Abstract
Here we report a 33-years-old woman with hereditary spherocytosis and hemochromatosis due to homozygosity for the C282Y mutation of the HFE gene. The coinheritance of both conditions led to severe iron overload and liver cirrhosis at young age. The patient was treated by repeated phlebotomy, and reversibility of cirrhosis was documented by transient elastography. This report discusses the pathophysiology of iron accumulation in patients with hemolytic anemia combined with HFE C282Y homozygosity. The case indicates that patients with hematological disorders characterized by increased erythropoetic activity should be screened for HFE mutations.
Keywords: hereditary hemochromatosis, hereditary spherocytosis, iron overload, liver cirrhosis
Introduction
Hereditary spherocytosis (HS) is a common disorder characterized by corpuscular hemolytic anemia of varying severity with increased red cell osmotic fragility and presence of spherocytes. In Western Europe, the estimated prevalence of HS is about 1: 5,000 [1]. The molecular basis of HS is an inherited deficiency or dysfunction of one of the proteins of the erythrocyte membrane: ankyrin, anion exchanger 1, α-spectrin, β-spectrin, or pallidin (protein 4.2) [2]. These proteins connect the membrane skeleton to the lipid bilayer. Anemia, jaundice and splenomegaly are the most common clinical features of HS. Most affected individuals present with dominantly inherited mild or moderate forms; severe HS occurs in approximately 5% of cases [3].
Hereditary hemochromatosis is an autosomal recessive disorder that is due to the C282Y mutation of the HFE gene in most patients [4]. The mutation causes dysregulation of iron homoeostasis and an increase of intestinal iron absorption. The clinical manifestations are related to excessive iron deposition, particularly in liver, heart, pancreas, and pituitary gland. Hereditary hemochromatosis represents one of the most common inherited diseases in Caucasian populations in the United States and Western Europe, with a frequency of about 5 per 1,000 (0.5 percent) for the homozygous carrier state [5].
Hemochromatosis in combination with spherocytosis was first described by Lawrence in 1949 [6]. About 20 cases with iron overload and HS have been reported since [7-17]. Here we report a case of a young woman with coinheritance of HS and genetically proven hemochromatosis. The two conditions led to severe iron overload and liver cirrhosis at young age.
Case presentation
The 33-years-old woman with mild hemolytic anemia presented with severe iron overload in March 2003. Her blood tests revealed a transferrin saturation of 90%, a serum iron level of 2,070 μg/l, and aferritin level of 2,920 ng/ml. There was no history of prolonged iron intake, blood transfusions or excessive alcohol abuse. The patient reported one severe hemolytic attack with a hemoglobin decrease to 80 g/l, which was associated with viral infection in January 2003. Hematological findings at hospital admission two months later were as follows: hemoglobin 128 g/l, hematocrit 36.3%, reticulocytes 424/nl (normal range 20 -90/nl), spherocytes on the peripheral blood smear. Abnormal laboratory values included elevated total serum bilirubin (27 mg/l), elevated lactate dehydrogenase activity (314 U/l) and haptoglobin concentrations below the detection limit as signs of hemolysis as well as slightly elevated alanine aminotransferase activity (40 U/l). Abdominal ultrasound showed hepatosplenomegaly.
Based on the presence of spherocytes and increased red cell osmotic fragility, and in the absence of any other cause of the hemolytic anemia, the diagnosis of spherocytosis was established. Because of the high levels of serum iron and ferritin and the markedly elevated transferrin saturation index, we suspected coexisting hereditary hemochromatosis. The genetic analysis (PCR) revealed homozygosity for the HFE mutation C282Y, which is present in 69 -100% of patients with HH [4].
In May 2003, percutaneous liver biopsy showed complete liver cirrhosis with severe hepatocellular siderosis. From May 2003 to June 2004 she underwent phlebotomies twice weekly. Serum ferritin was maintained below 50 μg/l, and liver function tests were normal. Since June 2004 monthly maintenance phlebotomies have been continued.
In June 2007, we performed transient elastography (Fibroscan®), which revealed a liver stiffness of 8.1 kPa, consistent with moderate fibrosis (stage F2) (Figure 1) [18,19]. This indicates that continuous phlebotomy over 4 years has led to regression of fibrosis, since transient elastography can exclude cirrhosis with high accuracy [20,21].
Figure 1.
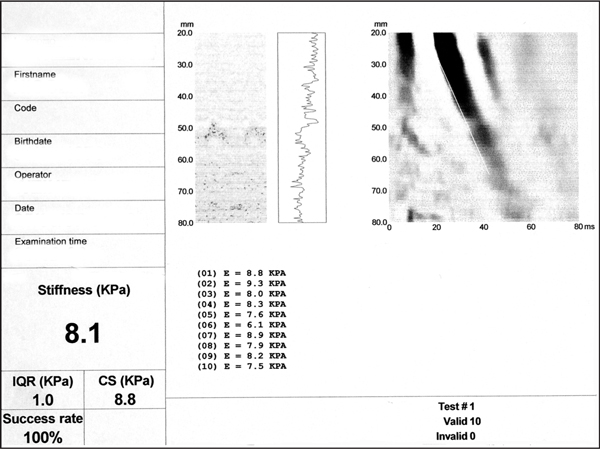
Transient elastography. The liver stiffness index (8.1 kPa) is consistent with fibrosis stage F2 (moderate fibrosis).
Discussion
Our patient presented with a combination of HS and genetically proven hemochromatosis. These coexisting conditions led to severe iron overload and liver cirrhosis at young age. The patient suffered from a mild form of HS, hence no splenectomy was performed. Despite regular iron loss owing to her menstrual cycles, mild hemolysis and the absence of iron intake, the patient developed manifest hemochromatosis with liver damage.
In light of the recent insights into the molecular regulation of iron metabolism [22,23] several explanations might account for the excessive iron storage in this patient. Firstly, iron absorption in patients with hemolytic anemia can be inappropriately stimulated despite massive iron overload. This results from the dominant effect of the erythropoetic demand for iron over the inhibitory signals of excessive iron stores mediated by hepcidin, which is the principal regulator of iron metabolism in humans [23,24]. Hepcidin acts by inhibiting intestinal iron absorption and iron release from macrophages. However in the presence of systemic iron overload, urinary hepcidin concentrations are low in patients with thalassemia and congenital dyserythropoetic anemia [25]. Secondly, our patient carries the homozygous C282Y mutation of the HFE gene, which is involved in the regulation of hepcidin synthesis. It has been demonstrated that mice and humans with homozygous HFE-related hemochromatosis display inappropriately low hepatic expression and serum levels of hepcidin [26]. Moreover, overexpression of hepcidin in HFE deficient mice prevents or delays hepatic iron accumulation [27,28].
Only 24 cases of congenital spherocytosis associated with iron overload have been reported in the literature [7-17]. The association of some other hemolytic anemias, including acanthocytosis, sickle cell anemia and thalassemia, and hereditary hemochromatosis has also been described [29-31]. This case of severe iron overload and liver damage in a young female with coexisting HS and hereditary hemochromatosis further supports a synergistic effect of both conditions. This is the first report to demonstrate regression of cirrhosis in a patient with HS and hemochromatosis, as documented by transient elastography. The concept that fibrosis can regress when the initial disease is controlled or cured is supported by ample clinical evidence and has been highlighted recently [32]. Of note, the reversibility of liver fibrosis was demonstrated in a recent study of 36 patients with hemochromatosis undergoing phlebotomy [33]: regression of fibrosis of by at least 2 METAVIR units was observed in 69% of patients with F3 fibrosis and 35% of patients with cirrhosis. Thus, screening for hemochromatosis is recommended in all individuals with increased erythropoetic activity.
References
- Eber SW, Pekrun A, Neufeldt A, Schröter W. Prevalence of increased osmotic fragility of erythrocytes in German blood donors: screening using a modified glycerol lysis test. Ann Hematol. 1992;64(2):88–92. doi: 10.1007/BF01715351. [DOI] [PubMed] [Google Scholar]
- Delaunay J. Molecular basis of red cell membrane disorders. Acta Haematol. 2002;108(4):210–8. doi: 10.1159/000065657. [DOI] [PubMed] [Google Scholar]
- Bolton-Maggs PH, Stevens RF, Dodd NJ, Lamont G, Tittensor P, King MJ. General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis and management of hereditary spherocytosis. Br J Haematol. 2004;126(4):455–74. doi: 10.1111/j.1365-2141.2004.05052.x. [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Edwards CQ, Griffen LM, Goldgar D, Drummond C, Skolnick MH, Kushner JP. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Eng J Med. 1998;318(21):1355–62. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- Lawrence RD. Haemochromatosis in three families and in a woman. Lancet. 1949;1(18):736. doi: 10.1016/s0140-6736(49)92301-6. [DOI] [PubMed] [Google Scholar]
- Barry M, Scheurer PJ, Scherlock S, Ross CF, Williams R. Hereditary spherocytosis with secondary hemochromatosis. Lancet. 1968;2(7566):481–5. doi: 10.1016/s0140-6736(68)90648-x. [DOI] [PubMed] [Google Scholar]
- Brandenberg JB, Demarmels Biasiutti F, Lutz HU, Wuillemin WA. Hereditary spherocytosis and hemochromatosis. Ann Hematol. 2002;81(4):202–9. doi: 10.1007/s00277-002-0432-0. [DOI] [PubMed] [Google Scholar]
- Ichiche M, Lacor P, Hoorens A, Brande JV, Brussaard H, Vanstraelen D. Congenital spherocytosis with hereditary hemochromatosis without pathogenic mutations in the HFE gene. Eur J Intern Med. 2004;15(7):460–2. doi: 10.1016/j.ejim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Fargion S, Cappellini MD, Piperno A, Panajotopoulos N, Ronchi G, Fiorelli G. Association of hereditary spherocytosis and idiopathic hemochromatosis. A synergistic effect in determining iron overload. Am J Clin Pathol. 1986;86(5):645–9. doi: 10.1093/ajcp/86.5.645. [DOI] [PubMed] [Google Scholar]
- Lopez DE, Kohan M, Ferreno D, Raffa MP, Prytyka A. Hemochromatosis associated with hereditary spherocytosis. Acta Gastroenterol Lationoam. 1997;27(4):267–70. [PubMed] [Google Scholar]
- Mohler DN, Wheby MS. Case report: hemochromatosis heterozygotes may have significant iron overload when they also have hereditary spherocytosis. Am J Med Sci. 1986;292(5):320–4. doi: 10.1097/00000441-198611000-00014. [DOI] [PubMed] [Google Scholar]
- Montes-Cano MA, Rodríguez-Muñoz F, Franco-Osorio R, Núñez-Roldán A, González-Escribano MF. Hereditary spherocytosis associated with mutations in HFE gene. Ann Hematol. 2003;82(12):769–72. doi: 10.1007/s00277-003-0733-y. [DOI] [PubMed] [Google Scholar]
- O'Mahony S, O'Brien PA, Whelton MJ. Genetic hemochromatosis and congenital spherocytosis. Lancet. 1987;1(8527):282. doi: 10.1016/s0140-6736(87)90108-5. [DOI] [PubMed] [Google Scholar]
- Takegoshi T, Nishino T, Tanino M, Nonokura A, Otha G. An autopsy case of hemochromatosis and hepatoma combined with hereditary spherocytosis. Jpn J Med. 1984;23(1):48–52. doi: 10.2169/internalmedicine1962.23.48. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Scott PJ, North JDK. Hemochromatosis in association with hereditary spherocytosis. Arch Intern Med. 1967;120(6):701–7. doi: 10.1001/archinte.1967.00300050057009. [DOI] [PubMed] [Google Scholar]
- Zimelman AP, Miller A. Primary hemochromatosis with hereditary spherocytosis. Arch Intern Med. 1980;140(7):983–4. doi: 10.1001/archinte.1980.00040020983025. [DOI] [PubMed] [Google Scholar]
- Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56(7):968–73. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold JF, Morin S, Taylor-Robinson SD. Transient elastography for the assessment of chronic liver disease: ready for the clinic? World J Gastroentrol. 2007;13(36):4791–7. doi: 10.3748/wjg.v13.i36.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55(3):403–8. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and metaanalysis. Clin Gastroenterol Hepatol. 2007;5(10):1214–20. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Molecular insights into the pathogenesis of hereditary haemochromatosis. Gut. 2006;55(4):564–8. doi: 10.1136/gut.2005.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Kaplan J. Hepcidin regulation: ironing out the details. J Clin Invest. 2007;117(7):1755–8. doi: 10.1172/JCI32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program. 2006;29-35:507. doi: 10.1182/asheducation-2006.1.29. Review. [DOI] [PubMed] [Google Scholar]
- Papanikolaou G, Tzillianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg JP, Sakellaroopoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105(10):4103–5. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–73. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- Viatte L, Nicolas G, Lou DQ, Bennoun M, Lesbordes-Brion JC, Canonne-Hergaux F, Schönig K, Bujard H, Kahn A, Andrews NC, Vaulont S. Chronic hepcidin induction causes hyposideremia and alters the pattern of cellular iron accumulation in hemochromatotic mice. Blood. 2006;107(7):2952–8. doi: 10.1182/blood-2005-10-4071. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Fargion S, Sampietro M, Graziadei G, Fiorelli G. Nontransfusional iron overload thalassemia intermedia: role of the hemochromatosis allele (letter) Blood. 1998;92(11):4479–80. [PubMed] [Google Scholar]
- Conrad ME. Sickle cell disease and hemochromatosis. Am J Hematol. 1991;38(2):150–2. doi: 10.1002/ajh.2830380217. [DOI] [PubMed] [Google Scholar]
- Hitchins R, Naughton L, Kerlin P, Cobbcroft R. Spur cell anemia (acanthocytosis) complicating idiopathic hemochromatosis. Pathology. 1988;20(1):59–61. doi: 10.3109/00313028809085198. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Reversibility of hepatic fibrosis and cirrhosis--is it all hype? Nat Clin Pract Gastroenterol Hepatol. 2007;4(5):236–7. doi: 10.1038/ncpgasthep0813. [DOI] [PubMed] [Google Scholar]
- Falize L, Guillygomarc'h A, Perrin M, Lainé F, Gyuader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44(2):472–7. doi: 10.1002/hep.21260. [DOI] [PubMed] [Google Scholar]


