Abstract
Background
Progesterone administration prior to intravaginal challenge with pathogenic SIVmac239 decreases the protective efficacy of live attenuated vaccines in rhesus macaques.
Methods
To determine if progesterone alters the efficacy of live attenuated vaccines through local or systemic effects, seven male rhesus macaques were immunized with SHIV89.6 and then challenged intravenously with SIVmac239. Three of these animals were treated with Depo-Provera 30 days prior to the SIV challenge.
Results
The SHIV animals had significantly lower plasma viral RNA levels than the unimmunized control monkeys, but the Depo-Provera treated, SHIV-immunized animals did not. Despite the lack of protection, the Depo-Provera SHIV animals had strong SIV specific T-cell responses. However, altered patterns of NK frequency and CD38 T-cell expression prior to SIV challenge were observed in Depo-Provera SHIV animals.
Conclusions
Depo-Provera eliminates live-attenuated lentivirus vaccine efficacy in male rhesus monkeys through systemic effects on antiviral immunity and/or viral replication.
Keywords: contraception, inflammation, live-attenuated vaccine, progesterone, T-cell activation
Introduction
Although elusive, a vaccine against HIV remains the best hope for stopping the AIDS pandemic. HIV is transmitted by sexual contact and the goal of a vaccine is to prevent establishment of systemic infection after sexual contact. In the SIV model of AIDS, only live-attenuated viruses provide protection against intravaginal virus inoculation and thus understanding the mechanisms of protection that are elicited by attenuated lentivirus infections would be a major advance in HIV vaccine development. Using attenuated SHIV89.6 as a vaccine virus, we have consistently protected rhesus macaques from uncontrolled challenge virus replication after intravaginal SIVmac239 inoculation [1, 6, 7, 23].
We have also shown that Depo-Provera blunts live-attenuated SHIV89.6 mediated protection from intravaginal challenge with SIVmac239 [3]. Depo-Provera administration leads to vaginal epithelial thinning in macaques and increased susceptibility to vaginal SIV transmission [19] and this may explain the reduced protection of SHIV infection against vaginal SIV challenge. However, progestins also disrupt immune responses [24] and increase expression of CCR5 on CD4+ T cells [29] that could decrease vaccine mediated protection from vaginal SIV transmission. Understanding how Depo-Provera decreases attenuated lentivirus-mediated protection is important for understanding the nature of protective anti-HIV immunity and also because hormonal contraceptives, comprised of progestins and estrogens, are among the most extensively used family planning methods world wide and they could alter the effectiveness of an AIDS vaccine. In fact, the use of hormonal contraception at the time of HIV-infection in women has been reported to increase plasma vRNA set point and to lead to persistently higher viremia [13]. However, the mechanism involved in the increased susceptibility to HIV infection is unclear as no clinically significant thinning of the epithelium has been found in women using progestin contraceptives [20] that is equivalent to the epithelial thinning in macaques [19].
Sex steroid hormones regulate the physiological activity of the immune system by interacting with a variety of critical cell types including lymphocytes and monocyte/macrophages [26]. Ovarian hormones in women and monkeys have a dramatic effect on immunity, and the physiology of immune cells varies in a regular pattern during the menstrual cycle with the nadir of B-cell responsiveness occurring during the luteal phase of the menstrual cycle when progesterone is the highest [14, 15]. Progesterone suppresses cell-mediated immunity and promotes a switch from Th1 towards Th2 type immune responses [10], and these effects may help to maintain a successful pregnancy. Progesterone has also been reported to inhibit T-cell proliferation and CTL activity [18], induce pro-inflammatory effects in neutrophils and decrease NK cell activity [5]. However, results of studies on the role of progesterone in immunity are often conflicting or inconclusive (reviewed in [5]). Finally, the differences in virus load between men and women in the course of the HIV-1 infection suggest a role for gender specific factors, including ovarian hormones in AIDS progression [25].
The objective of the present study was to determine whether vaccine failure after progesterone administration to female rhesus monkeys immunized with attenuated SHIV89.6 and challenged intravaginally with SIVmac239 could be due to systemic effects on antiviral immunity or viral replication. To address this question we infected seven male rhesus macaques with attenuated SHIV89.6 and treated three of them with Depo-Provera 30 days before intravenous (IV) challenge with SIVmac239. We found that Depo-Provera significantly increased viral replication in SHIV89.6 immunized monkeys following IV SIVmac239 challenge. Thus Depo-Provera decreased vaccine efficacy in male rhesus monkeys challenged IV with SIVmac239. We conclude that exogenous progestins altered systemic innate and adaptive immune responses to the SIV challenge or promoted viral replication in immunized animals and, indeed, we observed altered T-cell activation in the Depo-Provera treated monkeys. These findings show that, in male rhesus monkeys, exogenous progestins can eliminate attenuated lentivirus-mediated protection from intravenous SIV challenge through systemic effects on the host virus relationship.
Materials and methods
Animals
Adult male rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC) in accordance with the regulations of the American Association for Accreditation of Laboratory Animal Care standards. The experiments were approved by the Institutional Animal Use and Care Committee of the University of California, Davis. The animals were from 5 to 12 years old. All animals were negative for antibodies to HIV-2, SIV, type-D retrovirus, and simian T-cell lymphotropic virus type 1 at the time the study was initiated. In SIV-infected animals at the CNPRC, AIDS is defined as weight loss of >15% in 2 weeks, opportunistic infections which do not respond to therapy or persistent leucopenia (total leukocytes of <3000/μl blood).
SHIV89.6 immunization and Depo-Provera administration
Seven adult male macaques were immunized by IV inoculation with a previously described stock of live attenuated SHIV89.6 [1]. Approximately one year after SHIV89.6 inoculation, and 4 weeks before challenge with SIV, a single 30 mg dose of Depo-Provera [3] was administered by intramuscular injection to three macaques randomly selected from the group of seven macaques (Table 1). Hereafter, these three macaques are referred as ‘Depo-Provera SHIV macaques’ and the four Depo-Provera-naïve, SHIV-immunized macaques are referred as ‘SHIV macaques’.
Table 1.
Summary of study organization, characteristics of the male rhesus monkeys, treatments and peak plasma vRNA levels after SIV challenge
| Animal number | Origin | Age at challenge | Mamu- A*01 status | Attenuated SHIV89.6 infection | Depo-Provera treatment | Peak plasma vRNAIoglO (copies/ml) |
|---|---|---|---|---|---|---|
| 34569 | Ch | 7 year 2 months | − | + | None | 3.53 |
| 34484 | Ch | 8 year 5 months | − | + | None | 4.48 |
| 34537 | Ch | 7 year 11 months | − | + | None | 2.93 |
| 34549 | Ch | 6 year | − | + | None | 5.74 |
| 30706 | 1/8 Ch | 7 year 1 months | − | + | + | 4.71 |
| 34573 | Ch | 9 year 6 months | − | + | + | 4.76 |
| 32212 | In | 5 year 1 months | + | + | + | 3.79 |
| 34554 | Ch | 7 year | − | − | None | 6.91 |
| 34566 | Ch | 6 year 2 months | − | − | None | 7.23 |
| 32383 | 1/8 Ch | 5 year 1 months | − | − | None | 7.34 |
| 33583 | Ch | 9 year 11 months | − | − | None | 6.95 |
Ch, Chinese-origin rhesus monkeys; In, Indian-origin rhesus monkeys.
Intravenous SIVmac239 inoculation
The pathogenic SIVmac239 stock used in the present study was produced in rhesus peripheral blood mononuclear cells (PBMC) as previously described [23] and contained approximately 105 TCID50/ml. The virus challenge of the monkeys consisted of a single IV inoculation with 1 ml of the SIVmac239 stock diluted 1:100 in sterile saline to produce an inoculum containing 103 TCID50. Four vaccine-naïve control male macaques were challenged IV with SIVmac239 contemporaneously with the seven SHIV-immunized macaques. Blood samples were collected at regular intervals post challenge (PC). Six months after IV SIVmac239 challenge, all the monkeys were necropsied and blood and tissues were collected.
PBMC isolation
Peripheral blood mononuclear cells were isolated from heparinized blood using Lymphocyte Separation Medium (ICN Biomedicals, Aurora, OH, USA). PBMC samples were frozen in 10% DMSO (Sigma, St Louis, MO, USA)/90% newborn calf serum (Gemini Bio-Products, Calabasas, CA, USA) and stored in liquid nitrogen until future analysis in immunological and virological assays [23].
Plasma vRNA measurement
Plasma samples were analyzed for viral RNA (vRNA) by a quantitative branched DNA (bDNA) assay [9]. Virus load in plasma samples is reported as vRNA copy numbers per ml of plasma. The detection limit of this assay is 125 vRNA copies/ml plasma.
Measurement of anti-SIV IgG antibody titers
SIV-specific IgG binding antibody titers in plasma were measured using ELISA plates coated with detergent-disrupted SIVmac251 as previously described [16]. The results of the anti-SIV IgG ELISA are reported as the highest dilution of a sample that produced optical density values above the cut-off value.
SIV-specific IFN-γ ELISPOT assay
As described previously [1, 27], the number of IFN-γ secreting cells in cryo-preserved PBMC responding to a SIVmac239 Gag p27 peptide pool was determined using an IFN-γ ELISPOT kit (U-CyTech, Utrecht University, Utrecht, the Netherlands). Negative controls consisted of cells that were cultured in medium only. A sample was considered positive only if the frequency of IFN-γ secreting cells in a well exceeded 50/1 × 106 PBMC and if the number of positive IFN-γ spot-forming cells (SFC) was greater than the mean of the matched medium-only wells ±2 standard deviation (SD). Data are reported as the number of IFN-γ SFC per 1 × 106 PBMC and the background IFN-γ spot numbers in medium-only wells were subtracted from the spot numbers of SIV peptide-stimulated wells. In addition to stimulating each PBMC sample with PMA/ionomycin, PBMC from monkeys infected with SIVmac239 delta nef and known to have strong anti-SIV p27-specific IFN-γ responses were included as positive controls in every assay. Further, PBMC samples from SIV naïve monkeys were included as negative controls in every assay. All the positive and negative controls gave appropriate results in all experiments.
Flow cytometric analysis of cell populations in blood
Blood was collected at frequent intervals and the percentage of CD3+ CD4+ and CD3+CD8+ T cells, and of the CD20+ B cells, within the lymphocyte population was determined by flow cytometric analysis using a FACSCalibur (Becton Dickinson Immunocytometry Systems, Milpitas, CA, USA) and rhesus macaque-reactive antibodies anti-CD3-peridinin chlorophyll protein (PerCP) clone No. SP34, anti-CD4-phycoerytherin (PE) clone No. L-200, CD8-fluorescein isothiocyanate (FITC) clone No. SK1 and CD20- allophycocyanin (APC) clone No. L27. Additional combinations of surface markers were performed simultaneously in the same blood samples: CD3, CD4 and CD8 with CD62L-PE (clone No. SK11); CD25-PE (clone No. 2A3); or CD3 with either CD4 or CD8 only in combination with HLA-DR-PE/CD38-FITC (clone No. G46-6 and OKT10 respectively); CD16-FITC/CD8beta-PE (clone No. 3G8 and 2ST8.5H7 respectively); CCR5-PE/CD95-FITC (clone No. 3A9 and DX2 respectively). The anti-CD38 was produced by culture of the hybridoma OKT10 from ATCC and generously provided by R. Reyes. All other antibodies were purchased from Pharmingen (Pharmingen/Becton-Dickinson, San Diego, CA, USA) except CD20-APC, CD62L and CD25-PE, which were from Becton-Dickinson (Becton-Dickinson, San Jose, CA, USA).
Intracellular staining for Ki67 in lymph nodes
Lymph node cell suspensions were labeled with two different cocktails: (i) anti-CD3-Pacific Blue® (Becton and Dickinson, San Jose, CA, USA), anti-CD4-Cy55 PerCp, anti-CCR5-PE, anti-CD25-Cy7PE, anti-CXCR3-APC (clone No. IC6), anti-HLA-Dr-Cy7 APC (clone No. L243); (ii) anti-CD3-Cy55 PerCp, anti-CD8-Alexa Fluor 700, anti-CD28-PE (clone No. L293), anti-CCR7-Cy7 PE (clone No. 3D12), anti-CD38 (conjugated in-house to Cascade Blue® using a Xenogen labeling kit, Molecular Probes, Eugene, OR, USA), anti-CXCR3-APC and anti-HLA-Dr-Cy7 APC (all Mabs except anti-CD38 were from Becton Dickinson). Samples were permeabilized with 0.5% saponin for intracellular staining with Ki67-FITC (clone no. B56, BD) and incubated for 15 minutes at room temperature. After washing with the permeabilizing buffer, cells were fixed with 1% paraformaldehyde. Data was acquired using a FACSAria flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Inc., Ashland, OR, USA) and Macintosh G5 computers (Apple Inc, Cupertino, CA, USA). At least 100,000 small lymphocyte events were collected from each tube analyzed.
Statistical analysis
To determine if the mean plasma vRNA levels among the three monkey groups were significantly different, the vRNA data was used to calculate the area under the curve (AUC) for animal. The mean AUC for each group was analyzed by a Kruskall–Wallis test, and to determine if the difference between the vRNA levels of all possible pairs of groups was significant, a Dunn's multiple comparison post hoc test was used. All the above calculations were done using Prism 4.0 software (Graph Pad Inc, San Diaego, CA, USA) and a Macintosh G5 computer (Apple Inc.). The remaining data sets were analyzed by applying linear and nonlinear random-effects models to address the statistical dependencies of the repeated measures (Davidian & Giltinan, 1995; Laird & Ware, 1982) using SAS (SAS Institute, Cary, NC, USA) version 9.1. Any missing data were assumed to be missing at random. Unless otherwise noted, a two-tailed test of significance was used.
Results
Depo-Provera eliminates live-attenuated SHIV-mediated control of virus replication after intravenous SIV challenge of male rhesus macaques
Seven male rhesus macaques were IV inoculated with SHIV89.6 [17] and 2 weeks later all the animals had peak plasma vRNA levels of approximately 106 vRNA copies/ml that decreased to undetectable levels between 16–24 weeks after immunization (data not shown). Depo-Provera was administered to three randomly selected animals four weeks prior to IV SIVmac239 challenge at 52 weeks post-immunization. In addition to the four SHIV immunized and three Depo-Provera SHIV immunized monkeys, four vaccine-naïve control monkeys were also IV inoculated with SIV (103 TCID50). Note that Depo-Provera administration had no detectable effect on the plasma viremia associated with chronic SHIV89.6 infection as all animals had undetectable plasma vRNA 4-week prior to, and on the day of, SIV challenge (Fig. 1).
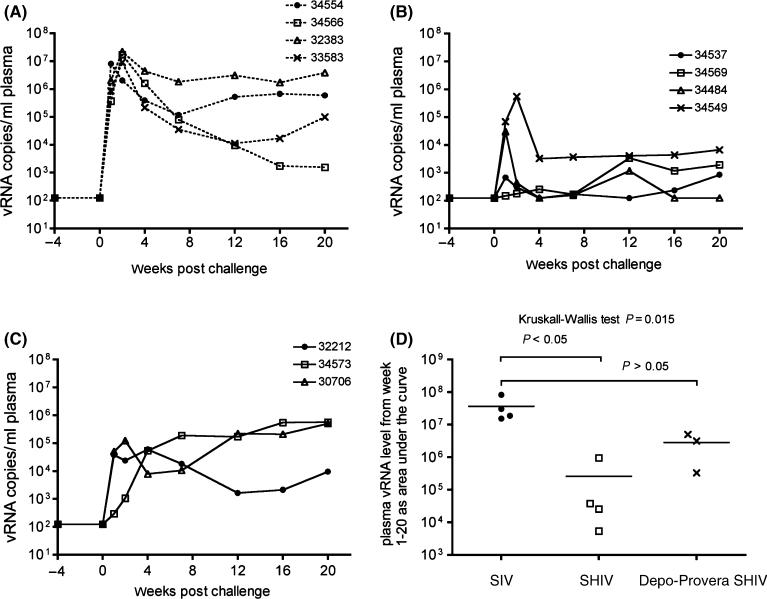
Fig. 1.
Plasma viral RNA (vRNA) levels after intravenous SIVmac239 challenge. (A) SIV control macaques, (B) SHIV macaques and (C) Depo-Provera SHIV macaques. In panel D, the vRNA levels over the entire 20 weeks of follow up were transformed into areas under the curve (AUC) and the mean AUC of the three groups of animals were compared using a Kruskall–Wallis test and then a pair-wise comparison between the groups of immunized animals and the control group was performed using Dunn's multiple comparisons test. P-values are noted in the Figure.
All four vaccine-naïve control animals had high peak plasma vRNA levels (107 vRNA copies/ml) between 1 and 2 weeks PC that decreased by varying degrees to a set point at 7–12 weeks PC (Fig. 1A). All seven SHIV89.6 immunized animals had vRNA detected in plasma collected from 1 to 4 weeks PC, and most immunized animals (six of seven) had a distinct peak in vRNA levels (105–106 copies/ml) between 1 and 2 weeks PC (Fig. 1B and C).
From 4 weeks PC on, two distinct plasma vRNA patterns were detected among the SHIV-immunized monkeys. One group of SHIV–immunized animals had PC plasma vRNA set point levels <104 vRNA copies/ml and these animals are considered to be ‘protected’. The other group of SHIV-immunized animals had PC plasma vRNA set point levels >104 vRNA copies/ml and these animals are considered to be ‘unprotected’.
Based on these criteria, all four SHIV immunized animals were protected from uncontrolled SIVmac239 replication through 20 weeks PC, while only one of Depo-Provera SHIV macaque (32212) was similarly protected. Of note, monkey 32212 was the only Mamu-A*-01-positive Indian-origin rhesus macaque among the study animals (Table 1). Plasma vRNA levels in one of the vaccine-naïve controls monkeys (34566) declined to <104 copies/ml at 12 weeks PC, while the three other control animals maintained high levels of plasma vRNA (106 vRNA copies/ml) until the end of the study.
Plasma vRNA data was used to calculate the AUC of the vRNA for each animal. The mean vRNA AUC of the three animal groups were significantly different (P = 0.015) from one another (Fig. 1D). Further, the SHIV monkey group had a significantly lower (P < 0.05) mean plasma vRNA AUC value than the vaccine naïve control animal group (Fig. 1D). However, the mean plasma vRNA AUC value of the SHIV-Depo-Provera monkey group was not significantly different (P > 0.05) from the vaccine naïve control animal group. Thus, prior infection with SHIV89.6 significantly reduced virus replication after IV SIV challenge of male rhesus macaques, but Depo-Provera administration eliminates the protection from uncontrolled challenge virus replication that an attenuated SHIV89.6 infection normally provides.
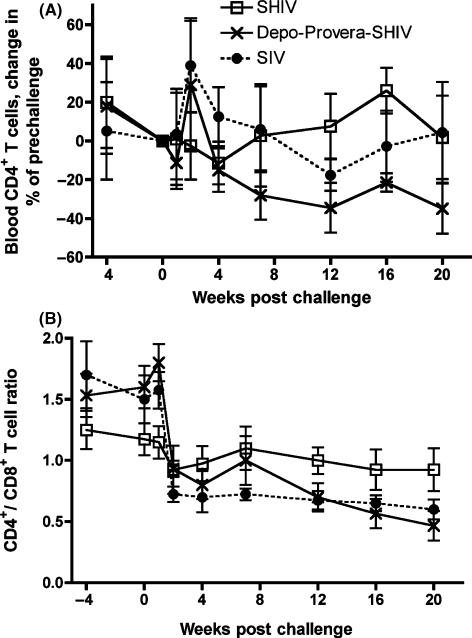
Consistent with their significantly higher plasma vRNA levels, as a group, the Depo-Provera SHIV macaques had a significantly more rapid decline in the frequency of CD4+ T cells (P = 0.044, one-tailed analyses), and the CD4+/CD8+ T-cell ratio (P = 0.002, one-tailed analyses), in blood after challenge compared to the SHIV macaques (Fig. 2).
Fig. 2.
Changes in peripheral CD4+ T-cell populations after intravenous SIVmac239 challenge. (A) % change in mean number of CD4+ T cells relative to levels on the day of challenge (P = 0.044 SHIV vs. SIV, one-tailed analyses), (B) mean CD4+/CD8+ ratios (P = 0.002 SHIV vs. SIV, one-tailed analyses). (•) SIV vaccine-naïve control macaques (n = 4); ( ) SHIV macaques (n = 4) and (
) SHIV macaques (n = 4) and ( ) Depo-Provera SHIV macaques (n = 3).
) Depo-Provera SHIV macaques (n = 3).
SIV-specific plasma antibody titers were not affected by Depo-Provera administration
All SHIV89.6-immunized macaques developed plasma anti-SIV binding antibodies by 4 weeks PC, and these responses persisted throughout the chronic phase of SHIV89.6 infection, even when vRNA was undetectable in the plasma (data not shown). At the time of administration with Depo-Provera (1 year after immunization with SHIV89.6), serum anti-SIV binding antibody titer ranged from 1:400 to 1:8000 and no changes were observed after Depo-Provera administration. SIVmac239 IV challenge induced a rapid increase in serum anti-SIV binding antibodies titers by 2 weeks PC; and these titers were similarly high in both immunized groups (data not shown).
Effect of Depo-Provera on SIV-specific T-cell responses
Peripheral blood mononuclear cells samples from the first 4 weeks PC were unavailable for all animals for these assays, but a summary of the results from all samples that were available is provided in Table 2. Depo-Provera treated SHIV monkeys had relatively strong SIV Gag specific IFN-γ Enzyme linked immuno spot (ELISPOT) responses (Table 2) but inconsistent positive anti-SIV T-cell proliferative responses compared to SHIV monkeys (data not shown). Over the entire 20-week PC observation period, the proportion of positive SIV Gag-specific IFN-γ ELISPOT responses was lower among samples collected from SHIV monkeys (45%) than from Depo-Provera SHIV monkeys (81%) (Table 2). Thus the ELISPOT responses tended to be more consistent and stronger in the SHIV Depo-Provera monkeys than the SHIV monkeys. Both SHIV and Depo-Provera SHIV macaques developed and maintained strong and consistent SIV-specific T-cell proliferative responses by 2 weeks PC (data not shown). After 7 weeks PC, SHIV macaques showed an increase in the strength of the SIV-specific T-cell proliferative responses, while the responses in the Depo-Provera SHIV macaques declined coincident with the decline in total circulating CD4+ T cell. However there was higher variability among the T-cell proliferative responses of the Depo-Provera SHIV macaques compared to the SHIV macaques.
Table 2.
Proportion of SHIV immunized monkeys with SIV Gag-peptide specific IFN- γ spot forming cells in PBMC
| Weeks after intravenous SIV challenge |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 0 | 1 | 2 | 4 | Cumulative Response | 7 | 12 | 16 | 20 | Cumulative Response |
| SHIV | 0/3a | 1/3 100b | ND | 1/2 40 | 2/8c (25%) | 2/4 91 (±21) | 1/2 211 | 1/3 162 | 3/3 158 (±8) | 9/20d (45%) |
| Depo-SHIV | 1/3 155 | 0/2 | 3/3 132 (±49) | 2/2 272 (±133) | 6/10 (60%) | 3/3 231 (±70) | 2/2 230 (±36) | 3/3 228 (±75) | 3/3 293 (±135) | 17/21 (81%) |
The number of animals with positive IFN-γ ELISPOT responses/ total number of animals tested.
Corrected (peptide stimulated wells – media only wells) mean number of IFN-γ spot forming cells/106 PBMC in positive samples (±SEM).
The total number of samples with positive IFN-γ ELISPOT responses/ total number of PBMC samples collected between week 0 and week 4 PC.
The total number of samples with positive IFN-γ ELISPOT responses/ total number of PBMC samples collected between week 0 and week 20 PC.
Effect of Depo-Provera on NK cell frequency
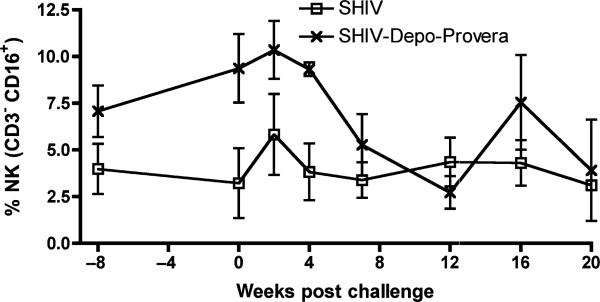
As an immunosuppressive role has been attributed to progesterone, effects on innate antiviral cell mediated immune cell populations were assessed by determining NK cell frequency in PBMC. We defined NK cells as CD3-CD16+CD8+/– lymphocytes. Depo-Provera administration increased NK cell frequency in attenuated SHIV89.6 infection. On the day of the SIV challenge, NK cell frequency was higher (P = 0.028) and following SIV challenge the frequency decreased significantly faster (P = 0.016) in Depo-Provera SHIV macaques than in the SHIV monkeys (Fig. 3). Eventually the NK cell frequencies in the two vaccine groups became similar.
Fig. 3.
Frequency of natural killer lymphocytes in the peripheral blood after intravenous inoculation with SIVmac239. ( ) SHIV macaques (n = 4) and (
) SHIV macaques (n = 4) and ( ) Depo-Provera SHIV macaques (n = 3). NK cells are defined as CD3–CD16+CD8+/– lymphocytes. P-values are: P = 0.028 on the day of challenge and P = 0.016 for the overall change of rate.
) Depo-Provera SHIV macaques (n = 3). NK cells are defined as CD3–CD16+CD8+/– lymphocytes. P-values are: P = 0.028 on the day of challenge and P = 0.016 for the overall change of rate.
Effect of Depo-Provera on T-cell activation
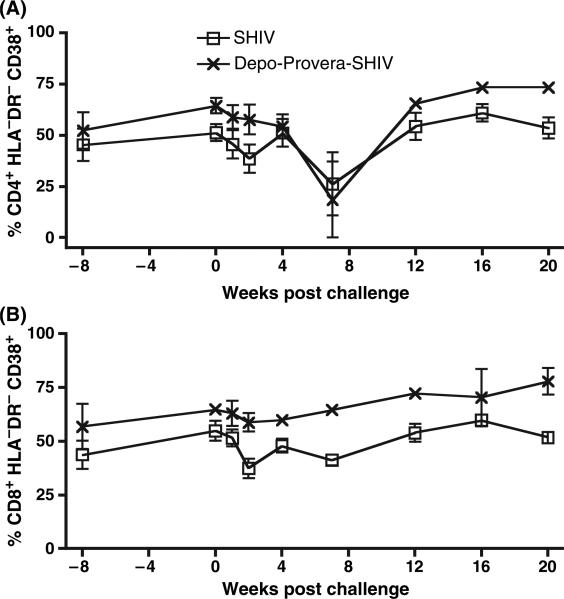
To determine the effect of exogenous progestins on T-cell activation, the frequency of T cells expressing several activation markers was assessed by flow cytometry. The frequency of T cells expressing CD38 but not HLA-DR tended to be higher in the Depo-Provera SHIV group compared to the SHIV group (Fig. 4). In fact, on the day of challenge the frequency of CD8+ T cells expressing CD38 but not HLA-DR was significantly higher in the Depo-Provera SHIV group compared to the SHIV group (P = 0.029, Fig. 4B) and the same trend was observed for the CD4+ T cells (Fig. 4A).
Fig. 4.
Frequency of activated T cells in peripheral blood after intravenous inoculation with SIVmac239. ( ) SHIV macaques (n = 4) and (
) SHIV macaques (n = 4) and ( ) Depo-Provera SHIV macaques (n = 3). (A) Percent of CD3+CD4+ T cells expressing CD38 but not HLA-DR. (B) Percent of CD3+CD8+ T cells expressing CD38 but not HLA-DR (P = 0.029 on the day of challenge).
) Depo-Provera SHIV macaques (n = 3). (A) Percent of CD3+CD4+ T cells expressing CD38 but not HLA-DR. (B) Percent of CD3+CD8+ T cells expressing CD38 but not HLA-DR (P = 0.029 on the day of challenge).
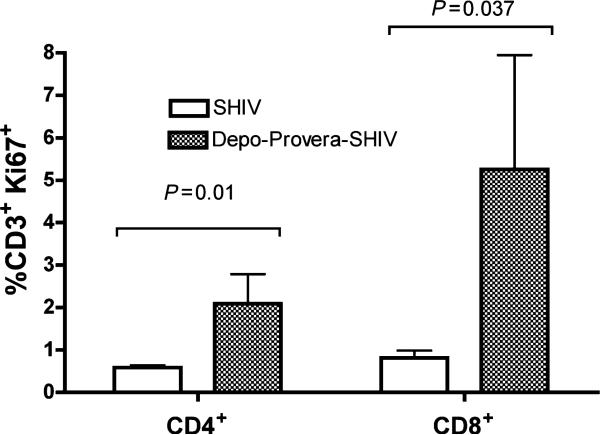
The frequency of T lymphocytes expressing Ki-67, a nuclear protein that is expressed in dividing T cells from S phase to mitosis of the cell cycle and on activated T cells blocked at G0, [31],[8] in axillary lymph nodes (LN) at week 12 PC was measured (Fig. 5). Both Ki67+CD4+ T and Ki67+CD8+ T cells were significantly more frequent in the axillary LN of the Depo-Provera SHIV macaques compared to SHIV macaques (P = 0.01 and P = 0.037 respectively).
Fig. 5.
Frequency of Ki67+ CD3+ T cells within the lymphocyte population in the axillary lymph after intravenous inoculation with SIVmac239 at 12 weeks PC. ( ) SHIV macaques (n = 4) and (
) SHIV macaques (n = 4) and ( ) Depo-Provera SHIV macaques (n = 3). P-values are noted in the Figure.
) Depo-Provera SHIV macaques (n = 3). P-values are noted in the Figure.
Discussion
This study demonstrates that Depo-Provera acts systemically to decrease live-attenuated lentivirus vaccine efficacy in male rhesus macaques. The Depo-Provera treated SHIV-immunized monkeys had increased viral replication and faster loss of CD4+ T cells than the SHIV-immunized monkeys that did not receive Depo-Provera. However it remains to be determined if in female rhesus monkeys the loss of vaccine efficacy [2] is due to factors beyond the effect of Depo-Provera on the genital tract. Although the primary endpoint of this study was a comparison of PC plasma vRNA levels among SHIV-immunized and control monkey groups, a number of significant changes in innate antiviral immunity and T-cell activation in the monkeys that were treated with Depo-Provera were observed. Thus, on the day of SIV challenge, Depo-Provera had increased frequency of NK cells and altered pattern of T-cell activation in SHIV-immunized male rhesus macaques. This set of aberrant systemic anti-viral immune responses is likely responsible for the detrimental effect of Depo-Provera on vaccine efficacy.
Progesterone has direct effects on activated T lymphocytes that express intracellular progesterone receptors. Upon binding, the progesterone/progesterone receptor complex translocates to the nucleus to function as a transcription factor directly regulating expression of the genes that contain the appropriate binding site (reviewed in [4]). The steroid/steroid receptor complex can interact with nuclear factor-kB (NF-kB) and inhibit its transactivating activity (reviewed in [22]). Thus, progesterone could be expected to reduce the expression of pro-inflammatory cytokines and perhaps, indirectly suppress HIV replication by sequestering NF-kB. Endogenous progestins are highest during pregnancy, a period when the female must not reject the fetal allograft, and during the luteal phase of the menstrual cycle when immunoglobulin secretion is suppressed in the B cells of women [15] and female macaques [14]. While progesterone can have immunosuppressive effects, there was no evidence of suppressed adaptive antiviral immunity in the Depo-Provera treated monkeys. Compared to the untreated SHIV-immunized monkeys, the Depo-Provera treated SHIV monkeys had a significantly increased frequency of NK lymphocytes in blood on the day of challenge. In the present study samples from all seven animals were not available until 7 weeks PC, thus it is not possible to comment on the relative strength of the antiviral immune responses at 1 week PC in the Depo-Provera treated male monkeys. However, a much higher proportion of PBMC samples collected from Depo-Provera SHIV monkeys had positive anti-p27 Gag IFN-γ ELISPOT responses from the day of challenge to the week 20 PC. We previously reported that SHIV-immunized monkeys had significantly weaker IFN-γ ELISPOT responses to SIV Gag peptides than Depo-Provera SHIV-immunized female rhesus macaques at 1 week, but not at 2 and 4 weeks, after intravaginal SIVmac239 challenge [2].
The Depo-Provera treated SHIV-immunized monkeys had an increased frequency of CD4+CD38+HLA-DR– T cells and CD8+ CD38+ HLA-DR– T cells compared to untreated animals in PBMC at the day of challenge. As activated CD4+ T cells are capable of supporting high-level viral replication (reviewed in [11]), the abnormally high level of CD4+ T-cell activation in the Depo-Provera treated SHIV-immunized monkeys directly contributes cellular substrate to support challenge virus replication. Thus increased CD4+ T-cell activation may play a major role in the loss of vaccine efficacy in Depo-Provera treated SHIV-immunized male rhesus macaques. Depo-Provera treated SHIV-immunized monkeys also had aberrant activation of CD8+ T cells characterized by an increased frequency of Ki67+ CD8+ T cells and CD8+ CD38+ HLA-DR– T cells compared to untreated animals. Thus, despite possessing a population of activated CD8+ T cells and large numbers of SIV Gag-specific IFN- γ secreting T cells, the Depo-Provera treated SHIV-immunized monkeys could not control viral replication. A plausible explanation for this apparent conundrum is that CD38+ expression confers increased apoptosis-sensitivity to HIV-specific CD8+ T cells [30]. Consistent with this hypothesis is the observation that generalized immune activation and accelerated T cell turnover in HIV-patients has been associated with the chronic expansion of CD8+ T cells that cannot properly differentiate, may revert into resting central memory cells, do not produce IL-2 and are more susceptible to apoptosis [28].
The finding that Depo-Provera administration results in loss of vaccine efficacy in SHIV-immunized male rhesus monkeys challenged IV with SIV mac239 demonstrates that Depo-Provera has profound effects on the systemic host response to lentiviral infections. Systemic effects may contribute to loss of protection in SHIV-immunized female rhesus monkeys challenged intravaginary with SIVmac239 [2]. In the male monkeys in this study the systemic effects of Depo-Provera included abnormal activation profiles in both CD4+ and CD8+ T cell subsets. Taken together, this evidence suggests that SHIV-immunized monkeys have a blunted inflammatory response to SIV and that Depo-Provera administration produced an enhanced or aberrant inflammatory response to the challenge virus in the treated SHIV-immunized monkeys. Thus the ability to maintain a relatively low level of inflammation in response to the viral challenge seems to be a key feature of the protective effect of attenuated SHIV 89.6. Depressed inflammation in response to SIV infection also seems to play a key role in the lack of disease in SIV-infected African green monkeys [12]. Because the proportion of monkeys protected from SIVmac239 challenge by prior attenuated SHIV89.6 infection can be altered by administration of exogenous hormones it is possible to conclude that host MHC phenotype is not the sole determinant of protection in this attenuated lentiviral vaccine system. Further, the ability to pharmacologically alter the proportion of protected animals is a powerful tool for studying the nature of protective immunity in attenuated AIDS vaccine virus systems. Because the decrease in vaccine efficacy in the male monkeys was due to systemic effects, Depo-Provera could be expected to have a similar effect on HIV vaccines in women despite the fact that Depo-Provera has minimal effect on vaginal epithelial thickness in women [21]. Studies are underway to determine if Depo-Provera directly affects systemic immunity and challenge virus replication in intravenously challenged SHIV-immunized female rhesus macaques. However, at this point it seems reasonable to conclude that to avoid one confounding factor, women using hormonal contraception should be excluded from efficacy trails of candidate HIV vaccines.
Acknowledgments
The authors thank the Immunology Core Laboratory and Primate Services Unit at the CNPRC and Ding Lu and Lili Guo for excellent technical assistance. This work was supported by Public Health Services grants U51RR00169, from the National Center for Research Resources; and P01 AI066314 and R01 AI44480 from the National Institute of Allergy and Infectious Diseases.
References
- 1.Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of Depo-Provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–36. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 5.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 6.Busch M, Abel K, Li J, Piatak M, Jr, Lifson JD, Miller CJ. Efficacy of a SHIV 89.6 proviral DNA vaccine against mucosal SIVmac239 challenge. Vaccine. 2005;23:4036–47. doi: 10.1016/j.vaccine.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Busch M, Lu D, Fritts L, Lifson JD, Miller CJ. Comparison of virology and immunology in SHIV 89.6 proviral DNA and virus-inoculated rhesus macaques. J Med Primatol. 2003;32:240–6. doi: 10.1034/j.1600-0684.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 8.Combadere B, Blanc C, Li T, Carcelain G, Delaugerre C, Calvez V, Tubiana R, Debre P, Katlama C, Autran B. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur J Immunol. 2000;30:3598–603. doi: 10.1002/1521-4141(200012)30:12<3598::AID-IMMU3598>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Dailey PJ, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J Med Primatol. 1995;24:209. [Google Scholar]
- 10.Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005;97:389–96. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–34. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 12.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavreys L, Baeten JM, Kreiss JK, Richardson BA, Chohan BH, Hassan W, Panteleeff DD, Mandaliya K, Ndinya-Achola JO, Overbaugh J. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis. 2004;189:303–11. doi: 10.1086/380974. [DOI] [PubMed] [Google Scholar]
- 14.Lu FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu FX, Ma Z, Moser S, Evans TG, Miller CJ. Effects of ovarian steroids on immunoglobulin-secreting cell function in healthy women. Clin Diagn Lab Immunol. 2003;10:944–9. doi: 10.1128/CDLI.10.5.944-949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Kiyono H, Lu D, Kawabata S, Torten J, Srinivasan S, Dailey PJ, McGhee JR, Lehner T, Miller CJ. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. Aids. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Brosio PB, Lafaile M, Li J, Collman RG, Sodroski J, Miller CJ. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J Virol. 1996;70:3045–50. doi: 10.1128/jvi.70.5.3045-3050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannel DN, Falk W, Yron I. Inhibition of murine cytotoxic T cell responses by progesterone. Immunol Lett. 1990;26:89–94. doi: 10.1016/0165-2478(90)90181-o. [DOI] [PubMed] [Google Scholar]
- 19.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 20.Mauck C. Overview of why hormones may be an issue. J Acquir Immune Defic Syndr. 2005;38(Suppl 1):S11–2. doi: 10.1097/01.qai.0000167026.95406.93. [DOI] [PubMed] [Google Scholar]
- 21.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 22.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 23.Miller CJ, McChesney MB, Lü XS, Dailey PJ, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously-infected with SHIV are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–21. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison CS, Richardson BA, Celentano DD, Chipato T, Mmiro F, Mugerwa R, Padian NS, Rugpao S, Salata RA. Prospective clinical trials designed to assess the use of hormonal contraceptives and risk of HIV acquisition. J Acquir Immune Defic Syndr. 2005;38(Suppl 1):S17–8. doi: 10.1097/01.qai.0000167029.41149.ad. [DOI] [PubMed] [Google Scholar]
- 25.Napravnik S, Poole C, Thomas JC, Eron JJ., Jr Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31:11–9. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen T. Hormonal regulation of immune responses. Ann Med. 1994;26:255–8. doi: 10.3109/07853899409147900. [DOI] [PubMed] [Google Scholar]
- 27.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003;282:103–15. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, Kaech SM, Weintrob A, Altman JD, Sodora DL, Feinberg MB, Silvestri G. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174:2900–9. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 29.Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski MA, Garcia P. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–90. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar PA, Mounzer KC, Altman JD, Katsikis PD. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8+ T cells. Blood. 2007;109:2505–13. doi: 10.1182/blood-2006-05-021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]