Abstract
Mastocytosis is a clonal disease of the hematopoietic stem cell. The condition consists of a heterogeneous group of disorders characterized by a pathological accumulation of mast cells in tissues including the skin, bone marrow, liver, spleen and the lymph nodes. Mastocytosis is a rare disease which occurs both in children and adults. Childhood onset mastocytosis is usually cutaneous and transient while in adults the condition commonly progresses to a systemic form. The heterogeneity of clinical presentation of mastocytosis is typically related to the tissue mast cell burden, symptoms due to the release of mast cell mediators, the type of skin lesions, the patient's age at the onset and associated haematological disorders. Therefore, a multidisciplinary approach is recommended. The present article provides an overview of clinical symptoms, diagnostic criteria and treatment of mastocytosis to facilitate the diagnosis and management of mastocytosis patients in clinical practice.
Keywords: mastocytosis, children, adults, classification, clinics, treatment
Introduction
Mastocytosis is defined as a heterogeneous group of disorders characterized by an accumulation of mast cells (MC) in one or more organs, particularly in the skin, the bone marrow, liver, spleen and lymph nodes [1–4]. The clinical presentation and the course of mastocytosis are variable, ranging from pure cutaneous involvement (cutaneous mastocytosis – CM) to different systemic forms (systemic mastocytosis – SM). Mastocytosis occurs in both children and adults. In about 15% of all patients with mastocytosis the disease is congenital and approximately 50% of all patients develop the first symptoms before the age of 2 years [5–7]. The condition is a rare clonal disease of the haematopoietic stem cell [8–10]. Genetic findings indicate different pathogenetic patterns of mastocytosis. Adult patients, particularly those with systemic variants of the disease, usually express activating mutations of the stem cell factor (SCF) receptor KIT, mostly in exon 17 (D816V). The interaction between SCF and its cognate receptor, the KIT receptor tyrosine kinase, plays a crucial role in the regulation of MC growth and differentiation [2, 5, 10]. In contrast to adult-onset mastocytosis, the majority of paediatric and familial mastocytosis cases seem to lack codon 816 mutations [5]. Therefore childhood-onset mastocytosis has been considered a reactive disease. Results of a recent study on the genetic background of childhood mastocytosis in which the entire KIT gene sequence from skin biopsies was analysed support the idea that paediatric mastocytosis is also a clonal disease associated with D816V and other activating KIT mutations [11]. However, it is not certain if individual KIT mutations are necessary and sufficient to cause MC transformation [2]. The proliferation of MC is regulated not only by SCF (and KIT) but also by other cytokines such as IL-4, IL-6, IL-10 and IL-13. A recent genetic study suggested that the 1112C/T IL13 gene polymorphism and the resulting ‘hypertranscription’ may predispose to the development of SM [12]. Dysregulation of MC apoptosis may also be considered in pathogenesis of mastocytosis. Both up-regulation of antiapoptotic protein Bcl-2 in aggressive mastocytosis and up-regulation of antiapoptotic protein Bcl-X in bone marrow of patients with indolent mastocytosis have been reported [5, 13, 14].
The clinical presentation of the disease first of all depends on the tissue MC burden and systemic symptoms due to the release of MC mediators [3, 15, 16]. Mast cells are multipotent effector cells of the immune system which produce histamine, tryptases, chymase, carboxypeptidase A, heparin, chondroitin sulfate glycosaminoglycans, prostaglandin D2, leukotrienes (LTC4, LTD4, LTE4), vascular endothelial growth factor (VEGF), platelet-activating factor (PAF), multifunctional cytokines (TNF-α, TNF-β, SCF, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, GM-CSF,) and chemokines (IL-8, MCP-1, MIP-1α) [3, 16]. Although the complete role of each MC-dependent mediator in the pathogenesis of mastocytosis remains unclear, these mediators are responsible for numerous clinical symptoms [3, 16]. Flushing, itching, blistering, diarrhoea, abdominal pain, vomiting, hypotension, headache and bone pain are all the most frequently reported MC mediator-related symptoms [15, 16]. This explains the heterogeneity of clinical manifestations of mastocytosis. These nonspecific mediator-related symptoms can be mimicked by other diseases. Therefore, diagnostic procedures of mastocytosis should include histological, immunohistochemical, and molecular examinations.
Despite its clinical forms, mastocytosis differs in the age of onset. Therefore the condition may be divided into childhood onset mastocytosis and adult onset mastocytosis [3–7]. The age of mastocytosis onset is very important because it has prognostic implications [17]. The majority of children with CM experience spontaneous resolution of skin lesions by adolescence, whereas adult-onset mastocytosis is chronic and tends to progress to the systemic form [5, 7, 16, 17]. The WHO classification defines 7 disease variants (Table I) [18].
Table I.
Classification of mastocytosis according WHO recommendations
| Cutaneous mastocytosis (CM) |
| Indolent systemic mastocytosis (ISM) |
| SM with an associated clonal haematological |
| non-MC-lineage disease (SM-AHNMD) |
| Aggressive SM (ASM) |
| MC leukaemia (MCL) |
| MC sarcoma (MCS) |
| Extracutaneous mastocytoma |
This article provides a brief review of the literature concerning clinical aspects of mastocytosis in children and adults. It is particularly intended for physicians involved in the care of patients with this unusual disorder. Because of the various clinical presentations of the disease, mastocytosis is in the scope of interest of dermatologists, allergists, haematologists and paediatricians. We present recent consensus statements on diagnosis and treatment recommendations from a practical viewpoint to facilitate the evaluation and workup of suspected mastocytosis patients.
Cutaneous mastocytosis
The most common clinical presentation of mastocytosis is the cutaneous form [5]. Approximately two-thirds of all CM cases occur in children [2, 4]. Most children have mastocytosis limited to the skin [6, 7, 19]. In adults skin lesions are usually the first sign of systemic disease [1, 16, 17]. Therefore, a consensus has been reached to apply SM criteria in all adult patients before establishing the final diagnosis (CM or SM) and to use the checkpoint ‘mastocytosis in the skin’ (MIS) instead of CM in pre-diagnostic cases [1]. ‘Mastocytosis in the skin’ is recognized on the basis of typical morphology of skin lesions, positive Darier's sign and histopathology of a skin sample. The histopathological substrate of all lesions of CM is an infiltrate by MC that can be visualized using basic dyes such as Giemsa, toluidine blue, Astra blue or by immunocytochemical stains revealing tryptase [19, 20]. Anti-KIT (CD117) staining has a high specificity and high sensitivity for MC in paraffin sections. This staining is against c-kit membrane receptor and allows MC to be identified even after degranulation [19]. Therefore c-kit immunostaining is used preferentially in routine workup. In doubtful cases a KIT mutation at codon 816 should be examined in the affected skin [19].
Based on the patterns of skin lesions, three major clinical manifestations of CM are distinguished by WHO: maculopapular type (MPCM), diffuse cutaneous mastocytosis (DCM) and solitary mastocytoma of the skin [1, 18]. Mechanical irritation of skin lesions leads to a release of mast cell mediators and thus to reddening and urticarial swelling. This reaction is known as Darier's sign and is pathognomonic for all forms of CM [5, 19–21]. The frequency of CM clinical forms in children is reported differently in the literature, which may be partially due to various classifications of CM [1, 6, 20–22]. Traditionally, MPCM has been named urticaria pigmentosa (UP) and the nodular lesions have been defined as multiple mastocytoma. In 2002 a modified consensus proposal concerning classification of CM was presented by Hartmann and Henz (Table II) [21].
Table II.
Classification of cutaneous mastocytosis according to Hartmann and Henz [21]
| Maculopapular cutaneous mastocytosis |
| Plaque type cutaneous mastocytosis |
| Nodular cutaneous mastocytosis/mastocytoma |
| Diffuse cutaneous mastocytosis |
| Telangiectatic cutaneous mastocytosis |
This classification is detailed with respect to clinical appearance, the course and prognosis of different disease entities. From a dermatological perspective, the authors propose to consider maculopapular, plaque and telangiectatic forms as separate entities. They underline that the maculopapular form occurring in children as well as in adults rarely resolves spontaneously, in contrast to the plaque form, which usually disappears by puberty [21, 23]. Maculopapular CM and mastocytoma are reported to be the most common clinical types (47-75% and 17-51%, respectively) in contrast to DCM, which is a rare finding (1-5%) [5, 20–24]. As far as morphology of skin lesions is concerned, very distinct presentations of CM have been observed. MPCM is characterized by small, brown macules and papules (Figure 1). This type is the most common clinical manifestation of adult onset CM. The plaque form consists of slightly tan-to-orange, flat, slightly elevated plaques up to several centimetres in diameter (Figure 2). The condition usually manifests in infancy. Telangiectatic form (telangiectasia macularis eruptiva perstans) is a rare clinical presentation occurring mainly in adults, which consists of red and brown telangiectatic macules. This form is considered to be limited to the skin. However, systemic involvement also may occur [20, 23]. Mastocytoma is a solitary nodular lesion which may be present at birth or appears within the first 3 months of life and resolves spontaneously with age (Figure 3). Multiple nodular lesions are very rare [20–24]. Diffuse cutaneous mastocytosis is the most severe form of CM, which presents as erythroderma involving almost the entire skin [20, 23, 24] (Figure 4). In this form the clinical features occur at birth or in early infancy. Widespread haemorrhagic blisters may be the first clinical presentation of DCM. Bullous lesions do not represent a specific subset of the CM phenotype [6, 20]. Bullae can occur in all forms of CM, usually in infants, and are related to mast cell load in the skin. The cases in which bullae are a prominent feature are more likely to be associated with systemic involvement and a higher risk of anaphylactic reaction [20, 25, 26]. Due to a widespread and heavy mast cell load in the entire skin, children with DCM may have flushing, itching, hypotension, anaphylactic shock, diarrhoea and gastrointestinal bleeding [20, 21, 27]. Systemic involvement and a fatal outcome have been reported in some DCM cases [25–27]. Blistering and severe mast cell related symptoms have a tendency to decrease or even disappear in time. A generalized thickening of the skin with leather-grain appearance and the pronounced Darier's sign are prominent features of the disease in teenagers and adults with DCM [24, 28].
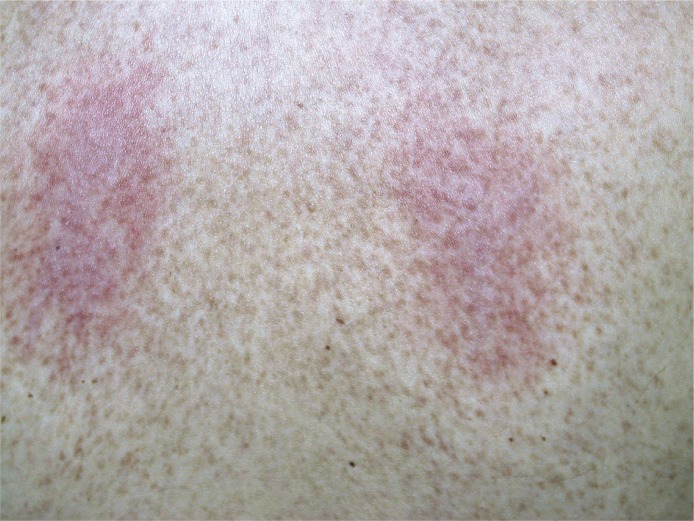
Figure 1.
Maculopapular cutaneous mastocytosis/urticaria pigmentosa
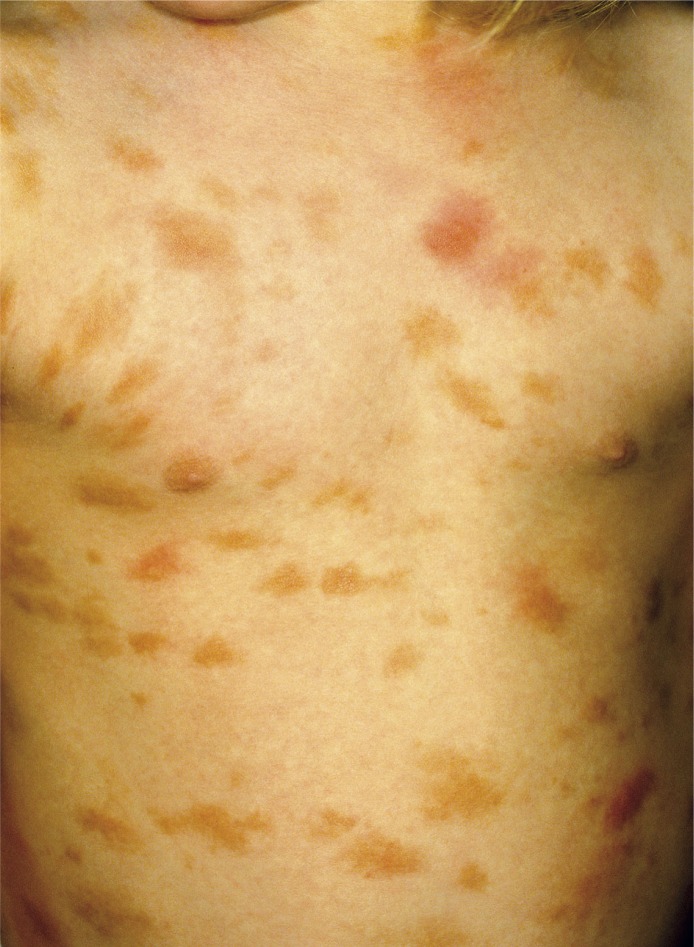
Figure 2.
Maculopapular cutaneous mastocytosis/plaque type. Darier's sign is visible
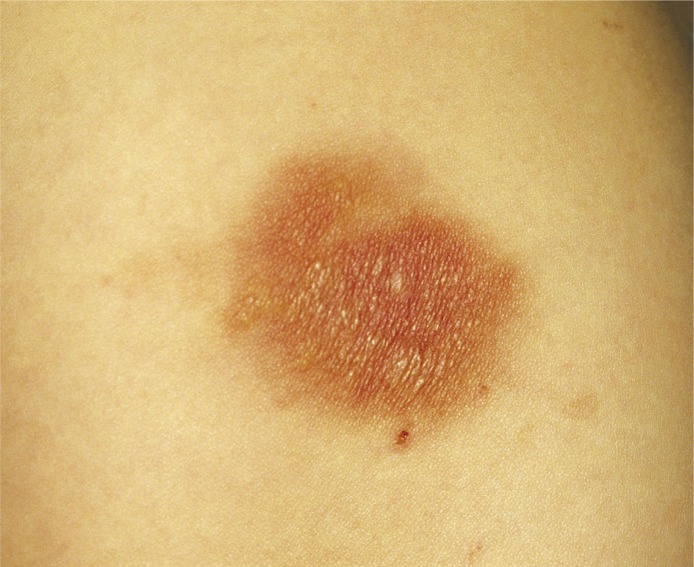
Figure 3.
Mastocytoma
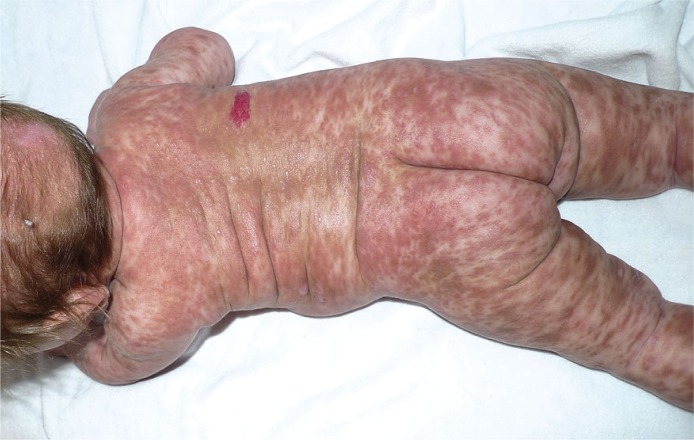
Figure 4.
Diffuse cutaneous mastocytosis
Apart from clinical appearance, a substantial heterogeneity in clinical presentation may be observed among patients with a similar form of the disease. Moreover, CM cases differ in the extent and activity of skin lesions. Therefore scoring of the CM index (SCORMA Index) was designed in order to assess the extent and activity of skin lesions. It is based on a semi-quantitative analysis of the extent, intensity and subjective complains [29, 30]. The extent of the skin abnormality is evaluated in part A as the percentage of exposed skin. The intensity of the condition is dealt with in part B. A lesion of typical morphology, representing the majority of lesions, is judged on pigmentation, vesiculation, elevation, and Darier's sign. Each item is scored from 0 to 3, where 0 is for an absent item and 3 represents the highest severity. In part C, five subjective symptoms – provocative factors, flushing, diarrhoea, pruritus and local bone pain – are scored by patients from 0 to 10 using a visual analogue scale. The formula A/5 + 5B + 2C/5 is used to calculate the final SCORMA score. The value of the SCORMA score ranges from 5.2 to 100. This method provides standardized information on the severity of CM and imposes no burden on the patient, which is particularly important in children [19]. The SCORMA Index is comparable with that used for atopic dermatitis (SCORAD) and psoriasis (PASI) and its clinical applications have been reported [29–31].
Systemic mastocytosis
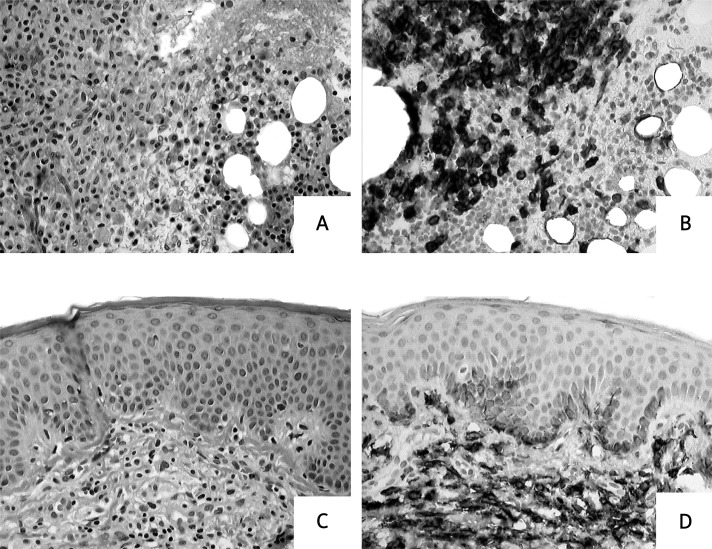
Systemic mastocytosis is the predominant form of mastocytosis in adult patients whereas in children systemic involvement is a very rare finding [3–5, 32]. Therefore, paediatric SM is usually presented as such in case reports [3, 5, 25–27]. In one of the largest paediatric studies, involving 173 children with mastocytosis, only two cases of systemic involvement were found [24]. In the majority of cases SM is recognized on the basis of careful histopathological examination of bone marrow. However, other internal organs might also be involved, particularly the liver, spleen, gastrointestinal tract and the lymph nodes [1–5, 32]. The application of molecular criteria is crucial for establishing the diagnosis of SM in order to distinguish mastocytosis from reactive MC hyperplasia or myelomastocytic disorders [1, 35–40]. Systemic mastocytosis is defined by major and minor SM criteria (Table III) [2, 18]. If at least one major and one minor or at least three minor SM criteria are fulfilled, the diagnosis is SM. The histopathological examination of the bone marrow and the skin samples taken from a patient with SM is presented in Figure 5. It is noteworthy that SM so far has not been diagnosed as often as it should, particularly in patients with CM, because of avoidance of careful systemic mastocytosis workup including bone marrow examination. Therefore, we present the routine work-up of cases with suspected mastocytosis in adults (Table IV) and children (Table V) according to recent consensus statements and recommendations [1–3, 16, 19, 40–43].
Table III.
Diagnostic criteria for SM according to WHO recommendations [18]
| Major |
| Multifocal dense infiltrates of MC in the bone marrow or other extracutaneous organs, confirmed by special stains such as MC tryptase (>15 MC aggregating) |
| Minor |
| In MC infiltrates in the bone marrow or other Extracutaneous organs, >25% of MC are spindle-shaped Or otherwise atypical; or in bone marrow smears, >25% of MC are spindle shaped or otherwise atypical |
| Activating point mutation of KIT in codon 816 is present in extracutaneous organs |
| MCs in extracutaneous organs (CD117) co-express either CD2 or CD25, or both, as determined by flow cytometry |
| Serum tryptase is persistently >20 ng/ml (does not count in patients who have an associated clonal haematological non-MC disease; AHNMD) |
Figure 5.
Histopathological examination of the bone marrow and the skin samples. Biopsies from a patient with systemic mastocytosis. A, B – Bone marrow biopsy reveals focal mast cell accumulation. This fulfils the criterion of systemic mastocytosis (more than 15 mast cells per aggregate). Haematoxylin and eosin (A) and CD117 staining (B). C, D – Perivascular mast cell infiltrates in the upper portion of the dermis. Haematoxylin and eosin (C) and CD117 staining (D)
Table IV.
Diagnostic algorithm for adult mastocytosis patients
| 1. History (the onset and duration of the disease, provoking factors, presence of mediator-related symptoms and anaphylaxis, mastocytosis in family) |
| 2. Physical examination (inspection of the skin, Darier's sign, organomegaly: liver, spleen, lymph nodes) |
| 3. Skin biopsy |
| 4. Peripheral blood analysis and serum biochemistry |
| 5.Liver function tests |
| 6. Serum tryptase levels |
| 7. Abdomen ultrasound (or other imaging studies depending on individual presentation) |
| 8. Bone densitometry |
| 9. Bone marrow trephine biopsy (>2 cm). Antibodies against CD25, CD 117, and tryptase should be applied; in SM-AHNMD further immunohistochemical staining |
| 10. Blood and bone marrow smears |
| 11. Flow cytometric analysis of bone marrow MC for the presence of CD2 and CD25 |
| 12. Genetic examination – determination of KIT mutation (D816V) in bone marrow MC |
Table V.
Diagnostic algorithm for children with mastocytosis
| Steps 1-7 are the same for children and adults |
| Bone densitometry in selected cases with unexplainable bone pain |
| Screening for SM including bone marrow biopsy, flow cytometric analysis of bone marrow MC and determination of KIT mutation (D816V) should be done only in cases with suspected haematological disease or suspected SM. Indications to perform these examinations are: |
| Serum tryptase levels exceeding 100 ng/ml or rising |
| Clinically significant abnormalities in the peripheral blood |
| Organomegaly (liver, spleen, or lymph nodes) |
A bone marrow examination should be performed in all adult patients suspected of mastocytosis even if the serum tryptase is normal (approximately 11 ng/ml) [33–40]. Serum tryptase levels closely correlate with the course of mastocytosis and therefore are used preferentially for the diagnosis and follow-up of mastocytosis patients [1, 31–33]. In adults the final diagnosis should be established after checking for SM criteria, whereas in children with tryptase < 100 ng/ml the diagnosis of CM may be decided upon without bone marrow biopsy, unless other signs of SM are present. Tryptase levels exceeding 100 ng/ml or rising are the indication for bone marrow examination regardless of age [1, 19]. Systemic mastocytosis can be diagnosed when focal compact infiltrates with a significant proportion of spindle-shaped MC are present or in cases with exclusive occurrence of round MC after demonstration of CD25 expression by the MC [41]. Peripheral blood is not acceptable as an alternative to bone marrow when searching for KIT mutations. The presence of KIT D816V in the skin is indicative of MIS, but not diagnostic for SM [1].
Mastocytosis is a progressive disease in a considerable number of cases, particularly in adults. Therefore follow-up in all mastocytosis patients is recommended [1–3]. Generally check-up once a year is sufficient [1, 5, 19, 42]. However, in some symptomatic, severe or progressive cases an individual scheme of visits is necessary. History, physical examination, peripheral blood analysis, determination of serum tryptase levels, serum biochemistry, liver function tests and abdomen ultrasound are routinely performed procedures during follow-up. Bone marrow examinations and other investigations should be undertaken or repeated depending on individual presentation, course and treatment of the disease [1, 5, 19, 42].
Indolent systemic mastocytosis (ISM) is the most common presentation of the disease in adults. This form of SM is characterized by low MC burden (not exceeding 30% in the bone marrow), presence of mediator-related symptoms and skin involvement, particularly maculopapular lesions [1, 2, 4]. In the majority of ISM cases the prognosis is good. Spontaneous resolution of skin lesions in adult patients is rare and may be associated with transformation into an aggressive variant of the disease [34]. Isolated bone marrow mastocytosis (the absence of skin lesions), well-differentiated SM and smoldering SM (SSM) are subsets of ISM [41, 42]. Smoldering SM is diagnosed in patients with B-findings (borderline, benign) including hypercellular marrow, dysplasia, huge MC marrow infiltration (>30%), serum tryptase >200 ng/ml, and organomegaly (spleen, liver, lymph nodes), without impaired organ function [1, 41, 42]. Well-differentiated SM presents as an exclusively round-cell type of the disease with compact multifocal MC infiltrates without expression of CD25. This subset is characterized by a point mutation outside codon 816 of KIT [41].
Systemic mastocytosis with an associated clonal haematological non-MC-lineage disease (SM-AHNMD) concerns approximately one-third of SM cases [1, 2]. Myeloproliferative and myelodysplastic disorders are the most common diseases [35–37]. Both the SM component and AHNMD component of the disease should be diagnosed and classified according to WHO criteria [1, 18, 37]. In patients with eosinophilia and the FIP1L1-PDGFRA fusion gene and/or CHIC2 deletion, the diagnosis is SM with chronic eosinophilic leukaemia (SM-CEL). In patients with CEL-related organopathy, but no marker of monoclonal eosinophils, the final diagnosis is SM with ‘hypereosinophilic syndrome’ (SM-HES) [1, 37].
Aggressive SM (ASM) is characterized by progressive growth of neoplastic MC in diverse organs leading to organopathy. C-findings (consider cytoreduction with chemotherapy or with targeted drugs) are indicative of aggressive disease. They are the result of a clinically relevant impairment or loss of organ function due to local MC infiltrates. C-findings include marked cytopenia, osteolysis (or osteoporosis) with pathological fractures, hepatomegaly with impaired liver function and ascites or portal hypertension, splenomegaly with hypersplenism, and malabsorption with weight loss. Patients with ASM have poor prognosis [1, 16, 40–43]. In some ASM cases eosinophilia and lymphadenopathy may occur.
MC leukaemia (MCL) is a rare disease variant that can arise de novo or evolve from pre-existing mastocytosis [2]. The condition is an aggressive haematological malignancy characterized by circulating MC, rapidly progressive organopathy, high proportion of MC in the bone marrow smears (over 20% atypical MC), fatal disease progression and a short survival in most cases [35–40].
Mast cell sarcoma and extracutaneous mastocytoma are solid MC tumours of malignant and benign nature, respectively. They are extremely rare forms of mastocytosis [2, 18].
SM-AHNMD, ASM and isolated bone marrow mastocytosis patients might often not exhibit MIS. Patients with MCL usually present without skin signs and symptoms [1]. Systemic mastocytosis without MIS is very difficult to diagnose. It is therefore of particular importance to be aware of the possibility of an underlying mastocytosis in patients with unexplained episodes of hypotension, anaphylactic shock, idiopathic flushing, diarrhoea, headache, and other symptoms that might be due to a release of MC mediators [37].
Treatment of mastocytosis
The pathogenetic complexity and biological heterogeneity of mastocytosis variants make the therapy of the condition difficult. Despite recent advances in understanding the pathophysiology of mastocytosis, no standard treatment exists [42]. To choose the appropriate treatment strategy it is essential to screen for MC mediator-related symptoms, B and C findings, evidence for MCL, osteopathy, the presence of cutaneous symptoms and coexisting or associated diseases [1, 42]. Key issues concerning therapy of mastocytosis will be addressed in this section. Independent of the disease variant, all patients require avoidance of factors triggering mediator release and symptomatic therapy to control mediator-related symptoms [1–3, 42, 43]. Possible triggering factors include physical stimuli (heat, cold, mechanical irritation of skin lesions), emotional stress, drugs (aspirin and other non-steroidal anti-inflammatory drugs, alcohol, morphine, derivatives, polymyxin-B, amphotericin B, some drugs used in general anaesthesia, radiographic dyes, dextromethorphan, β-adrenergic blockers, α-adrenergic and cholinergic receptor antagonists), venoms, polymers (dextran, gelatine) and biological response modifiers [3, 19]. The basis of the symptomatic therapy is a combination of histamine receptor (HR1 and HR2) antagonists [19, 42, 43]. In more resistant cases MC-stabilizing agents may be considered. Glucocorticosteroids (GCs) are occasionally ordered for treating recurrent hypotensive episodes, ascites, and diarrhoea with malabsorption [1–3]. In cases of gastrointestinal symptoms HR2 antagonists, proton pump inhibitors and oral cromolyn sodium are recommended [2, 3, 42, 43]. All patients are advised to carry epinephrine self-injectors because systemic hypotension/anaphylactic reactions may occur in those with any of the mastocytosis variants [1–3, 42, 43]. The basis of MIS therapy is ultraviolet (UV) light irradiation [1, 44]. Both oral and bath PUVA (psoralen plus UVA radiation) therapies are used. Narrowband UVB phototherapy and topical GCs are advised in patients with skin lesions. Systemic GCs should be considered in cases with extensive blistering. A novel option in CM therapy is raft modulators such as topical miltefosine, which inhibits mediator release from human MC and reduces MC-driven skin inflammatory responses [45]. Specific immunotherapy is usually recommended in patients with IgE-dependent anaphylaxis [42, 46]. Therapy with bisphosphonates should be advised in cases of severe osteopenia, osteoporosis or long-term GCs treatment [42, 43]. Nonspecific cytoreductive therapy should be considered in patients with C findings suffering from the aggressive and leukemic variants or MC sarcoma [40, 42, 43]. Therapy with IFN-α, cladribine, cytarabine, fludarabine and hydroxyurea have been reported in this group of patients [42, 43, 47]. No standard treatment has been published [42]. Glucocorticosteroids show some activity in SSM and ASM [47]. IFN-α may be effective in patients with severe osteopathy and osteolysis [40]. In rapidly progressing ASM, MCL and MC sarcoma polychemotherapy and, in responding patients, haematopoietic stem cell transplantation must be considered [40, 42, 43]. In cases of SM-AHNMD, both components of the disease have to be diagnosed, graded and treated separately [42, 47]. Novel approaches in treatment of mastocytosis are targeted therapies, which may be considered in patients with the most aggressive forms of the disease [48–50]. Imatinib, masitinib and bafetinib are agents unable to block the kinase activity of KIT D816V, whereas midostaurin and dasatinib have clear inhibitory activity against KIT D816V [42, 43, 48–50]. Targeted drugs can be used alone, in combinations employing agents directed against KIT D816V and against other relevant, KIT-independent kinases or in association with chemotherapy [42]. Targeting antibodies directed against various surface molecules and proapoptotic agents are promising candidates for novel therapeutic strategies [42, 49]. Current studies focus on combined drugs targeting different molecular pathways to improve the therapy of the most advanced MC disorders [48–50].
In conclusion, clinical heterogeneity is a hallmark of mastocytosis. Variable cutaneous presentation or the absence of typical skin symptoms, different course of the disease in children and adults, internal organ involvement and numerous mediator-related symptoms may all cause mastocytosis to be confused with a variety of endocrinological, vascular, or immunologic disorders. Therefore a multidisciplinary approach is recommended in diagnosis and management of mastocytosis patients. Although great progress has been made in revealing the pathogenesis of the disease and establishing diagnostic and treatment recommendations, mastocytosis still remains a challenge for physicians and scientists.
References
- 1.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 2.Pardanani A, Akin C, Valent P. Pathogenesis, clinical features, and treatment advances in mastocytosis. Best Pract Res Clin Haematol. 2006;19:595–615. doi: 10.1016/j.beha.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81:677–90. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 4.Horny HP, Sotlar K, Valent P, Hartmann K. Mastocytosis a disease of the hematopoietic stem cell. Dtsch Arztebl Int. 2008;105:686–92. doi: 10.3238/arztebl.2008.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682–95. doi: 10.1046/j.1365-2133.2001.04123.x. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann K, Metcalfe DD. Pediatric mastocytosis. Hematol Oncol Clin North Am. 2000;14:625–40. doi: 10.1016/s0889-8588(05)70299-9. [DOI] [PubMed] [Google Scholar]
- 7.Carter MC, Metcalfe DD. Paediatric mastocytosis. Arch Dis Child. 2002;86:315–9. doi: 10.1136/adc.86.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longley BJ, Jr, Metcalfe DD, Tharp M, et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA. 1999;96:1609–14. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations. Proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25:571–6. doi: 10.1016/s0145-2126(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 10.Sotlar K, Colak S, Bache A, et al. Variable presence of KIT(D816V) in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD) J Pathol. 2010;220:586–95. doi: 10.1002/path.2677. [DOI] [PubMed] [Google Scholar]
- 11.Bodemar CH, Hermine O, Palmerini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–15. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 12.Nedoszytko B, Niedoszytko M, Lange M, et al. Interleukin 13 promoter gene polymorphism – 1112C/T is associated with the systemic form of mastocytosis. Allergy. 2009;64:287–94. doi: 10.1111/j.1398-9995.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann K, Hermes B, Rappersberger K, Sepp N, Mekori YA, Henz BM. Evidence for altered mast cell proliferation and apoptosis in cutaneous mastocytosis. Br J Dermatol. 2003;149:554–9. doi: 10.1046/j.1365-2133.2003.05598.x. [DOI] [PubMed] [Google Scholar]
- 14.Cervero C, Escribano L, San Miguel JF, et al. Expression of bcl-2 by human bone marrow mast cells and its overexpression in mast cell leukemia. Am J Hematol. 1999;60:191–5. doi: 10.1002/(sici)1096-8652(199903)60:3<191::aid-ajh4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Lange M, Niedoszytko M, Nedoszytko B, Glen J. Aethiopathogenesis of mastocytosis: current options. Post Dermatol Alergol. 2009;26:142–5. [Google Scholar]
- 16.Valent P, Akin C, Sperr WR, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 17.Middelkamp Hup MA, Heide R, Tank B, Mulder PGH, Oranje AP. Comparison of mastocytosis with onset in children and adults. J Eur Acad Dermatol Venereol. 2002;16:115–20. doi: 10.1046/j.1468-3083.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 18.Valent P, Horny HP, Li CY, et al. Mastocytosis (Mast cell disease). World Health Organization (WHO) classification of tumors. Pathology and genetics. In: Jaffe ES, Haris NL, Stein H, Vardiman JW, editors. Tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 291–302. [Google Scholar]
- 19.Heide R, Beishuizen A, de Groot H, et al. Mastocytosis in children: a protocol for management. Pediatr Dermatol. 2008;25:493–500. doi: 10.1111/j.1525-1470.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 20.Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519–28. doi: 10.1016/s0145-2126(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann K, Henz BM. Classification of cutaneous mastocytosis: a modified consensus proposal. Luek Res. 2002;26:483–4. doi: 10.1016/s0145-2126(01)00157-6. [DOI] [PubMed] [Google Scholar]
- 22.Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, Gutierrez-Castrellon P, Ruiz-Maldonado R. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285–90. doi: 10.1111/j.1468-3083.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann K, Henz BM. Cutaneous mastocytosis – clinical heterogeneity. Int Arch Allergy Immunol. 2002;127:143–6. doi: 10.1159/000048187. [DOI] [PubMed] [Google Scholar]
- 24.Hannaford R, Rogers M. Presentation of cutaneous mastocytosis in 173 children. Australas J Dermatol. 2001;42:15–21. doi: 10.1046/j.1440-0960.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 25.Waxtein LM, Vega-Memije ME, Cortes-Franco R, Domingues-Soto L. Diffuse cutaneous mastocytosis with bone marrow infiltration in a child: a case report. Pediatr Dermatol. 2000;3:198–201. doi: 10.1046/j.1525-1470.2000.01751.x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy M, Walsh D, Drumm B, Watson Bullous mastocytosis: a fatal outcome. Pediatr Dermatol. 1999;16:452–5. doi: 10.1046/j.1525-1470.1999.00116.x. [DOI] [PubMed] [Google Scholar]
- 27.Shah PY, Sharma V, Worobec AS, Metcalfe DD, Zwick DC. Congenital bullous mastocytosis with myeloproliferative disorder and c-kit mutation. J Am Acad Dermatol. 1998;39:119–21. doi: 10.1016/s0190-9622(98)70413-x. [DOI] [PubMed] [Google Scholar]
- 28.Heide R, Zuidema E, Beishuizen , et al. Clinical aspects of diffuse cutaneous mastocytosis in children: two variants. Dermatology. 2009;219:309–15. doi: 10.1159/000243808. [DOI] [PubMed] [Google Scholar]
- 29.Heide R, Middelkamp Hup MA, Mulder PGH, Oranje AP. Clinical scoring of cutaneous mastocytosis. Acta Derm Venereol. 2001;81:273–6. doi: 10.1080/00015550152572912. [DOI] [PubMed] [Google Scholar]
- 30.Heide R, van Dorn K, Mulder PG, et al. Serum tryptase and SCORMA (SCORing MAstocytosis) Index as disease severity parameters in childhood and adult cutaneous mastocytosis. Clin Exp Dermatol. 2008;34:462–8. doi: 10.1111/j.1365-2230.2008.03005.x. [DOI] [PubMed] [Google Scholar]
- 31.Lange M, Renke J, Glen J, Niedoszytko M, Nedoszytko B. Serum tryptase, interleukin 6 and SCORMA Index as disease severity parameters in childhood mastocytosis. Post Dermatol Alergol. 2010;27:338–45. [Google Scholar]
- 32.Metcalfe DD, Akin C. Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk Res. 2001;25:577–82. doi: 10.1016/s0145-2126(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 33.Brockow K, Akin C, Huber M, Metcalfe DD. Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: relationship to symptomatology, tryptase levels, and bone marrow pathology. J Am Acad Dermatol. 2003;48:508–16. doi: 10.1067/mjd.2003.98. [DOI] [PubMed] [Google Scholar]
- 34.Brockow K, Scott LM, Worobec AS, Kirshenbaum A, Akin C, Huber M. Regression of urticaria pigmentosa in adult patients with systemic mastocytosis: correlation with clinical patterns of disease. Arch Dermatol. 2002;138:785–90. doi: 10.1001/archderm.138.6.785. [DOI] [PubMed] [Google Scholar]
- 35.Sperr WR, Horny HP, Lechner K, Valent P. Clinical and biologic diversity of leukemias occurring in patients with mastocytosis. Leuk Lymphoma. 2000;37:473–86. doi: 10.3109/10428190009058500. [DOI] [PubMed] [Google Scholar]
- 36.Horny HP, Sotlar K, Sperr WR, Valent P. Systemic mastocytosis with associated clonal haematological non-mast cell lineage diseases: histopathological challenge. J Clin Pathol. 2004;57:604–8. doi: 10.1136/jcp.2003.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: Delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3–11. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Feger F, Dumas AR, Leriche L, Valent P, Arock M. Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol. 2002;127:110–4. doi: 10.1159/000048179. [DOI] [PubMed] [Google Scholar]
- 39.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocysis: the role of KIT mutations. Br J Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 40.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosios and related mast cell didorders: current treatment options and propose response criteria. Leuk Res. 2003;27:635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 41.Horny P. Mastocytosis an unusual clonal disorder of bone marrow-derived hematopoietic progenitor cell. Am J Clin Pathol. 2009;132:438–47. doi: 10.1309/AJCPPXHMN5CJOXHZ. [DOI] [PubMed] [Google Scholar]
- 42.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf K. Treatment of cutaneous mastocytosis. Int Arch Immunol. 2002;127:156–9. doi: 10.1159/000048190. [DOI] [PubMed] [Google Scholar]
- 45.Hartmann K, Siebenhaar F, Belloni B. Effects if topical treatment with raft modulator miltefosine and clobetasol in cutaneous mastocytosis: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2010;162:185–90. doi: 10.1111/j.1365-2133.2009.09434.x. [DOI] [PubMed] [Google Scholar]
- 46.Niedoszytko M, de Monchy J, van Doormaal JJ, Jassem E, Oude Elbering JN. Mastocytosis and insect venom allergy: diagnosis, safety, and efficacy of venom immunotherapy. Allergy. 2009;64:1237–45. doi: 10.1111/j.1398-9995.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 47.Valent P, Akin C, Sperr WR, et al. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005;46:35–48. doi: 10.1080/10428190400010775. [DOI] [PubMed] [Google Scholar]
- 48.Gleixner KV, Mayerhofer M, Aichberger KJ, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2Cda) and evaluation of cooperative drug effects. Blood. 2006;107:752–9. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 49.Aichberger KJ, Gleixner KV, Mirkina I, et al. Identification of proapoptotic Bim as a tumor suppressor in neoplastic mast cells: role of KIT D816V and effects of various targeted drugs. Blood. 2009;114:5342–51. doi: 10.1182/blood-2008-08-175190. [DOI] [PubMed] [Google Scholar]
- 50.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–91. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]