Abstract
AIM: To investigate the clinicopathologic features which predict surgical overall survival in patients with proximal gastric carcinoma involving the esophagus (PGCE).
METHODS: Electronic pathology database established in the Department of Pathology of the Nanjing Drum Tower Hospital was searched for consecutive resection cases of proximal gastric carcinoma over the period from May 2004 through July 2009. Each retrieved pathology report was reviewed and the cases with tumors crossing the gastroesophageal junction line were selected as PGCE. Each tumor was re-staged, following the guidelines on esophageal adenocarcinoma, according to the 7th edition of the American Joint Commission on Cancer Staging Manual. All histology slides were studied along with the pathology report for a retrospective analysis of 13 clinicopathologic features, i.e., age, gender, Helicobacter pylori (H. pylori) infection, surgical modality, Siewert type, tumor Bormann’s type, size, differentiation, histology type, surgical margin, lymphovascular and perineural invasion, and pathologic stage in relation to survival after surgical resection. Prognostic factors for overall survival were assessed with uni- and multi-variate analyses.
RESULTS: Patients’ mean age was 65 years (range: 47-90 years). The male: female ratio was 3.3. The 1-, 3- and 5-year overall survival rates were 87%, 61% and 32%, respectively. By univariate analysis, age, male gender, H. pylori, tumor Bormann’s type, size, histology type, surgical modality, positive surgical margin, lymphovascular invasion, and pT stage were not predictive for overall survival; in contrast, perineural invasion (P = 0.003), poor differentiation (P = 0.0003), > 15 total lymph nodes retrieved (P = 0.008), positive lymph nodes (P = 0.001), and distant metastasis (P = 0.005) predicted poor post-operative overall survival. Celiac axis nodal metastasis was associated with significantly worse overall survival (P = 0.007). By multivariate analysis, ≥ 16 positive nodes (P = 0.018), lymph node ratio > 0.2 (P = 0.003), and overall pathologic stage (P = 0.002) were independent predictors for poor overall survival after resection.
CONCLUSION: Patients with PGCE showed worse overall survival in elderly, high nodal burden and advanced pathologic stage. This cancer may be more accurately staged as gastric, than esophageal, cancer.
Keywords: Cancer, Esophagus, Gastroesophageal junction, Staging, Stomach
INTRODUCTION
Carcinoma in the gastroesophageal junction (GEJ) region can be sub-grouped by the Siewert classification system into 3 types on the basis of the distance between tumor epicenter and the GEJ line[1,2]. Type I carcinomas are centered in the distal esophagus, 1-5 cm above the GEJ. They arise largely from Barrett’s esophagus (BE), may or may not invade the GEJ, and are commonly reported as Barrett’s adenocarcinoma. Type II tumors straddle the GEJ line and are believed to be true GEJ cancers with epicenter within 1 cm above and 2 cm below the GEJ. Type III tumors are sub-cardial gastric cancers with epicenter 2-5 cm below the GEJ that is crossed as they grow proximally.
Although the Siewert classification system has been widely used internationally, its prognostic value has been challenged[3-5]. In Asian countries, type I GEJ cancer is rare and types II and III carcinomas behave similarly[5,6]. In China, almost all GEJ carcinomas arise in the proximal stomach with a stable or slightly increased incidence in recent years[7-10]. In our most recent study comparing clinicopathologic features of GEJ cancer between Chinese patients treated in Nanjing, China, and American patients treated in Boston, the United States, we showed that almost all GEJ cancers in Chinese patients were Siewert types II and III tumors[11], unlike those seen in American patients in which the distribution of these three types of tumors was almost evenly[12]. Their tumors were not BE-related, but associated with proximal gastritis with Helicobacter pylori (H. pylori) infection and a better overall survival rate, despite a larger tumor size and more advanced pathologic stages at diagnosis[11]. Surprisingly, the studies on factors predicting post-operative overall survival in Chinese patients with proximal gastric carcinoma involving the esophagus (PGCE) are scarce. The purpose of the present study was to investigate clinicopathologic features that may predict overall survival after surgical resection in Chinese patients with PGCE who were treated at a single high-volume tertiary medical center in Nanjing, China.
MATERIALS AND METHODS
Selection of patients
A total of 177 consecutive resection cases of histopathologically confirmed proximal gastric carcinoma were identified through a search of the computerized pathology database established in the Department of Pathology of the Nanjing Drum Tower Hospital in Nanjing, China, over the period from May 2004 through July 2009. Each pathology report was reviewed (by Huang Q) for cases with tumors crossing the GEJ line. Inclusion criteria were: (1) a tumor with epicenter in the proximal stomach within 5 cm below the GEJ and invading into the distal esophagus, which corresponded to Siewert types II and III tumors; (2) no chemotherapy or radiation therapy before the surgical resection; and (3) the availability of follow-up information through telephone interviews (by Feng AN) to the patient or family members. Exclusion criteria consisted of: (1) the tumor not crossing the GEJ; and (2) the patient lost to follow-up. Following a standard comprehensive surgical pathology processing protocol, all resection specimens were evaluated for the Bormann’s gross type and surgical margins. The GEJ line, defined by the proximal end of gastric longitudinal mucosal folds, was evaluated in each case. The tumor epicenter location and its distance from the GEJ were recorded. The number of overall survival months after surgery was calculated until May 2010, based on whether the patient was alive or had died of any cause. For all selected patients, medical records and pathology reports were re-evaluated for demographic and clinicopathologic information, tumor stage, surgical approach (total or partial gastrectomy or Ivor-Lewis procedure), completeness of resection, and histopathology of the tumor. The study protocol was approved by the Medical Ethics Committee of the Nanjing Drum Tower Hospital in Nanjing, China.
Tumor staging
All tumors were staged with the esophageal cancer staging criteria, according to the 7th edition of the American Joint Commission on Cancer Staging Manual (AJCC 7)[13]. The status of regional involved lymph nodes in the para-distal esophageal, para-cardial and peri-gastric regions was determined microscopically. The lymph nodes in the celiac axis region, including the left gastric artery, celiac artery, hepatic and splenic hila, etc., were identified by the surgeon during the operation, submitted as separate specimens and examined microscopically. Lymph node metastasis was determined by the routine histological examination. In this study, since PGCE was classified as GEJ cancer and staged as esophageal cancer, metastases to celiac axis regional nodes were considered as distant metastasis[13,14].
The proximal, distal and radial margins of resection were routinely inked for microscopic examination and classified as negative or positive if there was histological evidence of carcinoma present at, or within 1 mm of the inked resection edge. Lymphovascular and peri-neural invasion was assessed microscopically on routine histology sections. Suspected distant tumor metastasis in the liver or other organs detected and biopsied intraoperatively was confirmed microscopically.
Statistical analysis
All patients’ demographic and tumor gross characteristics were considered categorical variables except for age, overall survival month, number of lymph nodes retrieved and number of lymph nodes involved, which were classified as continuous variables. All statistical analysis was carried out (by Shi J) using the SPSS software (SPSS Inc., version 15.0, Chicago, IL, United States). Specific comparison between groups was performed using χ2 and Student t tests. Patient overall survival rates after surgical resection were estimated with the Kaplan-Meier method and the log rank test. The patients who were alive at the last follow-up were censored for calculation of the overall survival rates. Cox multivariate proportional hazards regression models were used to assess the overall survival power of these parameters. The P value of < 0.05 was considered statistically significant.
RESULTS
Clinicopathologic characteristics
A total of 142 cases were eligible for this study. The patients’ demographics, clinicopathologic characteristics, and the results of univariate overall survival analysis were shown in Table 1. Most (82%) tumors were type II of the Siewert classification, while there was no type I tumor in this cohort. The mean patient age was 65 years (range: 47-90). The male-female ratio was 3.3. The number of months of patient follow-up after surgical resection was 29 ± 17 mo (mean ± SD, range: 1-70 mo). By the time of the last follow-up, 58 (41%) patients had died and the remaining survivors were censored. Overall, the 1-, 3- and 5-year overall survival rates were 87%, 61% and 36%, respectively. Among the demographic and pathologic variables, perineural invasion and poor tumor differentiation were associated with worse overall survival (Table 1). None of the surgical modalities were found significant for overall survival prediction. The status of H. pylori infection, tumor gross type, gender, age, tumor size, lymphovascular invasion, and even positive surgical margins were not significant for overall survival prediction by univariate analysis.
Table 1.
Demographic, clinical, and pathological features predicting overall survival in patients with proximal gastric carcinoma involving esophagus
| Characteristics | Number of patients (%) | Months after surgery (mean ± SD) | P value | 1-yr survival % | 3-yr survival % | 5-yr survival % |
| Age (yr) | 0.108 | |||||
| < 70 | 97 (68.3) | 30 ± 17 | 91.1 | 65.3 | 40.1 | |
| ≥ 70 | 45 (31.7) | 27 ± 19 | 77.0 | 49.3 | 17.3 | |
| Gender | 0.594 | |||||
| Male | 109 (76.8) | 28 ± 17 | 71.1 | 61.1 | 23.1 | |
| Female | 33 (23.2) | 30 ± 18 | 88.3 | 60.1 | 48.1 | |
| Helicobacter pylori infection | 0.400 | |||||
| Negative | 80 (56.3) | 30 ± 18 | 87.3 | 67.6 | 29.4 | |
| Positive | 62 (43.7) | 27 ± 17 | 87.1 | 50.8 | 32.3 | |
| Surgical modality | 0.950 | |||||
| Partial gastrectomy | 112 (78.9) | 29 ± 18 | 85.7 | 68.6 | 35.1 | |
| Total gastrectomy | 21 (14.8) | 28 ± 18 | 76.2 | 65.3 | 0.0 | |
| Ivor-Lewis procedure | 9 (6.3) | 29 ± 17 | 89.2 | 60.6 | 34.3 | |
| Siewert type | 0.444 | |||||
| Type II | 116 (81.7) | 29 ± 17 | 86.8 | 63.8 | 27.2 | |
| Type III | 26 (18.3) | 33 ± 19 | 88.5 | 50.2 | 40.2 | |
| Bormann’s type | 0.380 | |||||
| Polypoid | 2 (1.4) | 27 ± 26 | 100.0 | 50.1 | 50.1 | |
| Fungating | 17 (12.0) | 34 ± 20 | 83.3 | 70.9 | 53.2 | |
| Ulcerated | 96 (67.6) | 28 ± 17 | 88.0 | 56.3 | 26.8 | |
| Flat | 27 (19.0) | 26 ± 18 | 86.5 | 66.6 | 29.6 | |
| Tumor size (cm) | 0.087 | |||||
| < 3 | 19 (13.4) | 30 ± 17 | 87.0 | 72.1 | 17.2 | |
| 3.1-7.9 | 112 (78.9) | 30 ± 17 | 89.7 | 61.5 | 38.5 | |
| > 8 | 11 (7.7) | 18 ± 15 | 63.6 | 26.5 | 0.0 | |
| Surgical margin | 0.165 | |||||
| Negative | 126 (88.7) | 30 ± 17 | 88.8 | 60.6 | 30.1 | |
| Positive | 16 (11.3) | 23 ± 19 | 74.5 | 66.2 | 33.1 | |
| Lymphovascular invasion | 0.050 | |||||
| Negative | 56 (39.4) | 33 ± 18 | 90.9 | 65.6 | 31.2 | |
| Positive | 86 (60.6) | 27 ± 17 | 84. | 57.6 | 31.7 | |
| Perineural invasion | 0.003 | |||||
| Negative | 55 (38.7) | 35 ± 18 | 92.7 | 63.4 | 41.3 | |
| Positive | 87 (61.3) | 26 ± 17 | 83.6 | 59.4 | 19.3 | |
| Tumor differentiation | 0.0003 | |||||
| Well | 1 (0.7) | 57 | 100.0 | 100.0 | 100.0 | |
| Moderately | 70 (49.3) | 34 ± 17 | 95.2 | 69.3 | 44.2 | |
| Poorly | 70 (49.3) | 24 ± 16 | 81.3 | 52.7 | 11.3 | |
| Undifferentiated | 1 (0.7) | 4 | 0.0 | 0.0 | 0.0 | |
| Tumor histopathology | 0.548 | |||||
| Adenocarcinoma (nitric oxide synthase) | 112 (78.9) | 31 ± 17 | 89.6 | 71.0 | 34.2 | |
| Adenocarcinoma with micropapillary feature | 24 (16.9) | 24 ± 10 | 100 | 0.0 | 0.0 | |
| Adenosquamous carcinoma | 9 (6.3) | 28 ± 15 | 88.9 | 33.3 | 0.0 | |
| Mucinous carcinoma | 9 (6.3) | 24 ± 14 | 88.9 | 30.5 | 30.5 | |
| Signet ring cell carcinoma | 23 (16.2) | 23 ± 19 | 69.3 | 58.5 | 39.0 | |
| Carcinoma with neuroendocrine features | 7 (4.9) | 28 ± 17 | 100.0 | 50.0 | 25.0 | |
| Carcinoma with mixed types | 37 (26.1) | 24 ± 13 | 86.3 | 50.1 | 50.1 | |
| Pathologic T stage | 0.400 | |||||
| 1A | 2 | 38 ± 27 | 100.0 | 50.0 | 50.0 | |
| 1B | 2 | 47 ± 3 | 100.0 | 100.0 | 100.0 | |
| 2 | 12 | 27 ± 17 | 83.3 | 72.9 | 54.7 | |
| 3 | 125 | 29 ± 17 | 87.0 | 59.8 | 29.2 | |
| 4A | 0 | 0 | - | - | - | |
| 4B | 1 | 18 | 100.0 | 0.0 | 0.0 | |
| Pathologic N stage | 0.001 | |||||
| 0 | 36 | 35 ± 17 | 94.4 | 72.0 | 53.5 | |
| 1 | 22 | 32 ± 17 | 91.3 | 70.1 | 70.1 | |
| 2 | 35 | 30 ± 17 | 85.3 | 64.7 | 15.9 | |
| 3A | 35 | 25 ± 17 | 85.3 | 57.4 | 14.4 | |
| 3B | 14 | 15 ± 9 | 70.7 | 41.3 | 10.3 | |
| Pathologic M stage | 0.005 | |||||
| M0 | 130 | 28 ± 18 | 88.3 | 64.5 | 32.5 | |
| M1 | 12 | 12 ± 4 | 75.0 | 13.1 | 13.1 | |
| Overall stage | 0.012 | |||||
| 1A | 4 | 42 ± 16 | 100.0 | 75.0 | 75.0 | |
| 1B | 7 | 30 ± 15 | 100.0 | 100.0 | 100.0 | |
| 2A | 26 | 34 ± 19 | 88.5 | 63.0 | 42.0 | |
| 2B | 26 | 32 ± 17 | 84.6 | 66.8 | 60.7 | |
| 3A | 29 | 31 ± 17 | 93.1 | 72.6 | 19.8 | |
| 3B | 29 | 27 ± 18 | 86.2 | 62.9 | 15.3 | |
| 3C | 9 | 18 ± 10 | 76.2 | 15.2 | 15.2 | |
| 4 | 12 | 16 ± 9 | 75.0 | 13.1 | 13.1 |
Pathologic staging
Following the AJCC 7 staging guidelines, the vast majorities (88%) of tumors were skewed to pT3 and only a few staged at pT1 (n = 4), pT2 (n = 12) and pT4 (n = 1). The pT stage was found not to be a relevant factor for overall survival prediction. In contrast, positive lymph node metastasis were detected in 106 (75%) cases and significantly associated with worse overall survival (Table 2). Twelve of 142 patients (8%) had distant metastasis at the time of operation and showed significantly worse overall survival.
Table 2.
Univariate analysis of overall survival in patients with lymph node metastasis
|
Overall survival |
|||||
| Characteristic | No. of patients (%) | HR | 95% CI1 | P value | |
| Celiac axis lymph node | |||||
| Negative | 130 (92) | 1.00 | |||
| Positive | 12 (8) | 3.33 | 1.40 | 7.95 | 0.007 |
| Perineural invasion | |||||
| Negative | 55 (38.7) | 1.00 | |||
| Positive | 87 (61.3) | 1.48 | 0.54 | 4.11 | 0.447 |
| Number of nodes retrieved/case | |||||
| 10 | 21 (15) | 1.00 | |||
| ≥ 11 | 121 (85) | 1.48 | 0.72 | 3.06 | 0.290 |
| ≤ 15 | 46 (32) | 1.00 | |||
| ≥ 16 | 96 (68) | 2.29 | 1.24 | 4.22 | 0.008 |
| ≤ 23 | 100 (70) | 1.00 | |||
| ≥ 24 | 42 (30) | 1.15 | 0.65 | 2.03 | 0.627 |
| Number of positive nodes/case | |||||
| ≤ 6 | 58 (41) | 1.00 | |||
| ≥ 7 | 48 (34) | 1.72 | 1.20 | 2.46 | 0.003 |
| ≤ 15 | 92 (65) | 1.00 | |||
| ≥ 16 | 14 (10) | 2.30 | 1.40 | 3.77 | 0.001 |
| Lymph node ratio | |||||
| 0 | 36 (25) | 1.00 | |||
| ≤ 0.2 | 40 (28) | 1.34 | 0.61 | 2.97 | 0.467 |
| > 0.2 | 66 (46) | 2.28 | 1.15 | 4.51 | 0.018 |
Hazards ratios (HR), 95% confidence interval (CI) and P values for postoperative time to recurrence and overall survival were adjusted according to important clinical characteristics. Survival time was defined as the period from the surgical treatment to endpoint of follow-up.
Lymph node status with overall survival
The mean number of lymph nodes retrieved per case was 21 (range: 4-66). The presence of lymph node metastasis was found in 106 (75%) cases. There were 12 patients (8%) with cancer metastasis in celiac axis lymph nodes, which significantly predicted worse overall survival by univariate analysis (Table 2). There was a statistically significant overall survival difference between patients with a total number of retrieved lymph nodes ≤ 15 and ≥ 16 (Table 2). The numbers of positive lymph nodes more than 7 and 16 were worse overall survival predictors. The ratio of the number of positive nodes to the total number of nodes retrieved, i.e., the lymph node ratio, was significantly associated with worse overall survival (Tables 2 and 3, Figure 1).
Table 3.
Independent overall survival predictors retained by multivariate analysis
| Factor | No. of patients |
Multivariate analysis |
|||
| HR | 95% CI | P value | |||
| Celiac axis lymph node | Compared to pN0 | ||||
| Negative | 130 | ||||
| Positive | 12 | 1.76 | 0.68 | 4.57 | 0.246 |
| Number of positive nodes/case | Compared to pN0 | ||||
| LN+ ≥ 7 | 48 | 1.34 | 0.6 | 2.96 | 0.467 |
| LN+ ≥ 16 | 14 | 2.77 | 1.15 | 4.51 | 0.018 |
| Lymph node ratio | Compared to pN0 | ||||
| ≤ 0.2 | 40 | 3.8 | 1.28 | 11.26 | 0.016 |
| > 0.2 | 66 | 7.79 | 2.05 | 29.57 | 0.003 |
| Overall stage pIV | Compared to pI | ||||
| 12 | 18.43 | 2.27 | 145.62 | 0.002 | |
| Tumor differentiation poorly differentiated | Compared to well differentiated | ||||
| 70 | 1.42 | 0.79 | 2.55 | 0.243 | |
LN: Lymph node; HR: Hazards ratio; CI: Confidence interval.
Figure 1.
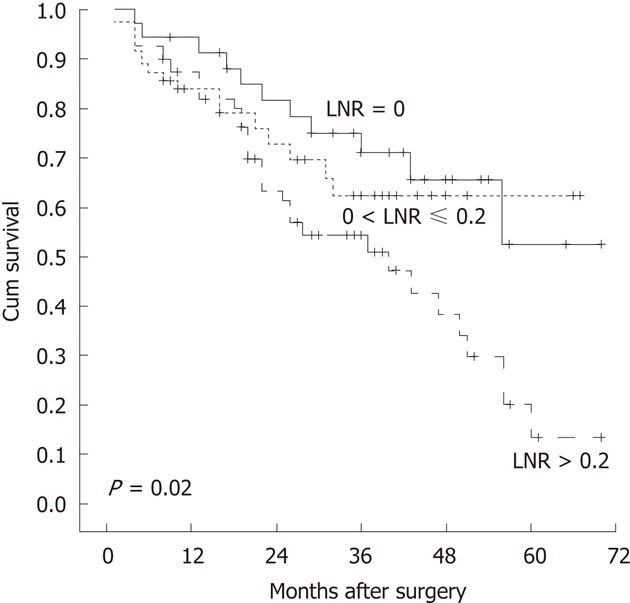
Kaplan-Meier overall survival curves of patients stratified by various lymph node ratios. The difference in overall survival among lymph node ratio (LNR) groups was statistically significant.
Significance of the lymph node ratio on overall survival
Among 3 different lymph node ratio groups, the lymph node ratios were significantly associated with the worse overall survival (Tables 2 and 3). The overall survival status was better illustrated on a Kaplan-Meier plot among groups with lymph node ratios > 0.2, compared to that with the ratio ≤ 0.2 (Figure 1). We found that the relative risk for poor overall survival was 37-fold when the lymph node ratio was over 0.4 and 75-fold when over 0.5, compared with the ratio at 0.
Independent factors predicting overall survival retained at multivariate analysis
By multivariate analysis, over 16 positive lymph nodes per case, lymph node ratio > 0.2, and the overall pathologic stage were found to be independent factors for predicting worse surgical overall survival (Table 3).
DISCUSSION
In this study, the factors predicting overall surgical survival in Chinese patients with PGCE are similar to those of gastric cancers but different from those of GEJ cancers reported in patients from Western countries[15-18]. We show that in Chinese patients, type I GEJ carcinomas remain vanishingly rare and PGCE tumors are mostly as type II and some as type III GEJ cancers. The factors predicting surgical overall survival are comparable to those reported in Japan[6] and China Taiwan[5]. Importantly, nodal burden in PGCE correlates highly with post-operative overall survival, as seen in gastric cancer. Nodal metastasis in the celiac axis region is a significant predictor of worse overall survival. Finally, the independent risk factors for worse overall survival in our patients include tumor metastasis in more than 16 nodes, the lymph node ratio > 0.2, distant metastasis, and overall pathologic stage; in contrast, the prognostic factors, such as > 70 years, BE, the male gender, tumor histology type, the surgical resection method, and even positive resection margin, etc., are not significant for predicting overall survival in Chinese patients with PGCE, which are predictive in type I GEJ carcinomas in Western patients[15,19], probably because of the rarity of the type I GEJ carcinomas in Chinse patients. Our results would impact upon surgical management of Chinese patients with PGCE, if confirmed in a larger prospective trial(s).
Accurate GEJ cancer staging is difficult. The AJCC 7 staging system requires the use of the esophageal scheme for pathologic staging of this group of cancers, regardless of the location of tumor epicenters. This new mandate is controversial. In a recent study, we showed that PGCE staged with the gastric cancer staging rules was better stratified, especially for the pN and pIII stages, compared to the use of the esophageal scheme that showed an erroneously better overall survival in the patients staged at pIIIA than those at pIA and pIIB[20]. The results shown in this study further substantiate the above conclusion and lent support to the contention that the current AJCC 7 cancer staging system for GEJ cancer needs to be modified when applied to Chinese patients with PGCE.
Lymph node metastasis in gastric cancer has been shown to be more significant in predicting overall survival than tumor invasion depth.This concept was confirmed in this study. For instance, we found no significant differences in overall survival among patients with different pT stages by either univariate or multivariate analysis. In contrast, the number of positive lymph nodes dictated patient overall survival[16]. Furthermore, the total number of nodes retrieved (> 15) had a significant overall survival predictive value, as suggested by others[17]. This may have resulted in a more precise evaluation of positive nodes and thus more accurate pN staging. In the current series, the overall survival was significantly worse in the patients with considerable nodal burden such as more than 7 positive nodes and higher lymph node ratios. Apparently, a rich lymphatic network in the GEJ region, and/or protein lytic enzymes secreted by neoplastic cells, may facilitate the lymphatic dissemination of neoplastic cells to intra-abdominal nodes[16,21]. It was reported that even for pT1b GEJ cancers, 30% of cases with positive nodes had a 5-year overall survival rate of only 33%[18]. Moreover, patients with primary tumors in the middle to lower esophagus and the proximal stomach were found to have nodal diseases within the abdomen at rates of as high as 45% to 93%[22,23]. Taken together, our data emphasize the important overall survival predictive value for a thorough abdominal nodal dissection in Chinese patients with PGCE[21].
The significance of the ratio between involved and retrieved lymph nodes for overall survival prediction has not been described in patients with PGCE. Our results add into a growing body of evidence for the value of a lymph node ratio in predicting overall survival of patients with PGCE. In the late 1990s, Siewert et al[24] published their findings of prognostic significance of the lymph node ratio in gastric cancer in a large German cohort of 1654 cases. Their conclusion was repeatedly confirmed by the studies in gastric cancer patients in Japan[25,26], China Taiwan[5], mainland China[27,28], South Korea[29], Spain[30] and Italy[31]. The advantage of the use of this parameter in patients with PGCE lies upon its ease to use, irrespective of the surgical methods used by different surgeons and various types of resection specimens from different patients[24]. In reality, the number of retrieved lymph nodes is largely influenced by the extent of lymph node dissection by different surgeons, whose nodal dissection skills vary[32,33]. It was reported that patients with a lymph node ratio smaller than 0.2 had a better overall survival rate[33,34], which is also our experience. As the lymph node ratio increases, so is the increased relative risk of poor overall survival. Our data show that in patients with PGCE, the lymph node ratio could be used clinically as a powerful overall survival predictor.
The significance of metastatic nodal disease in the celiac axis region in patients with GEJ cancers for overall survival prediction remains obscure due to limited studies in the literature[18,21]. When nodal metastasis is discovered in this region, some studies classify it as a pM1a disease of distal esophageal or GEJ cancers[14], according to the 6th AJCC cancer staging system. The overall survival predictive value of positive celiac lymph nodes in proximal gastric cancer stays controversial[18,35-37]. It was reported that the patients with undetected celiac nodal disease at the time of surgical resection were subsequently found to have celiac nodal involvement with overall survival similar to that of patients with stage III disease[21]. In our previous[28] and current studies on PGCE, nodal disease in the celiac axis region was also an important overall survival predictor by univariate analysis, which, however, did not reach a statistically significant level by multivariate analysis, probably due to the small sample size. Nevertheless, it appears that the site of nodal disease may be as important as the number of nodal metastasis in predicting overall survival for patients with PGCE.
The major limitations of this study are several. First, the sample size of the current cohort was relatively small and most cancer cases were advanced and staged at pIII. There were only a few cases staged at pI or pIV, which might have contributed to the lack of significance for the tumor pT stage in overall survival prediction. Second, the patient follow-up was carried out by telephone interview only. This might have invited inaccurate and biased results. At present, an accurate electronic patient medical record system has not been established in China and the government death record of citizens is not available to the public. Therefore, telephone interview has been the primary tool to collect the overall survival information. Finally, because of the retrospective nature of the study, the methods for surgical resection, lymph node retrieval, and specimen dissection were not standardized, which might have caused inconsistent nodal retrieval results. However, in over 75% of cases in this study, the number of lymph nodes retrieved per case was over 21. Therefore, the overall data quality should be reasonably solid and reliable.
In conclusions, PGCE, like gastric cancer, has similar overall survival after resection in patients with no or minimal nodal burden in this cohort of consecutively treated Chinese patients. However, the elderly patients over 70 years and those with considerable nodal diseases including celiac nodal metastasis, distant metastasis, and advanced summary pathologic stage fare worse in overall survival after resection. Application of the AJCC 7 esophageal staging scheme to PGCE may be less accurate in predicting overall survival than applying the gastric staging scheme[20]. Because of the small sample size and a single institution experience, further larger, prospective studies are required to validate our findings in the Chinese patient population.
COMMENTS
Background
In China, almost all gastroesophageal junction (GEJ) carcinomas arise in the proximal stomach with a stable or slightly increased incidence in recent years. In our most recent study comparing clinicopathologic features of GEJ cancer between Chinese patients treated in Nanjing, China, and American patients treated in Boston, the United States, the authors showed that GEJ cancers in Chinese patients were unlike those seen in American patients. However, the studies on factors predicting post-operative overall survival in Chinese patients with proximal gastric carcinoma involving the esophagus (PGCE) are scarce.
Research frontiers
The purpose of the present study was to investigate clinicopathologic features that may predict overall survival after surgical resection in Chinese patients with PGCE who were treated at a single high-volume tertiary medical center in Nanjing, China.
Innovations and breakthroughs
In this study, the factors predicting overall surgical survival in Chinese patients with PGCE are similar to those of gastric cancers but different from those of GEJ cancers reported in patients from Western countries. The authors show that in Chinese patients, type I GEJ carcinomas remain vanishingly rare and PGCE tumors are mostly as Siewert type II and some as type III GEJ cancers. The factors predicting surgical overall survival are comparable to those reported in Japan and Taiwan. Importantly, nodal burden in PGCE correlates highly with post-operative overall survival, as seen in gastric cancer. Nodal metastasis in the celiac axis region is a significant predictor of worse overall survival. Finally, the independent risk factors for worse overall survival in our patients include tumor metastasis in more than 16 nodes, the lymph node ratio > 0.2, distant metastasis and overall pathologic stage; in contrast, the prognostic factors, such as > 70 years, Barrett's esophagus, the male gender, tumor histology type, the surgical resection method, and even positive resection margin, etc., are not significant for predicting overall survival in Chinese patients with PGCE, which are predictive in Siewert type I GEJ carcinomas in Western patients, probably because of the rarity of the Siewert type I GEJ carcinomas in Chinse patients.
Applications
Their results would impact upon surgical management of Chinese patients with PGCE, if confirmed in a larger prospective trial(s). The authors suggest that the GEJ cancer in Chinese patients be treated as gastric cancer.
Peer review
This study was designed as the next step to examine the clinicopathologic features that may predict survival after surgical resection of proximal gastric carcinoma, keeping in mind that this tumor type is rare in Chinese patients. The manuscript is well written and methodology is accurately described. By multivariate analysis, > 16 positive nodes, lymph node ratio > 0.2, distant metastasis and summary pathologic stage were found to be independent predictors for poor survival. The authors stress that the lymph node ratio could be used as a reliable survival predictor, as this parameter is not influenced by the surgical methods and the number of retrieved lymph nodes.
Footnotes
Supported by Key Grants from the Science and Technology Development Project of the Nanjing City, No. ZKX05013 and ZKX07011; A Special Grant from the Nanjing Drum Tower Hospital, Nanjing, China
Peer reviewers: Mark de Ridder, MD, PhD, Dienst Radiotherapie, UZ Brussel, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium; Liza N van Steenbergen, PhD, Comprehensive Cancer Centre South, Eindhoven Cancer Registry, 5600 AE Eindhoven, The Netherlands
S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ. Carcinoma of the gastroesophageal junction: classification, pathology and extent of resection. Dis Esophagus. 1986;9:173–182. [Google Scholar]
- 3.de Manzoni G, Pedrazzani C, Pasini F, Di Leo A, Durante E, Castaldini G, Cordiano C. Results of surgical treatment of adenocarcinoma of the gastric cardia. Ann Thorac Surg. 2002;73:1035–1040. doi: 10.1016/s0003-4975(01)03571-8. [DOI] [PubMed] [Google Scholar]
- 4.Mariette C, Castel B, Toursel H, Fabre S, Balon JM, Triboulet JP. Surgical management of and long-term survival after adenocarcinoma of the cardia. Br J Surg. 2002;89:1156–1163. doi: 10.1046/j.1365-2168.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 5.Fang WL, Wu CW, Chen JH, Lo SS, Hsieh MC, Shen KH, Hsu WH, Li AF, Lui WY. Esophagogastric junction adenocarcinoma according to Siewert classification in Taiwan. Ann Surg Oncol. 2009;16:3237–3244. doi: 10.1245/s10434-009-0636-9. [DOI] [PubMed] [Google Scholar]
- 6.Ichikura T, Chochi K, Sugasawa H, Mochizuki H. Proposal for a new definition of true cardia carcinoma. J Surg Oncol. 2007;95:561–566. doi: 10.1002/jso.20727. [DOI] [PubMed] [Google Scholar]
- 7.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 8.Huang Q, Zhang LH. The histopathologic spectrum of carcinomas involving the gastroesophageal junction in the Chinese. Int J Surg Pathol. 2007;15:38–52. doi: 10.1177/1066896906295998. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LH, Huang Q. Changes in Incidences of Gastric Cardiac-gastroesophageal Junctional and Sub-cardiac Carcinomas in Nanjing of China: 20-year Retrospective Study from a Single Tertiary Medical Center. N Am J Med Sci. 2009;2:35–38. [Google Scholar]
- 10.Huang Q, Fan XS, Feng AN, Qiang Z, Yu CG, Mashimo H, Lauwers GY. Distal Esophageal Adenocarcinoma Remains Rare Among Chinese Population: A Clinicopathologic Study of 211 Resection Cases. Gastroenterology. 2011;140:S670. [Google Scholar]
- 11.Huang Q, Fan X, Agoston AT, Feng A, Yu H, Lauwers G, Zhang L, Odze RD. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology. 2011;59:188–197. doi: 10.1111/j.1365-2559.2011.03924.x. [DOI] [PubMed] [Google Scholar]
- 12.Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg. 2006;95:260–269. doi: 10.1177/145749690609500409. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. Esophagus and Esophagogastric Junction. In: AJCC Cancer Staging Manual., editor. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 14.Rizk NP, Venkatraman E, Bains MS, Park B, Flores R, Tang L, Ilson DH, Minsky BD, Rusch VW. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 15.Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–367; discussion 360-367. doi: 10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagarde SM, ten Kate FJ, Reitsma JB, Busch OR, van Lanschot JJ. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2006;24:4347–4355. doi: 10.1200/JCO.2005.04.9445. [DOI] [PubMed] [Google Scholar]
- 17.Lagarde SM, Reitsma JB, de Castro SM, Ten Kate FJ, Busch OR, van Lanschot JJ. Prognostic nomogram for patients undergoing oesophagectomy for adenocarcinoma of the oesophagus or gastro-oesophageal junction. Br J Surg. 2007;94:1361–1368. doi: 10.1002/bjs.5832. [DOI] [PubMed] [Google Scholar]
- 18.Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 19.Whitson BA, Groth SS, Li Z, Kratzke RA, Maddaus MA. Survival of patients with distal esophageal and gastric cardia tumors: a population-based analysis of gastroesophageal junction carcinomas. J Thorac Cardiovasc Surg. 2010;139:43–48. doi: 10.1016/j.jtcvs.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Huang Q, Shi J, Feng A, Fan X, Zhang L, Mashimo H, Cohen D, Lauwers G. Gastric cardiac carcinomas involving the esophagus are more adequately staged as gastric cancers by the 7th edition of the American Joint Commission on Cancer Staging System. Mod Pathol. 2011;24:138–146. doi: 10.1038/modpathol.2010.183. [DOI] [PubMed] [Google Scholar]
- 21.Schomas DA, Quevedo JF, Donahue JM, Nichols FC, Romero Y, Miller RC. The prognostic importance of pathologically involved celiac node metastases in node-positive patients with carcinoma of the distal esophagus or gastroesophageal junction: a surgical series from the Mayo Clinic. Dis Esophagus. 2010;23:232–239. doi: 10.1111/j.1442-2050.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Sons HU, Borchard F. Cancer of the distal esophagus and cardia. Incidence, tumorous infiltration, and metastatic spread. Ann Surg. 1986;203:188–195. doi: 10.1097/00000658-198602000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama H, Tsurumaru M, Kawamura T, Ono Y. Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg. 1981;194:438–446. doi: 10.1097/00000658-198110000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 26.Saito H, Fukumoto Y, Osaki T, Yamada Y, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol. 2008;97:132–135. doi: 10.1002/jso.20929. [DOI] [PubMed] [Google Scholar]
- 27.Yu JW, Wu JG, Zheng LH, Zhang B, Ni XC, Li XQ, Jiang BJ. Influencing factors and clinical significance of the metastatic lymph nodes ratio in gastric adenocarcinoma. J Exp Clin Cancer Res. 2009;28:55. doi: 10.1186/1756-9966-28-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Li YF, Sun XW, Chen YB, Li W, Xu DZ, Guan XX, Huang CY, Zhan YQ, Zhou ZW. Prognosis of 980 patients with gastric cancer after surgical resection. Chin J Cancer. 2010;29:923–930. doi: 10.5732/cjc.010.10290. [DOI] [PubMed] [Google Scholar]
- 29.Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323–329. doi: 10.1007/s00268-001-0227-9. [DOI] [PubMed] [Google Scholar]
- 30.Santiago JR, Osorio J, Gutierrez I, Perez N, Mufioz E, Veloso E, Marco C. Prognostic usefulness of lymph node ratio in understaged gastric cancer. Hepatogastroenterology. 2009;56:1557–1561. [PubMed] [Google Scholar]
- 31.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 32.Lerut T, Coosemans W, Decker G, De Leyn P, Moons J, Nafteux P, Van Raemdonck D. Extended surgery for cancer of the esophagus and gastroesophageal junction. J Surg Res. 2004;117:58–63. doi: 10.1016/j.jss.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett‘s cancer. World J Surg. 2003;27:1052–1057. doi: 10.1007/s00268-003-7060-2. [DOI] [PubMed] [Google Scholar]
- 34.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 35.Steup WH, De Leyn P, Deneffe G, Van Raemdonck D, Coosemans W, Lerut T. Tumors of the esophagogastric junction. Long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J Thorac Cardiovasc Surg. 1996;111:85–94; discussion 94-95. doi: 10.1016/S0022-5223(96)70404-X. [DOI] [PubMed] [Google Scholar]
- 36.Lerut T, Coosemans W, Decker G, De Leyn P, Ectors N, Fieuws S, Moons J, Nafteux P, Van Raemdonck D. Extracapsular lymph node involvement is a negative prognostic factor in T3 adenocarcinoma of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 2003;126:1121–1128. doi: 10.1016/s0022-5223(03)00941-3. [DOI] [PubMed] [Google Scholar]
- 37.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


