Abstract
Introduction
Low-grade systemic inflammation plays an important role in the pathogenesis and natural history of chronic obstructive pulmonary disease (COPD) and peripheral arterial disease (PAD). The aim of the study was to analyze plasma concentrations of selected markers of inflammation in patients suffering from PAD with or without coexistent COPD.
Material and methods
Thirty patients (6 women) with advanced PAD (at least IIb stage according to Fontaine scale) hospitalized due to critical limb ischemia were examined. In all patients spirometry was performed to confirm or exclude COPD. Plasma concentration of IL-6, IL-8 and TNF-α was measured using ELISA method. Statistical analysis was performed according to COPD status and according to smoking status independently.
Results
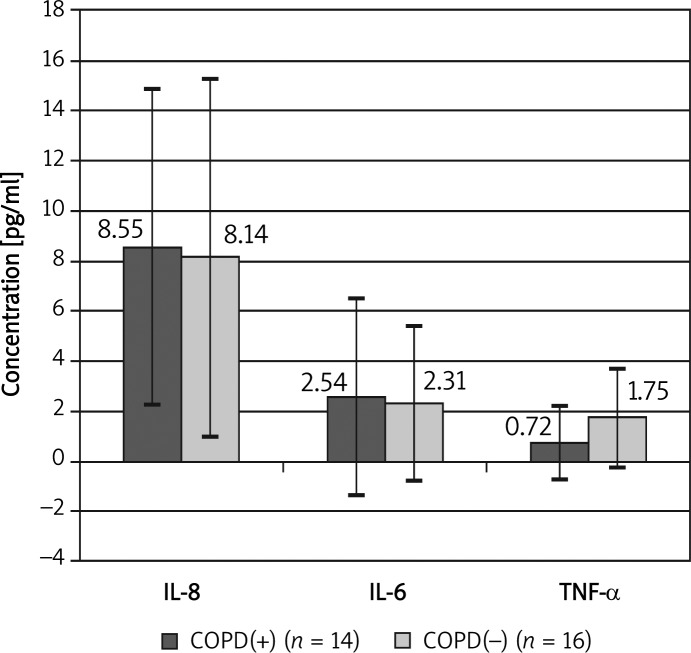
In the whole group of patients with PAD, COPD was recognized in 14 cases (for the first time in 10 cases). All patients were smokers (46.7% current, 53.3% ex-smokers). We found a significant correlation between FEV1%N (percent of norm of first second expiratory volume) and the number of years of smoking (r = –0.39; p < 0.05). We found similar concentrations of IL-6 (2.54 pg/ml vs. 2.31 pg/ml), IL-8 (8.55 pg/ml vs. 8.14 pg/ml, TNF-α (0.72 pg/ml vs. 1.75 pg/ml) in the COPD(+) group in comparison to the COPD(–) group (differences were not significant). We observed significant positive correlations (p < 0.05) between concentrations of measured markers and significant negative correlations between pain free walking distance and these markers.
Conclusions
Our study confirmed coexistence of PAD with COPD. The character of inflammation is similar in these smoking-related diseases
Keywords: chronic obstructive pulmonary disease, peripheral arterial disease, systemic inflammation, of interleukin-8, of interleukin-6, tumour necrosis factor-α
Introduction
Cigarette smoking is a major risk factor of many serious and fatal diseases worldwide. Among them chronic obstructive pulmonary disease (COPD) is nowadays one of the biggest health problems with more than 300 million people suffering from this disease [1, 2]. In COPD the inflammatory process is initiated by environmental fumes, mostly by tobacco smoke – about 90% of patients suffering from COPD are smokers [3]. Smoking is also a major risk factor of coronary heart disease and other manifestations of atherosclerosis. In the last few years coexistence of COPD and cardiovascular diseases in relation to cigarette smoke has been discussed [4–8]. Peripheral arterial disease (PAD) is a manifestation of atherosclerosis which, similarly to COPD, is very strongly connected with cigarette smoking. According to Coughlin et al., 76% of people suffering from PAD are current or ex-smokers [9].
Many studies show that PAD is associated with systemic inflammation and endothelial dysfunction [10–12]. It is also known that COPD is not only a pulmonary disease but is characterized by development of low-grade inflammation in the whole organism [4]. Some observations indicate a possible epidemiological and pathophysiological connection between PAD and COPD. The inflammatory process seems to be similar in both diseases also at the molecular level. Many studies show that the same markers (chemokines, inflammatory cytokines, acute phase proteins and activators of coagulation) are involved in the pathogenesis of both diseases [13]. However, there is a lack of studies describing the coexistence of PAD and COPD. In our earlier study we showed that COPD was a frequent disease but it was underdiagnosed in smokers with PAD for many reasons [14]. We proved in our prior article that the Medical Research Council (MRC) dyspnea scale is useless in COPD patients with coexistence of PAD. Moreover, heart diseases, which are very frequent in this group, can delay the COPD diagnosis being considered as the primary cause of dyspnea. The question about the particular differences in the inflammatory process between the two diseases is still open. The answer can be useful in understanding processes which cause development of PAD and COPD and in the future it will allow appropriate treatment of these coexisting diseases.
The aim of the study was to assess the coexistence of PAD and COPD in the group of smokers and to evaluate differences in systemic inflammation between patients suffering from peripheral arterial disease and COPD compared to the group with peripheral arterial disease only. For this purpose concentrations of the selected markers interleu-kin-6 (IL-6), IL-8, tumour necrosis factor-α (TNF-α) and fibrinogen were measured.
Material and methods
The study group consisted of 30 patients hospitalized due to critical limb ischemia in a surgery clinic. Patients were aged from 50 to 75 years (mean age 62 ±7 years); 24 were men and 6 women.
Advanced peripheral arterial disease was diagnosed in all investigated patients and the diagnosis of PAD was confirmed by angio-computed tomography in all cases [15]. Clinical stage of the disease was assessed according to the Fontaine scale [16] – all included patients had at least stage IIb of PAD (pain-free walking distance less than 200 m). There were 5 patients in stage II, 15 in stage III, and 10 in stage IV. It was the first hospitalization due to critical limb ischemia in 11 cases (36.7%).
Surgical interventions included angioplasties with or without stent implantation in 8 cases (26.7%), thrombectomy in 4 cases (13.3%), and various types of arterial bypass in 18 cases (60%). There were no amputations.
Another inclusion criterion was history of smoking cigarettes, more than 10 pack-years. In our group 14 patients (47%) were current smokers, and 16 patients (53%) were ex-smokers.
Patients suffering from acute infections, diabetes, autoimmune diseases, with diagnosed neoplasm, treated with immunosuppression, or with contraindications to spirometry were excluded.
The COPD was diagnosed using GOLD criteria and recommendations of the Polish Respiratory Society [1, 2]. We used spirometric criteria according to the last document considering that FEV1%FVC ratio lower than the lower limit of normal (less than 5th percentile) confirmed COPD.
All patients were asked about symptoms of COPD: chronic cough, sputum production, effort dyspnea and, if necessary, for history of COPD. Information about other diseases (especially cardiovascular diseases) and current treatment was also collected. The spirometry test was performed using a LungTest 1000 spirometer, MES Kraków. If the pseudo-Tiffeneau ('Tiffeneau index') (FEV1/FVC) was below the lower limit of the normal value (5th percentile), the bronchodilatation test was performed (administration of inhaled 400 µg of salbutamol and after 15 min another spirometry test). To exclude any influence of the surgical intervention or acute phase of the disease, spirometry was performed 3 days after it.
All patients continued their therapy of prior diseases: coronary artery disease, heart failure, arterial hypertension, metabolic syndrome. We did not include in our investigation patients who were treated with corticosteroids.
Five milliliters of blood from each patient was collected at the same time of the day. Blood samples were drawn into tubes with EDTA and were next centrifuged. The plasma was separated in aliquots and frozen at –70°C. The plasma concentrations of IL-6 and TNF-α were measured by the ELISA method using a Human Immunoassay kit (R&D Systems, USA) according to the prescription by the producer. For measurement of IL-8 concentration the ELISA chemiluminescence method was used (R&D Systems, USA).
The study was approved by the Ethics Committee of the Medical University of Warsaw. All the patients gave their informed consent.
Statistical analysis
For data comparison the Mann-Whitney U test was used; p < 0.05 was regarded as significant. The relationships between the data were examined by the Spearman rank correlation coefficient. Correlations with both R ≥ 0.4 and p < 0.05 were considered relevant. The program Statistica 8.0 was used.
Results
The COPD was diagnosed in 14 patients (46.6% of the group). In 10 patients COPD was diagnosed for the first time during this study.
Studied patients were divided into two groups according to COPD status: with (COPD +) and without coexistence of COPD (COPD–). Patients’ characteristics are presented in Table I. There were no significant differences between the two groups in the studied variables.
Table I.
Characteristics of study group according to coexistence of PAD and COPD
| Parameter | COPD(+) (n = 14) | COPD(–) (n = 16) | Significance |
|---|---|---|---|
| Age [years] | 62.4 ±8.3 | 61.0 ±4.9 | NS |
| Male/female | 13/1 | 11/5 | |
| BMI [kg/m2] | 22.8 ±3.6 | 24.7 ±3.6 | NS |
| Active smoker | 6 (42.9%) | 8 (50%) | NS |
| Number of smoking years | 40.4 ±6.4 | 38.0 ±5.9 | NS |
| Number of pack-years | 57.2 ±14.4 | 49.1 ±20.6 | NS |
| Arterial hypertension | 8 (57%) | 11 (68.8%) | NS |
| Coronary artery disease | 6 (42.9%) | 7 (43.8%) | NS |
| Heart failure | 4 (28.6%) | 5 (31.3%) | NS |
| Metabolic syndrome | 2 (14.3%) | 4 (25%) | NS |
| Cholesterol serum | 165.4 ±69.6 | 149.9 ±47.3 | NS |
| TG [mg/dl] | 107.93 ±42.8 | 118.94 ±41.4 | NS |
| HDL [mg/dl] | 71.79 ±27.5 | 68.76 ±31.2 | NS |
| LDL [mg/dl] | 121.64 ±40.2 | 106.44 ±25.4 | NS |
| Pain-free walking distance [m] | 60.7 ±66 | 69.4 ±43 | NS |
The mean postbronchodilator FEV1%FVC in patients with COPD was 74.2 ±9.6% and in the group without COPD was 81 ±6.5% (p < 0.05). The mean FEV1%N in the COPD group was also lower than in the group without COPD (81.9 ±28.3% vs. 94.5±14%) (p < 0.05). COPD stage I was diagnosed in 11 patients, in 1 case stage II, in 2 stage III.
The mean serum concentration of IL-6 was 2.42 ±3.44 pg/ml, of IL-8 8.33 ±6.64 pg/ml, of TNF-α 1.27 ±1.68 pg/ml in the whole investigated group. The mean concentration of fibrinogen was 515 ±194 mg/dl and was elevated in 67% of patients.
Because of the small number of patients with advanced COPD it was impossible to evaluate statistically the level of investigated markers in each stage of COPD separately. Only the mean values of markers according to the stage of COPD are shown in Table II.
Table II.
Concentration of inflammatory markers according to COPD severity
| Stage of COPD | IL-6 [ng/ml] | IL-8 [ng/ml] | TNF-α [ng/ml] | Fibrinogen [mg/ml] |
|---|---|---|---|---|
| I (n = 11) | 2.38 | 9.32 | 0.52 | 551 |
| II (n = 1) | 0 | 6.51 | 0 | 678 |
| III (n = 2) | 4.72 | 5.35 | 2.21 | 557 |
We found similar concentrations of IL-6, IL-8 and TNF-α in the group with PAD COPD(+) compared with the group with PAD only (Figure 1) (no statistically significant differences between groups). The concentration of fibrinogen was also similar in patients with PAD COPD(+) compared with PAD only: 561 ±210 mg/ml vs. 482 ±180 mg/ml (no statistically significant differences between groups).
Figure 1.
Comparison of concentration of interleukins in patients with and without COPD
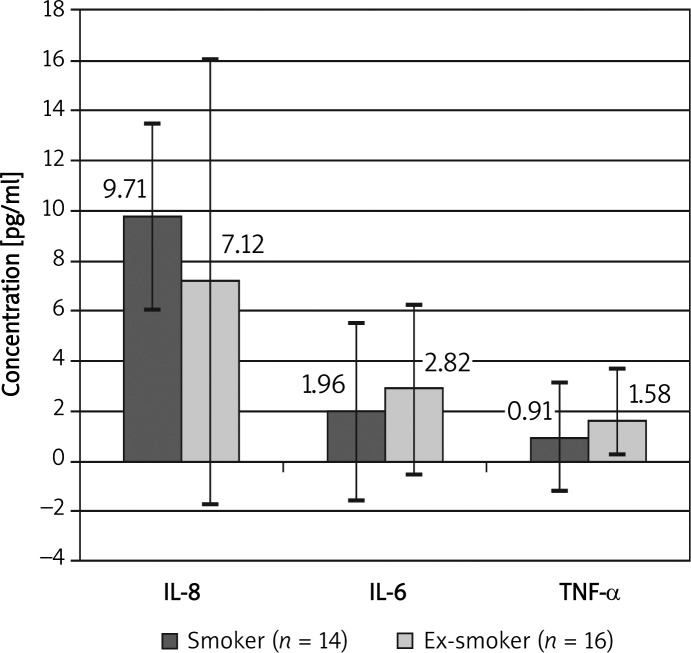
Analysis of the concentration of inflammatory markers according to the smoking status did not reveal any significant differences. However, concentration of IL-8 was slightly higher in the group of smokers than in ex- smokers, whereas the concentrations of IL-6 and of TNF-α were higher in the ex-smoker group than in current smokers (Figure 2). The concentration of fibrinogen was similar in current smoking patients and in ex-smokers: 528 ±209 mg/ml vs. 506 ±189 mg/ml (no statistically significant differences between groups).
Figure 2.
Comparison of concentration of interleukins in smokers and ex-smokers
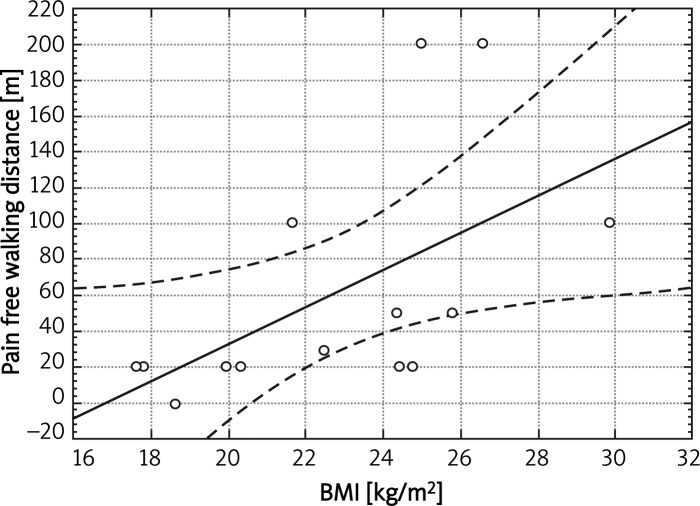
We found a significant negative correlation between the number of smoking years and FEV1%N value (r = –0.39) in the whole group of patients, and in the subgroup of active smokers the correlation was even stronger (r = –0.69). We revealed a positive correlation between BMI and pain-free walking distance in the group with COPD (r = 0.71, p < 0.05) (Figure 3).
Figure 3.
Correlation between pain-free walking distance and BMI in group with COPD (r = 0.71, p < 0.05)
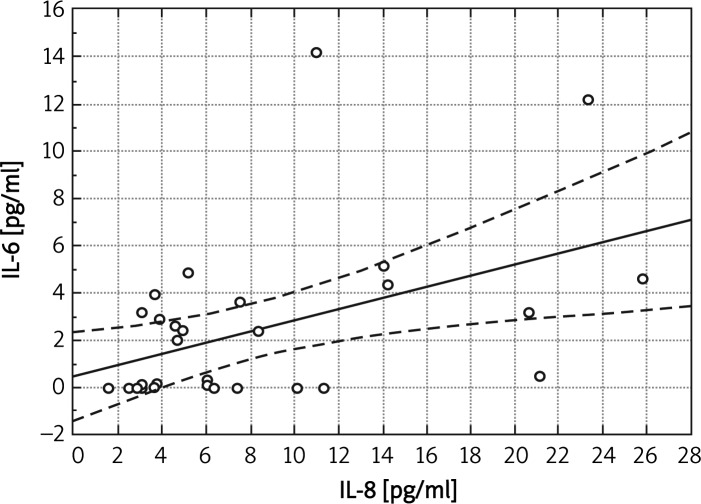
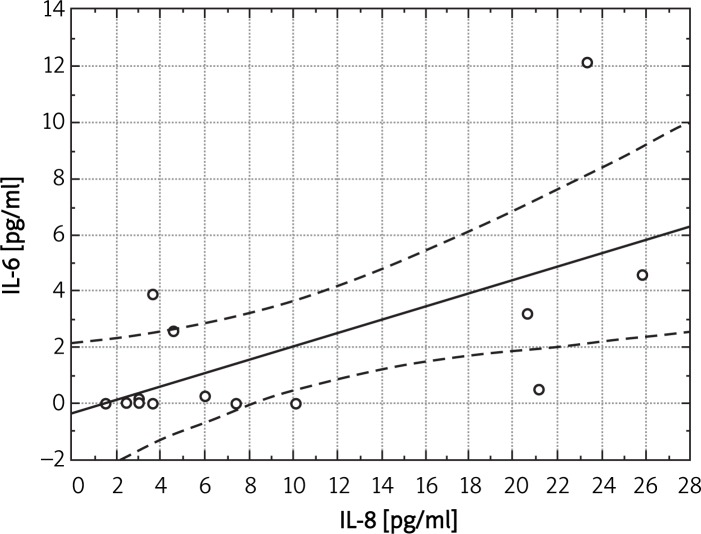
We found statistically significant correlations between concentration of IL-8 and concentration of fibrinogen (r = 0.407, p < 0.05) or concentration of IL-6 (r = 0.44, p < 0.05) in the whole group (Figure 4). In the group with PAD only we noted a positive correlation between concentration of IL-8 and fibrinogen (r = 0.68, p < 0.05).
Figure 4.
Correlation between IL-6 and IL-8 in the whole group (r = 0.44, p < 0.05)
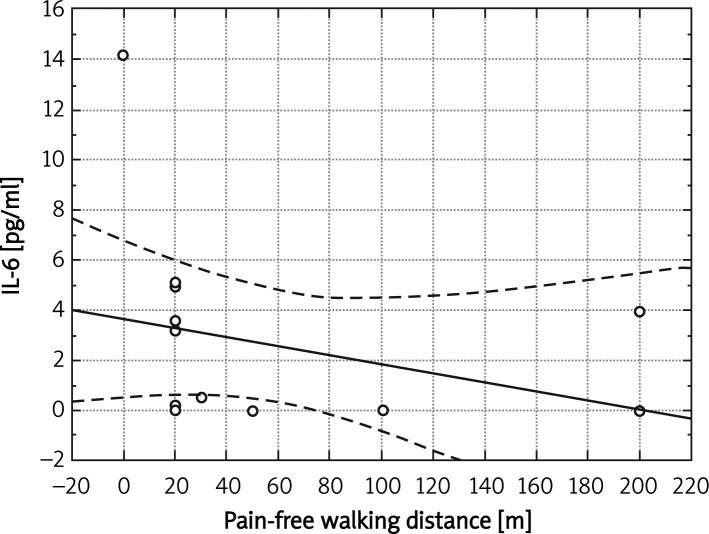
We found a significant negative correlation between the pain-free walking distance and IL-8 concentration in the whole study group (r = –0.41, p < 0.05). In the group with coexistence of PAD and COPD there was a negative correlation of pain-free walking distance with plasma concentration of IL-6 (r = –0.58, p < 0.05) (Figure 5).
Figure 5.
Correlation between IL-6 and pain-free walking distance in group with COPD (r = –0.58, p < 0.05)
The strongest correlations were observed in the group of current smokers: between the concentrations of IL-8 and IL-6 (r = 0.64, p < 0.05) (Figure 6), IL-8 and TNF-α (r = 0.64, p < 0.05), IL-8 and fibrinogen (r = 0.60, p < 0.05), and of IL-6 and TNF-α (r = 0.53, p < 0.05). In this group a negative correlation of IL-8 with pain-free walking distance was also significant (r = –0.59, p < 0.05). These correlations were not observed in the group of patients who gave up smoking.
Figure 6.
Correlation between IL-6 and IL-8 in group of current smokers (r = 0.64, p < 0.05)
Discussion
Our study is one of the first to investigate the problem of coexistence of two smoking related diseases, PAD and COPD. In our earlier article we showed that this coexistence is common but still underdiagnosed [14]. The present study seems to be the first in which the authors attempt to evaluate differences between the inflammatory processes in PAD and COPD in the group of smokers. We found slightly elevated concentration of IL-6, IL-8 and fibrinogen in patients with coexistence of PAD and COPD and we confirmed a role of smoking in the enhancement of systemic inflammation in these patients.
The first important issue was to collect an adequate study group, in which only the main diseases, COPD and PAD, will have an influence on the level of analyzed markers. This is the reason why patients with acute infections, diabetes, autoimmune diseases, with diagnosed neoplasm, or treated with immunosuppression were excluded. It is concordant with the observations of Pinto-Plata et al., who found that the level of IL-6 and IL-8 rise during exacerbation of COPD (IL-6: 6.38 pg/ml during exacerbation vs. 2.80 pg/ml in the stable period of the disease, IL-8: 8.18 pg/ml vs. 3.72 pg/ml) [17]. In the review by Wouters et al. many other markers were shown to be also elevated during COPD exacerbations [18]. The data from the literature show similarly significant changes of the level of markers investigated by us in neoplasm [19] and diabetes [20]. It was also important to include only patients with a similar stage of PAD and to investigate them in a comparable stage of the disease. For this purpose only patients with advanced PAD were included in the study group and blood was collected at the same time: 3 days after surgery or acute ischemia, to reduce the influence of acute ischemia on studied variables.
Interleukin-6 plasma concentration found in our study (2.31 ±3.09 pg/ml) was comparable to the result of Tzoulaki et al. (IL-6: 3.3 pg/ml) in the group of patients with symptomatic PAD [21]. They also excluded patients with active inflammatory processes but did not exclude individuals with diabetes. In that study IL-6 was the only marker which was a predictor of ankle-brachial index (ABI) change at 5-and 12-year follow-up. These results showed that IL-6 is a suitable marker to monitor the inflammatory process in PAD. The level of this cytokine is also elevated in COPD and in the study of Pinto-Plata et al. the IL-6 concentration was 2.80 pg/ml in the stable period of the disease, which was similar to our finding of 2.54 ±3.9 pg/ml in the group with COPD and PAD [17]. Similar concentrations of IL-6 in patients with airflow limitation were presented Sunyer et al. [22]. Our study confirmed that IL-6 was a good indicator of inflammation in both COPD and PAD, but was not specific for either of these diseases.
The studies of Pedersen et al. showed that plasma concentrations of IL-6 increased markedly in the circulation during and just after exercise and had an anti-inflammatory effect [23–26]. The capacity for exercise was considerably limited in patients with PAD and severe COPD and this was a possible explanation why we did not observe significant changes of the level of IL-6 in our study. Moreover, we found a negative correlation of IL-6 concentration with pain-free walking distance. This observation was similar to Tzoulaki et al., who found a trend in the relation between PAD severity (measured as a decrease of ABI) and IL-6 concentration [21]. Thus our observations confirmed the role of IL-6 as a marker of inflammation in PAD.
We observed increased concentration of fibrinogen, which in 67% of cases exceeded the reference value, especially in those with coexisting PAD/COPD and in current smokers. The activation of coagulation is known to play a role in the pathogenesis of atherosclerosis in PAD [27]. But also in COPD elevated concentration of fibrinogen was observed [28]. The correlation of fibrinogen and IL-8 concentration in our study confirmed the relation of systemic inflammation with coagulation in PAD and COPD [13, 18].
Surprisingly, the TNF-α level was low and did not differ between groups in our study. This marker was presented as crucial in the pathogenesis of systemic inflammation in many chronic diseases [7, 8, 11, 29]. The lack of significant increase of TNF-α may have resulted from the facts that our patients were in the stable stage of the disease and several exclusion criteria were used. The TNF-α is a central cytokine in the loss of muscle mass in COPD. Muscle wasting and low body mass index (BMI) were described as closely connected with systemic inflammation in COPD, and also as negative prognostic factors [30]. We found a significant relation of the level of IL-6 with BMI and a negative relation of the pain-free walking distance with concentration of IL-6 and of IL-8. Thus, our observations added new evidence supporting similarity of the character of the inflammatory process in PAD and COPD.
Higashimoto et al. found that the level of IL-6, IL-8 and fibrinogen in serum of patients with COPD did not differ between current smokers and ex-smokers [31]. Only the level of TNF-α was significantly higher in the group of smokers in his study. We also did not reveal any significant differences in the level of investigated markers. However, the strong, significant correlations between these markers which were observed in the group of current smokers by us showed that smoking had a greater influence on systemic inflammation than the disease process per se.
Taken together, the results of our study confirmed a high prevalence of COPD among patients with PAD with some similarities in the inflammatory process of these diseases with a fundamental role of tobacco smoke.
Acknowledgments
Supported by Grant of Medical University of Warsaw, No. 1WU/NM1/07.
References
- 1. Available at: www.goldcopd.com: 2009.
- 2.Pierzchala W. Recommendations of Polish Society of Lung Diseases about diagnosis and therapy of chronic obstructive pulmonary disease (COPD) [Polish] Pneumonol Alergol Pol. 2010;78:318–47. [PubMed] [Google Scholar]
- 3.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–12. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 5.Iversen KK, Kjaergaard J, Akkan D, et al. Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med. 2008;264:361–9. doi: 10.1111/j.1365-2796.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 6.Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007;49:171–80. doi: 10.1016/j.jacc.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Maccallum PK. Markers of hemostasis and systemic inflammation in heart disease and atherosclerosis in smokers. Proc Am Thorac Soc. 2005;2:34–43. doi: 10.1513/pats.200406-036MS. [DOI] [PubMed] [Google Scholar]
- 8.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8:706–11. doi: 10.1016/j.ejheart.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin PA, Gulati V, Mavor AI, Gough MJ, Homer-Vanniasinkam S. Risk factor awareness in patients with peripheral arterial disease. J Cardiovasc Surg (Torino) 2007;48:735–40. [PubMed] [Google Scholar]
- 10.Nylaende M, Kroese A, Stranden E, et al. Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc Med. 2006;11:21–8. doi: 10.1191/1358863x06vm662oa. [DOI] [PubMed] [Google Scholar]
- 11.Signorelli SS, Mazzarino MC, Di PL, et al. High circulating levels of cytokines (IL-6 and TNFalpha), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med. 2003;8:15–9. doi: 10.1191/1358863x03vm466oa. [DOI] [PubMed] [Google Scholar]
- 12.Silvestro A, Scopacasa F, Ruocco A, et al. Inflammatory status and endothelial function in asymptomatic and symptomatic peripheral arterial disease. Vasc Med. 2003;8:225–32. doi: 10.1191/1358863x03vm503oa. [DOI] [PubMed] [Google Scholar]
- 13.Hunninghake DB. Cardiovascular disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:44–9. doi: 10.1513/pats.200410-050SF. [DOI] [PubMed] [Google Scholar]
- 14.Sleszycka J, Wozniak K, Banaszek M, Wiechno W, Domagala-Kulawik J. Prevalence and difficulties in chronic obstructive pulmonary disease diagnosis in patients suffering from severe peripheral arterial disease [Polish] Pol Merkur Lekarski. 2009;27:92–6. [PubMed] [Google Scholar]
- 15.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–97. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 16.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–296. [PubMed] [Google Scholar]
- 17.Pinto-Plata VM, Livnat G, Girish M, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest. 2007;131:37–43. doi: 10.1378/chest.06-0668. [DOI] [PubMed] [Google Scholar]
- 18.Wouters EF, Groenewegen KH, Dentener MA, Vernooy JH. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc. 2007;4:626–34. doi: 10.1513/pats.200706-071TH. [DOI] [PubMed] [Google Scholar]
- 19.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients: a summary of published results. Int J Colorectal Dis. 2010;25:135–40. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 20.Signorelli SS, Mazzarino MC, Spandidos DA, Malaponte G. Proinflammatory circulating molecules in peripheral arterial disease. Int J Mol Med. 2007;20:279–86. [PubMed] [Google Scholar]
- 21.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 22.Sunyer J, Pistelli R, Plana E, et al. Systemic inflammation, genetic susceptibility and lung function. Eur Respir J. 2008;32:92–7. doi: 10.1183/09031936.00052507. [DOI] [PubMed] [Google Scholar]
- 23.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans; effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–5. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–51. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski KA. Short period of exercise causes rapid increase of serum interleukin 6 with no effect on its soluble receptor. Arch Med Sci. 2009;5:364–70. [Google Scholar]
- 27.Tzoulaki I, Murray GD, Price JF, et al. Hemostatic factors, inflammatory markers, and progressive peripheral athero-sclerosis: the Edinburgh Artery Study. Am J Epidemiol. 2006;163:334–41. doi: 10.1093/aje/kwj051. [DOI] [PubMed] [Google Scholar]
- 28.Wedzicha JA, Seemungal TA, Maccallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–5. [PubMed] [Google Scholar]
- 29.Wainwright G. Cholestrol-lowering therapy and cell membranes. Stable plaque at the expense of unstable membranes? Arch Med Sci. 2009;5:289–95. [Google Scholar]
- 30.Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:5s–13s. doi: 10.1183/09031936.03.00004603a. [DOI] [PubMed] [Google Scholar]
- 31.Higashimoto Y, Yamagata Y, Taya S, et al. Systemic inflammation in chronic obstructive pulmonary disease and asthma: similarities and differences. Respirology. 2008;13:128–33. doi: 10.1111/j.1440-1843.2007.01170.x. [DOI] [PubMed] [Google Scholar]