Abstract
Objective
To evaluate the performance of total PSA (tPSA), the free/total PSA ratio (f/tPSA), complexed PSA (cPSA) and the complexed/total PSA ratio (c/tPSA) in prostate cancer detection.
Methods
Frozen sera of 442 patients have been analysed for tPSA, free PSA (fPSA) and cPSA. 131 patients had prostate cancer and 311 patients benign prostatic hyperplasia.
Results
Differences in the distribution of the biomarkers were seen as follows: tPSA, cPSA and c/tPSA were significantly higher in the PC group, and f/tPSA was significantly higher in the BPH group. In the tPSA-range of 0-4 ng/ml none of the biomarkers showed a significant difference in the distribution between both groups. In the tPSA-ranges of 0-10 ng/ml, 2-10 ng/ml, 4-10 ng/ml and <10 ng/ml, f/tPSA showed the highest specificity at high sensitivtities, followed by c/tPSA, cPSA, and tPSA, respectively. In tPSA-ranges greater than 10 ng/ml, cPSA offered the best discriminatory ability. GPSA compared to tPSA offered better specificity at high sensitivities in all tPSA-ranges.
Conclusion
F/tPSA offers the best ability to distinguish between both groups in lower tPSA-ranges, followed by c/tPSA. CPSA compared to tPSA offers a better ability to discriminate between both groups in all PSA-ranges and could be used as an initial test for PC.
Keywords: Prostate cancer, tPSA, cPSA, ratio f/tPSA, ratio c/tPSA, detection of prostate cancer, benign hyperplasia of the prostate
Introduction
Prostate cancer (PC) is the most diagnosed cancer in men. In the European Union, prostate cancer accounts for approximately 11% of all cancers and 9% of all cancers deaths [1]. The diagnosis of PC is based on a combination of digital-rectal examination (DRE), testing of the prostate specific antigen (PSA) and the transrectal ultrasound guided biopsy (TRUS-biopsy). Testing of PSA and the digital-rectal examination are both limited with regard to their low sensitivity and specificity. Consequently, patients are often undergoing histological examination by prostate biopsy because of an elevated PSA or an abnormal DRE without having prostate cancer. A serum PSA measurement of 4 ng/ml is often still regarded as the threshold above which prostate biopsy is performed. With this cut-off of PSA, sensitivities of 80% to 91% and specificities from 28% to 85% can be found. Nevertheless there are several studies in the literature which have shown 22% to 24% of men with prostate cancer in a PSA value between 2.5 to 4 ng/ml. Approximately 70% of them have a clinically significant PC [2,3]. Clinically significant PCs are mostly cancers which are localised in the prostate and have a potential to be treated curatively.
Due to this limitation, there is a constant or continuous effort to improve the diagnostic tools for the detection of prostate cancer. The most promising approach to improve the specificity of PSA, particularly in the range lower 10 ng/ml, is the measurement of molecular isoforms of PSA. These are the disengaged free PSA (fPSA) and the complexed PSA (cPSA) bound to α1-antichymotrypsin. Several studies have shown an increase in sensitivities and specificities, primarily in the PSA range of lower 10 ng/ml, for the ratio of free to total PSA (f/tPSA). They affirm that f/tPSA can better distinguish between patients with prostate cancer from patients with a benign hyperplasia of the prostate. For cPSA, the data in the actual literature are inconsistent. Some offer an improvement in the early detection of PC and others do not [4,5].
The aim of our study was to evaluate the diagnostic performance of tPSA, cPSA and the associated f/tPSA and c/tPSA ratios for differentiating between patients with PC and BPH.
Materials, Methods and Statistics
Patients
Serum samples were obtained from a total of 442 patients in the Department of Urology, Munich, Germany. The study was performed with retrospective sera in accordance with the ethical standards of the Helsinki Declaration 1985. All patients have been divided into two groups.
Benign hyperplasia of the prostate
This group included 311 patients (median age: 66, range: 26-89). The diagnosis was established clinically by digital rectal examination (enlargged, confined and indolent with smooth surface and no indurations) and transrectal ultrasound (enlarged organ without hypoechoic areas suggestive of PC). The histological examination was performed ultrasound guided prostate biopsy.
Prostate cancer group
A total of 131 patients have been enrolled (median age: 66, range 47-92). The histopathologically diagnosis of prostate cancer has been established by examination of the prostate after biopsy or radical prostatectomy. The cancer stage was assigned according the TNM-System. The histological grade was classified as grades 1, 2 and 3 and by the Gleason-score.
Sample collection
Blood samples were taken before any diagnostic or therapeutic measures involving the prostate. The blood samples were collected in evacuated tubes (S-Monovette 10m, sarstedt GmbH, Sarstedt, Germany) and centrifuged at 1600 g for at least 15 minutes at 4C. Samples were stored at -80C within 3 hours after collection and not thawed before analysis. All samples have been analyzed at the Institute of Clinical Chemistry, Hospital of the University of Munich - Campus Grosshadern, Munich, Germany.
Assays
Identification of tPSA and fPSA were performed with the Roche Elecsys PSA immunoassay (ECLIA, Roche Diagnostics GmbH, Mannheim, Germany) on the fully automated Elecsys analyser 1010 according to the instruction of the manufactures. CPSA was determined by the immunoassay for the Bayer ADVIA centaur. Also in the measurement of cPSA the instruction of the manufactures were fully followed. These measurements were performed in the year 2006.
Statistical Analysis
Data were analyzed using statistical SAS software (SAS V9.1, SAS Institute Inc., Cary, NC). The Wilcoxon rank-sum test and the correlation coefficients according to Spearman (rs) were calculated. The diagnostic validity was evaluated by the receiver operating characteristic (ROC) curves and the area under the curve (AUC). P values ≤ 0.05 were considered to reflect statistical significance.
Results
Distribution of the different PSA forms and their ratios with range and median
TPSA was distributed in the BPH group with a range of 0.1-39.1 ng/ml (median of 3.2 ng/ml) and in the PC group with a range of 0.1-1347 ng/ml (median of 13.7 ng/ml), respectively. The distribution of the ratio f/tPSA in the group with BPH was 0.02-1.0 (median 18%) and 0.03-1.0 (10%) respectively. The dispersion of cPSA in the BPH group was 0.01-32 ng/ml (median 1.7 ng/ml) and 0.01-989 ng/ml (median 9.02 ng/ml) in the group with PC. For the distribution of c/tPSA a range of 0.07-0.91 (median 0.057) was found for the BPH group and a range of 0.02-0.89 (median 0.66) for the PC group, respectively.
The concentrations of tPSA, its ratio f/tPSA, cPSA and the ratio c/tPSA were significantly different between both study groups. TPSA, cPSA and c/tPSA were significantly higher in the PC group and, as expected, f/tPSA was significantly higher in the BPH group.
Relationship of tPSA, f/tPSA, cPSA and c/tPSA amongst each other and to tumor staging and grading
CPSA values (rs = 0.983 and rs = 0.991) for BPH and PC patients were more closely related to tPSA values than to fPSA values (rs = 0.854 and rs = 0,841). There was no direct relationship between tPSA, cPSA, f/tPSA and the ratio c/tPSA to tumor stage. In consideration of the relationship between the different PSA forms and their ratios as well as the tumour grading, the ratio f/tPSA had the best power in differentiation between the BPH-group and low grade tumors, followed by cPSA, tPSA and c/tPSA, respectively.
In regards to the high grade tumors, cPSA had the best power to discriminate between both groups, followed by tPSA, f/tPSA, and c/tPSA, respectively.
Analysis in sum and in respect to different tPSA-ranges
In the present study ROC analyses have been made in the total range and in taking different tPSA-ranges into account. The Receiver Operating Characteristic Curves (ROC) and their area under the curves (AUC) are shown in Figure 1 for the whole patient collective. The areas under the ROC curves were 0.786 for tPSA, 0.799 for cPSA, 0.759 for f/tPSA and 0.698 for c/tPSA. Figures 2 to 6 show the different areas under the ROC curves in respect to different tPSA ranges. Figure 2 for the range 0-4 ng/ml, Figure 3 for the range 2-10 ng/ml, Figure 4 for 4-10 ng/ml tPSA and Figure 5 shows the range of tPSA 0 to 10 ng/ml. In Figure 6, all patients with a tPSA value of greater than 10 ng/ml are considered. As illustrated in Figure 2, there are no significant differences in the distribution of all parameters between both study groups (p > 0.05). In the tPSA range of 2-10 ng/ml, 4-10 ng/ml and 0-10 ng/ml significant distribution between patients with BPH and PC could be found except for tPSA in the range of 4-10 ng/ml tPSA. Considering the range of tPSA greater than 10 ng/ml, all parameters have been significantly distributed in respect to both study groups (Figure 6).
Figure 1.
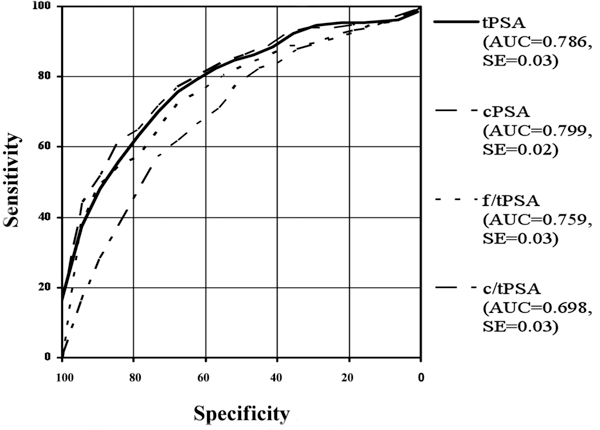
Roc-Analysis of different PSA-ranges: includes the whole collective.
Figure 2.
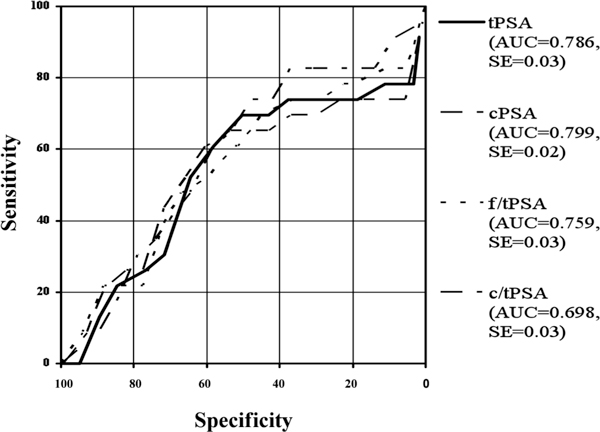
Roc-Analysis of different PSA-ranges: tPSA-ranges 0-3.9 ng/ml.
Figure 6.
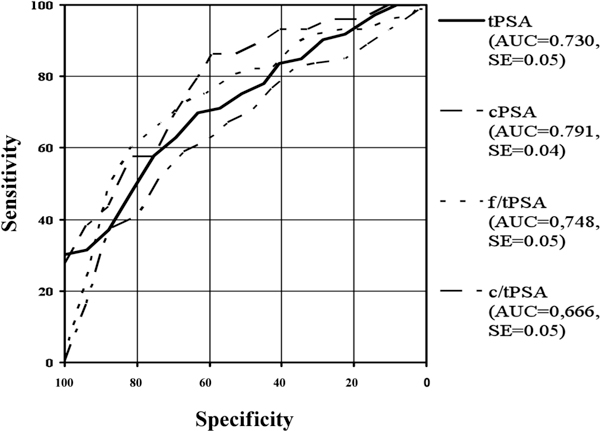
Roc-Analysis of different PSA-ranges: >10 ng/ml tPSA.
Figure 3.
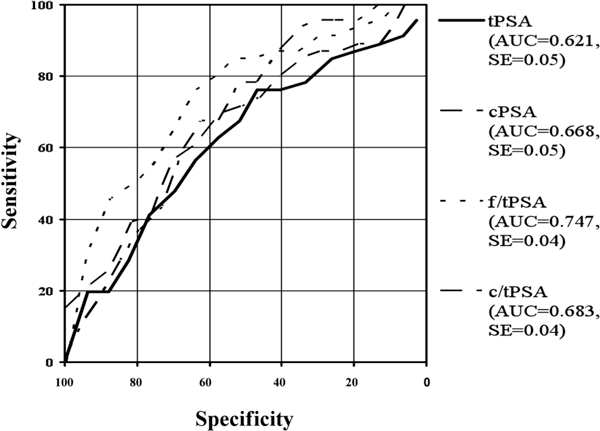
Roc-Analysis of different PSA-ranges: 2-10 ng/ml tPSA.
Figure 4.
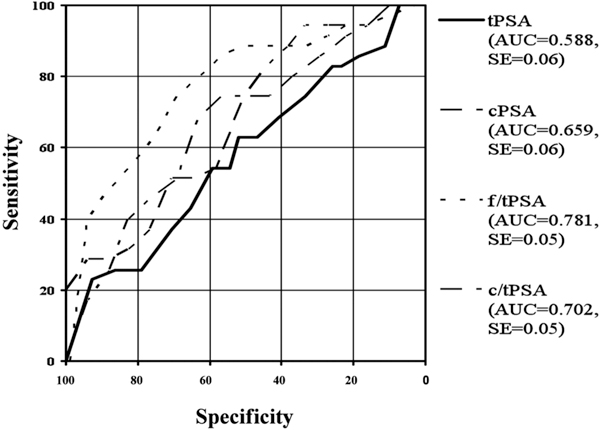
Roc-Analysis of different PSA-ranges: includes tPSA-range 4-10 ng/ml.
Figure 5.
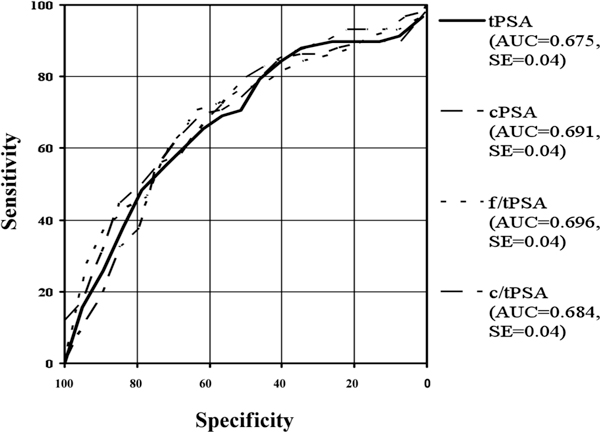
Roc-Analysis of different PSA-ranges: 0-10 ng/ml.
Discussion
The identification of tPSA, the digital-rectal examination and the ultrasonography of the prostate are still the recommended analytic methods to decide whether or not a patient should undergo a biopsy of the prostate for detection prostate cancer [6]. All examinations are limited because of their deficient sensitivity and specificity. TO improve the early detection of PC, various PSA forms seem to be an established way for the early detection of PC. It is of great interest to the urologist to avoid unnecessary biopsies and anticipate the distress of treated patients [7,8]. Serum PSA has an acceptable sensitivity but also has a low specificity - the reason for this being an increased level of PSA in the serum of patients with benign prostatic disease [9]. Other concepts to enhance the diagnostic validity of PSA have been suggested. These include age-related tPSA references ranges, and the velocity and density of PSA, particularly in a tPSA overlapping range [10].
TPSA, cPSA, f/tPSA and c/tPSA in comparison to the overall study group
Using a cut-off value of 4 ng/ml tPSA, our data shows similar sensitivities and specificities of tPSA in comparison to other studies. We found a sensitivity of 82% and a specificity of 58%. The positive predictive value was 45% and the negative predictive value was 89%. Tanguay et al. reported from 535 patients, 204 patients had histologically proven prostate cancer and the remaining 311 patients had benign prostate hyperplasia. At a cut-off of 4 ng/ml, the sensitivity was 87% with a specificity of 27% [11]. Other studies with a cut-off of 4 ng/ml had sensitivtities from 80-91% and specificities from 28-85% [12-14]. A possible reason for the wide range of specificity could be the use of different assays [15].
Compared to Tanguay et al., the ratio f/tPSA showed no benefit in discriminating between patients with prostate cancer and patients with a benign prostatic hyperplasia [11]. Sensitivity was 82%, specificity 55%, the positive predictive value was 43% and the negative predictive value was 87%. These data agree with other published data [16]. It has been shown in numerous studies that the ratio f/tPSA in addition to tPSA has the best ability to discriminate between patients with PC and without PC especially in ranges lower than 10 ng/ml tPSA [17,18]. These findings can be outlined by our data.
In contrasting tPSA to the α1-antichymotrypsin bound cPSA showed at a sensitivity of 82%, a specificity of 60%. The positive predictive value was 46% and the negative predictive value was 89%. Unnecessary biopsies of 5 patients with BPH could have been avoided using cPSA instead of tPSA. The data for using cPSA instead of tPSA are still controversial. Stenman et al. were the first investigators to note that cPSA is the major form of PSA with PC [7]. Brawer et al. showed in a patient population of 300 patients that the AUC with 0.722 of cPSA was higher than the AUC with 0.688 of tPSA. Considering a sensitivity of 95%, the specificity was 26.7% and 4.9% higher than the specificity of tPSA [12]. Our data showed an AUC for cPSA of 0.798 and an AUC for tPSA of 0.785. Another study by Brawer showed a significantly higher specificity for cPSA in comparison to tPSA in a study population of 657 patients altogether [13]. Other studies could not find a benefit for use of cPSA instead of tPSA in the detection of prostate cancer. Miller at al. published the same discriminating potency for cPSA than for tPSA [19]. Okihara et al. found no advantage in the discrimination between patients with PC and patients with benign disease of the prostate in important clinical sensitivity levels [20].
We also examined the ratio of c/tPSA in our study. In comparison to tPSA, cPSA and the ratio f/tPSA, sensitivity of c/tPSA is lower and offers no advantage in the discrimination of both study groups in respect to the whole study population. These data are in contrast to the published data from Jung et al. [21]. Jung et al. found the same diagnostic power for the ratio c/tPSA as for the ratio of f/tPSA. Okihara et al. had shown no increase in the discrimination power between both study groups in comparison to the ratio f/tPSA. But the ratio of c/tPSA did show better sensitivities and specificities than tPSA and cPSA [20].
TPSA, cPSA, f/tPSA and c/tPSA in comparison to different tPSA ranges
It is well known that the discrimination between patients with and without prostate cancer in tPSA ranges of <10 ng/ml is often difficult and this is part of the major problem in deciding if a biopsy of the prostate is necessary or not. In the tPSA range of <4.0 ng/ml our data shows no discrimination power between study groups. This has already been published by Lein et al. who compared two study groups with and without prostate cancer in a PSA-range of lower than 4.0 ng/ml. Lein et al. also found no discrimination power of all biomarkers in their study population [22]. In contrast, Okihara et al. did find a benefit of cPSA against the ratio f/tPSA in the tPSA-range of < 4.0 ng/ml [23]. Our data do not agree with theirs. Interestingly, we found in the tPSA-range lower than 4.0 ng/ml 4 patients with a prostate cancer in the t4-stadium and 3 patients in t3-stadium. 6 patients had a Gleason score ≥ 7. Similar findings were published by thompson et al. 2004. They showed the presence of inappropriate tumors in PSA-ranges lower 4 ng/ml [24].
TO consider the tPSA-range of 4-10 ng/ml, our data show the best discrimination power by the ratio of f/tPSA. In the range of 4-10 ng/ml we found 35 patients with a prostate cancer and 81 patients with BPH in our study. For example, \at a sensitivity of 85%, the ratio of f/tPSA was 58%, followed by the ratio c/tPSA with a specificity of 40%. The specificity of cPSA in respect to a sensitivity of 85% was 28%. The specificity of tPSA was 19%. Interestingly, tPSA was not able to discriminate between both study groups (AUC = 0.588, p > 0.05). These findings are in accordance with published data from Catalona et al. Catalona et al. also found the best discrimination power to be in the range of 4-10 ng/ml for f/tPSA (1998). Brawer published the advantage of f/tPSA against cPSA in total and in the range of 4-10 ng/ml. The diagnostic sensitivity was significantly better in both PSA-ranges [13]. The benefit of the use of f/tPSA is obvious. However there exist some limitations which should be considered. The use of different assays for the identification of tPSA and fPSA leads to different cut-off-values [25]. The age of the patients must be taken into consideration [26]. FPSA is a very unstable marker. For example the concentration of fPSA decreases by frozen storage [27]. This fact must be considered in our data as well.
Taking the ranges of 2-10 ng/ml and <10 ng/ml tPSA into account for the best discrimination between both study groups was achieved by the ratio f/tPSA, followed by the ratio c/tPSA, cPSA and than tPSA. In the range of 2-10 ng/ml our data are analogous to the range of 4-10 ng/ml, a diagnostic benefit for cPSA compared to tPSA. These data agree with other published data [28]. In published data from Jung et al. the ratio c/tPSA had a better discrimination power than cPSA and tPSA in lower tPSA-ranges. Our data confirm this findings [21].
In tPSA ranges of >10 ng/ml cPSA offered the best discriminatory power between both study groups, followed by tPSA, the ratios f/tPSA and the ratio c/tPSA, respectively. The diagnostic power of cPSA in comparison to tPSA was also found by Mitchell et al. in these tPSA-ranges [29].
Conclusion
In conclusion, our data show the limitation of the different biomarkers and their ratios in total and in different PSA-ranges in respect to high sensitivities. In the ranges of 2-10 ng/ml, 4-10 ng/ml and <10 ng/ml the ratio of f/tPSA provides the best results in discrimination between both study groups. Without doubt the ratio f/tPSA can be an additional tool for the decision of whether or not a biopsy of the prostate should be performed in lower tPSA-ranges. The instability of the free PSA and the particular claim in the determination must be mentioned. The ratio of c/tPSA offered better specificities in lower tPSA-ranges than tPSA and cPSA, but its limited practicality as a result of the need for two different assays. The α1-antichymotrypsin bound cPSA was superior to tPSA in all tPSA-ranges and could be used as a single test in the detection of prostate cancer. Because of the aforementioned limitations of the different biomarkers, further studies are necessary to find improved markers for the detection of prostate cancer to reduce the number of patients who undergo a prostate biopsy without having prostate cancer.
Abbreviations
PC: prostate cancer; BPH: benign hyperplasia of the prostate; PSA: prostate specific antigen; tPSA: total PSA; cPSA: complexed PSA; fPSA: free PSA; f/tPSA: ratio free to total PSA; c/tPSA: ratio complexed PSA to total PSA.
Acknowledgements
We thank K. Hofmann for excellent technical assistance.
References
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. Eau guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum psa concentrations of 2.6 to 4.0 ng/ml and benign prostate examination. Enhancement of specificity with free psa measurements. Jama. 1997;277:1452–1455. doi: 10.1001/jama.1997.03540420048028. [DOI] [PubMed] [Google Scholar]
- Babaian RJ, Johnston DA, Naccarato W, Ayala A, Bhadkamkar VA, Fritsche HH Jr. The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/ml: Relation to biopsy strategy. J Urol. 2001;165:757–760. doi: 10.1016/S0022-5347(05)66519-6. [DOI] [PubMed] [Google Scholar]
- Parsons JK, Brawer MK, Cheli CD, Partin AW, Djavan R. Complexed prostate specific antigen (psa) reduces unnecessary prostate biopsies in the 2.6-4.0 ng/ml range of total psa. BJU Int. 2004;94:47–50. doi: 10.1111/j.1464-410X.2004.04899.x. [DOI] [PubMed] [Google Scholar]
- Parsons JK, Partin AW. Applying complexed prostate-specific antigen to clinical practice. Urology. 2004;63:815–818. doi: 10.1016/j.urology.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. [eau guidelines on prostate cancer] Actas Urol Esp. 2009;33:113–126. doi: 10.1016/s0210-4806(09)74110-5. [DOI] [PubMed] [Google Scholar]
- Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: Assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]
- Woodrum DL, Brawer MK, Partin AW, Catalona WJ, Southwick PC. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5–12. doi: 10.1016/S0022-5347(01)63996-X. [DOI] [PubMed] [Google Scholar]
- Catalona WJ. Screening for prostate cancer. Lancet. 1994;343:1437. [PubMed] [Google Scholar]
- Ukimura O, Durrani O, Babaian RJ. Role of psa and its indices in determining the need for repeat prostate biopsies. Urology. 1997;50:66–72. doi: 10.1016/S0090-4295(97)00116-7. [DOI] [PubMed] [Google Scholar]
- Tanguay S, Begin LR, Elhilali MM, Behlouli H, Karakiewicz PI, Aprikian AG. Comparative evaluation of total psa, free/total psa, and complexed psa in prostate cancer detection. Urology. 2002;59:261–265. doi: 10.1016/S0090-4295(01)01497-2. [DOI] [PubMed] [Google Scholar]
- Brawer MK, Meyer GE, Letran JL, Bankson DD, Morris DL, Yeung KK, Allard WJ. Measurement of complexed psa improves specificity for early detection of prostate cancer. Urology. 1998;52:372–378. doi: 10.1016/S0090-4295(98)00241-6. [DOI] [PubMed] [Google Scholar]
- Brawer MK, Cheli CD, Neaman IE, Goldblatt J, Smith C, Schwartz MK, Bruzek DJ, Morris DL, Sokoll LJ, Chan DW, Yeung KK, Partin AW, Allard WJ. Complexed prostate specific antigen provides significant enhancement of specificity compared with total prostate specific antigen for detecting prostate cancer. J Urol. 2000;163:1476–1480. doi: 10.1016/S0022-5347(05)67646-X. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Raha K, Bhunia CL, Bhattachary DK. The usefulness of prostate specific antigen density as a screening method for prostatic carcinoma. J Indian Med Assoc. 2001;99:627–628. 630. [PubMed] [Google Scholar]
- Reiter W, Stieber P, Schmeller N, Nagel D, Schambeck C, Fateh-Moghadam A. Is free prostate-specific antigen helpful in the differential diagnosis of benign hyperplasia and cancer of the prostate? Tumour Biol. 1997;18:80–87. doi: 10.1159/000218017. [DOI] [PubMed] [Google Scholar]
- Trinkler FB, Schmid DM, Hauri D, Pei P, Maly FE, Sulser T. Free/total prostate-specific antigen ratio can prevent unnecessary prostate biopsies. Urology. 1998;52:479–486. doi: 10.1016/S0090-4295(98)00157-5. [DOI] [PubMed] [Google Scholar]
- Lein M, Stephan C, Jung K, Schnorr D, Loening S. Relation of free psa/total psa in serum for differentiating between patients with prostatic cancer and benign hyperplasia of the prostate: Which cutoff should be used? Cancer Invest. 1998;16:45–49. doi: 10.3109/07357909809039753. [DOI] [PubMed] [Google Scholar]
- Espana F, Royo M, Martinez M, Enguidanos MJ, Vera CD, Estelles A, Aznar J, Jimenez-Cruz JF, Heeb MJ. Free and complexed prostate specific antigen in the differentiation of benign prostatic hyperplasia and prostate cancer: Studies in serum and plasma samples. J Urol. 1998;160:2081–2088. doi: 10.1016/S0022-5347(01)62248-1. [DOI] [PubMed] [Google Scholar]
- Miller MC, O'Dowd GJ, Partin AW, Veltri RW. Contemporary use of complexed psa and calculated percent free psa for early detection of prostate cancer: Impact of changing disease demographics. Urology. 2001;57:1105–1111. doi: 10.1016/S0090-4295(01)00953-0. [DOI] [PubMed] [Google Scholar]
- Okihara K, Cheli CD, Partin AW, Fritche HA, Chan DW, Sokoll LJ, Brawer MK, Schwartz MK, Vessella RL, Loughlin KR, Johnston DA, Babaian RJ. Comparative analysis of complexed prostate specific antigen, free prostate specific antigen and their ratio in detecting prostate cancer. J Urol. 2002;167:2017–2023. doi: 10.1016/S0022-5347(05)65075-6. discussion 2023-2014. [DOI] [PubMed] [Google Scholar]
- Jung K, Elgeti U, Lein M, Brux B, Sinha P, Rudolph B, Hauptmann S, Schnorr D, Loening SA. Ratio of free or complexed prostate-specific antigen (psa) to total psa: Which ratio improves differentiation between benign prostatic hyperplasia and prostate cancer? Clin Qiem. 2000;46:55–62. [PubMed] [Google Scholar]
- Lein M, Kwiatkowski M, Semjonow A, Luboldt HJ, Hammerer P, Stephan C, Klevecka V, Taymoorian K, Schnorr D, Recker F, Loening SA, Jung K. A multicenter clinical trial on the use of complexed prostate specific antigen in low prostate specific antigen concentrations. J Urol. 2003;170:1175–1179. doi: 10.1097/01.ju.0000087560.30497.4e. [DOI] [PubMed] [Google Scholar]
- Okihara K, Ukimura O, Nakamura T, Mizutani Y, Kawauchi A, Naya Y, Uchida M, Ogiwara T, Miki T. Can complexed prostate specific antigen enhance prostate cancer detection in japanese men? Eur Urol. 2004;46:57–64. doi: 10.1016/j.eururo.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- Junker R, Brandt B, Zechel C, Assmann G. Comparison of prostate-specific antigen (psa) measured by four combinations of free psa and total psa assays. Clin Chem. 1997;43:1588–1594. [PubMed] [Google Scholar]
- Veltri RW, Miller MC, O'Dowd G J, Partin AW. Impact of age on total and complexed prostate-specific antigen cutoffs in a contemporary referral series of men with prostate cancer. Urology. 2002;60:47–52. doi: 10.1016/S0090-4295(02)01695-3. [DOI] [PubMed] [Google Scholar]
- Sokoll LJ, Bruzek DJ, Dua R, Dunn W, Mohr P, Wallerson G, Eisenberger M, Partin AW, Chan DW. Short-term stability of the molecular forms of prostate-specific antigen and effect on percent complexed prostate-specific antigen and percent free prostate-specific antigen. Urology. 2002;60:24–30. doi: 10.1016/s0090-4295(02)01723-5. [DOI] [PubMed] [Google Scholar]
- Roddam AW, Duffy MJ, Hamdy FC, Ward AM, Patnick J, Price CP, Rimmer J, Sturgeon C, White P, Allen NE. Use of prostate-specific antigen (psa) isoforms for the detection of prostate cancer in men with a psa level of 2-10 ng/ml: Systematic review and meta-analysis. Eur Urol. 2005;48:386–399. doi: 10.1016/j.eururo.2005.04.015. discussion 398-389. [DOI] [PubMed] [Google Scholar]
- Mitchell ID, Croal BL, Dickie A, Cohen NP, Ross I. A prospective study to evaluate the role of complexed prostate specific antigen and free/total prostate specific antigen ratio for the diagnosis of prostate cancer. J Urol. 2001;165:1549–1553. doi: 10.1016/S0022-5347(05)66346-X. [DOI] [PubMed] [Google Scholar]


