Abstract
Introduction
The aim of this prospective study was to evaluate the diagnostic accuracy of dual-source computed tomography coronary angiography (CTCA) compared to intravascular ultrasound (IVUS) for the detection of restenosis in patients who underwent coronary stenting for bifurcation left main (LM) stenosis.
Material and methods
Twenty-four patients underwent percutaneous intervention of the LM and were subsequently examined (median 9.2 months after procedure) using IVUS and CTCA for the detection of restenosis.
Results
Significant restenosis was detected according to IVUS examination in 6 patients (25%) and 8 segments (13%). Based on segment analysis, sensitivity, specificity, positive and negative predictive values of CTCA for the detection of restenosis were 89%, 68%, 32%, 97%, respectively. There was moderate to good correlation between the minimal luminal area (MLA), measured by CTCA and IVUS for LM, the left anterior descending artery (LAD) and the left circumflex artery (LCx) (r=0.64, p<0.01; r=0.49, p=0.03; r=0.76, p<0.01, respectively). A Bland-Altman analysis showed that the MLAs measured by CTCA were underestimated in all segments (mean difference 1.67 ±2.2 mm2 for LM; 2.0 ±2.0 mm2 for LAD; 1.79 ±1.79 mm2 for LCx). An ROC analysis of the MLAs derived by CTCA for detecting significant stenosis was performed. The area under the curve for all analysed segments was 0.73.
Conclusions
The present study demonstrates that in patients after LM bifurcation stenting CTCA performs well in the exclusion of in-segment restenosis. However, due to the low positive predictive value of CTCA, the finding of any restenosis should be confirmed by invasive examination.
Keywords: stent, left main stenosis, intervention, computed tomography
Introduction
Percutaneous coronary intervention (PCI) with stent implantation is an alternative to surgical revascularization in some patients with significant stenosis of the left main coronary artery (LM) [1–3]. Although there have been many improvements in the quality of stents and a higher rate of drug-eluting stent use, approximately 10% of patients still develop restenosis, which might be associated with clinically significant consequences [1, 3]. Interestingly, the risk of restenosis is mainly influenced by lesion location. Stenting of ostial and body LM stenosis was found to have a very low incidence of restenosis, which was 1–3% at the 1-year follow-up [4, 5]. On the other hand, stenting for LM bifurcation was associated with a six-fold higher risk of target vessel revascularization [5], and these results suggested the need for meticulous follow-up after PCI [1, 3, 6].
Recently, dual-source computed tomography angiography (CTCA) with improved temporal resolution has provided a new non-invasive tool for detecting in-stent restenosis [7]. However, the detection and quantification of in-stent restenosis is still much more difficult than in native coronary arteries, which is mainly due to the presence of metal artefacts interfering with the correct interpretation of luminal area [8–10].
The aim of this pilot study was to evaluate the diagnostic performance of CTCA for the detection of restenosis in patients after percutaneous treatment of an LM bifurcation lesion. Intravascular ultrasound (IVUS) was used as the gold standard.
Material and methods
Patients
This study was conducted prospectively; 24 patients with above 50% stenosis of the unprotected LM bifurcation treated by a single coronary stent implantation from February 2008 to December 2009 were enrolled. Patients with ST-elevation myocardial infarction and a need for the implantation of more than one stent were excluded. Intravascular ultrasound (IVUS) and CTCA follow-up examinations were recommended to all patients. Written informed consent was obtained from each patient and the local ethics committee approved all study protocols.
Intervention
Direct coronary stenting with the implantation of only one stent covering the LM and proximal parts of the left anterior descending artery (LAD) was the technique used in this study [6, 7, 11]. Pre-dilation of the lesion was performed if needed. Final kissing balloon post-dilation was used. Intravascular ultrasound was used at the operator's discretion. Twenty patients (83%) had stenosis of type 1-1-1 according to the Medina classification [12]. The following drug-eluting and bare-metal stents with a nominal diameter of 3.5–4.5 mm were used: Xience Prime Coronary Stent System (Abbott Vascular, Santa Clara, CA, USA), BioMatrix (Biosensors, Morges, Switzerland), and Coroflex® Blue (B. Braun, Melsungen, Germany). Two bare-metal stents and 22 drug-eluting stents were used. After PCI, aspirin, clopidogrel, statins and other optimal medical treatments were prescribed to all patients.
Computed tomography angiography examination
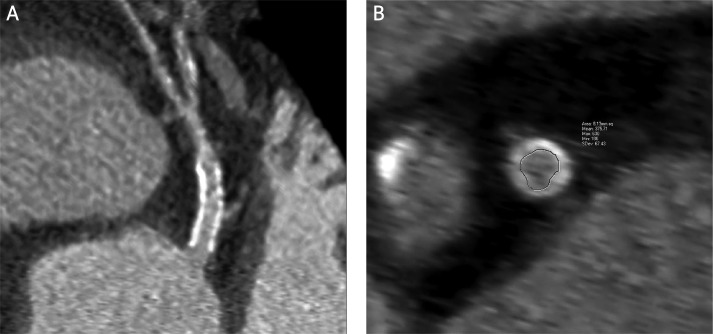
All CT examinations were performed on a dual-source CT scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany) with 70 to 100 ml of iodixanol (Iomeron, 400 mg/ml, Altana-Bracco, Germany) followed by 40 ml of saline solution [13]. Two doses of sublingual isosorbide dinitrate spray were used at the start of the examination. No β-blockers were given before examination. The examination focused on three coronary segments: the whole LM, the proximal part of the LAD and the left circumflex artery (LCx). The LAD and LCx were evaluated in 1-cm-long segments distal to the stent. Images were reconstructed with a slice thickness of 0.6 mm and reconstruction increment of 0.3 mm using a sharp convolution kernel (B46f). AqNET software (version 1.8.4.5., TeraRecon, USA) was used for the evaluation and measurements. Identification of the minimal lumen was made from longitudinal images of curved planar reconstructions and measurements of the minimal luminal area were performed manually from images perpendicular to the arterial axis (Figure 1). Data sets were analysed by a single, experienced radiologist blinded to the clinical data.
Figure 1.
Assessment of a coronary artery stent by CTCA. A– Longitudinal view, B– minimal luminal area measurement
Intravascular ultrasound examination
Coronary angiography was performed 1 day after CTCA. Several angiograms of the left coronary artery were acquired. Subsequently, IVUS was performed using the Volcano Endosonics System (Volcano Therapeutics Inc., CA, USA). After the intracoronary administration of 500 µg of isosorbide dinitrate, the ultrasound catheter (Eagle Eye Platinum, Volcano Therapeutics Inc., CA, USA) was advanced 10 mm beyond the stent to both the LAD and LCx, and was subsequently slowly withdrawn (0.5 mm/s) [14]. Images were recorded continuously and recorded on CDs. The IVUS data were analysed by an experienced technician and confirmed by an invasive cardiologist blinded to all clinical and CTCA data. Quantitative analysis was based on the finding showing the most severe narrowing in the LM, LAD and LCx. Location of significant lesions was also compared with CTCA assessment.
The minimal luminal area (MLA) was assessed by CTCA and IVUS. The IVUS examination was considered as the gold standard. Significant restenosis was defined as MLA < 6 mm2 in the LM and < 4 mm2 in the LAD or LCx [15]. Segments with severe restenosis, according to coronary angiography, did not have to be examined by IVUS and were considered significantly stenosed.
Statistical analysis
Continuous variables were expressed as mean±SD, and categorical variables as counts and percentages. Sensitivity, specificity, positive predictive value, negative predictive value, accuracy (with 95% confidence intervals) and the likelihood ratio of CTCA for the detection of restenosis were assessed against IVUS as the gold standard. The MLAs of the LM, LAD and LCx measured by CTCA and IVUS were correlated by means of a Bland-Altman analysis and Pearson's correlation coefficient. Receiver-operating-characteristic (ROC) analysis was used to determine the optimal cut-off value for detecting significant restenosis. The area under the ROC curve (AUC) provided a measure of the overall accuracy that was independent of the decision criteria by plotting true-positive rates against false-positive rates as the cut-off level of the model varied. The optimal cut-off value for detecting significant stenosis (based on MLA) was defined as the point with the highest sum of sensitivity and specificity. A p-value of below 0.05 was considered statistically significant. The statistical software Stata 9.2 (StataCorp LP, College Station, TX, USA) was used.
Results
Immediate results
A total of 24 patients safely underwent PCI with implantations of 24 stents with a nominal diameter of 3.5–4.5 mm (Table I). There were no significant complications of these interventions and residual stenosis was < 20% in all cases.
Table I.
Baseline and procedural characteristics (n=24)
| Variable | Value |
|---|---|
| Age [years] (range) | 66±11 (46-88) |
| Men [%] | 58 |
| BMI [kg/m2] | 28±5 |
| Previous myocardial infarction [%] | 38 |
| Current smoker [%] | 21 |
| Hypertension [%] | 71 |
| Total plasma cholesterol [mmol/l; mg/dl] | 4.4 ±1.4; 170.1 ±54.1 |
| LDL cholesterol [mmol/l; mg/dl] | 2.3 ±1.0; 88.9 ±38.7 |
| HDL cholesterol [mmol/l; mg/dl] | 1.2 ±0.4; 46.4 ±15.5 |
| Plasma triglyceride [mmol/l; mg/dl] | 1.8 ±1.6; 159.4 ±141.7 |
| Diabetes mellitus [%] | 42 |
| β-Blockers [%] | 71 |
| Calcium channel blockers [%] | 25 |
| Angiotensin-converting inhibitors [%] | 92 |
| Clopidogrel [%] | 88 |
Follow-up
Follow-up examinations were performed on all patients. The median length between PCI and the follow-up examination was 9.2 months (3–12 months). No patients died during follow-up. Twenty-four follow-up examinations occurred safely and without any complications. One examination was performed on a patient with atrial fibrillation. All segments were classified as evaluable by CTCA. However, three LAD segments (13%) were not evaluable by IVUS because of a failure of the smooth IVUS catheter passing along the coronary wire into the LAD. In 7 cases (29%), we were not able to examine the LCx by IVUS because of a failure to smoothly pass the IVUS catheter through the stent into the LCx. According to coronary angiography there were two borderline ostial LCx stenoses present in these cases. Hence, 62 coronary segments (24 in LM, 21 in LAD and 17 in LCx) were evaluable for both CTCA and IVUS. Significant restenosis, according to IVUS examination, was detected in 6 patients (25%) and 8 segments (13%); these patients were subsequently treated by re-PCI. The good capability of CTCA in correctly identifying patients with significant restenosis was counter-balanced by a false-positive rate of 27% (17/62). The sensitivity, specificity, predictive values, accuracy and likelihood ratio of CTCA for detecting significant restenosis are summarized in Table II.
Table II.
Diagnostic performance of CTCA in detecting significant stenosis
| Parameter | LM (n=24) | LAD (n=21) | LCx (n=17) | All segments (n=62) | All patients (n=24) |
|---|---|---|---|---|---|
| Sensitivity (95% CI) | 100% (29.2–100) | 100% (15.8–100) | 75% (19.4–99.4) | 89% (51.8–99.7) | 86% (42.1–99.6) |
| Specificity (95% CI) | 74% (52.8–91.8) | 68% (43.5–87.4) | 54% (25.1–80.8) | 68% (53.7–80.1) | 41% (18.4–67.1) |
| Positive predictive value (95% CI) | 38% (8.5–75.5) | 25% (3.2–65.1) | 33% (7.5–70.1) | 32% (14.9–53.5) | 38% (15.2–64.6) |
| Negative predictive value (95% CI) | 100% (79.4–100) | 100% (75.3–100) | 88% (47.4–99.7) | 97% (85.8–99.9) | 88% (47.4–99.7) |
| Accuracy (95% CI) | 79% (62.7–95.3) | 71% (50.5–91.5) | 59% (35.6–82.4) | 71% (59.7–82.3) | 54% (34.1–73.9) |
| Likelihood ratio | 4.2 | 3.2 | 1.6 | 2.8 | 1.5 |
| Cut-off value [mm2] | <6.19 | <4.78 | <4.97 | <5.29 | |
| SE 46% | SE 67% | SE 75% | SE 64% | ||
| SP 88% | SP 90% | SP 65% | SP 77% | ||
| Area under curve | 0.6788 | 0.8204 | 0.7243 | 0.7293 |
SE – sensitivity, SP – specificity
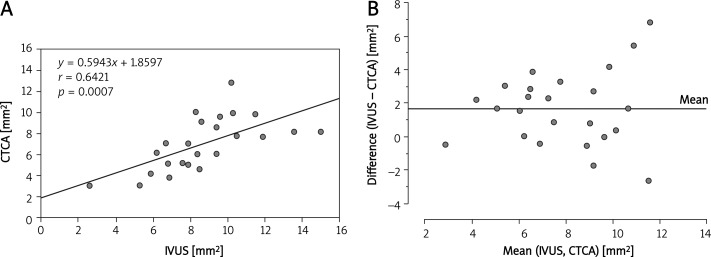
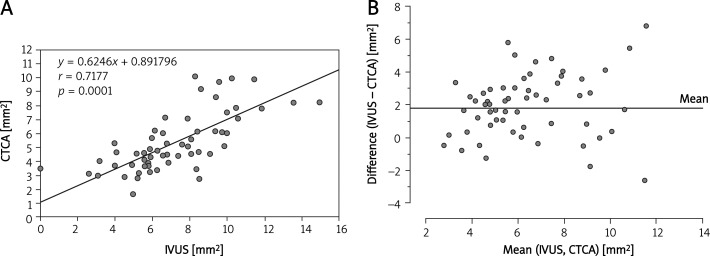
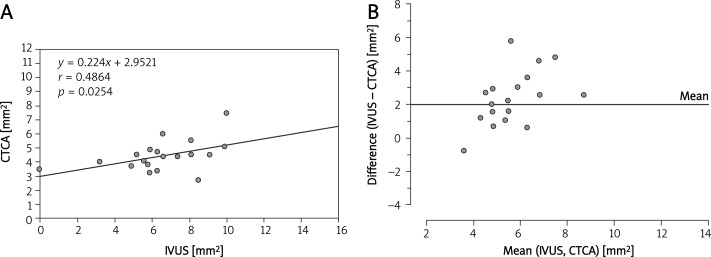
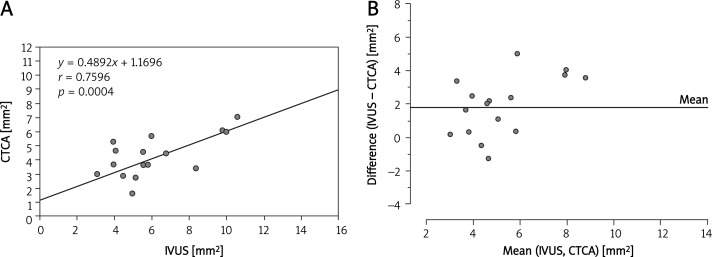
Correlations between the MLAs of LM, LAD and LCx measured by IVUS and CTCA are summarized in Figures 2–5. A Bland-Altman analysis showed that the MLAs measured by CTCA were underestimated (mean difference 1.67 ±2.2 mm2 for LM; 2.0 ±2.0 mm2 for LAD; 1.79 ±1.79 mm2 for LCx).
Figure 2.
A– Correlation between IVUS and CTCA measurement of minimal luminal area in the stented left main coronary artery. B– Bland-Altman analysis of differences between the minimal luminal area measured by CTCA and IVUS
Figure 5.
A– Correlation between IVUS and CTCA measurement of minimal luminal area in all measured segments. B– Bland-Altman analysis of differences between the minimal luminal area measured by CTCA and IVUS
Figure 3.
A– Correlation between IVUS and CTCA measurement of minimal luminal area in the stented left anterior descending artery. B– Bland-Altman analysis of differences between the minimal luminal area measured by CTCA and IVUS
Figure 4.
A– Correlation between IVUS and CTCA measurement of minimal luminal area in the left circumflex artery. B– Bland-Altman analysis of differences between the minimal luminal area measured by CTCA and IVUS
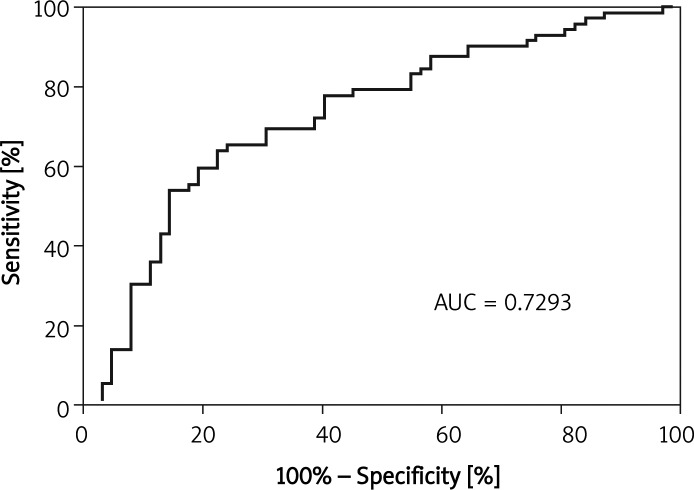
An ROC analysis of the MLAs derived by CTCA for detecting significant stenosis was performed. The AUC for all analysed segments was 0.73 (Figure 6). The other AUCs and the best cut-off values with their sensitivity and specificity are summarized in Table II.
Figure 6.
Diagnostic value of CTCA measurement for assessment significant restenosis in all segments expressed by ROC curve
Discussion
The high diagnostic performance of CTCA in the detection of significant coronary artery disease is well established [16]. However, the potential role of CTCA in the detection of LM bifurcation restenosis is unclear. To the best of our knowledge, this is the only study to evaluate the diagnostic performance of CTCA in patients after percutaneous coronary intervention (PCI) of an LM bifurcation lesion with the use of only one stent. The results of this study demonstrate that in patients who underwent examinations 9 months (3–12 months) after PCI the occurrence of in-segment restenosis was present in 25% according to IVUS. CTCA had a high negative predictive value (97%) and a low positive predictive value (32%) for the detection of significant restenosis. It is likely that patients with no or minimal restenosis on CTCA do not need further investigation. Therefore, from a clinical point of view, these results suggest that CTCA might be a suitable gatekeeper to conventional angiography or IVUS in patients after LM bifurcation stenting.
Bifurcation lesions provide a challenge for both establishing a correct diagnosis and its management. In particular, errors in patients with LM in-segment restenosis may have significant clinical consequences. Unfortunately, eccentric coronary plaques, coronary calcification or the overlap of coronary branches negatively influence accurate evaluations of LM morphology on coronary angiography [17]. Additionally, coronary angiography of LM is limited to the evaluation of true luminal size because of the lack of a reference segment for comparison, which can result in underestimations of the severity of stenosis [3]. Therefore, it was necessary to compare CTCA results with IVUS, which is considered as the gold standard in the evaluation of coronary anatomy.
The recently used single or dual-source CT scanners have a high potential for the exclusion of significant coronary artery disease [16]. However, the more accurate stent visualization and restenosis quantification allowed by increased spatial and temporal resolution is also very promising for patients with a potentially higher rate of restenosis. Although it is not known whether it is necessary to routinely follow up all patients after bifurcation stenting of the LM, it is believed that current CT scanners may offer an elegant and non-invasive method for the exclusion of significant restenosis [1, 16]. In patients with a borderline LM stenosis in a study published by Dragu et al., a high degree of correlation was found between multidetector CT coronary angiography and IVUS regarding the assessment of MLA (r=0.93, p<0.01) [16], but in the stented segments the coronary lumen visualization was much worse [16]. This fact was also confirmed by the present study as we demonstrated a good correlation between MLA measurements in the non-stented LCx (r=0.76, p<0.01) and only moderate correlations in the stented LM and LAD (r=0.64, p<0.01 and r=0.49, p=0.03).
All CT examinations were performed with 70 to 100 ml of iodixanol (Iomeron, 400 mg/ml, Altana-Bracco, Germany) followed by 40 ml of saline solution. Similarly, all coronary angiographies were performed with the same contrast medium (50–100 ml). Therefore, there was no difference in the risk of contrast-induced nephropathy between the two methods.
The accurate CTCA quantification of restenosis is dependent not only on a higher spatial and temporal resolution, but also on the solution of further technical issues including the presence of stent-associated high-density artefacts (blooming artefacts), coronary calcifications, the luminal diameter of the coronary stent used and the heart rate of the patient [8–10, 16–19]. All of these factors cause a lowering of the measured MLA, which was also confirmed by the present study, and the mean difference between MLAs measured by CTCA and IVUS was 1.8 mm2. Therefore, the systematic underestimation of MLA by CTCA in patients after PCI should be taken into account in clinical practice.
Maintz et al. [20] demonstrated that stent lumen visibility largely varies depending on the stent type. Magnesium was a far more favourable stent material with regard to the quality of imaging when compared with the more common currently used stent materials (steel, cobalt-chromium and tantalum). The magnesium stent exhibited a lumen visibility of 90%, whereas the majority of the other stents exhibited a lumen visibility of 50–59% [20]. This fact may play a significant role in the future because biodegradable magnesium alloy coronary stents are a promising solution for long-term adverse events caused by interactions between the coronary artery wall and permanent stent platforms of drug-eluting stents [21]. The stents used in this study were made of steel and a cobalt-chromium alloy.
This single-centre study must be understood in the context of its limitations. First, PCI for LM bifurcation lesions is technically demanding and the selection of an appropriate stenting technique is dependent on plaque morphology and distribution. The optimal treatment for LM bifurcation lesions is not known, but the current consensus prefers a single-stent strategy that covers the lesion from the LM to LAD with optional treatment of the LCx. This simple stenting approach to bifurcation lesions might be associated with a lower rate of restenosis [22] and this technique was used in all patients included in the present study. Therefore, these results must be interpreted cautiously and any generalizations should also be made with caution. Second, since the stents were implanted from the LM to the LAD in 7 cases (29%), we were not able to pass the IVUS catheter through the stent into the LCx. Therefore, we are aware that the real rate of restenosis in the LCx might be higher than was reported. Similarly, in 3 cases (13%), the proximal segment of the LAD was not evaluable for the IVUS catheter. Third, another limitation of this study was the small number of patients included. Therefore, it was impossible to perform a reasonable subgroup analysis.
In conclusion, the present pilot study demonstrates that, in patients after LM bifurcation stenting, CTCA performs well for the exclusion of in-segment restenosis. Therefore, patients with no or minimal restenosis on CTCA would not need further invasive examinations. However, due to the low positive predictive value of CTCA, the finding of any restenosis should be confirmed by invasive examination. More prospective, multi-centre, and long-term data are needed to determine a definite role of CTCA in detection and quantification of in-stent LM restenosis.
Acknowledgments
This study was supported by a grant from the Ministry of Health of the Czech Republic No. 00064203. The authors thank Eva Hansvenclova for her assistance in the data collection and study analysis.
References
- 1.Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–55. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 2.Park DW, Kim YH, Yun SC, et al. Long-term outcomes after stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 10-year results of bare-metal stents and 5-year results of drug-eluting stents from the ASAN-MAIN (ASAN Medical Center-Left MAIN Revascularization) Registry. J Am Coll Cardiol. 2010;56:1366–75. doi: 10.1016/j.jacc.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 3.Park SJ, Kim YH. Percutaneous coronary intervention for unprotected left main coronary artery stenosis. World J Cardiol. 2010;2:78–88. doi: 10.4330/wjc.v2.i4.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chieffo A, Park SJ, Valgimigli M, et al. Favorable long-term outcome after drug-eluting stent implantation in nonbifurcation lesions that involve unprotected left main coronary artery: a multicenter registry. Circulation. 2007;116:158–62. doi: 10.1161/CIRCULATIONAHA.107.692178. [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M, Malagutti P, Rodriguez-Granillo GA, et al. Distal left main coronary disease is a major predictor of outcome in patients undergoing percutaneous intervention in the drug-eluting stent era: an integrated clinical and angiographic analysis based on the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) and Taxus-Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registries. J Am Coll Cardiol. 2006;47:1530–7. doi: 10.1016/j.jacc.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Biondi-Zoccai GG, Giraudi E, Moretti C, et al. Impact of routine angiographic follow-up after percutaneous coronary drug-eluting stenting for unprotected left main disease: the Turin Registry. Clin Res Cardiol. 2010;99:235–42. doi: 10.1007/s00392-009-0112-3. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese F, Weustink AC, Van Mieghem C, et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart. 2008;94:848–54. doi: 10.1136/hrt.2007.126474. [DOI] [PubMed] [Google Scholar]
- 8.Carrabba N, Bamoshmoosh M, Carusi LM, et al. Usefulness of 64-slice multidetector computed tomography for detecting drug eluting in-stent restenosis. Am J Cardiol. 2007;100:1754–8. doi: 10.1016/j.amjcard.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Andreini D, Pontone G, Bartorelli AL, et al. Comparison of feasibility and diagnostic accuracy of 64-slice multidetector computed tomographic coronary angiography versus invasive coronary angiography versus intravascular ultrasound for evaluation of in-stent restenosis. Am J Cardiol. 2009;103:1349–58. doi: 10.1016/j.amjcard.2009.01.343. [DOI] [PubMed] [Google Scholar]
- 10.Gilard M, Cornily JC, Rioufol G, et al. Noninvasive assessment of left main coronary stent patency with 16-slice computed tomography. Am J Cardiol. 2005;95:110–12. doi: 10.1016/j.amjcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 11.Veselka J, Mates M, Tesař D, et al. Direct stenting without predilatation: a new approach to coronary intervention. Coron Artery Dis. 2000;11:503–7. doi: 10.1097/00019501-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Medina A, Suárez de Lezo J, Pan M. A new classification of coronary bifurcation lesions. Rev Esp Cardiol. 2006;59:183. [PubMed] [Google Scholar]
- 13.Veselka J, Zemánek D, Duchoňová R, et al. Coronary angiography and dual-source computed tomography are complementary methods in diagnosis of significant stenosis of the right coronary artery originating from the left aortic sinus. Cent Eur J Med. 2008;3:111–4. [Google Scholar]
- 14.Cervinka P, Costa MA, Angiolillo DJ, et al. Head-to-head comparison between sirolimus-eluting and paclitaxel-eluting stents in patients with complex coronary artery disease: an intravascular ultrasound study”. Catheter Cardiovasc Interv. 2006;67:846–51. doi: 10.1002/ccd.20755. [DOI] [PubMed] [Google Scholar]
- 15.Shao JH, Aronow WS, Ravipati G, et al. Prevalence of minimal luminal cross sectional area of coronary arteries < 4 mm2 determined by intravascular ultrasound in patients with coronary artery calcium scores of 0-100, 100-200, 200-300, and > 400 determined by cardiac computed tomography. Arch Med Sci. 2009;5:172–4. [Google Scholar]
- 16.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Oviedo C, Maehara A, Mintz GS, et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–12. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]
- 18.Dragu R, Kerner A, Gruberg L, et al. Angiographically uncertain left main coronary artery narrowings: correlation with multidetector computed tomography and intravascular ultrasound. Int J Cardiovasc Imag. 2008;24:557–63. doi: 10.1007/s10554-007-9290-0. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Almutairi AM. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: a meta-analysis. Eur J Radiol. 2010;73:266–73. doi: 10.1016/j.ejrad.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Maintz D, Burg MC, Seifarth H, et al. Update on multidetector coronary CT angiography of coronary stents: in vitro evaluation of 29 different stent types with dual-source CT. Eur Radiol. 2009;19:42–9. doi: 10.1007/s00330-008-1132-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, Petrini L, Gastaldi D, et al. Finite element shape optimization for biodegradable magnesium alloy stents. Ann Biomed Eng. 2010;38:2829–40. doi: 10.1007/s10439-010-0057-8. [DOI] [PubMed] [Google Scholar]
- 22.Colombo A, Moses JW, Morice MC, et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation. 2004;109:1244–49. doi: 10.1161/01.CIR.0000118474.71662.E3. [DOI] [PubMed] [Google Scholar]