Abstract
Visceral leishmaniasis (VL) is a serious lethal parasitic disease caused by Leishmania donovani in Asia and by Leishmania infantum chagasi in Southern Europe and South America. VL is endemic in 47 countries with an annual incidence estimated to be 500,000 cases. This high incidence is due in part to the lack of an efficacious vaccine. Here, we introduce an innovative approach to directly identify parasite vaccine candidate antigens that are abundantly produced in vivo in humans with VL. We combined RP-HPLC and mass spectrometry and categorized three L. infantum chagasi proteins, presumably produced in spleen, liver, and bone marrow lesions and excreted in the patients’ urine. Specifically, these proteins were the following: Li-isd1 (XP_001467866.1), Li-txn1 (XP_001466642.1), and Li-ntf2 (XP_001463738.1). Initial vaccine validation studies were performed with the rLi-ntf2 protein produced in E. coli mixed with the adjuvant BpMPLA-SE. This formulation stimulated potent Th1 response in BALB/c mice. Compared to control animals, mice immunized with Li-ntf2 + BpMPLASE had a marked parasite burden reduction in spleens at 40 days post-challenge with virulent L. infantum chagasi. These results strongly support the proposed antigen discovery strategy of vaccine candidates to kala-azar and opens novel possibilities for vaccine development to other serious infectious diseases.
Keywords: Leishmania infantum chagasi, vaccine, ntf2, kala-azar, visceral leishmaniasis, urine
INTRODUCTION
Visceral leishmaniasis (VL) or kala-azar is a worldwide protozoal vector borne disease, endemic in 47 countries with approximately 200 million people at risk of infection and with an annual incidence estimated to be 500,000 cases (http://www.doctorswithoutborders.org/publications/reports/2010/MSF-Kala-Azar-Fact-Sheet.pdf). In addition to being human disease, VL is a zoonotic infection as well. Foxes and domestic dogs are major vertebrate reservoirs of the parasite. Canine VL (CVL) is widely distributed in Latin America and southern Europe (1, 2). In the USA the potential for CVL to be a significant problem has been recently highlighted (3–5).
Acquired resistance to leishmaniasis is mediated by T cells. T cell deficient mice rapidly succumb after infection with most species of Leishmania and adoptive transfer of normal T cells confers resistance to the animals. Moreover, patients with AIDS are highly susceptible to VL either as a result of concurrent infection or as a reactivation of older sub-clinical infection (6). Among the T cells CD4+ (Th1) are crucial for resistance and CD8+ T cells seem to participate more in the memory events of the immune response involved in parasite elimination (6–11).
Although little success has been achieved in vaccine development to VL, vaccine candidates have been tested in mice and dogs and proven to induce some level of protection (12–20). In contrast, vaccination against cutaneous leishmaniasis (CL) has been practiced for centuries. Deliberate inoculation of virulent organisms from the pus of an active lesion was an ancient practice, a process known as leishmanization, has proven to be efficacious and is still used in some countries, notably Uzbekistan (21). Vaccination using crude antigen preparation obtained from promastigote forms of various species of Leishmania (cutaneous and visceral complexes) have been tested in human clinical trials in both the Old and New World. The results vary from 0–75% efficacy against CL and only modest protection against VL (22–25). Although none of these vaccine approaches is ideal, their results do support the proposal that induction of protection against leishmaniasis (CL and VL) is feasible and can be achieved with either a viable or a sub-unit vaccine. Indeed, a recombinant protein vaccine for CL, developed by our group that induces excellent protection in the mouse and monkey models of CL (21, 26–28) is currently in human clinical trials (29).
Here, we describe an innovative approach for the direct identification of VL vaccine candidate molecules that are produced in vivo during disease and that are present in bodily fluids of patients with VL. This approach led to the identification of several polypeptides of L. infantum chagasi, one of which has been extensively studied and is reported here. Importantly, this antigen Li-ntf-2 (NCBI accession XP_001463738.1), tested as a vaccine candidate formulated with the adjuvant BpMPLA-SE, induced marked parasite burden reduction in a mouse model of VL.
MATERIAL AND METHODS
Human samples
A total of seven urine samples were evaluated in this study. These samples were collected from seven patients diagnosed with VL based on the following criteria: a clinical course consistent with VL (e.g., fever, anemia, hepatosplenomegaly), and confirmatory laboratory findings (identification of Leishmania in bone marrow aspirates). Two patients were from the University Hospital, University of Brasília Medical School (Brasília, DF, Brazil) and five patients were from the Natan Portela Institute of Tropical Diseases, Federal University of Piauí, Teresina, PI, Brazil. Urine donation protocol was approved by the Investigational Review Boards and Ethics Committees of both University hospitals. Urines were frozen immediately after collection and sent frozen to The Forsyth Institute, Cambridge, MA.
Mass spectroscopy analysis
Individual samples (15 ml) were concentrated using Centricon P3 (3kDa cutoff filters) to ~200–300µl. Urine samples were then submitted to SDS-PAGE followed by Coomassie staining. Bands were excised from the gel and submitted for mass spectroscopy analysis at the Taplin Mass Spectrometry Facility, Harvard Medical School, Boston, MA. Gel bands were then trypsin-digested into peptides. Peptides were analyzed by nano-scale liquid chromatography coupled to a tandem mass spectrometer. Eluted peptides first had their molecular masses measured, then were fragmented, and finally the fragment masses were measured. The specific fragmentation pattern was computer-searched against predicted tryptic peptides from all known proteins from genome sequencing projects of human and Leishmania. The power of the technique is in its redundancy. Because many peptides are generated from the initial gel band, multiple matches to the protein of interest are detected. In this way, the protein identity is completely unambiguous. Peptide score cutoff values were chosen at Xcorr of 1.8 for singly charged ions, 2.5 for doubly charged ions, and 3.0 for triply charged ions, along with deltaCN values of 0.1, and RSP values of 1. The cross correlation values chosen for each peptide assure a high confidence match for the different charge states, while the deltaCN cutoff insures the uniqueness of the peptide hit. The RSP value of 1 insured that the peptide matched the top hit in the preliminary scoring and that the peptide fragment file only matched to one protein hit.
Animals
Female BALB/c mice and LVG Golden Syrian hamsters were purchased from Charles River Laboratories (Wilmington, MA) and kept under specific pathogen-free conditions. Animals were used at 12 weeks of age. All experiments were carried out under the guidelines of the Institutional Animal Care and Use Committee at the Forsyth Institute.
Parasite
The L. infantum chagasi strain used in these studies was kindly supplied by Dr. Mary E. Wilson (University of Iowa, Iowa City, IA) and was maintained in vivo in hamsters. Metacyclic promastigotes of the parasite were used for challenge infections. For challenge infection of BALB/c mice parasites were isolated from the spleen of hamster and cultured in Schneider’s medium (Invitrogen, Carlsbad, CA) supplemented with 20% FBS (Hyclone, Thermo Scientific, Rockford, IL) and 2mM L-glutamine (Gibco-Invitogen, Carlsbad, CA) for 7–10 days at 26°C.
Cloning of Leishmania infantum chagasi gene, protein expression and purification
Oligonucleotide PCR primers were designed to amplify the full-length open reading frame of the target gene from genomic DNA of L. infantum chagasi. The forward primer contained an Nde I restriction site at the ATG initiation codon followed by sequences derived from the gene. The reverse primer included a Bam HI restriction site followed by a stop codon and sequences from the gene. The resultant PCR product was digested with restriction enzymes and subcloned into pET-14b expression vector, which was similarly digested for directional cloning. Alternatively, the Li-ntf-2 DNA sequence was codon optimized for expression in E. coli containing the same restriction enzyme sites (Nde I and Bam HI). The DNA fragment was synthesized (Blue Heron, Bothel, WA) and the synthetic gene was sub-cloned into pET-14b as well. A ligated pET-14b vector was subsequently used to transform E. coli BL21(DE3)pLysS host cells (Novagen, Madison, WI) for expression. Recombinant protein was obtained and purified from 100ml of IPTG-induced batch cultures by affinity chromatography using QIAexpress Ni-NTA agarose matrix (QIAGEN, Chatsworth, CA) as described (30, 31). The yields of recombinant protein were 10–20mg per liter of induced bacterial culture, and purity was assessed by SDS-PAGE followed by Coomassie blue staining. Endotoxin level present in the in the purified recombinant protein preparations was measured by the Limulus Amebocyte Lysate assay (Lonza, Walkersville, MD) and shown to be 2.89 EU/ml.
Western Blot
Purified recombinant proteins (100 ng) and whole antigen extract from Leishmania infantum chagasi were fractionated by SDS-PAGE (4–20% gradient gel) and transferred to polyvinylidene fluoride membrane (PVDF, Millipore, Medford, MA). Crude Leishmania chagasi lysates were prepared from amastigotes freshly recovered from spleen of infected hamster, or after promastigote parasites were cultured for 7–10 days in complete Schneider’s medium at 26°C (promastigote lysate preparation). The blots were blocked overnight at 4°C with Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 1% bovine serum albumin (BSA) and subsequently probed with antigen specific rabbit antisera and pre-immune rabbit. After several rinses with TBS-T, goat anti-rabbit IgG labeled with horseradish peroxidase (ThermoScientific Pierce, Rockford, IL) was added. After additional washings, bound conjugates were detected using ECL enhanced chemiluminescence system (Amersham/GE Healthcare, Piscataway, NJ) and proteins were visualized by autoradiography (Kodak BioMax, Rochester, NY).
Immunization regimen and challenge
Groups of 10 mice were injected subcutaneously as follow: control mice were injected with saline plus adjuvant only (20µg/ml), and immunized mice were injected with recombinant protein (5µg/mouse) in presence of 20µg/ml of the adjuvant BpMPLA-SE (Institute Butantan, São Paulo, Brazil). BpMPLA-SE is an oil-in water emulsion containing monophosphoryl lipid A (MPLA) derived from Bordetella pertussis. The B. pertussis MPLA-SE is a stable oil in water emulsion that contains squalene (the oil phase) and the surfactant Tween 80. A detailed description of this adjuvant formulation and its biological and immunological properties has been described elsewhere (32, 33). Mice in each group were vaccinated three times with same doses of inoculums following three weeks intervals. Ten days after the last immunization three mice from each group were euthanized to carry out T cell immunogenicity assays, and four weeks after immunization remainder mice were challenged intravenously with 10×106 L. infantum chagasi promastigotes to assess vaccination efficacy.
In vitro cytokine response assay
Ten days after the last boost spleens were excised and splenocytes harvested using 70µm cell strainers. After centrifugation over Histopaque (Sigma, St. Louis, MO) and washings, mononuclear cell suspensions were prepared in RPMI supplemented with 10% FBS (Hyclone), 100µg/ml streptomycin, 100U/ml penicillin, 25mM HEPES, 2mM L-glutamine, 0.05mM 2-ME (all Sigma). Cell viability was estimated with Trypan Blue 0.4% (Sigma). 2×105 cells were added to the wells of a 96-well flat-bottomed culture microplate (Costar, Lowell, MA). Cells were stimulated for 72h with 5 µg/ml of recombinant protein. Supernatants were collected and frozen until use. Cells cultured in presence of ConA (5µg/ml) or complete medium alone were included as controls. Cytokine (IFN-γ and IL-4) concentration in the supernatants was measured using specific sandwich ELISA kit (R&D Systems, Minneapolis, MN).
Flow cytometry and intracellular cytokine staining assay
Ten days after the last boost mice were sacrificed, spleens were excised and splenocytes were obtained as described above. Cells from both immunized and control mice were counted and adjusted to 2×107 cells/ml in RPMI 1640 medium (Gibco-Invitrogen) supplemented with 10 % FBS, 25 mM HEPES, 2 mM L-glutamine, 20 U of penicillin/ml, 20 µg of streptomycin/ml. Two million cells (100 µl) from individual mice were incubated with medium or recombinant protein (2 µg/ml) plus Golgi Stop (2 µl/ml) on a 96-cell culture plate. As positive control, splenocytes were incubated with PMA (2 µg/ml), ionomycin (10 µg/ml) and Golgi Stop. The cells were incubated at 37°C for 6h and transferred to 4°C overnight. The following day, cells were washed with 2% FBS in PBS followed by incubation with monoclonal antibodies specific for cell surface molecules for an additional 30 min. Permeabilization was performed for one hour with Cytofix/Cytoperm solution (BD Biosciences). Cells were washed with 1X Perm/Wash buffer (BD Biosciences) and then stained with anti-cytokine mAb (anti-IFN-γ or anti IL-4). After an additional washing with 1X Perm/Wash buffer, the cells were fixed in 2% formaldehyde-PBS. Samples were collected on FACSAria III instrument (BD Biosciences, San José, CA) and analyzed using FlowJo software (Tree Star). The following antibodies from BD Biosciences, San José, CA were used: anti-CD3e-FITC (145–2C11); anti-IFN-γ-APC (XMG1.2); anti-IL-4-PE (BVD4-1D11); and anti-CD4-APC-Cy7 (GK1.5).
Parasite quantification
The load of Leishmania in the spleen was estimated by limiting dilution assay. Forty days after challenge with L. infantum chagasi promastigotes animals were euthanized and spleens were removed and homogenized in complete Schneider's medium. Homogenates were 10-fold serially diluted in 12 replicates for each dilution in 96-well flat-bottomed microplates (one plate per spleen) and incubated for 7–10 days at 26°C. Number of viable parasites was calculated from the highest dilution in which parasites could be found under phase contrast light microscopy in any of the 12 replicates (34).
Antibody ELISA
IgG1 and IgG2a antibodies raised against L. infantum chagasi recombinant protein were titrated by standard ELISA protocol. High binding 96-well microplates (Costar) were coated with purified recombinant protein (2µg/ml) prepared in 0.2M sodium carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Wells were washed with PBS containing 0.05% Tween 20 (PBS-T) and blocked with 1% BSA in PBS-T for 2 hours. Serum samples were added at 2-fold serial dilutions in PBS-T containing 0.1% BSA and plates were incubated for 1 hour at RT. After another washing step, biotinylated rat anti-mouse IgG1 or IgG2a mAb (2µg/ml, BD Biosciences) was added and incubated for 1 hour. Streptavidin-HRP conjugate (BD Biosciences) was used at 1/2000 followed by addition of ready-to-use substrate solution containing tetramethylbenzidine (KPL, Gaithersburg, MD). Color development was stopped using 1N HCl. Optical density data were recorded at A450.
Statistical analysis
Data are presented as mean and SEM. Statistical significance was determined by the Student t test for comparison of two groups. Values of ρ < 0.05 were considered statistically significant.
RESULTS
Antigen discovery of L. infantum chagasi proteins in urine of VL patients
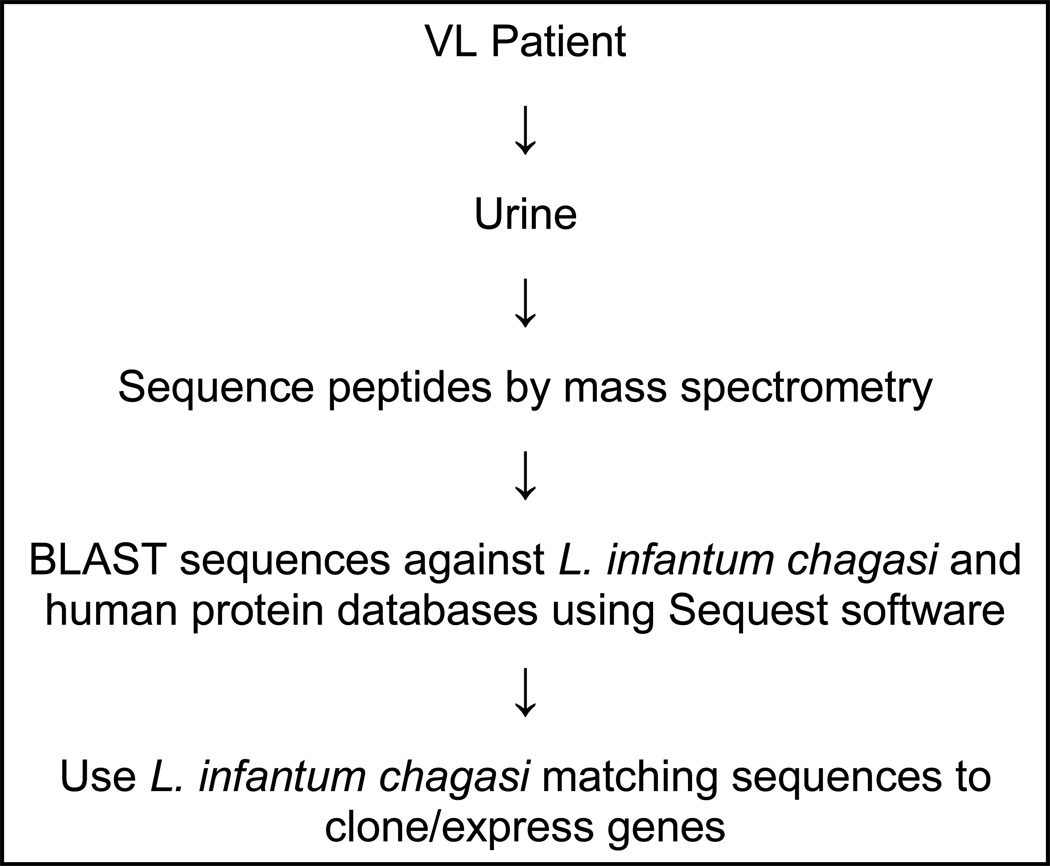
The general protocol to analyze the human urine samples from VL patients is illustrated in Fig. 1. Urines were initially collected from seven patients with active, culture-confirmed VL. These patients did not have any clinical signs or symptoms or laboratory findings compatible with renal or urinary tract abnormalities. None of the patients were under anti-leishmaniasis therapy at the time of urine collection. Individual urine samples were analyzed by mass spectrometry and generated approximately 400 peptide sequences. As expected, most sequences of the identified peptides had identical sequence homologies with that of human proteins. Importantly, eight peptide sequences that had no known homologies with human proteins had identical sequence homologies with the deduced sequences of three different L. infantum chagasi proteins. Figure 2 depicts the discovered proteins and highlights the peptides found in the patients’ urines. Importantly, these L. infantum chagasi peptides were identified in multiple urine samples, thus strongly validating these findings. Overall, these findings suggest that these proteins are abundant antigens of L. infantum chagasi produced during the disease and broadly present in human bodily fluids.
Figure 1. Schematic representation of antigen discovery strategy of L. infantum chagasi proteins produced in vivo and excreted in urine of patients with active VL.
Figure 2. L. infantum chagasi peptide sequences discovered in urine of VL patients.
Peptide sequences and positioning within the peptide donor protein sequences are highlighted in red/bold/underline. Note that several different peptides spanning at different sequence positions of the donor proteins were identified, which unambiguously define them as truly L. infantum chagasi peptides/proteins.
Both L. infantum chagasi iron superoxide dismutase 1 and L. infantum chagasi tryparedoxin 1 have been previously described and their immunological properties studied in some details (35–37). In contrast, little is known about the biology of the putative L. infantum nuclear transport factor 2 (Li-ntf2) (38) and no reports were found about the immunological significance of the molecule. Therefore, the present study concentrates on the evaluation of Li-ntf2 as a vaccine candidate for VL.
The open reading frame of the full-length gene coding Li-ntf2 was amplified by PCR and sub-cloned into the E. coli pET-14b expression vector. This cloning strategy resulted in no expression of protein. However, using a synthetic gene with optimized sequence for E. coli followed by sub-cloning in pET-14b resulted in excellent over-expression of the recombinant protein, which was then purified using the Ni-NTA agarose resin.
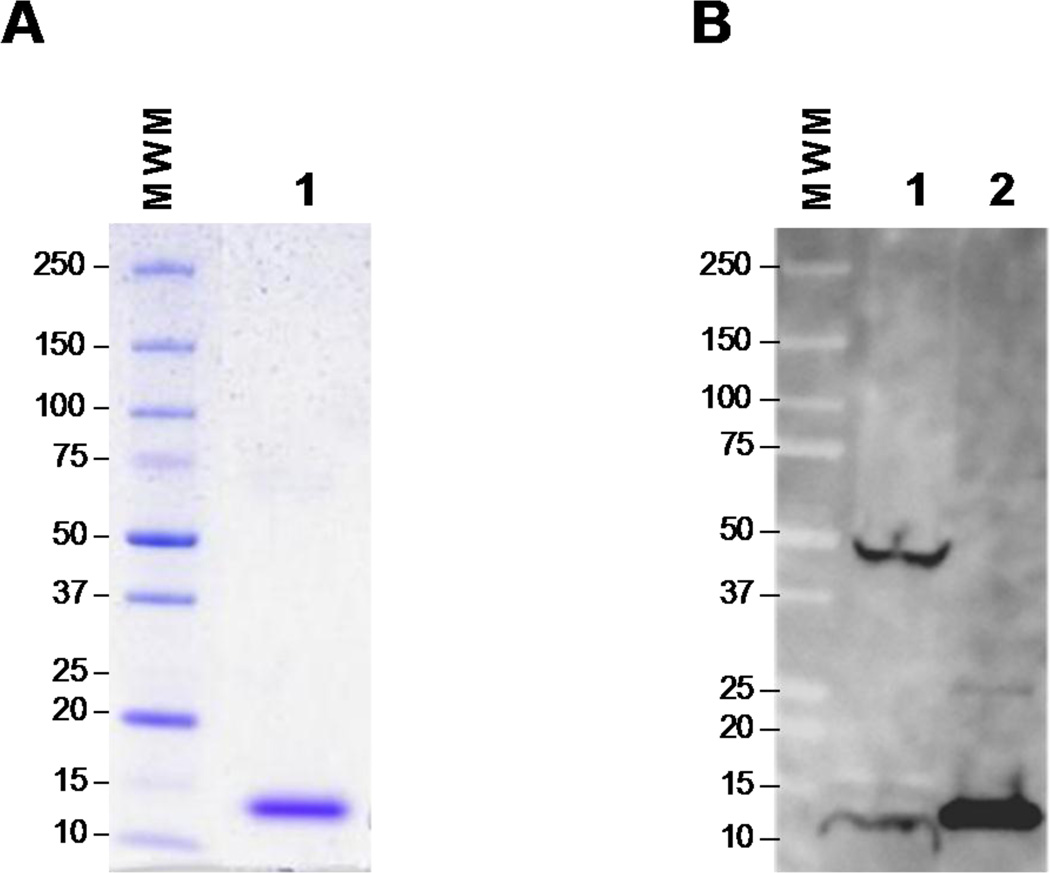
In order to validate the E. coli-expressed and purified protein (Fig. 3A) as true L. infantum chagasi protein, a crude lysate of the parasite and the recombinant protein were immunoblotted and then probed with specific polyclonal rabbit anti-recombinant Li-ntf2 antisera (Fig. 3B). The antiserum clearly recognized a protein band in the crude parasite lysate at similar molecular weight as the recombinant protein. Therefore, the results support that the recombinant Li-ntf2 is a replica of the native protein produced by L. infantum chagasi. A second band of MW ~45 kDa was also recognized by the antiserum. However, the nature of this band is not known. It is possible that the Li-ntf2 aggregates with itself, or is part of a larger protein complex, or a building block of a larger protein originated by post-translational modification.
Figure 3. Characterization of purified recombinant Li-ntf2.
Purity of the protein was evaluated by SDS-PAGE (4–20% gradient polyacrylamide gel) and Coomassie blue staining (A). Characterization was done by Western blot analysis (B) using a crude antigenic preparations of L. infantum promastigotes (lane 1) and purified recombinant Li-ntf2 (lane 2). Proteins were identified using specific rabbit anti-recombinant Li-ntf2 antiserum. Reactivity was detected with goat anti-rabbit IgG labeled with horseradish peroxidase followed by autoradiography. Numbers on the left side indicate the molecular weights of the markers.
Immunogenicity studies in mice
Because CD4+ T cells of the Th1 phenotype are crucial mediators of resistance to VL, we chose to immunize mice with a formulation containing the purified recombinant Li-ntf2 plus the adjuvant monophosphoryl lipid A (MPLA) from Bordetella pertussis emulsified with squalene (Butantan Institute, São Paulo, SP, Brazil). An MPL based adjuvant was chosen because this type of formulation has been shown to be safe and to preferentially stimulate Th1 responses (26, 29, 32, 33). Squalene is added to condition a stable oil-in-water emulsion (BpMPLA-SE) to further increase the stimulation of the immune system. Mice were immunized three times with 5µg of Li-ntf2 alone or mixed with 20µg of BpMPLA-SE. As control, another group of mice was immunized with saline + BpMPLA-SE. The choice of 20µg of BPMPLA-SE was based on initial dose response studies that indicated that this concentration, compared to 2.5µg, 5.0µg, and 10µg, induced the most robust immune response (not shown). Ten days after the last immunization mice were bled (for serologic studies) and sacrificed. Spleens were removed and splenic cell suspensions were prepared and stimulated for 6 hours with either medium (control) or purified recombinant Li-ntf2. Cells were harvested and cytokine responses (IFN-γ and IL-4) were measured by flow cytometry for intracellular cytokine staining (ICS). Culture supernatants from cells stimulated for 72 hours were used to measure total cytokine response by conventional capture ELISA.
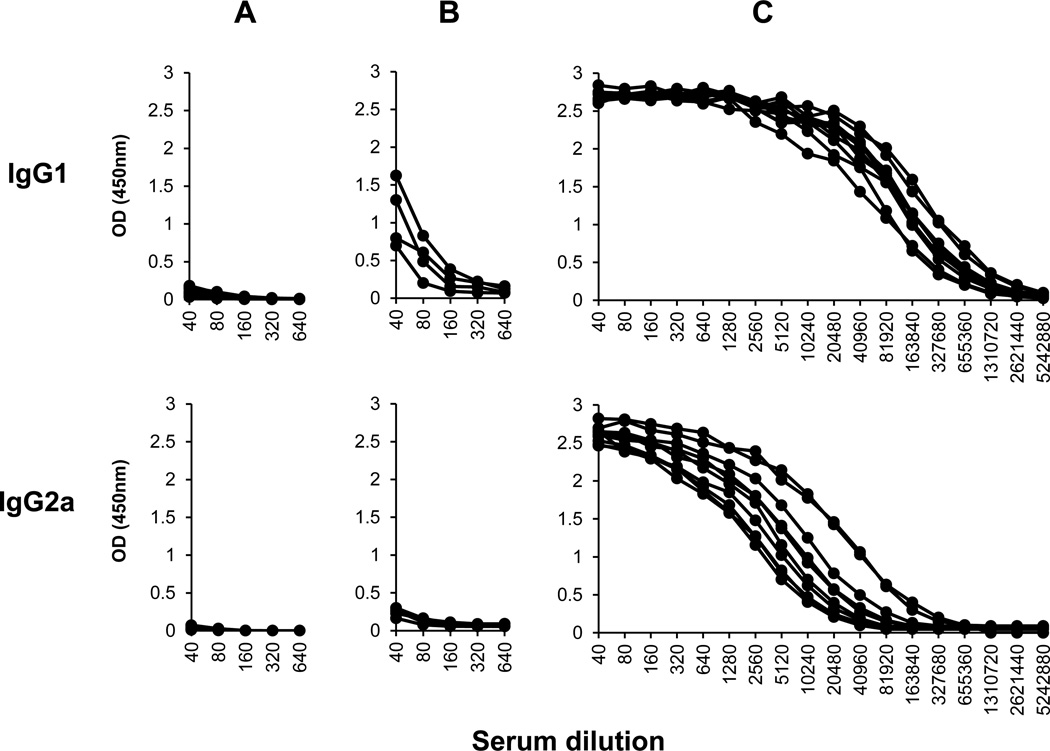
Figure 4 shows the serological titration of the IgG1 and IgG2a specific antibody response to the recombinant Li-ntf2. High titers of both IgG1 and IgG2a were generated after immunization with Li-ntf2 + BpMPLA-SE. In contrast, animals immunized with the protein alone (mixed with saline) produced low titers of IgG1 and no detectable levels of IgG2a. Because class switch to IgG2a is dependent of production of IFN-γ, this result is consistent with a stimulation of Th1 response by the antigen/adjuvant formulation.
Figure 4. Isotype specific antibody response of mice immunized with purified recombinant Li-ntf2 formulated with the adjuvant MPLA-SE.
Ten mice were immunized three times (three weeks apart) with saline plus the adjuvant BpMPLA-SE (A), or with the Li-ntf2 in saline (B), or with Li-ntf2 mixed with BpMPLA-SE (C). Animals were bled 10 days after the last immunization and antibody responses of IgG1 and IgG2a isotypes were measured by ELISA using biotinylated isotype rat mAbs specific for mouse IgG isotypes. The curves represent the responses of each individual mouse.
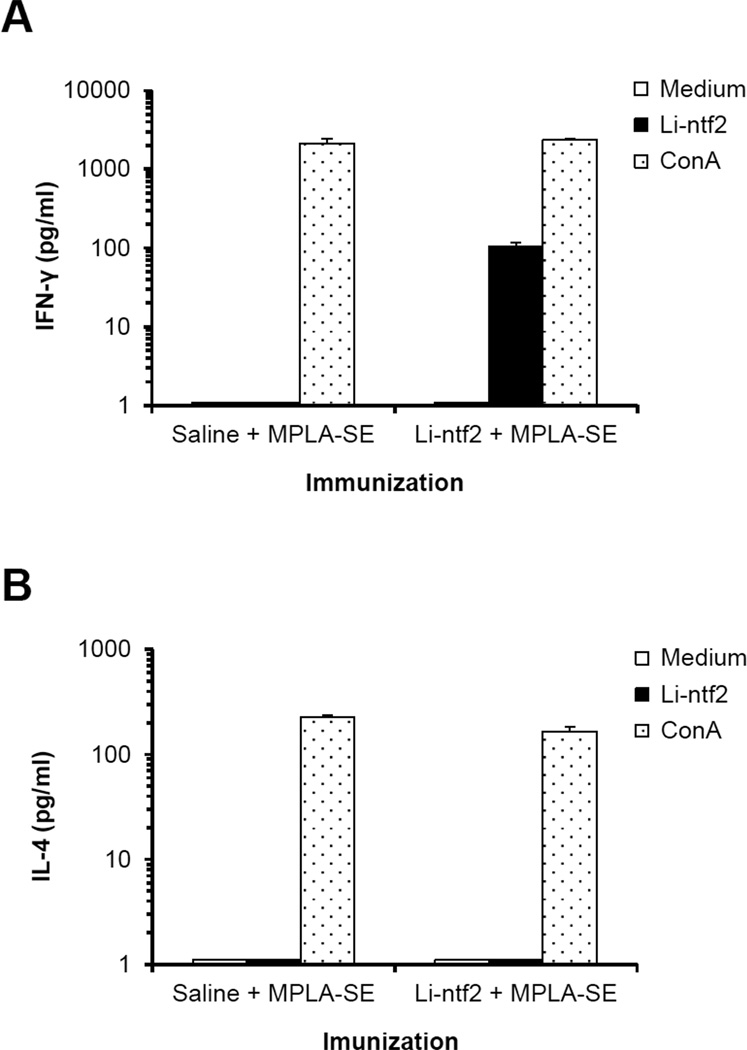
The antigen stimulated cytokine response of cells obtained from animals immunized with Li-ntf2 + BpMPLA-SE as well as with saline + BpMPLA-SE was initially tested in the culture supernatant of spleen cell cultures stimulated with Li-ntf2. Results are illustrated in Fig. 5. In the cell cultures obtained from antigen immunized mice and stimulated in vitro with Li-ntf2 the Th1 cytokine IFN-γ was readily detected in the culture supernatants. This cytokine was not detected in the culture supernatants of cells stimulated with medium alone nor in the cultures from spleen cells obtained from mice immunized with saline + BpMPLA-SE and stimulated in vitro with recombinant Li-ntf2. Moreover, no IL-4 could be detected in the supernatants of antigen-stimulated cultures. However, both IFN-γ and IL-4 were readily detected in the culture supernatants of cultures stimulated with the mitogen ConA thus confirming the potential of the cells to produce both cytokines. These results confirm the antigen specificity of the responses obtained from cells of mice immunized with recombinant Li-ntf2 plus BpMPLA-SE, i.e., the trace amount of LPS (<2,9 EU/ml) present in the antigen preparation is not responsible for the antigen induction of cytokine production by cells from immunized Li-ntf2-immunized mice. Finally, no IL-4 could be detected in any of the culture supernatants (not shown).
Figure 5. Cytokine response of spleen cells from mice immunized with purified recombinant Li-ntf2 formulated with the adjuvant BpMPLA-SE.
BALB/c mice (three) were immunized as described in Fig. 4. Spleen cells were harvested 10 days after the last immunization either with saline + BpMPLA-SE or purified recombinant Lintf2 + BpMPLA-SE. Cells were cultured for 72h in the presence of medium only, or with 5µg/ml of the recombinant protein, or with 5µg/ml of ConA;. Culture supernatants were collected and assayed for the presence of IFN-γ and IL-4 by sandwich ELISA.
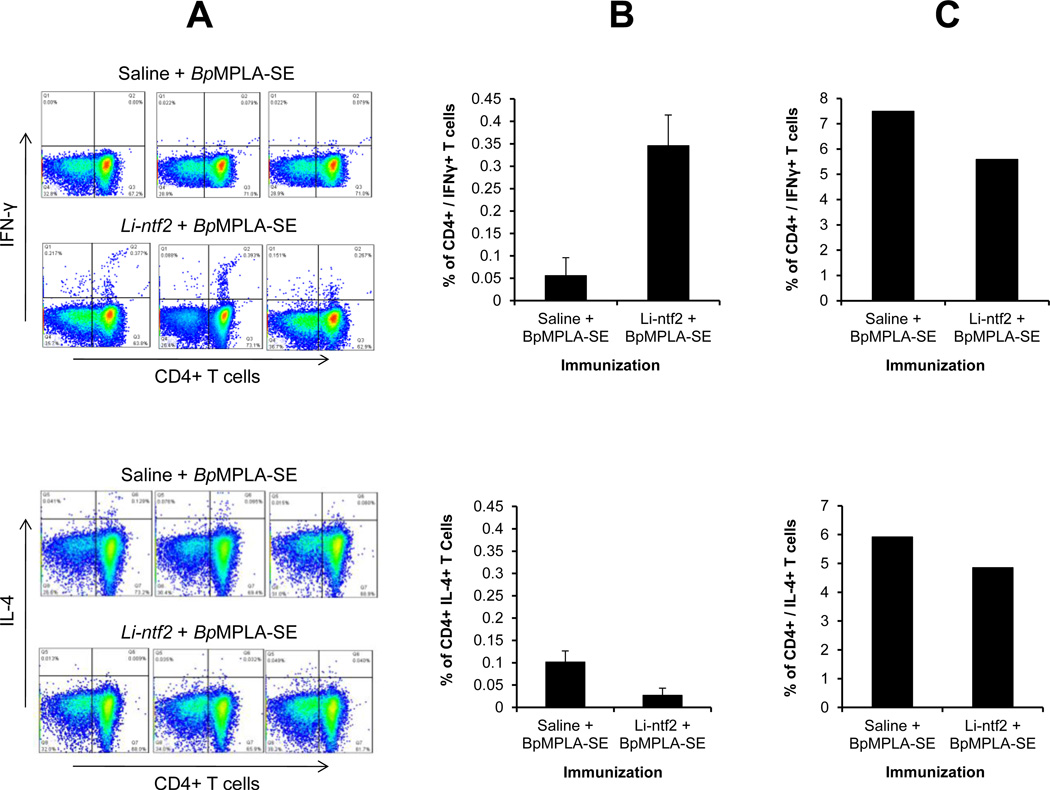
The confirmation that the IFN-γ detected in the culture supernatants was produced by CD4+ T cells was obtained by intracellular cytokine staining (ICS). The results are illustrated in Fig. 6. As can be seen, the results obtained using ICS clearly point to CD4+ T cells as an important source of IFN-γ produced upon stimulation with recombinant Li-ntf2. In the cell cultures obtained from antigen immunized mice and stimulated in vitro with Li-ntf2 the Th1 cytokine IFN-γ was readily detected (Fig. 6A, upper panel). In contrast no IL-4 producing cells could be detected (Fig. 6B, lower panel). As expected, no IFN-γ or IL-4 producing cells were detected in cells obtained from mice immunized with saline + BpMPLA-SE and re-stimulated in vitro with Li-ntf2. However, CD4+ T cells producing both IFN-γ and IL-4 were clearly detected in cultures stimulated with the polyclonal activators PMA + ionomicin (Fig. 6C). Together these results confirm that the immunization protocol using the adjuvant BpMPLA-SE induces a preferential Th1 response to the L. infantum chagasi vaccine candidate Li-ntf2.
Figure 6. Phenotype of CD4+ T cell response of mice immunized with purified recombinant Li-ntf2 formulated with the adjuvant BpMPLA-SE.
Three mice were immunized as described in Fig. 4. Spleen cells were harvested 10 days after the last immunization either with saline + BpMPLA-SE or purified recombinant Li-ntf2 + BpMPLA-SE. Production of IFN-γ (Upper panel) and IL-4 (Lower panel) by CD4+ T cells was determined by intracellular cytokine staining and analyzed by flow cytometry. (A), Dot plots represent the responses of individual mouse in each group after stimulation with Li-ntf2; (B) Percentage double positive CD4+ / IFN-γ+ T cells (upper panel) or CD4+ / IL-4+ T cells (lower panel) stimulated with the Li-ntf2 (average of responses of cells from three mice per group); and (C) Percentage double positive CD4+ / IFN-γ+ T cells (upper panel) or CD4+ / IL-4+ T cells (lower panel) stimulated with the polyclonal activators PMA + Ionomicin (average of responses of cells from three mice per group). Note the abundance of CD4+ / IFN-γ+ double positive cells and the scarce presence of CD4+ / IL4+ double positive cells in cultures obtained from mice immunized with Li-ntf2 + BpMPLA-SE and re-stimulated in vitro with Li-ntf2. Cells from both groups of immunized mice produced abundant quantities of both IFN-γ and IL-4 when stimulated with PMA + Ionomicin. Little or no cytokine producing cells were observed in the cell cultures from animals immunized with saline + BpMPLA-SE and equally re-stimulated in vitro with the antigen. Similarly, no cytokine producing cells were detected in any culture stimulated with medium only (not shown).
Protection studies in mice
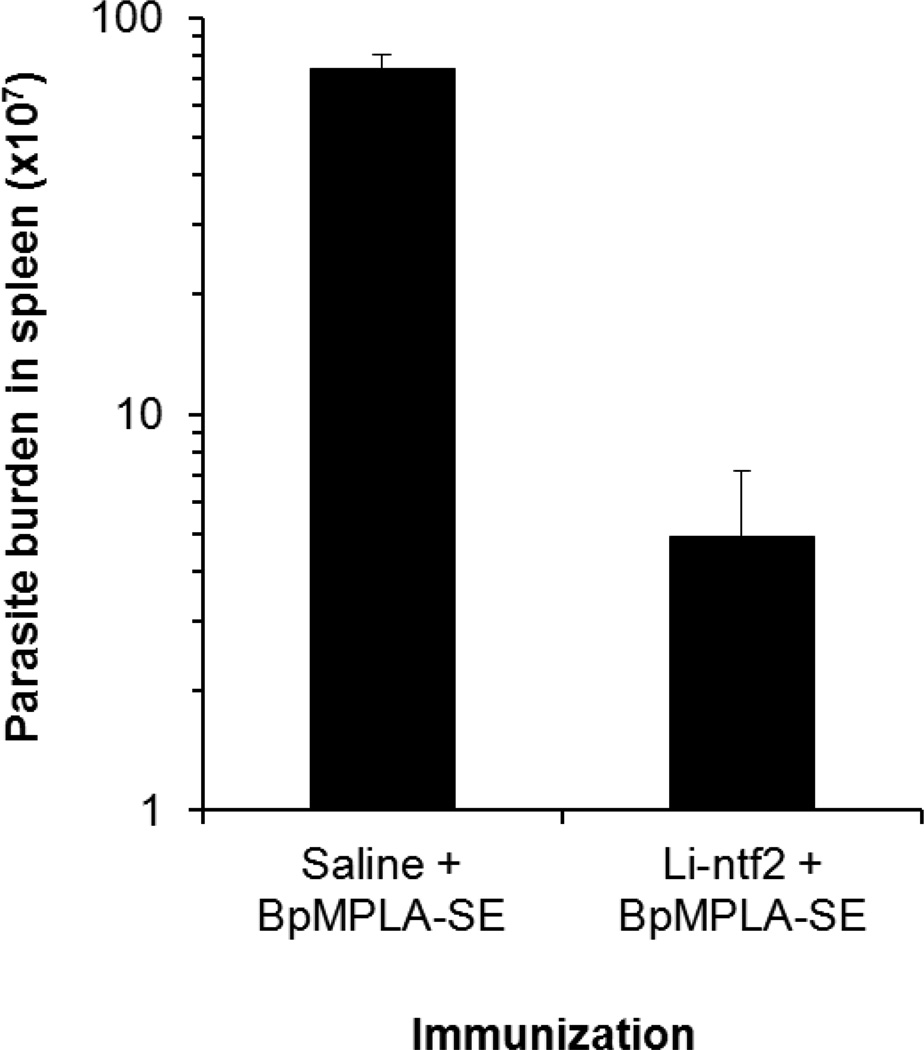
Although mice is not considered an ideal model of VL these animals have been used and accepted for initial evaluation and selection of vaccine candidate antigens for future validation in large animal models (e.g., dogs). Two groups of BALB/c mice were immunized three times (three weeks interval) with either 5µg of the recombinant Li-ntf2 mixed with 20µg of the adjuvant BpMPLA-SE or with saline mixed with 20µg of BpMPLA-SE. For these experiments, a group of animals immunized with Li-ntf2 alone was not included because the immunogenicity studies indicated that this formulation stimulated little or undetectable immune response. Thirty days after the last immunization mice were challenged i.v. with 10×106 promastigotes (stationary phase of growth) freshly derived from amastigotes isolated from the spleen of L. infantum chagasi-infected hamster. To determine protection, animals were sacrificed 40 days later and spleen homogenates were prepared in a serial dilution manner and incubated in Schneider’s medium for limiting dilution quantification. Parasite enumeration was performed one week later and results are expressed in Fig. 7. As can be seen immunization with recombinant Li-ntf2 plus BpMPLA-SE caused significant reduction in the parasite burden (1.3 log10) compared to animals immunized with saline + BpMPLA-SE. Therefore, these results support the hypothesis that abundant L. infantum chagasi antigens present in bodily fluids of humans during disease can be potential target vaccine candidates.
Figure 7. Vaccination of mice against VL with purified recombinant Li-ntf2 formulated with the adjuvant BpMPLA-SE.
BALB/c mice (7 animals per group) were immunized three times as described in Figure 4. As control, mice (n=7) were immunized with saline plus BpMPLA-SE. One month after the last immunization the animals were challenged i.v. with 10×106 promastigote forms (stationary phase of growth) of L. infantum chagasi. Parasite burden in the animals’ spleens was determined 40 days later by limiting dilution assay. Bars represent SEM of the results given by 7 mice per group. Student t test was used to compare the average of parasite burden observed for animals immunized with Li-nft2 + BpMPLA-SE with that of animals immunized with Saline + BpMPLA-SE. ρ value was <0.005.
DISCUSSION
Although much effort has been placed on the development of anti-VL human vaccine little or no success has been achieved. Two vaccines to canine visceral leishmaniasis (CVL) are commercially available in Brazil but these products have not been approved for human use due to low protection efficacy in dogs as well as complications with standard operating procedures for the manufacture of one of them, which is a glycoprotein fraction purified from L. donovani promastigotes (39, 40).
The barriers to achieve better success in vaccine development to VL are not entirely understood. However, most of the antigen discovery approaches tested so far have used antibodies and in some cases PBMC from patients with VL to identify parasite molecules of interest. Although these approaches have been validated for other diseases, including CL, we believe that they are not ideal for VL because VL patients have both massive hypergammaglobulinemia due to polyclonal activation of B cells and severe immunossupression of T cell responses. Consequently, these readouts do not necessarily reflect host resistance to L. donovani/infantum chagasi.
In this work we address this critical barrier of antigen discovery. Our target vaccine candidates are parasite molecules that are abundantly produced in vivo in human patients with active VL. The hypothesis of this proposal is that microbial antigens found in vivo in the bodily fluids of the host are potentially effective vaccine candidate molecules because they represent microbial molecules actively and continuously produced and released during disease, therefore by definition, good targets for vaccine development. Moreover, our former studies in tuberculosis confirm this hypothesis (31).
Thus, we combined RP-HPLC and mass spectrometry (MS) and categorized three distinct L. infantum chagasi proteins present in the urine of seven VL patients. The rationale to use urine as source of the pathogen’s antigens was based on the premise that L. infantum chagasi proteins or their breakdown products (peptides) produced in vivo, e.g., in spleen/liver/bone marrow lesions, would reach the host’s blood circulation and subsequently would be excreted in the patient’s urine. The three L. infantum chagasi proteins identified in the urines of seven patients with VL were: L. infantum chagasi iron superoxide dismutase, L. infantum chagasi tryparedoxin, and L. infantum chagasi nuclear transport factor 2 (Li-ntf2). The MS results were unambiguously conclusive that the peptides identified in the patients’ urines were indeed derived from the parasite’s proteins. This assertion is strongly supported by the facts that the inferred amino acid sequences of the derived peptides had very high XCorr (>3.0) and several different peptides found in the patients’ urines spanned at different sequence positions of the corresponding peptide donor proteins.
The concept of detecting microbial molecules in human bodily fluids of infected individuals particularly for diagnosis purposes has strong precedent. For example, molecules from numerous viruses (e.g., hepatitis A and B), bacteria (e.g., Streptococcus pneumonia, Streptococcus pyogenes, Legionella pneumophila), and parasites (e.g., Entamoeba histolytica) have long been described in various human samples (e.g., blood, mucous secretions, and feces) of patients suffering from the diseases caused by these microorganisms (41–46). Interestingly, some of these molecules have been successfully used as vaccines in humans (e.g., for hepatitis A and B). Regarding leishmaniasis, L. donovani polysaccharide antigens have also been described to be present in the urine of VL patients and have been used in an antigen detection assay for the diagnosis of this disease (47–52). However, because of the polysaccharide nature of these molecules they are, by definition, not rational antigens to be used as vaccine candidates to VL. Resistance to VL is T cell mediated and it is well known that polysaccharide antigens, in contrast to proteins, by in large do not induce T cell responses. Therefore, the proteins described in the present studies can be of great interest as vaccine candidates to VL.
The gene coding for the identified L. infantum chagasi proteins are also present, in various other members of the Trypanosomatidae family including L. donovani, L. major, L. brasiliensis, L. amazonensis, African trypanosomes, and Trypanosoma cruzi. However, at this point we have no information if these molecules are either abundantly produced in vivo or are present in bodily fluids of patients with the diseases caused by these parasites. Future investigation will be necessary to verify these possibilities.
The present studies were concentrated on the putative Li-ntf2 because little is known about the biological and immunological properties of this leishmanial molecule. Cloning of the gene and production and purification of the recombinant molecule was easily achieved. Approximately 20mg of purified protein was obtained per liter of induced E. coli culture broth. Importantly, Western blot analyzes using specific rabbit antiserum raised against the purified recombinant Li-ntf2 clearly showed that this molecule is constitutively produced by L. infantum chagasi. Two clear bands present in the parasite’s whole antigen preparation could be detected by the antiserum. One band that migrates at approximately 14 kDa position coincides with the predicted MW of the native Li-ntf2 (13.89 kDa). A second band migrates at ~45 kDa MW position. As expected, the recombinant Li-ntf2 migrates slightly slower than the native molecule, which is due to its slightly higher MW due to the presence of the 6 His tag. It is interesting to note that this band reacts stronger with the specific anti-Li-ntf2 antiserum than the band that matches the predicted MW of the native molecule. At this point, we have no experimental evidences about the nature of this molecule. One possibility is that the native molecule is either a homo or heteropolymer of Li-ntf2. However, further investigation will need to be carried out to support this possibility.
To verify the protection potential of Li-ntf2 we chose to formulate this antigen with a monophosphoryl lipid A (MPL) adjuvant. This choice was based in our former and successful experience with this type of adjuvant in vaccine development to cutaneous leishmaniasis (27, 53–55). MPL has proven to modulate the immune system to a predominant Th1 response (31, 56, 57), which is essential to control leishmaniasis and several other infectious processes. The MPL used in the present studies (BpMPLA-SE) is the monophosphoryl lipid A (MPLA) derived from Bordetella pertussis lipopolysaccharide containing squalene and one surfactant that facilitates the formation of a stable oil-in-water emulsion. This adjuvant has proven to induce potent immune response to influenza virus (including H5N1) vaccine candidates (32, 33). In the present studies we confirmed that this adjuvant formulated with recombinant Li-ntf2 induces in BALB/c mice a potent Th1 response to this protein.
This conclusion was reached based on several evidences. First, high titer of specific antibody response of both IgG1 and IgG2a isotypes was generated against Li-ntf2. This pattern of high level of IgG2a production is generally accepted as reliable surrogate of Th1 response (58). Because IL-4 is known for many years to promote immunoglobulin class switching to IgG1, an antibody response that included this isotype of immunoglobulin was believed in the past to be a surrogate of the Th2 response. However, later evidences demonstrated that IgG1 antibodies are divided in two distinct sub-families of molecules (59). One that is dependent on IL-4 (Th2 associated) and another that is dependent on IL-12 and IFN-γ (Th1 associated). Therefore, the generation of high titers of IgG1 antibody can only be interpreted as a surrogate of a polarized Th2 response in the absence of IgG2a antibody. In contrast, because the class switch to IgG2a is solely dependent on IFN-γ, high titers IgG2a antibody, whether or not associated with IgG1 antibody has been generally accepted as a good surrogate of a typical Th1 response. Second, in vitro antigen stimulation of cells obtained from mice immunized with Li-ntf2 + BpMPLA-SE clearly indicated that IFN-γ was readily produced by CD4+ T cells. In contrast IL-4 producing CD4+ T cells could not be detected. Together, these findings clearly point to a typical Th1 phenotype of immune response induced by the antigen/adjuvant formulation.
The protection experiments in the mouse model were carried out using an identical immunization protocol used for the immunogenicity studies. Although the mice were challenged i.v. using a high inoculum (10×106 promastigote forms of the parasite at stationary phase of growth) of L. infantum chagasi freshly derived from amastigote forms isolated from infected hamsters, excellent protection was achieved. Over 1.3 log reduction in parasite burden was observed in vaccinated animals compared to controls. This level of protection is comparable and even superior to most VL vaccine reported to date (14–17, 60). The reasons to explain the difficulty to elicit a more complete protection in this animal model are not known. One possibility to explain this limitation is that most regimens of immunization do not necessarily generate CD8+ T cell responses to parasite antigens, and these cells seem to also participate in immunity/memory mechanisms of protection against Leishmania (11). Indeed, this possibility has been evaluated in more details in infectious caused by other pathogens such as Mycobacterium tuberculosis and Plasmodium. Interestingly, using heterologous prime/boost protocols of immunizations that generate both CD4+ and CD8+ T cell responses to the vaccine candidate, better protection has been achieved than with immunizations that used conventional boosting strategies (61, 62). In a typical protocol, animals are primed with a vaccine candidate using an antigenic formulation that induces preferential CD4+ T cells (e.g., protein plus adjuvant) and are boosted using a formulation that stimulates preferentially CD8+ T cells (e.g., genetic immunizations using virus or plasmid DNA carriers of the vaccine candidate). Experiments designed to evaluate the efficacy of this strategy to improve the protection induced by Li-ntf2 are in progress.
Although further protection experiments using different protocols of immunization, larger animal samples, different animals models such as hamster and dogs need to be performed before the formulation Li-ntf2 + BpMPLA-SE can be tested in clinical trials. It is important to emphasize that the findings reported in this work strongly support the proposed antigen discovery strategy of vaccine candidates to VL. Moreover, and equally important, this simple but innovative approach of antigen discovery opens novel and broader possibilities for vaccine development to other serious infectious diseases like tuberculosis, trypanosomiasis, malaria, and AIDS.
Acknowledgments
Financial support: This work was supported in part by NIH grant R43AI084259-01 awarded to CA.
Footnotes
Disclosures: SSK, LQ, KAK, FSK, FOS, DLC, DCJr, CHNC, and IR have no conflicts of interests. CA is an employee of DetectoGen Inc. and has no ownership or ownership option in the company. AC-N is a consultant for DetectoGen Inc.
REFERENCES
- 1.Belazzoug S. Leishmaniasis in Mediterranean countries. Vet Parasitol. 1992;44:15–19. doi: 10.1016/0304-4017(92)90139-z. [DOI] [PubMed] [Google Scholar]
- 2.Dujardin JC, Campino L, Canavate C, Dedet JP, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y, Boelaert M. Spread of vector-borne diseases and neglect of Leishmaniasis, Europe. Emerg Infect Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, Petersen CA. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis. 2011;5:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddlestone SM. Visceral leishmaniasis in a dog from Maryland. J Am Vet Med Assoc. 2000;217:1686–1688. 1659. doi: 10.2460/javma.2000.217.1686. [DOI] [PubMed] [Google Scholar]
- 5.Gaskin AA, Schantz P, Jackson J, Birkenheuer A, Tomlinson L, Gramiccia M, Levy M, Steurer F, Kollmar E, Hegarty BC, Ahn A, Breitschwerdt EB. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34–44. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Montalban C, Martinez-Fernandez R, Calleja JL, Garcia-Diaz JD, Rubio R, Dronda F, Moreno S, Yebra M, Barros C, Cobo J. Visceral leishmaniasis (kala-azar) as an opportunistic infection in patients infected with the human immunodeficiency virus in Spain. Rev Infect Dis. 1989;11:655–660. doi: 10.1093/clinids/11.4.655. [DOI] [PubMed] [Google Scholar]
- 7.Locksley RM, Heinzel FP, Holaday BJ, Mutha SS, Reiner SL, Sadick MD. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res Immunol. 1991;142:28–32. doi: 10.1016/0923-2494(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 8.Melby PC. Recent developments in leishmaniasis. Curr Opin Infect Dis. 2002;15:485–490. doi: 10.1097/00001432-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Muller I, Pedrazzini T, Farrell JP, Louis J. T-cell responses and immunity to experimental infection with Leishmania major. Annu Rev Immunol. 1989;7:561–578. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- 10.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 11.Stager S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 12.Palatnik-de-Sousa CB, Silva-Antunes I, Morgado AA, Menz I, Palatnik M, Lavor C. Decrease of the incidence of human and canine visceral leishmaniasis after dog vaccination with Leishmune in Brazilian endemic areas. Vaccine. 2009;27:3505–3512. doi: 10.1016/j.vaccine.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes AP, Costa MM, Coelho EA, Michalick MS, de FE, Melo MN, Luiz TW, Resende DM, Hermont V, Abrantes CF, Gazzinelli RT. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine. 2008;26:5888–5895. doi: 10.1016/j.vaccine.2008.05.095. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovaniinfections. Vaccine. 2001;20:59–66. doi: 10.1016/s0264-410x(01)00322-x. [DOI] [PubMed] [Google Scholar]
- 15.Goto Y, Bhatia A, Raman VS, Liang H, Mohamath R, Picone AF, Vidal SE, Vedvick TS, Howard RF, Reed SG. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clin Vaccine Immunol. 2011;18:1118–1124. doi: 10.1128/CVI.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafati S, Nakhaee A, Taheri T, Taslimi Y, Darabi H, Eravani D, Sanos S, Kaye P, Taghikhani M, Jamshidi S, Rad MA. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23:3716–3725. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Dondji B, Perez-Jimenez E, Goldsmith-Pestana K, Esteban M, Mahon-Pratt D. Heterologous prime-boost vaccination with the LACK antigen protects against murine visceral leishmaniasis. Infect Immun. 2005;73:5286–5289. doi: 10.1128/IAI.73.8.5286-5289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, Coler RN, Vedvick TS, Reed SG. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–7045. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25:7450–7458. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, Laughlin EM, Coler RN, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Sundar S, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29:3531–3537. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 21.Modabber F, Reed S, Campos-Neto A. Vaccines against Leishmania. In: Levine Myron M, Kaper James B, Rappuoli Rino, Liu Margaret A, Good Michael F., editors. New Generation Vaccines. 3rd. ed. New York, Basel: Mar Dekker, Inc.; 2004. pp. 903–914. [Google Scholar]
- 22.Genaro O, de Toledo VP, da Costa CA, Hermeto MV, Afonso LC, Mayrink W. Vaccine for prophylaxis and immunotherapy, Brazil. Clin Dermatol. 1996;14:503–512. doi: 10.1016/0738-081x(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 23.Gunders AE, Naggan L, Michaeli D. Follow-up study of a vaccination programme against cutaneous leishmaniasis. I. Vaccination with a 5 year-old human strain of L. tropica from the Negev. Trans R Soc Trop Med Hyg. 1972;66:235–238. doi: 10.1016/0035-9203(72)90152-6. [DOI] [PubMed] [Google Scholar]
- 24.Khalil EA, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, Musa B, Ibrahim ME, Kamil AA, Elsheikh M, Babiker A, Modabber F. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356:1565–1569. doi: 10.1016/s0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, Momeni AZ, Dowlati Y, Godal T, Zicker F, Smith PG, Modabber F. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540–1543. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 26.Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Skeiky YA, Reed SG, Grimaldi G., Jr Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos-Neto A. Anti-leishmania vaccine. In: Farrell Jay P., editor. Leishmania. vol. 4. Kluwer Academic Publishers; 2002. pp. 169–190. [Google Scholar]
- 28.Kenney RT, Sacks DL, Sypek JP, Vilela L, Gam AA, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481–4488. [PubMed] [Google Scholar]
- 29.Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Pine SO, Cowgill KD, Reed SG, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 30.Kashino SS, Pollock N, Napolitano DR, Rodrigues V, Jr, Campos-Neto A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: an innovative and alternative approach of antigen discovery of useful microbial molecules. Clin Exp Immunol. 2008;153:56–62. doi: 10.1111/j.1365-2249.2008.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Kashino SS, Zhang Y, Daifalla N, Rodrigues V, Jr, Reed SG, Campos-Neto A. Cloning of the gene encoding a protective Mycobacterium tuberculosis secreted protein detected in vivo during the initial phases of the infectious process. J Immunol. 2005;175:5298–5305. doi: 10.4049/jimmunol.175.8.5298. [DOI] [PubMed] [Google Scholar]
- 32.Miyaki C, Quintilio W, Miyaji EN, Botosso VF, Kubrusly FS, Santos FL, Iourtov D, Higashi HG, Raw I. Production of H5N1 (NIBRG-14) inactivated whole virus and split virion influenza vaccines and analysis of immunogenicity in mice using different adjuvant formulations. Vaccine. 2010;28:2505–2509. doi: 10.1016/j.vaccine.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Quintilio W, Kubrusly FS, Iourtov D, Miyaki C, Sakauchi MA, Lucio F, Dias SC, Takata CS, Miyaji EN, Higashi HG, Leite LC, Raw I. Bordetella pertussis monophosphoryl lipid A as adjuvant for inactivated split virion influenza vaccine in mice. Vaccine. 2009;27:4219–4224. doi: 10.1016/j.vaccine.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 34.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 35.Cabral SM, Silvestre RL, Santarem NM, Tavares JC, Silva AF, Cordeiroda-Silva A. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin-10 and specific immunoglobulin. Immunology. 2008;123:555–565. doi: 10.1111/j.1365-2567.2007.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin C, Longoni SS, Urbano J, Minaya G, Mateo H, de Diego JA, Rosales MJ, Perez-Cordon G, Romero D, Sanchez-Moreno M. Enzyme-linked immunosorbent assay for superoxide dismutase-excreted antigen in diagnosis of sylvatic and Andean cutaneous leishmaniasis of Peru. Am J Trop Med Hyg. 2009;80:55–60. [PubMed] [Google Scholar]
- 37.Ismail SO, Skeiky YA, Bhatia A, Omara-Opyene LA, Gedamu L. Molecular cloning, characterization, and expression in Escherichia coli of iron superoxide dismutase cDNA from Leishmania donovani chagasi. Infect Immun. 1994;62:657–664. doi: 10.1128/iai.62.2.657-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Casanova M, Portales P, Blaineau C, Crobu L, Bastien P, Pages M. Inhibition of active nuclear transport is an intrinsic trigger of programmed cell death in trypanosomatids. Cell Death Differ. 2008;15:1910–1920. doi: 10.1038/cdd.2008.132. [DOI] [PubMed] [Google Scholar]
- 39.Palatnik-de-Sousa CB, Barbosa AF, Oliveira SM, Nico D, Bernardo RR, Santos WR, Rodrigues MM, Soares I, Borja-Cabrera GP. FML vaccine against canine visceral leishmaniasis: from second-generation to synthetic vaccine. Expert Rev Vaccines. 2008;7:833–851. doi: 10.1586/14760584.7.6.833. [DOI] [PubMed] [Google Scholar]
- 40.Saraiva EM, de Figueiredo BA, Santos FN, Borja-Cabrera GP, Nico D, Souza LO, de OM-A, de Souza EP, Fampa P, Parra LE, Menz I, Dias JG, Jr, de Oliveira SM, Palatnik-de-Sousa CB. The FML-vaccine (Leishmune) against canine visceral leishmaniasis: a transmission blocking vaccine. Vaccine. 2006;24:2423–2431. doi: 10.1016/j.vaccine.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Schaffner A, Michel-Harder C, Yeginsoy S. Detection of capsular polysaccharide in serum for the diagnosis of pneumococcal pneumonia: clinical and experimental evaluation. J Infect Dis. 1991;163:1094–1102. doi: 10.1093/infdis/163.5.1094. [DOI] [PubMed] [Google Scholar]
- 42.Socan M, Marinic-Fiser N, Kese D. Comparison of serologic tests with urinary antigen detection for diagnosis of legionnaires' disease in patients with community-acquired pneumonia. Clin Microbiol Infect. 1999;5:201–204. doi: 10.1111/j.1469-0691.1999.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 43.Helbig JH, Uldum SA, Bernander S, Luck PC, Wewalka G, Abraham B, Gaia V, Harrison TG. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires' disease. J Clin Microbiol. 2003;41:838–840. doi: 10.1128/JCM.41.2.838-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundy MS. Preliminary observations using a multi-layer ELISA method for the detection of Entamoeba histolytica trophozoite antigens in stool samples. Trans R Soc Trop Med Hyg. 1982;76:396–400. doi: 10.1016/0035-9203(82)90199-7. [DOI] [PubMed] [Google Scholar]
- 45.Pillai S, Mohimen A. A solid-phase sandwich radioimmunoassay for Entamoeba histolytica proteins and the detection of circulating antigens in amoebiasis. Gastroenterology. 1982;83:1210–1216. [PubMed] [Google Scholar]
- 46.Karki BM, Parija SC. Co-agglutination test for the detection of circulating antigen in amebic liver abscess. Am J Trop Med Hyg. 1999;60:498–501. doi: 10.4269/ajtmh.1999.60.498. [DOI] [PubMed] [Google Scholar]
- 47.Attar ZJ, Chance ML, El-Safi S, Carney J, Azazy A, El-Hadi M, Dourado C, Hommel M. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78:11–16. doi: 10.1016/s0001-706x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 48.Diro E, Techane Y, Tefera T, Assefa Y, Kebede T, Genetu A, Kebede Y, Tesfaye A, Ergicho B, Gebre-Yohannes A, Mengistu G, Engers H, Aseffa A, Desjeux P, Boelaert M, Hailu A. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Trans R Soc Trop Med Hyg. 2007;101:908–914. doi: 10.1016/j.trstmh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Riera C, Fisa R, Lopez P, Ribera E, Carrio J, Falco V, Molina I, Gallego M, Portus M. Evaluation of a latex agglutination test (KAtex) for detection of Leishmania antigen in urine of patients with HIV-Leishmania coinfection: value in diagnosis and post-treatment follow-up. Eur J Clin Microbiol Infect Dis. 2004;23:899–904. doi: 10.1007/s10096-004-1249-7. [DOI] [PubMed] [Google Scholar]
- 50.Salam MA, Khan MG, Mondal D. Urine antigen detection by latex agglutination test for diagnosis and assessment of initial cure of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105:269–272. doi: 10.1016/j.trstmh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Sarkari B, Chance M, Hommel M. Antigenuria in visceral leishmaniasis: detection and partial characterisation of a carbohydrate antigen. Acta Trop. 2002;82:339–348. doi: 10.1016/s0001-706x(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 52.Sundar S, Agrawal S, Pai K, Chance M, Hommel M. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. Am J Trop Med Hyg. 2005;73:269–271. [PubMed] [Google Scholar]
- 53.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, Modabber F, Campos-Neto A, Reed SG. Immunization with a Polyprotein Vaccine Consisting of the T-Cell Antigens Thiol-Specific Antioxidant, Leishmania major Stress-Inducible Protein 1, and Leishmania Elongation Initiation Factor Protects against Leishmaniasis. Infect Immun. 2002;70:4215–4225. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Expert Rev Vaccines. 2003;2:239–252. doi: 10.1586/14760584.2.2.239. [DOI] [PubMed] [Google Scholar]
- 55.Skeiky YA, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, Webb JR, Campos-Neto A, Reed SG. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20:3292–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 56.Fujiwara RT, Vale AM, Franca da Silva JC, Da Costa RT, Quetz JS, Martins Filho OA, Reis AB, Correa OR, hado-Coelho GL, Bueno LL, Bethony JM, Frank G, Nascimento E, Genaro O, Mayrink W, Reed S, Campos-Neto A. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential vaccine candidates for canine visceral leishmaniasis. Vet Res. 2005;36:827–838. doi: 10.1051/vetres:2005033. [DOI] [PubMed] [Google Scholar]
- 57.Miret J, Nascimento E, Sampaio W, Franca JC, Fujiwara RT, Vale A, Dias ES, Vieira E, Da Costa RT, Mayrink W, Campos NA, Reed S. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26:1585–1594. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 59.Faquim-Mauro EL, Coffman RL, Abrahamsohn IA, Macedo MS. Cutting edge: mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–3576. [PubMed] [Google Scholar]
- 60.Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–7071. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 61.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, Dicks MD, Faber BW, de Cassan SC, Folgori A, Nicosia A, Gilbert SC, Hill AV. Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]