Abstract
Introduction
Urotensin II (UII) is a vasoactive peptide secreted by endothelial cells. Increased plasma UII concentration was observed in patients with heart failure, liver cirrhosis, diabetic nephropathy and renal insufficiency. In patients with myocardial infarction both increased and decreased plasma UII concentrations were demonstrated. The aim of this study was to analyze whether plasma UII concentration reflects the severity of acute coronary syndrome (ACS).
Material and methods
One hundred and forty-nine consecutive patients with ACS, without age limit, were enrolled in the study. In all patients plasma concentration of creatinine, creatine kinase isoenzyme MB (CK-MB), troponin C, N-terminal prohormone of brain natriuretic peptide (NT-pro BNP), and UII were assessed, and echocardiography was performed in order to assess the degree of left ventricular hypertrophy, ejection fraction (EF) and mass (LVM).
Results
In patients with the highest risk (TIMI 5-7) plasma UII concentration was significantly lower than in those with low risk (TIMI 1-2): 2.61±1.47 ng/ml vs. 3.60±2.20 ng/ml. Significantly lower plasma UII concentration was found in patients with increased concentration of troponin C (2.60±1.52 ng/ml vs. 3.41±2.09 ng/ml). There was a significant negative correlation between plasma UII concentration and TIMI score or concentration of troponin C, but not CK-MB. Borderline correlation between plasma UII and ejection fraction (R = 0.157; p=0.063) or NT-proBNP (R = − 0.156; p=0.058) was found.
Conclusions
Decreased plasma urotensin II concentration in patients with ACS could be associated with more severe injury of myocardium.
Keywords: urotensin II, acute coronary syndrome
Introduction
Urotensin II (UII) is an 11-amino acid peptide discovered as a neurosecretory product in teleost fish, with a sequence homologous to somatostatin [1]. Its main source in humans and its clinical significance are still uncertain. Urotensin II is released by endothelium, across the heart, kidney, head and neck, liver, lower limbs and pulmonary circulation [2]. The response to UII administration in vivo and in vitro is differentiated in vascular beds [3]. Urotensin II is considered to be a more potent vasoconstrictor and cardiostimulant than endothelin-1, but in some conditions it becomes a vasodilator [4–7]. Furthermore, UII has an inotropic effect in humans and rats [8]. Increased plasma UII concentration was observed in patients with diabetes mellitus and end-stage kidney disease [9–11].
Acute myocardial infarction is the leading cause of morbidity and mortality. As vasoconstriction is involved in unfavorable myocardial and vascular remodeling, changes of UII secretion after myocardial infarction may deteriorate or counterbalance its clinical course. Khan et al. demonstrated an increase in UII concentration in patients with acute myocardial infarction, but simultaneously its lower values in patients with adverse clinical outcome [12]. Opposite results were described by Joyal et al. [13]. They compared plasma UII concentration in patients with acute coronary syndrome (ACS), stable angina and in healthy controls. Lower plasma UII levels in patients with ACS were in negative correlation with systemic blood pressure. Moreover, in patients with heart failure, increased UII expression in human cardiomyocytes and vascular smooth muscle cells was demonstrated [14].
As the results of the performed studies are ambiguous, we decided to investigate whether plasma UII concentration reflects the severity of acute coronary syndrome.
Material and methods
One hundred and forty-nine consecutive patients without age limit (78 females, 71 males) admitted to the Cardiology Department with acute coronary syndrome were enrolled in the study. The study complied with the Declaration of Helsinki and was approved by the local ethics committee and written informed consent was obtained from patients. Acute coronary syndromes were classified based on TIMI scores [15]. Detailed clinical anamnesis concerning cardiovascular and kidney disease was obtained on admission to the Department of Cardiology (Table I). In all patients serum concentration of glucose, creatinine, creatine kinase isoenzyme MB (CK-MB) and troponin C was immediately assessed. In addition, blood samples were drawn into plastic vials containing EDTA. Plasma samples were stored at –40°C until the time of the assay (cystatin C, NT-proBNP, folic acid, vitamin B12, homocysteine, UII).
Table I.
Characteristics of patients with acute coronary syndrome. Mean values ± SD
| Age [years] | 65±12 |
| Gender [M/F] | 72/78 |
| BMI [kg/m 2 ] | 29.4±4.9 |
| BMI > 30 kg/m 2 [%] | 43.3 |
| Smokers [%] | 58.0 |
| Diabetes mellitus [%] | 32.0 |
| Arterial hypertension [%] | 72.0 |
| Time since the diagnosis of hypertension [years] | 11.5±8.3 |
| Blood pressure at admission > 140/90 mm Hg [%] | 56.7 |
| Coronary artery disease (CAD) [%] | 62.0 |
| Time since the diagnosis of CAD [years] | 10.6±8.0 |
| Episodes of myocardial infarction [%] | 30.0 |
| PTCA with or without stent implantation [%] | 21.3 |
| CABG [%] | 4.7 |
| Left ventricular insufficiency [%] | 4.0 |
| eGFR [ml/min/1.73 m 2 ] | 72.4±25.1 |
| eGFR< 60 ml/min/1.73 m 2 [%] | 29.3 |
| Medication: | |
| β-Blockers [%] | 54.0 |
| Calcium antagonists [%] | 19.3 |
| Angiotensin-converting enzyme inhibitors [%] | 56.0 |
| Diuretics [%] | 26.0 |
| Antiplatelet [%] | 50.7 |
| Statins [%] | 31.3 |
BMI – body mass index, PTCA – percutaneous coronary angioplasty, CABG – coronary artery bypass grafting, eGFR – estimated glomerular filtration rate
In all patients abdominal ultrasound examination was performed in order to measure kidney length and echocardiography to assess the degree of left ventricular hypertrophy, ejection fraction (EF) and left ventricular mass (LVM). Transthoracic echocardiography was performed using the Aloka 4000 instrument (2D-mode, M-mode, conventional and color-labeled Doppler flow). The measurement of LVM was performed in parasternal projection, longitudinal axis, M-mode presentation. Left ventricular mass was calculated according to Penn's equation [16], as follows: LVM [g] = (1.04 × [LVEDD + IVSDD + PWDD] – LVEDD) – 13.6, where: LVEDD – left ventricular end-diastolic diameter, IVSDD – interventricular septum end-diastolic diameter.
All patients with ACS diagnosed with diminished glomerular filtration rate, albuminuria or proteinuria were later referred to the Nephrology Outpatient Clinic in the Department of Nephrology, Endocrinology and Metabolic Disorders for further, more detailed diagnosis of renal disorders.
Laboratory measurements
Serum concentrations of creatinine, glucose and CK-MB activity (Hitachi 902 instrument, Roche Diagnostic GmbH, Mannheim, Germany) as well as serum troponin C level (enzyme immunoassay method – MEIA, AxSYM instrument, Abbott Laboratories, Abbott Park, IL, USA) were estimated in the hospital laboratory. Qualitative dipstick test (Micral-Test, Roche Diagnostics, Mannheim, Germany) was used to assess urine albumin level.
Plasma cystatin C (BioVendor, Modřice, Czech Republic), urotensin II (Phoenix Peptide, Belmont, California, USA) and NT-proBNP (Roche Diagnostics, Mannheim, Germany) were measured by enzyme-linked immunosorbent assay (ELISA), while serum folic acid and vitamin B12 concentrations (MP Biomedicals DiaSorin, Germany) were measured by radioimmunoassay in the laboratory of the Department of Nephrology, Endocrinology and Metabolic Disorders, Medical University of Silesia, Katowice, Poland.
Plasma concentration of homocysteine was assessed by microparticle enzyme immunoassay (MEIA) (Abbott Laboratories, Abbott Park, Illinois, USA) in the biochemistry lab in Sosnowiec, Medical University of Silesia, Poland.
Kidney function expressed as estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) formula [17].
Statistical analysis
All presented data are expressed as means± standard deviation. Analyses were performed using the Statistica 7.0 (StatSoft Polska, Kraków, Poland) software. Normality of distribution was tested with Kolmogorov-Smirnov test. Student's t-test for dependent and independent variables, Mann-Whitney U pair-wise comparison for independent variables, and Wilcoxon pair-wise comparison for dependent variables were used as appropriate. χ2 test and χ2 test with Yates's correction were used to compare distributions between groups. Correlation coefficients were calculated according to Spearman. Values of p below 0.05 were considered as statistically significant. All tests were two-tailed.
Results
Patients’ characteristics
Sixty-two percent out of 149 patients referred to the hospital with acute coronary symptoms had previously diagnosed coronary artery disease. Thirty percent had a history of myocardial infarction and 26% of myocardial revascularization (Table I). Only 4% were previously diagnosed with left ventricular insufficiency. Thirty two percent of patients were treated for diabetes mellitus.
Arterial hypertension was previously diagnosed in 72% of patients. On admission 63% of them had higher blood pressure than recommended, while only 8.8% were receiving any antihypertensive medicine before the occurrence of ACS.
Thirty-seven patients (24.8%) were admitted with high risk of myocardial infarction or death within 14 days (TIMI risk score at least 5 points). This group of patient was significantly older, had higher prevalence of coronary artery disease, lower EF and markedly elevated concentration of NT-proBNP (Table II). Also kidney excretory function expressed both as eGFR and cystatin C concentration was significantly worse in patients with high TIMI risk score. 54.1% of patients from this group were suffering from diabetes mellitus.
Table II.
Characteristics of patients with acute coronary syndrome stratified according to TIMI score
| Parameter | TIMI 1–2 (N=22) | TIMI 3–4 (N=91) | TIMI 5–7 (N=37) |
|---|---|---|---|
| Age [years] | 56±12 | 65±10# | 71±14# |
| BMI [kg/m2] | 28.7±4.0 | 29.8±4.7 | 29.1±6.0 |
| Diabetes mellitus [%] | 0 | 52.7 | 54.1 |
| Arterial hypertension [%] | 40.9 | 78.0** | 75.7** |
| Systolic blood pressure [mm Hg] | 152±31 | 151±24 | 148±27 |
| Diastolic blood pressure [mm Hg] | 90±14 | 90±14 | 90±15 |
| Coronary artery disease (CAD) [%] | 18.2 | 64.8# | 81.1# |
| Left ventricular insufficiency [%] | 0 | 3.3 | 8.1 |
| Ejection fraction [%] | 57.3±7.5 | 52.0±6.1** | 47.1±9.4# |
| Left ventricular mass [g] | 275±117 | 316±85** | 330±90** |
| Hemoglobin [g/dl] | 13.7±1.6 | 14.2±1.2 | 13.7±2.3 |
| LDL cholesterol [mmol/l] | 3.45±1.19 | 3.09±1.08 | 2.81±1.06 |
| HDL cholesterol [mmol/l] | 1.45±0.36 | 1.36±0.41 | 1.42±0.41 |
| Triglycerides [mmol/l] | 1.67±0.77 | 1.82±1.17 | 1.56±0.67 |
| Homocysteine [µmol/l] | 15.7±7.1 | 16.7±6.6 | 16.6±4.8 |
| Vitamin B12 [pg/ml] | 266±126 | 319±148 | 364±318 |
| Folic acid [ng/ml] | 4.93±2.37 | 6.51±3.69 | 7.10±6.26 |
| eGFR [ml/min/1.73 m2] | 81±30 | 73±22 | 66±28* |
| Cystatin C [mg/l] | 1.19±0.31 | 1.49±0.52** | 1.70±0.57# |
| CRP [mg/l] | 1.7±2.5 | 1.3±3.3 | 2.4±4.3 |
| CK-MB [U/l] | 22±9 | 31±41 | 36±36* |
| Troponin C [ng/ml] | 1.97±4.12 | 2.55±7.63 | 2.66±3.92 |
| NT-proBNP [pg/ml] | 750±261 | 1240±2399* | 5490±6870# |
| Urotensin II [ng/ml] | 3.60±2.20 | 3.14±1.99 | 2.61±1.47* |
Statistical significance vs. TIMI 1-2
p < 0.05
p < 0.01
p < 0.001
BMI – body mass index, eGFR – estimated glomerular filtration rate
Plasma urotensin II concentrations
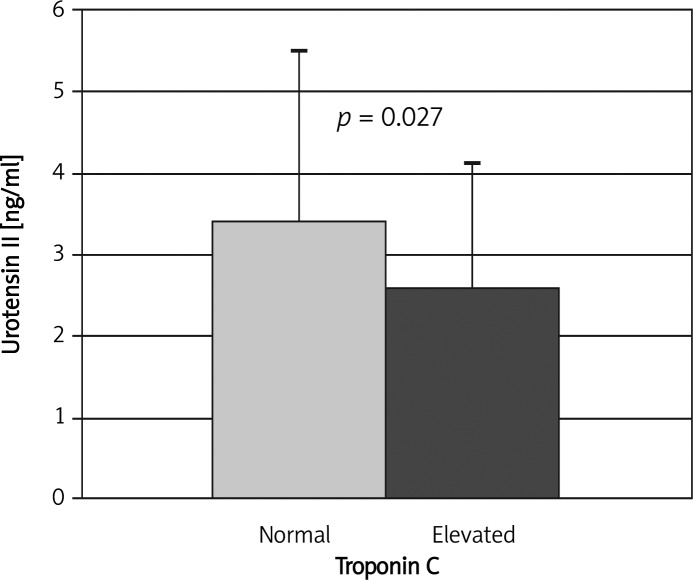
Mean plasma UII concentration in the whole group of patients with ACS was 3.08±1.92 ng/ml. Slightly, almost significantly higher values were observed in men than in women (3.39±2.02 vs. 2.80±1.79 ng/ml; p=0.07). Similar values were found in patients with and without arterial hypertension (2.99±1.85 vs. 3.30±2.10 ng/ml; NS). In patients with the highest risk (TIMI 5-7) plasma UII concentration was significantly lower (p=0.045) than in patients with low risk (TIMI 1-2) – Table II. Moreover, significantly lower plasma UII concentration was found in patients with increased troponin C concentration (2.60±1.52 vs. 3.41±2.09 ng/ml, p=0.027 – Figure 1).
Figure 1.
Urotensin II concentration in patients with elevated and normal plasma troponin C concentration
There was a significant negative correlation between plasma UII concentration and TIMI score (R = − 0.184; p=0.025) or concentration of troponin C (R= − 0.229; p=0.005), but not CK-MB (R=0.014; p=0.87). A borderline correlation between plasma UII and EF (R=0.157; p=0.063) or NT-proBNP (R= − 0.156; p=0.058) was found. There was no correlation between plasma UII concentration and LVM as well as systolic or diastolic blood pressure. Plasma UII concentration was not related to eGFR, while it correlated negatively with serum homocysteine concentration (R= − 0.234; p=0.004).
In the model of multiple stepwise backward regression analysis including plasma UII concentration as a dependent variable and eGFR, ejection fraction, concentration of troponin C and proBNP as independent variables, logarithm of troponin C plasma concentration (β = − 0.0754, p = 0.004) could explain 14.1% of plasma UII concentration variability.
Discussion
The present study demonstrates that patients with ACS and the highest risk of myocardial infarction or death within 14 days (TIMI 5-7) have significantly lower UII concentration than patients with low risk (TIMI 1-2), and is the first to prove a negative relationship between UII concentration and troponin C level and TIMI score.
The first authors investigating plasma levels of UII in patients with ACS were Joyal et al. [13]. They found lower plasma UII concentration in 54 patients with ACS compared with patients with stable angina and healthy controls. Moreover, patients with systolic dysfunction accompanying ACS had decreased mean plasma UII concentration in comparison to patients with preserved left ventricular function. The second study to focus on plasma UII concentration in patients with ACS was performed by Khan et al. [12]. Contrary to Joyal et al. and our results, they found raised plasma UII concentration in 129 patients with acute myocardial infarction as compared to healthy controls. In addition, partially in line with our findings, the authors demonstrated that lower than median UII concentration was an independent risk factor of adverse outcomes in patients with myocardial infarction. The authors suggested that elevated plasma UII concentration after myocardial infarction has a cardioprotective effect as a peripheral vasodilator. Somewhat contrary are the results obtained by Rdzanek et al., who did not observe any significant differences in plasma UII concentration in myocardial infarction survivors with and without hypertension [18]. These data are inconsistent with findings of Cheung et al. showing raised plasma UII concentration in patients with arterial hypertension [19].
We have demonstrated lower plasma UII concentration in patients with myocardial infarction than in other ACS patients, and the inverse relationship between plasma concentration of troponin C and UII (R= − 0.229; p = 0.005). Such a correlation was previously not examined either by Khan et al. or Joyal et al. [12, 13]. As the correlation is rather weak, we agree with their suggestions that plasma UII concentration is not a good marker of myocardial necrosis. On the other hand, the relationship between myocardial necrosis and plasma UII concentration may be indirect, reflecting worse hemodynamic function of the left ventricle. The rationale for such a hypothesis is the almost significant positive correlation between plasma UII and EF (R=0.157; p = 0.063) and negative with NT-proBNP (R= − 0.156; p = 0.058). Also Joyal et al. observed significantly lower plasma UII concentration in patients with lower EF.
It should be stressed that patients with the highest TIMI score had higher incidence of diabetes mellitus, arterial hypertension, coronary artery disease, higher LVM and lower EF that was associated with markedly elevated NT-proBNP. All these states are associated with chronic endothelial dysfunction, and usually increased plasma UII concentration [7, 20]. However, it is not proved whether acute damage of endothelium in patients with ACS is the cause of diminished release of UII as proposed by Joyal et al. [13]. Plasma UII concentration has not yet been assessed in patients with cardiac shock.
Based on our findings we suggest that diminished plasma UII concentration in patients with ACS could be associated with more severe injury of cardiac muscle and perhaps greater endothelial damage. Our hypothesis is backed up by the results described by Khan et al. [12], who demonstrated that plasma levels of UII below the median were associated with poorer survival and increased likelihood of adverse clinical outcome. Thus elevated levels of U II may have a cardioprotective effect. There are some data supporting that hypothesis. Stimulation of UII receptors localized in endothelial cells in animal models was followed by the release of nitric oxide and prostaglandins [21], even though the release of these potent vasodilators may be counterbalanced by the direct contractile influence of UII on smooth cells. The unanswered question is whether UII may act as a peripheral vasodilator after acute myocardial infarction unloading the injured myocardium – the more so, that it has been proven that UII has both positive inotropic and peripheral vasodilator effects [22, 23]. These findings suggest that in some circumstances, UII may be cardioprotective and explain the findings that patients with lower levels of UII were at highest risk (TIMI 5-7). However, further, larger studies are required to validate our hypothesis.
The limitation of our study is the lack of plasma samples extraction prior to ELISA, recommended, but not required by the kit manufacturer. This may have explained the relatively high plasma UII concentrations in our study.
In conclusion, decreased plasma UII concentration in patients with ACS could be associated with more severe injury of the myocardium.
Acknowledgments
The study was carried out as a research project supported by a grant from the State Committee of Scientific Research in Poland (N402 024 31/0919).
References
- 1.Pearson D, Shively JE, Clark BR, et al. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021–4. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YH, Yandle TG, Richards AM, Palmer SC. Urotensin II immunoreactivity in the human circulation: evidence for widespread tissue release. Clin Chem. 2009;55:2040–8. doi: 10.1373/clinchem.2009.131748. [DOI] [PubMed] [Google Scholar]
- 3.Ames RS, Aiyar NV, Ohlstein EH, Willette RN. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–74. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguire JJ, Kuc RE, Wiley KE, Kleinz MJ, Davenport AP. Cellular distribution of immunoreactive urotensin-II in human tissues with evidence of increased expression in atherosclerosis and a greater constrictor response of small compared to large coronary arteries. Peptides. 2004;25:1767–74. doi: 10.1016/j.peptides.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Russell FD, Meyers D, Galbraith AJ, et al. Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am J Physiol Heart Circ Physiol. 2003;285:1576–81. doi: 10.1152/ajpheart.00217.2003. [DOI] [PubMed] [Google Scholar]
- 6.Douglas SA, Dhanak D, Johns DG. From ‘gills to pills’: urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol Sci. 2004;25:76–85. doi: 10.1016/j.tips.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YC, Zhu YZ, Moore PK. The role of urotensin II in cardiovascular and renal physiology and diseases. Br J Pharmacol. 2006;148:884–901. doi: 10.1038/sj.bjp.0706800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell FD, Molenaar P, O'Brien DM. Cardiostimulant effects of urotensin-II in human heart in vitro. Br J Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Totsune K, Takahashi K, Arihara Z, Sone M, Ito S, Murakami O. Increased plasma urotensin II levels in patients with diabetes mellitus. Clin Sci. 2003;104:1–5. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 10.Kemp W, Krum H, Colman J, et al. Urotensin II: a novel vasoactive mediator linked to chronic liver disease and portal hypertension. Liver Int. 2007;27:1232–9. doi: 10.1111/j.1478-3231.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P, Malatino L. Urotensin II is an inverse predictor of incident cardiovascular events in end-stage renal disease. Kidney Int. 2006;69:1253–8. doi: 10.1038/sj.ki.5000114. [DOI] [PubMed] [Google Scholar]
- 12.Khan SQ, Bhandari SS, Quinn P, Davies JE, Ng LL. Urotensin II is raised in acute myocardial infarction and low levels predict risk of adverse clinical outcome in humans. Int J Cardiol. 2007;117:323–8. doi: 10.1016/j.ijcard.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Joyal D, Huynh T, Aiyar N, Guida B, Douglas S, Giaid A. Urotensin-II levels in acute coronary syndromes. Int J Cardiol. 2006;108:31–5. doi: 10.1016/j.ijcard.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359:1990–7. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- 15.Morrow DA, Antman EM, Parsons L, et al. Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA. 2001;286:1356–9. doi: 10.1001/jama.286.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Rdzanek A, Filipiak K, Karpiński G, Grabowski M, Opolski G. Exercise urotensin II Dynamics In myocardial infarction survivors with and without hypertension. Int J Cardiol. 2006;110:175–8. doi: 10.1016/j.ijcard.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Cheung BM, Leung R, Man YB, Wong LY. Plasma concentration of urotensin II is raised in hypertension. J Hypertens. 2004;22:1341–4. doi: 10.1097/01.hjh.0000125452.28861.f1. [DOI] [PubMed] [Google Scholar]
- 20.Russell F. Urotensin II in cardiovascular regulation. Vasc Health Risk Manag. 2008;4:775–85. doi: 10.2147/vhrm.s1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottrill FE, Douglas SA, Hiley CR, White R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br J Pharmacol. 2000;130:1865–70. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas S, Ashton D, Sauermelch C, et al. Human urotensin-II is a potent vasoactive peptide: pharmacological characterization in the rat, mouse, dog and primates. J Cardiovasc Pharmacol. 2000;26:163–6. doi: 10.1097/00005344-200036051-00051. [DOI] [PubMed] [Google Scholar]
- 23.Stirrat A, Gallagher M, Douglas A, et al. Potent vasodilatator response to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:925–8. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]