anal anastomosis (IPAA) was introduced in 1980[3]. Today, the Kock pouch is still appropriate for selected patients with ulcerative colitis (UC) and familial polyposis who are not candidates for IPAA or for those patients who have failed IPAA[4-6].
THE EVOLVING TECHNIQUE OF CONTINENT RESERVOIR
Kock’s original pouch design did not have a continence valve mechanism (Figure 1). A segment of ileum is opened longitudinally and sutured together to create U-shaped limbs. The pouch is formed with vertical folding. A corner of the reservoir is sutured to the skin of the abdominal wall. Next, an efferent limb is added to the reservoir. His initial intent was to use the rectus abdominis muscle to serve as a constricting force around the efferent loop of bowel and thus provide continence; some of the patients achieved continence. Later, a nipple valve was provided by retrograde intussusception of the efferent limb into the pouch (Figure 2). This configuration achieved complete continence. Many surgeons have adopted this technique[7,8]. All have expressed major concern in regard to the incidence of valve slippage. Dessusception of the valve (slippage) has been the most frequent complication in the long-term follow-up studies[9-14]. Total valve slippage renders the pouch incontinent; partial slippage makes intubation difficult or impossible.
Figure 1.
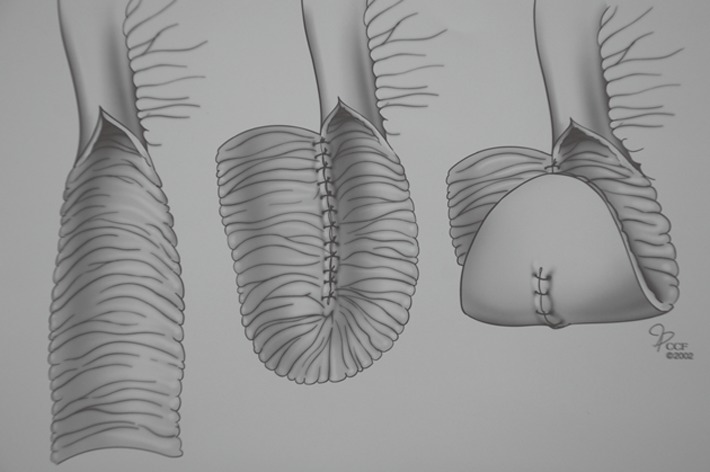
Original Kock pouch design without valve mechanism.
Figure 2.
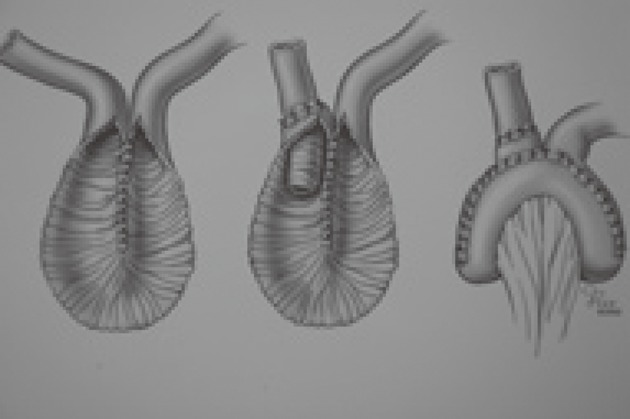
Continent Kock pouch.
In the following years, many modifications of operative technique were proposed to overcome this problem. Steichen introduced the use of staples for valve stabilization[15]. Fazio also described a stapled technique for nipple valve fixation[16]. These modifications have led to a decrease in the incidence of valve slippage (Figure 3). Later on, synthetic materials such as polypropylene (Marlex) or polyester (Mersilene) were also used to buttress the valve mechanism. The valve slippage problem was largely controlled but the use of these materials could lead to fistula formation[17,18].
Figure 3.
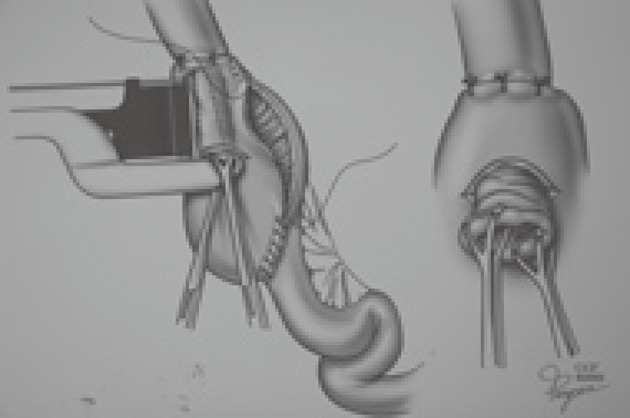
Stapled technique for nipple valve fixation.
Barnett introduced an isoperistaltic valve to avoid the slippage problem[19]. In his technique, an afferent limb of bowel is used to construct a nipple valve with isoperistaltic intussusception. He initially reported that none of his sixteen isoperistaltic CI patients needed reoperation for valve slippage during three years of follow-up. Barnett’s later works[20] and a Cleveland Clinic study[21] revealed that valve slippage occurs whether it is constructed in an isoperistaltic or anisoperistaltic fashion. The weakest point of the valve is on the mesenteric side where intussusception produces a large bulky mass that leads to slippage (Figure 4). Barnett was convinced that a support was absolutely necessary to avoid the problem and sought a suitable material that would not erode the valve. In 1986, he added an intestinal segment around the exit conduit to control valve slippage. This “living intestinal collar” buttressed the mesenteric side of the valve where slippage is initiated (Figure 5). The Barnett continent intestinal reservoir, including an intestinal collar and an isoperistaltic valve, has reduced the incidence of valve slippage and fistula formation[22]. Because of the complications related to valve intussusception, Stein developed a new design pouch incorporating a serosal lined ileal antireflux mechanism for urinary diversion after cystectomy in 1998[23]. Preliminary results of this innovative flap valve mechanism reservoir (T-pouch) indicated complete continence of the pouch but long-term results have not been reported[24].
Figure 4.
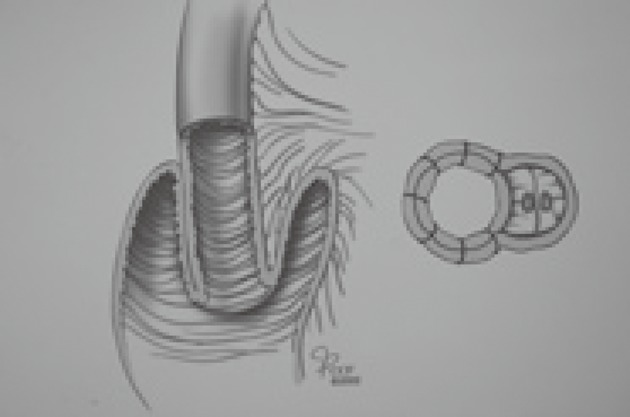
Valve intussusception produces a large bulky mass leading to slippage.
Figure 5.
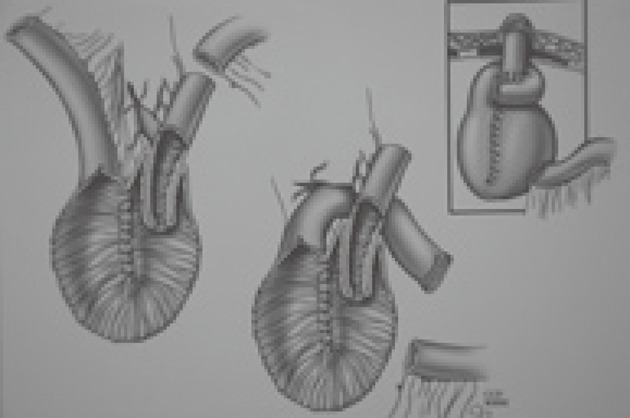
Barnett continent intestinal reservoir.
A novel CI has been reported by the authors[25]. The porcine model was used to create a valveless reservoir and its integrity was tested. Although promising results have been achieved with this simple technique, the method has not been tested in humans.
LONG-TERM RESULTS AND QUALITY OF LIFE
Berglund et al[26] and Ojerskog et al[27] published their long-term experiences of CI with or without valve mechanism. A total number of 435 patients had been provided with a CI; of these 50% had their pouch constructed at the time of proctocolectomy (one-stage operation). These pioneers of CI reported a high complication rate (23%) and operative mortality (4.3%) in their early series but these figures dropped to a complication rate of 8% with no mortality in the later period. The long-term complications of CI were mainly related to the valve mechanism, including sliding of the nipple valve, fistula formation, partial or total prolapse of the valve, necrosis of the valve and/or the outlet, stricture of the stoma and inflammatory changes in the reservoir (pouchitis). Valve revisions were performed within the first postoperative year in most of the patients. They concluded that patients with CI have perfect continence unless they develop complication in the valve mechanism.
The long-term results of CI were evaluated in 129 consecutive patients who had this procedure performed by one surgeon at the University of California, San Francisco, between 1975 and 1995[28]. The primary disease was UC in 119 (92%) patients, familial adenomatous polyposis (FAP) in 7 (5%), and multiple neoplasms in one (1%). Patient satisfaction, pouch durability and the details of the operation were evaluated using a modified 36-item short form health survey, phone calls, chart review and the personal records of the surgeon. Mean follow-up was 11.4 years (range: 1-21 years) and the late outcome was available for 85 patients (66%). Thirty-three of the patients (39%) had undergone an elective one-stage operation with total proctocolectomy and CI, whereas the remainder had undergone emergent and elective procedures prior to CI creation. Of these patients, 54 (64%) had a functional pouch, whereas 31 patients (36%) had their pouch excised because of complications mainly related to valve dysfunction. The important feature of this study was the overall pouch failure rate which was significantly higher for those of the patients who had their CI as a secondary procedure. Despite many revisions, 97% of the survey respondents who retained their pouch were very satisfied and considered their quality of life to be good or excellent.
The Cleveland Clinic study described 330 patients who underwent Kock pouch surgery between 1977 and 2001[21]. The mean patient age at the time of surgery was 34.9 years (range: 14-65 years) with a male to female ratio of 1:1.3. Indications for surgery included UC in 251 patients (76%); Crohn’s disease in 42 (12.7%); FAP in 23 (7%); indeterminate colitis in five (1.5%); and other diagnoses including colonic inertia, Hirschsprung disease, rectal villous adenoma, and imperforate anus in nine (2.8%). Patients were followed for a median of 11 years (range: 1-27 years); during this period, 51 (16.6%) patients had their Kock pouch excised. At 10 years, 87% of all patients maintained their pouches. At 20 years, 77% of all patients still had their pouches. The 10-year and 20-year continent pouch survival for UC and FAP disease patients were 92.5% and 83.3%, respectively. Patients with Crohn’s disease or indeterminate colitis had 10-year and 20-year pouch survival rates of 58.4% and 39.1%, respectively. The median complication-free pouch interval was 7 mo; the median revision-free pouch interval was 14 mo. Valve slippage was the most frequent complication leading to pouch revision (29.7%). The second most common complication was fistula formation (25.2%). The median time to valve slippage was two years. Parastomal hernia occurred in 15.5% of patients. Complete valve prolapse and stoma stricture were other complications. Patients had an average of 3.7 complications (range: 1-28 complications) and 2.9 pouch revisions (range: 1-27 pouch revisions) during their follow-up period. Patients undergoing staged procedures (such as prior restorative proctocolectomy, proctocolectomy with end ileostomy or ileorectal anastomosis) were 1.4 times more likely to undergo pouch revision compared with patients undergoing a single stage operation.
Quality of life for Kock pouch patients was evaluated in the Cleveland Clinic study. Patients with an end ileostomy were more than twice as likely to report social, work, and sexual restrictions compared with CI patients. End ileostomy patients reported higher antidiarrheal medication and fiber intake. A higher percentage of patients with CI reported having a better appetite. In addition, a higher proportion of end ileostomy patients reported having pouch-related complications. Finally, patients with CI were more likely to have the same procedure again and would recommend the procedure to someone else in comparison to the end ileostomy group of patients.
Wasmuth et al[29] evaluated 50 patients who underwent Kock CI between 1983 and 2002. The reoperation and pouch excision rates were 44% and 8%, respectively. Reoperation rate was higher among the patients having CI as a second procedure. The Mount Sinai group evaluated 31 patients who had revisions after more than 10 years of normal Kock pouch function[30]. Delayed pouch failure reasons were mostly related to the valve, and the pouch salvage rate was 93% with good functional outcome. Another study from Holland compared CI patients with a historical group of IPAA patients and end ileostomy patients[31]. They found that the quality of life of CI patients was not significantly better or worse than that of the patients with either a conventional ileostomy or an IPAA.
FUTURE OF THE CONTINENT ILEOSTOMY
CI studies have revealed that this procedure has many short and long-term complications, mainly related to the valve mechanism. Despite many revisions, patients who retained their pouch were very satisfied and considered their quality of life to be good or excellent. There is a need to develop a safe and reliable continent abdominal reservoir because there are still indications for the CI. These procedures have to be performed in specialized centers.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Victor W Fazio for his life-long dedication to pouch surgery and Mr. Joseph Pangrace of the Cleveland Clinic Department of Medical illustration.
Footnotes
Peer reviewer: Jingbo Zhao, Associate Professor, Mech-Sense, Science and Innovation Center, Aalborg Hospital, Sdr. Skovvej 15, 9000 Aalborg, Denmark
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
References
- 1.Kock NG. Intra-abdominal “reservoir” in patients with permanent ileostomy. Preliminary observations on a procedure resulting in fecal “continence” in five ileostomy patients. Arch Surg. 1969;99:223–231. doi: 10.1001/archsurg.1969.01340140095014. [DOI] [PubMed] [Google Scholar]
- 2.Brooke BN. The management of an ileostomy, including its complications. Lancet. 1952;2:102–104. doi: 10.1016/s0140-6736(52)92149-1. [DOI] [PubMed] [Google Scholar]
- 3.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusunoki M, Sakanoue Y, Shoji Y, Kusuhara K, Yamamura T, Utsunomiya J. Conversion of malfunctioning J pouch to Kock’s pouch. Case report. Acta Chir Scand. 1990;156:179–181. [PubMed] [Google Scholar]
- 5.Ecker KW, Haberer M, Feifel G. Conversion of the failing ileoanal pouch to reservoir-ileostomy rather than to ileostomy alone. Dis Colon Rectum. 1996;39:977–980. doi: 10.1007/BF02054684. [DOI] [PubMed] [Google Scholar]
- 6.Hultén L. Conversion of a pelvic pouch to a continent pouch (Kock pouch) Tech Coloproctol. 2001;5:192. doi: 10.1007/s101510100025. [DOI] [PubMed] [Google Scholar]
- 7.Beahrs OH. Use of ileal reservoir following proctocolectomy. Surg Gynecol Obstet. 1975;141:363–366. [PubMed] [Google Scholar]
- 8.Goligher JC, Lintott D. Experience with 26 reservoir ileostomies. Br J Surg. 1975;62:893–900. doi: 10.1002/bjs.1800621110. [DOI] [PubMed] [Google Scholar]
- 9.Schrock TR. Complications of continent ileostomy. Am J Surg. 1979;138:162–169. doi: 10.1016/0002-9610(79)90257-5. [DOI] [PubMed] [Google Scholar]
- 10.Failes DG. The continent ileostomy--an 11 year experience. Aust N Z J Surg. 1984;54:345–352. doi: 10.1111/j.1445-2197.1984.tb05331.x. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen HJ, Mäkitie A, Sivula A. Long-term results of continent ileostomy. Int J Colorectal Dis. 1986;1:40–43. doi: 10.1007/BF01648835. [DOI] [PubMed] [Google Scholar]
- 12.Fazio VW, Church JM. Complications and function of the continent ileostomy at the Cleveland Clinic. World J Surg. 1988;12:148–154. doi: 10.1007/BF01658045. [DOI] [PubMed] [Google Scholar]
- 13.Sjödahl R, Lemon E, Nyström PO, Olaison G. Complications, surgical revision and quality of life with conventional and continent ileostomy. Acta Chir Scand. 1990;156:403–407. [PubMed] [Google Scholar]
- 14.Köhler LW, Pemberton JH, Zinsmeister AR, Kelly KA. Quality of life after proctocolectomy. A comparison of Brooke ileostomy, Kock pouch, and ileal pouch-anal anastomosis. Gastroenterology. 1991;101:679–684. [PubMed] [Google Scholar]
- 15.Steichen FM, Loubeau J-M, Stremple JF. The continent ileal reservoir. Surgical Rounds. 1978:10–18. [Google Scholar]
- 16.Fazio VW, Tjandra JJ. Technique for nipple valve fixation to prevent valve slippage in continent ileostomy. Dis Colon Rectum. 1992;35:1177–1179. doi: 10.1007/BF02251973. [DOI] [PubMed] [Google Scholar]
- 17.Hultén L, Svaninger G. Facts about the Kock continent ileostomy. Dis Colon Rectum. 1984;27:553–557. doi: 10.1007/BF02555527. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JS, Williams SM. Fistula following continent ileostomy. Dis Colon Rectum. 1984;27:193–195. doi: 10.1007/BF02555675. [DOI] [PubMed] [Google Scholar]
- 19.Barnett WO. Improving the continent ileostomy. J Miss State Med Assoc. 1983;24:31–34. [PubMed] [Google Scholar]
- 20.Barnett WO. Current experiences with the continent intestinal reservoir. Surg Gynecol Obstet. 1989;168:1–5. [PubMed] [Google Scholar]
- 21.Nessar G, Fazio VW, Tekkis P, Connor J, Wu J, Bast J, Borkowski A, Delaney CP, Remzi FH. Long-term outcome and quality of life after continent ileostomy. Dis Colon Rectum. 2006;49:336–344. doi: 10.1007/s10350-005-0285-4. [DOI] [PubMed] [Google Scholar]
- 22.Mullen P, Behrens D, Chalmers T, Berkey C, Paris M, Wynn M, Fabito D, Gaskin R, Hughes T, Schiller D. Barnett continent intestinal reservoir. Multicenter experience with an alternative to the Brooke ileostomy. Dis Colon Rectum. 1995;38:573–582. doi: 10.1007/BF02054114. [DOI] [PubMed] [Google Scholar]
- 23.Stein JP, Lieskovsky G, Ginsberg DA, Bochner BH, Skinner DG. The T pouch: an orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836–1842. doi: 10.1016/S0022-5347(01)63170-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser AM, Stein JP, Beart RW. T-pouch: a new valve design for a continent ileostomy. Dis Colon Rectum. 2002;45:411–415. doi: 10.1007/s10350-004-6192-2. [DOI] [PubMed] [Google Scholar]
- 25.Nessar G, Remzi FH, Wu JS. Evolving technique for continent ileostomy: valveless pouch design. Tech Coloproctol. 2004;8:49–52; discussion 52. doi: 10.1007/s10151-004-0053-9. [DOI] [PubMed] [Google Scholar]
- 26.Berglund B, Brevinge H, Kock NG, Lindholm E. Expansion of various types of ileal reservoirs in situ. An experimental study in rats. Eur Surg Res. 1987;19:298–304. doi: 10.1159/000128713. [DOI] [PubMed] [Google Scholar]
- 27.Ojerskog B, Hällström T, Kock NG, Myrvold HE. Quality of life in ileostomy patients before and after conversion to the continent ileostomy. Int J Colorectal Dis. 1988;3:166–170. doi: 10.1007/BF01648361. [DOI] [PubMed] [Google Scholar]
- 28.Litle VR, Barbour S, Schrock TR, Welton ML. The continent ileostomy: long-term durability and patient satisfaction. J Gastrointest Surg. 1999;3:625–632. doi: 10.1016/s1091-255x(99)80085-7. [DOI] [PubMed] [Google Scholar]
- 29.Wasmuth HH, Svinsås M, Tranø G, Rydning A, Endreseth BH, Wibe A, Myrvold HE. Surgical load and long-term outcome for patients with Kock continent ileostomy. Colorectal Dis. 2007;9:713–717. doi: 10.1111/j.1463-1318.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 30.Denoya PI, Schluender SJ, Bub DS, Gorfine SR, Bauer JJ. Delayed Kock pouch nipple valve failure: is revision indicated? Dis Colon Rectum. 2008;51:1544–1547. doi: 10.1007/s10350-008-9350-0. [DOI] [PubMed] [Google Scholar]
- 31.Hoekstra LT, de Zwart F, Guijt M, Bakx R, Gerhards MF. Morbidity and quality of life after continent ileostomy in the Netherlands. Colorectal Dis. 2009;11:719–725. doi: 10.1111/j.1463-1318.2008.01674.x. [DOI] [PubMed] [Google Scholar]


