Abstract
Rationale: Our previous cross-sectional study showed that serum adiponectin is inversely associated with asthma among women. However, it is not known if serum adiponectin predicts future development of asthma or if asthma affects subsequent serum adiponectin concentrations among women.
Objectives: To determine longitudinal association between serum adiponectin and incident asthma among women.
Methods: We used data from examinations at Years 10, 15, and 20 of the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. In our primary analysis, the association of CARDIA Year 15 serum adiponectin concentration with Year 20 incident asthma was evaluated. In our secondary analysis, the converse direction, that is, the association of CARDIA Year 10 prevalent asthma with Year 15 serum adiponectin, was evaluated, using logistic regression techniques.
Measurements and Main Results: Our primary analysis included 1,450 women, mostly premenopausal. Multivariable analyses demonstrated that the lowest tertile of Year 15 serum adiponectin concentration (<7 mg/L) predicted significantly higher risk for incident asthma at Year 20 among women (odds ratio, 2.07; 95% confidence interval, 1.05, 4.10), and particularly among current smokers (interaction P = 0.051). Further, low serum adiponectin was more important than body mass index in predicting the risk for incident asthma among women. We also showed that the converse relationship was not true; that is, Year 10 prevalent asthma did not predict Year 15 serum adiponectin concentrations in women.
Conclusions: Serum adiponectin affects future risk for asthma in women and not vice versa. Measures that raise systemic adiponectin concentrations may lead to newer ways to prevent asthma among women, particularly among those who smoke.
Keywords: incident asthma, obesity, adiponectin, adipokine, women
At a Glance Commentary
Current Scientific Knowledge
A causative role for adiponectin in asthma has been established in mice. However, the adiponectin–asthma association is not established in humans.
What This Study Adds to the Field
In this longitudinal cohort, we demonstrate that low serum adiponectin concentrations, independent of obesity, predict higher risk for incident asthma among middle-aged women, particularly among current smokers. Measures that raise systemic adiponectin concentrations may lead to newer ways to prevent asthma among women and particularly those who smoke.
In 2005, more than 21 million people in the United States were estimated to be affected by asthma, amounting to 7.6% of the total population (1). Between 1980 and 1996, the prevalence of, and morbidity trends related to, asthma increased in the United States (2, 3). It is now well established that asthma is related to adiposity, particularly among women. Adipokines, proteins produced by adipose tissue, may regulate systemic inflammation and play a role in asthma (4–7). Adiponectin is one such adipokine with predominantly antiinflammatory effects. Adiponectin inhibits proinflammatory cytokines and endothelial adhesion molecules and induces antiinflammatory cytokines (8–11). Further, adiponectin regulates the proliferation and function of inflammatory cells including NK cells and T lymphocytes (12, 13).
Although visceral adipocytes are the most important source of adiponectin, serum adiponectin concentrations are reduced in obese subjects (14, 15). One possible explanation is that hypoxia-related necrosis of adipocytes activates macrophages in obese subjects (16). These activated macrophages produce tumor necrosis factor-α and IL-6, which in turn may directly inhibit the local production of adiponectin in a paracrine fashion (17). Adiponectin and all of the known receptors for adiponectin (AdipoR1, AdipoR2, T-cadherin, and calreticulin) are expressed on multiple cell types in the lung (18–20). Adiponectin is also transported from blood into the alveolar lining fluid via the T-cadherin molecule on the endothelium (20, 21).
A causative role for adiponectin in asthma has been better established in mice than in humans (22). Murine studies have, however, shown that this association is bidirectional, whereby exogenous adiponectin attenuates airway changes of asthma and allergen-induced bronchoprovocation decreases adiponectin concentrations (22). Although current human data remain inconclusive, our previous cross-sectional study shows that low serum adiponectin concentrations are associated with increased odds for prevalent asthma among women and not men (6). The direction of the adiponectin–asthma association in women is, however, not established. In other words, it is not known if low serum adiponectin predicts future risk for asthma or if presence of asthma lowers subsequent serum adiponectin concentrations among women. Our objective was to determine the longitudinal associations between serum adiponectin and incident asthma among women. If systemic adiponectin affects risk for incident asthma, measures that modify systemic adiponectin concentrations may lead to new preventive strategies for adult-onset asthma among women.
Methods
Study Design
This study used data from examinations at Years 10, 15, and 20 of the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) cohort in the United States and its Young Adult Longitudinal Trends in Antioxidants (YALTA) ancillary study. The CARDIA study, funded by the NHLBI, focuses on the development of cardiovascular disease. During 1985–1986, CARDIA randomly recruited 5,115 black and white men and women, aged 18 to 30 years, from the general population in Birmingham, Alabama; Chicago, Illinois; and Minneapolis, Minnesota; and from the membership of the Oakland Kaiser-Permanente Health Plan in Oakland, California. Follow-up examinations were completed 2, 5, 7, 10, 15, and 20 years later. Retention of CARDIA participants has been excellent, as 3,950, 3,672, and 3,549 survivors were respectively examined at Years 10, 15, and 20, constituting 78, 74, and 72%, respectively, from the baseline cohort. Self-reported information from subjects was obtained by trained interviewers using standardized questionnaires. Detailed methods, instruments, and quality control procedures are described at the CARDIA website (http://www.cardia.dopm.uab.edu/ex_mt.htm) and in other published reports (23, 24).
Our primary analysis evaluated the association of serum adiponectin concentration at CARDIA Year 15 examination with new cases of asthma (i.e., incident disease) among women at Year 20 examination. Our secondary analysis evaluated the converse direction, that is, the association of prevalent asthma at Year 10 examination with Year 15 serum adiponectin concentration among women.
Inclusion and Exclusion Criteria
The primary analysis included all women participants without prevalent asthma (as defined below) at CARDIA Year 15 examination (n = 1,450). The flowchart of subject inclusion and exclusion is depicted in Figure E1 in the online supplement. The secondary analysis included 1,455 women participants at Year 10 examination. To examine the longitudinal effect of asthma on serum adiponectin concentrations, cases of asthma newly diagnosed at Year 15 examination were excluded from the secondary analysis. Those with missing data for independent variables and covariates were excluded from all analyses.
Independent and Dependent Variables
Morning blood samples were collected after at least 8 hours of fasting at CARDIA Year 15 examination from seated participants with tourniquet use limited to 2 minutes to prevent hemoconcentration. Samples were then centrifuged, aliquoted, and frozen at −70°C within 90 minutes of the collection. Total adiponectin was measured in serum as part of the YALTA ancillary study by radioimmunoassay technique at Linco Research, Inc. (St. Charles, MO) using a rabbit polyclonal antibody and purified recombinant adiponectin standards with an effective range of 0.2 to 40 mg/L (25). Correlation between adiponectin concentrations measured in 407 paired serum samples in a blinded fashion was 0.91 and the interassay coefficient of variation for our laboratory was 8.8%. This assay measured total adiponectin and not its various isoforms.
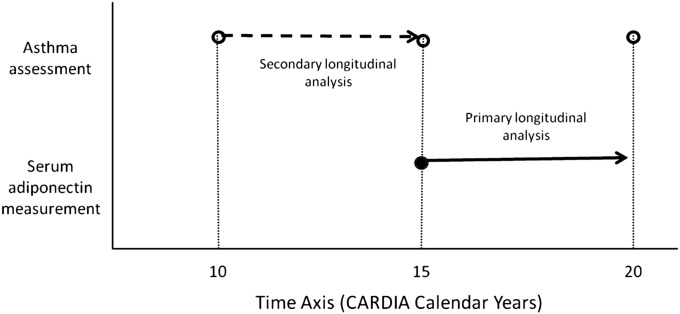
Asthma was defined by a self-reported provider diagnosis in the presence of either asthma symptoms in the year preceding the examination or verified use of asthma medications at the time of examination. Incident asthma, the primary dependent variable, was defined by the new occurrence of asthma at Year 20 examination. The time axis for the measurement of the dependent and independent variables in the primary and secondary analyses in the study are depicted in Figure 1.
Figure 1.
Selected time axis for the measurement of the dependent and independent variables in the primary and secondary analyses in the study.
Covariates
Covariates included age, race, body mass index (BMI), current smoking, history of diabetes, logarithmically transformed insulin resistance (defined by the homeostasis model assessment or HOMA) (26, 27), and logarithmically transformed physical activity score (based upon the questionnaire-assessed physical activity history score [28, 29]) at Year 15 examination, as well as self-report of hay fever, a surrogate marker of atopy (obtained at Year 0 examination). The above listed covariates were selected because they have been shown to affect either asthma status or serum adiponectin concentration (14, 30). Smoking was treated as a binary categorical variable, including those who currently smoked and those who were former/never smokers. BMI was calculated from height and weight measured by trained technicians using standardized equipment with participants wearing light clothing without shoes.
Statistical Analysis
We performed descriptive analyses (to calculate frequency distributions), univariate analyses (such as chi-square and t tests for categorical and continuous variables, respectively), and multivariable logistic regression analyses using incident asthma status at Year 20 examination as the dependent variable in the primary analysis and categories of serum adiponectin concentration at Year 15 examination as the dependent variable in the secondary analysis. Since adiponectin concentrations were not normally distributed and their association with risk for incident asthma was nonlinear (Figure E2), adiponectin concentrations were analyzed primarily as categories in the main text and as a logarithmically transformed continuous variable in the online supplement. Further, since the association between adiponectin and risk for incident asthma did not differ between second and third tertiles (Figure E2), participants in the lowest tertile of serum adiponectin concentration (<7 mg/L) were compared with the referent population (≥7 mg/L) formed by combining the two higher tertiles. The use of binary categories also conserved power, as compared with the corresponding linear variable. Consistent with our a priori hypothesis based on our previous study (6), we examined subgroups defined by self-reported menopausal status and performed formal tests for interaction. A two-sided P value of <0.05 was considered statistically significant. All statistical analysis was done using the Statistical Analysis Software (SAS) package version 9.1 (Cary, NC). This study was approved by the institutional review boards at University of New Mexico (Albuquerque, NM) and at each of the CARDIA study sites.
Results
Demographic Characteristics
The primary analysis included 1,450 women including 1,011 premenopausal women with 54 and 32 cases of incident asthma, respectively, at CARDIA Year 20 examination. Women with incident asthma at CARDIA Year 20 examination had significantly lower annual household income and higher rates of current smoking at CARDIA Year 15 examination than women without (Table 1). The two groups, however, did not differ with respect to BMI at Year 15 examination, change in BMI between examinations at Years 15 and 20, or past history of hay fever. Further, the characteristics of incident asthma cases in Table 1 for premenopausal women were similar to those for all women.
TABLE 1.
DISTRIBUTION OF SELECTED CHARACTERISTICS AMONG WOMEN WITH INCIDENT ASTHMA (AT CARDIA YEAR 20 EXAMINATION) AND CONTROLS
| Characteristics | Incident Asthma (n = 54) | Controls (n = 1,396) |
| Age, years | 45.4 ± 3.7 | 45.2 ± 3.7 |
| Race, % whites | 40.7 | 52.4 |
| Low annual household income, %, <$25,000 | 28.9* | 15.4 |
| Low educational status, %, ≤high school graduate | 25.9 | 18.5 |
| Lack of coverage for medical care, % | 5.6 | 10.4 |
| Difficult access to medical care, % | 9.3 | 9.1 |
| BMI, kg/m2 | 29.6 ± 7.4 | 28.6 ± 7.3 |
| 5-yr. (Year 20 − Year 15) change in BMI, kg/m2 | 0.6 ± 2.7 | 0.9 ± 3.0 |
| History of hay fever at year 0, % | 27.8 | 30.4 |
| Current smoker, % | 31.5* | 17.8 |
| History of diabetes mellitus, % | 5.6 | 7.5 |
| Premenopausal status at Year 15, % | 85.2 | 89.7 |
| Premenopausal status at Year 20, % | 72.7 | 76.1 |
| Geometric mean serum adiponectin, mg/L | 9.4 (5.2, 16.9)* | 11.2 (6.4, 19.5) |
| Low category of serum adiponectin, %, <7 mg/L | 29.6* | 16.4 |
| Geometric mean insulin resistance, HOMA units | 2.3 (1.2, 4.5) | 2.4 (1.3, 4.4) |
| Geometric mean physical activity score, exercise units | 213.3 (62.2, 731.8) | 166.0 (39.4, 699.9) |
| Prebronchodilator %FEV1/FVC ratio at Year 20 | 78.3 ± 8.4 | 79.7 ± 6.0 |
Definition of abbreviations: BMI = body mass index; CARDIA = Coronary Artery Risk Development in Young Adults; HOMA = homeostasis model assessment; FEV1/FVC = ratio of forced expiratory volume in 1 second to forced vital capacity.
Incident asthma was measured at CARDIA Year 20 examination; all other data are measured at CARDIA Year 15 examination, unless otherwise indicated. Data are presented as mean ± SD. Geometric mean is presented with 95% confidence interval in parentheses.
Distribution of above characteristics among men with incident asthma and controls is shown in Table E2.
Comparison between asthma and controls significant at P < 0.05.
Low Serum Adiponectin at Year 15 Predicted Increased Risk for Incident Asthma in Women at Year 20
Women with incident asthma at CARDIA Year 20 examination had significantly lower mean serum adiponectin concentrations at Year 15 examination than women without incident asthma (Table 1). In multivariable models, the low category of serum adiponectin concentration (defined as <7 mg/L) was associated with significantly higher risk for incident asthma among all women (odds ratio [OR], 2.07; 95% confidence interval [CI], 1.05, 4.10) and among premenopausal women (OR, 2.80; 95% CI, 1.17, 6.71; Table 2) compared with the high category. In alternative analyses with serum adiponectin as a logarithmically transformed continuous variable, similar results were seen (adjusted P = 0.04 for all women and 0.02 for premenopausal women; Table E1). However, the interaction between either menopausal status or atopic status and low serum adiponectin (among all women) on risk for incident asthma was not significant (Table 2). Details of additional nonsignificant interactions are provided in the online supplement.
TABLE 2.
ASSOCIATION BETWEEN THE LOW CATEGORY OF SERUM ADIPONECTIN CONCENTRATION AT CARDIA YEAR 15 EXAMINATION AND RISK FOR INCIDENT ASTHMA AT YEAR 20 EXAMINATION
| All Women |
Premenopausal Women |
Men |
|||||||
| n with Asthma/N | OR (95% CI) | P Value | n with Asthma/N | OR (95% CI) | P Value | n with Asthma/N | OR (95% CI) | P Value | |
| Unadjusted model | |||||||||
| Both current and not current smokers | 54/1,450 | 2.15 (1.18, 3.91) | 0.01 | 32/1,011 | 3.07 (1.47, 6.41) | 0.003 | 16/1,171 | 1.06 (0.39, 2.85) | 0.92 |
| Adjusted models | |||||||||
| Both current and not current smokers | 54/1,450 | 2.07 (1.05, 4.10) | 0.04 | 32/1,011 | 2.80 (1.17, 6.71) | 0.02 | 16/1,171 | 1.24 (0.42, 3.63) | 0.70 |
| Current smokers only | 17/265 | 5.07 (1.55, 16.59) | 0.007 | 10/152 | 8.89 (1.44, 54.95) | 0.02 | 3/246 | 1.73 (0.12, 24.99) | 0.69 |
| Not current smokers only | 37/1,185 | 1.13 (0.43, 2.97) | 0.82 | 22/859 | 1.58 (0.47, 5.29) | 0.47 | 13/925 | 0.68 (0.20, 2.25) | 0.53 |
Definition of abbreviations: CARDIA = Coronary Artery Risk Development in Young Adults; CI = confidence interval; OR = odds ratio.
Incident asthma and menopausal status were measured at CARDIA Year 20 examination; all other data are measured at Year 15 examination, unless otherwise indicated.
The adjusted models included age, race, body mass index (BMI), current smoking (where applicable), history of diabetes, logarithmically transformed insulin resistance, and logarithmically transformed physical activity score (all at Year 15 examination) and history of hay fever (at Year 0 examination). In the multivariable model, the only covariate with a significant main effect on incident asthma among women was current smoking (adjusted OR, 1.89; 95% CI, 1.03, 3.47; P = 0.04). Other covariates were not significant.
Participants in the lowest tertile of serum adiponectin concentration (<7 mg/L) were compared with the referent population comprising the top two tertiles pooled (adiponectin ≥7 mg/L).
Similar associations as above were noted when adiponectin was studied as a logarithmically transformed continuous variable (Table E1).
Smoking interactions: The two-way interaction for the adjusted analysis between current smoking status and low serum adiponectin category on incident asthma, as reflected in this table, was significant among premenopausal women (P = 0.048) and tended toward significance among all women (P = 0.051) but was not significant among men. The three-way interaction between sex, current smoking status, and low serum adiponectin category on incident asthma was not significant.
Other interactions: The interaction between sex and low serum adiponectin category on incident asthma among all subjects was not significant. Similar nonsignificant interactions were noted on incident asthma among women between low serum adiponectin category and each of the following variables: race, BMI, atopy, insulin resistance, physical activity, and menopause. Details are provided in the online supplement.
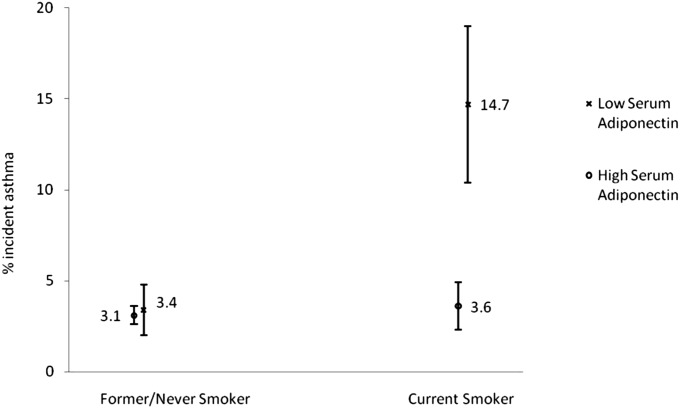
On the other hand, there was a significant interaction between current smoking and low serum adiponectin concentrations at Year 15 examination on incident asthma among all women (unadjusted P = 0.04; adjusted P = 0.051) and among premenopausal women (unadjusted P = 0.03; adjusted P = 0.048). In other words, low serum adiponectin was associated with higher risk for incident asthma among current smokers than former/never smokers, among both all women (Figure 2) and premenopausal women.
Figure 2.
Plot of interaction effects (unadjusted) between current smoking status and low serum adiponectin concentrations on incident asthma among all women. The risk for incident asthma with low serum adiponectin concentrations was substantially higher among female current smokers than former/never smokers. On the other hand, the risk for incident asthma with high serum adiponectin concentrations was similar between female current smokers and former/never smokers. Binomial confidence intervals are shown with the relative frequency estimates.
Interestingly, BMI at CARDIA Year 15 examination did not predict incident asthma among all women at Year 20 examination (Table 3). Change in BMI between examinations at Years 15 and 20 was not predictive either. Multivariable stepwise logistic regression analysis confirmed the relative importance of low serum adiponectin (P = 0.048) over BMI (P = 0.74) in predicting incident asthma among all women. Similar results were seen among female current smokers. Among female former/never smokers, we found that neither BMI nor adiponectin predicted incident asthma. Similar overall results were seen among premenopausal women.
TABLE 3.
ASSOCIATION BETWEEN BMI AT CARDIA YEAR 15 EXAMINATION AND RISK FOR INCIDENT ASTHMA AT YEAR 20 EXAMINATION
| All Women |
Premenopausal Women |
Men |
|||||||
| n with Asthma/N | OR (95% CI) | P Value | n with Asthma/N | OR (95% CI) | P Value | n with Asthma/N | OR (95% CI) | P Value | |
| Unadjusted model | |||||||||
| Both current and not current smokers | 54/1,450 | 1.09 (0.91, 1.30) | 0.34 | 32/1,011 | 1.14 (0.91, 1.41) | 0.26 | 16/1,171 | 1.23 (0.85, 1.80) | 0.28 |
| Adjusted models | |||||||||
| Both current and not current smokers | 54/1,450 | 1.15 (0.92, 1.45) | 0.21 | 32/1,011 | 1.19 (0.88, 1.61) | 0.27 | 16/1,171 | 1.38 (0.83, 2.30) | 0.22 |
| Current smokers only | 17/265 | 1.21 (0.80, 1.84) | 0.37 | 10/152 | 1.37 (0.78, 2.40) | 0.28 | 3/246 | 1.85 (0.49, 7.00) | 0.37 |
| Not current smokers only | 37/1,185 | 1.14 (0.86, 1.50) | 0.37 | 22/859 | 1.14 (0.78, 1.68) | 0.49 | 13/925 | 1.33 (0.75, 2.35) | 0.33 |
Definition of abbreviations: BMI = body mass index; CARDIA = Coronary Artery Risk Development in Young Adults; CI = confidence interval; OR = odds ratio.
Incident asthma and menopausal status were measured at CARDIA Year 20 examination; all other data are measured at Year 15 examination, unless otherwise indicated.
The adjusted models included age, race, current smoking, history of diabetes, logarithmically transformed insulin resistance, and logarithmically transformed physical activity score (at Year 15 examination) and history of hay fever (at Year 0 examination).
Odds ratios represent per 5 BMI units (in kg/m2). Similar results were obtained when BMI was studied as various categorical variables.
Change in BMI between examination at Years 15 and 20 did not predict incident asthma at Year 20 examination in any of the above categories. BMI at Year 15 also did not predict current asthma at Year 20 examination in any of the above categories. Merging men and women together did not affect results for either current or incident asthma.
Neither low serum adiponectin (studied as a categorical or a continuous variable) nor BMI predicted incident asthma in men, although sex–adiponectin and sex–BMI interactions on incident asthma did not reach statistical significance.
Asthma at Year 10 Did Not Predict Low Serum Adiponectin Concentrations in Women at Year 15
To eliminate the possibility that asthma predicts future low serum adiponectin concentrations, we evaluated the longitudinal association of prevalent asthma status at CARDIA Year 10 examination with categories of Year 15 adiponectin concentrations (<7 vs. ≥7 mg/L) among 1,455 women (including 112 women with Year 10 prevalent asthma, 248 women with lower Year 15 serum adiponectin, and 1,257 premenopausal women). In multivariable models similar to those used in our primary analyses, Year 10 prevalent asthma status did not significantly predict low Year 15 serum adiponectin concentrations in either all women (OR, 1.22; 95% CI, 0.71, 2.11; P = 0.48) or premenopausal women (OR, 1.13; 95% CI, 0.60, 2.12; P = 0.71; Table 4). Additional details are provided in the online data supplement.
TABLE 4.
ASSOCIATION BETWEEN PREVALENT ASTHMA AT CARDIA YEAR 10 EXAMINATION (PREDICTOR) AND RISK FOR LOW CATEGORY OF SERUM ADIPONECTIN CONCENTRATIONS AT YEAR 15 EXAMINATION (OUTCOME)
| All Women |
Premenopausal Women |
Men |
|||||||
| n with Low Adiponectin/N | OR (95% CI) | P Value | n with Low Adiponectin/N | OR (95% CI) | P Value | n with Low Adiponectin/N | OR (95% CI) | P Value | |
| Unadjusted model | |||||||||
| Both current and not current smokers | 248/1,455 | 1.53 (0.96, 2.42) | 0.07 | 203/1,257 | 1.39 (0.82, 2.36) | 0.22 | 481/1,164 | 0.53 (0.29, 0.98) | 0.04 |
| Adjusted models | |||||||||
| Both current and not current smokers | 248/1,455 | 1.22 (0.71, 2.11) | 0.48 | 203/1,257 | 1.13 (0.60, 2.12) | 0.71 | 481/1,164 | 0.54 (0.28, 1.04) | 0.07 |
| Current smokers only | 67/267 | 1.21 (0.46, 3.22) | 0.70 | 56/219 | 1.60 (0.55, 4.60) | 0.39 | 102/242 | 0.93 (0.27, 3.25) | 0.91 |
| Not current smokers only | 181/1,188 | 1.17 (0.60, 2.26) | 0.64 | 147/1,038 | 0.93 (0.42, 2.06) | 0.86 | 379/922 | 0.46 (0.21, 1.01) | 0.052 |
Definition of abbreviation: CARDIA = Coronary Artery Risk Development in Young Adults; CI = confidence interval; OR = odds ratio.
Prevalent asthma was measured at CARDIA Year 10 examination; all other data are measured at Year 15 examination, unless otherwise indicated.
The adjusted models included age, race, body mass index, current smoking, history of diabetes, logarithmically transformed insulin resistance, and logarithmically transformed physical activity score measured at CARDIA Year 15 examination and history of hay fever (at Year 0 examination). Low serum adiponectin concentration category was defined by the low category of serum adiponectin concentration (<7 mg/L) at Year 15.
Results were unchanged if covariates at CARDIA Year 15 examination were substituted with those at Year 10 examination instead.
Discussion
In this longitudinal cohort, we demonstrate that low serum adiponectin concentrations, independent of BMI, predict higher risk for incident asthma among middle-aged women, particularly among current smokers. We also show that the converse relationship, that is, prevalent asthma predicting future serum adiponectin concentrations, is not true among women. Thus, the findings of our longitudinal study suggest a clear direction to our previously described cross-sectional association between serum adiponectin and asthma among women (6). Although serum adiponectin concentrations and BMI are inversely correlated with each other (r of −0.33; P < 0.001), we found that low values of the former may be more important than high values of the latter in predicting the risk for incident asthma among female current smokers.
The literature pertaining to the adiponectin–asthma association is conflicting and confusing. Five human studies have analyzed the association between serum adiponectin and odds of prevalent asthma, independent of obesity (6, 31–34), of which three studies show no significant associations (32–34)(Table E3). These studies are limited by their smaller numbers of girls/women, modest effect sizes, and lower prevalence of asthma and obesity in populations outside the United States (32–34). The two positive studies, being cross-sectional in nature, are unable to establish the direction of the association (6, 31). Interestingly, a short longitudinal study of adolescents with moderate-to-severe asthma showed that low baseline serum adiponectin concentrations were associated with worse disease control among boys (sex interaction P < 0.10) (35). Further, mouse experiments support a bidirectional association—that is, allergen inhalation decreases serum adiponectin concentrations and exogenous adiponectin administration attenuates asthma (22). We had previously demonstrated in our small interventional study that acute bronchoprovocation by allergen inhalation did not affect serum adiponectin concentrations in sensitized human subjects with asthma (36). Our current longitudinal study thus confirms a unidirectional inverse association of serum adiponectin to incident asthma among women. Based upon our findings, we hypothesize that measures that raise systemic adiponectin concentrations may help prevent asthma onset among women, particularly among those who smoke. On the other hand, it is possible that this strategy may not benefit men, in light of our previously published finding that systemic adiponectin is adversely associated with asthma outcomes in men (37).
Compared with previous negative adiponectin–asthma studies (32–34), we speculate that our study had greater statistical power due to both a planned selection of large numbers of blacks and a fortuitous selection of large numbers of obese subjects and smokers, groups known to be associated with lower serum adiponectin concentrations (14). We further did not find a linear relationship between serum adiponectin and incident asthma, as seen in Figure E2. Since the depicted relationship may show a threshold effect, that is, an effect only seen with the lowest tertile of serum adiponectin concentration, it is important to have adequate numbers in this group for any study to demonstrate a significant effect on incident asthma. This may explain why our results may differ from previous negative studies in the field (32–34). Further, we demonstrate a greater asthma risk due to lower serum adiponectin concentrations among women who currently smoke than women who do not currently smoke, but the mechanistic basis for this interaction is not currently known.
Although we showed an association between low serum adiponectin and asthma among women, the sex-specific interaction was not significant (P = 0.24). In the absence of a sex-specific interaction, we cannot be certain that a similar effect is not seen among men. Our sex-specific analytic approach was primarily based upon our previously published finding of a sex-specific cross-sectional interaction of serum adiponectin on asthma outcomes in the same cohort (37). It is likely that our power for the interaction analysis in the current study was limited by the fewer cases of incident asthma among our otherwise equivalently sized sample of men (n = 16/1,171). Further, we did not find that combining both sexes increased our power. It should also be noted that serum adiponectin displays marked sexual dimorphism in its isoform profiles (38, 39). Compared with men, women have higher concentrations of the high-molecular-weight isoform (38, 39). The latter isoform is also the dominant isoform in murine lung (21) and may be the most biologically relevant isoform. Our study, however, did not measure adiponectin isoforms.
Interestingly, obese mice show airway responsiveness but without high eosinophil counts or atopic (TH2) cytokine expression in the airway (40). Leptin, another adipokine, stimulates lymphocytes toward a nonatopic (TH1) cytokine profile rather than an atopic (TH2) one (41). In a small cross-sectional study of German children, serum adiponectin was more strongly associated with nonatopic prevalent asthma than with atopic prevalent asthma (no interaction term reported), using a self-reported measure of atopy (Table E3) (31). However, using a similar measure to define atopy, our findings suggest that the association between serum adiponectin and incident asthma among women does not vary by atopic status. Since the predictor status of atopy for incident asthma in longitudinal studies weakens during adulthood as compared with childhood (42–45), it is possible that atopy may cease to be an effect modifier for the adiponectin–asthma association during adulthood.
We were surprised to find that BMI did not predict incident asthma in our analyses. These findings were thus contrary to our previous findings from the same cohort that demonstrated that baseline BMI and change in BMI predicted incident asthma in women between examinations at Years 0 and 10 (46). The absolute gain in BMI in kg/m2 for women between examination visits at Years 0 and 10 is 3.04 ± 3.64 (SD); between Years 10 and 15 is 1.44 ± 2.78; and between Years 15 and 20 is 0.86 ± 3.01. Thus, the decline in rate of gain in body mass with increasing age may make BMI at Year 15 and the change in BMI between Years 15 and 20 less predictive for incident asthma in women at Year 20 than was the case during the earlier period between Years 0 and 10. Further, it is also possible that obesity is a stronger predictor for incident asthma in younger (more sex hormonally active) women than in middle-aged women. Finally, while the previous analysis looked at accumulated incident asthma over examinations at Years 2, 7, and 10 (46), our current paper evaluated incident asthma at Year 20 examination. These differences may potentially explain the discrepant findings at different time points within the same cohort.
The strengths of our study include its focus on women, well-defined study population set within a cohort structure, and its clinical translational character, based on the recently published data on the role of systemic adiponectin in mouse and human asthma (6, 22, 37). Further, the results from our longitudinal analyses are supported by our previous cross-sectional analyses (6).
The study, however, has some limitations. Selection bias may occur if those measured for serum adiponectin were not representative of the CARDIA study population. However, our ad hoc analysis did not demonstrate that those measured were different from those not measured with respect to both obesity and asthma. Use of self-reported asthma diagnosis may result in misclassification. However, this misclassification bias is likely nondifferential (47). In addition, self-report may miss subjects with mild asthma. However, this is unlikely, given that most subjects with asthma in our study were of intermittent or mild persistent severity (48). In addition, self-reported asthma may include early chronic obstructive pulmonary disease, particularly among smokers. However, this seems less likely because most women with incident asthma in our study had normal FEV1/FVC ratio (Table 1). On the other hand, we cannot rule out the possibility of chronic bronchitis being misdiagnosed as asthma in our study. However, that error is likely to cause a nondifferential misclassification bias and is unlikely to produce a spurious result. Further, to confirm definitively that adiponectin has a different effect on asthma status between men and women, a statistically significant interaction is required. Our analysis may lack the statistical power to significantly detect this interaction. This study did not separately measure various serum adiponectin isoforms that may have varying in vivo activity in asthma. Interestingly, a recent study showed no significant correlation between airway and systemic concentrations of total adiponectin; the various isoforms were, however, not compared (49). Further, our definition of atopy was limited to self-reported hay fever at CARDIA Year 0 examination and was not confirmed by objective tests. Finally, adiponectin measurements were not repeated at other examination visits. A single assessment of a biomarker may be susceptible to short-term variation and may not reflect long-term exposure. However, studies suggest that serum concentrations of adiponectin are stable and that therefore, serum adiponectin is a good candidate for long-term epidemiologic risk assessment (50–52).
In summary, our longitudinal study demonstrates that low serum adiponectin predicts future risk for incident asthma among middle-aged women and not vice versa. Measures that raise systemic adiponectin concentrations may lead to newer ways to prevent asthma among women and particularly among those who smoke.
Supplementary Material
Acknowledgments
The authors thank William S. Beckett, M.D., M.P.H., Mount Auburn Hospital (Cambridge, MA) for his careful critique of the manuscript. The Coronary Artery Risk Development in Young Adults Study is conducted and supported by the NHLBI in collaboration with the Coronary Artery Risk Development in Young Adults Study Investigators.
Footnotes
Author Contributions: Conception and design: A.S., D.R.J.; analysis and interpretation: A.S., C.Q., M.S., B.T., M.W.S., L.J.S., D.R.J.; drafting the manuscript for important intellectual content: A.S., C.Q., M.S., B.T., M.W.S., L.J.S., D.R.J.
Supported by the CARDIA study contracts N01-HC-48047-50 and N01-HC-95095 and an ancillary study R01 HL 53560 (YALTA). A.S. is supported by 1K23HL094531-01 and DHHS/NIH/NCRR/GCRC grant # 5M01 RR00997.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201110-1767OC on April 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention. National health interview survey, 2001–2005 [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; c2005 [accessed 2011 Jan 1]. Available from: http://www.Cdc.Gov/nchs/nhis.Htm
- 2.Centers for Disease Control and Prevention Self-reported asthma prevalence and control among adults - United States 2001. MMWR Morb Mortal Wkly Rep 2003;52:381–384 [PubMed] [Google Scholar]
- 3.From the Centers for Disease Control and Prevention Self-reported asthma prevalence among adults–United States, 2000. JAMA 2001;286:1571–1572 [PubMed] [Google Scholar]
- 4.Fantuzzi G. Adipose tissue, adipokines and inflammation. J Allergy Clin Immunol 2005;115:911–919 [DOI] [PubMed] [Google Scholar]
- 5.Sprafka JM, Burke GL, Folsom AR, Hahn LP. Hypercholesterolemia prevalence, awareness, and treatment in blacks and whites: the Minnesota heart survey. Prev Med 1989;18:423–432 [DOI] [PubMed] [Google Scholar]
- 6.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR., Jr Association between asthma and serum adiponectin concentration in women. Thorax 2008;63:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood A, Camargo CA, Jr, Ford ES. Association between leptin and asthma in adults. Thorax 2006;61:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects lps-induced liver injury through modulation of tnf-alpha in kk-ay obese mice. Hepatology 2004;40:177–184 [DOI] [PubMed] [Google Scholar]
- 9.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 2004;316:924–929 [DOI] [PubMed] [Google Scholar]
- 10.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 2004;109:2046–2049 [DOI] [PubMed] [Google Scholar]
- 11.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines il-10 and il-1ra in human leukocytes. Biochem Biophys Res Commun 2004;323:630–635 [DOI] [PubMed] [Google Scholar]
- 12.Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, Lee MS, Lee JH, Yoon DY, Yoon SR, et al. Adiponectin is a negative regulator of nk cell cytotoxicity. J Immunol 2006;176:5958–5964 [DOI] [PubMed] [Google Scholar]
- 13.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, et al. Adiponectin inhibits the production of cxc receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 2008;102:218–225 [DOI] [PubMed] [Google Scholar]
- 14.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs DR., Jr Serum adiponectin in young adults–interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol 2004;14:492–498 [DOI] [PubMed] [Google Scholar]
- 15.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 16.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 17.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 2003;285:E527–E533 [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol 2009;182:684–691 [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi T, Misaki A, Fujita J, Sonobe H, Ohtsuki Y. T-cadherin (cdh13, h-cadherin) expression downregulated surfactant protein d in bronchioloalveolar cells. Virchows Arch 2001;438:370–375 [DOI] [PubMed] [Google Scholar]
- 20.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of acrp30/adiponectin. Proc Natl Acad Sci USA 2004;101:10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Hug C, Kasahara DI, Johnston RA, Williams AS, Verbout NG, Si H, Jastrab J, Srivastava A, Williams ES, et al. Impact of adiponectin deficiency on pulmonary responses to acute ozone exposure in mice. Am J Respir Cell Mol Biol 2010;43:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395 [DOI] [PubMed] [Google Scholar]
- 23.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116 [DOI] [PubMed] [Google Scholar]
- 24.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Jr, Liu K, Orden S, Pirie P, et al. Recruitment in the coronary artery disease risk development in young adults (CARDIA) study. Control Clin Trials 1987;8:68S–73S [DOI] [PubMed] [Google Scholar]
- 25.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46:459–469 [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 27.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, Quon MJ, Baron AD. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 2001;86:5457–5464 [DOI] [PubMed] [Google Scholar]
- 28.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755 [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Jacobs DR, Jr, Caspersen CJ, Gomez-Marin O, Knudsen J. Test-retest reliability of the Minnesota leisure time physical activity questionnaire. J Chronic Dis 1986;39:505–511 [DOI] [PubMed] [Google Scholar]
- 30.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol 2005;115:928–934 [DOI] [PubMed] [Google Scholar]
- 31.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol 2009;20:81–88 [DOI] [PubMed] [Google Scholar]
- 32.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol 2008;19:535–540 [DOI] [PubMed] [Google Scholar]
- 33.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, Viikari J, Raitakari OT. Obesity, adipokines and asthma. Allergy 2009;64:770–777 [DOI] [PubMed] [Google Scholar]
- 34.Sutherland TJ, Sears MR, McLachlan CR, Poulton R, Hancox RJ. Leptin, adiponectin, and asthma: findings from a population-based cohort study. Ann Allergy Asthma Immunol 2009;103:101–107 [DOI] [PubMed] [Google Scholar]
- 35.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol 2010;125:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood A, Qualls C, Seagrave J, Stidley C, Archibeque T, Berwick M, Schuyler M. Effect of specific allergen inhalation on serum adiponectin in human asthma. Chest 2009;135:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sood A, Dominic E, Qualls C, Steffes MW, Thyagarajan B, Smith LJ, Lewis CE, Jacobs DR., Jr Serum adiponectin is associated with adverse outcomes of asthma in men but not in women. Front Pharmacol 2011;2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab 2007;92:1857–1862 [DOI] [PubMed] [Google Scholar]
- 39.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 2005;280:18073–18080 [DOI] [PubMed] [Google Scholar]
- 40.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 2007;176:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol 2005;174:3137–3142 [DOI] [PubMed] [Google Scholar]
- 42.Illi S, von Mutius E, Lau S, Nickel R, Niggemann B, Sommerfeld C, Wahn U. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol 2001;108:709–714 [DOI] [PubMed] [Google Scholar]
- 43.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology 2009;14:814–821 [DOI] [PubMed] [Google Scholar]
- 44.Settipane GA, Greisner WA, III, Settipane RJ. Natural history of asthma: a 23-year followup of college students. Ann Allergy Asthma Immunol 2000;84:499–503 [DOI] [PubMed] [Google Scholar]
- 45.Basagana X, Sunyer J, Zock JP, Kogevinas M, Urrutia I, Maldonado JA, Almar E, Payo F, Anto JM. Incidence of asthma and its determinants among adults in Spain. Am J Respir Crit Care Med 2001;164:1133–1137 [DOI] [PubMed] [Google Scholar]
- 46.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 2001;164:2045–2050 [DOI] [PubMed] [Google Scholar]
- 47.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, Lemiere C, Sharma S, Field SK, Alvarez GG, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ 2008;179:1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Asthma Education and Prevention Program Expert panel report 3 (epr-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138 [DOI] [PubMed] [Google Scholar]
- 49.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma 2011;48:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan RC, Ho GY, Xue X, Rajpathak S, Cushman M, Rohan TE, Strickler HD, Scherer PE, Anastos K. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev 2007;16:1291–1293 [DOI] [PubMed] [Google Scholar]
- 51.Lukanova A, Soderberg S, Kaaks R, Jellum E, Stattin P. Serum adiponectin is not associated with risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2006;15:401–402 [DOI] [PubMed] [Google Scholar]
- 52.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem 2003;49:650–652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.