Abstract
The purpose of this study was to determine whether rhesus monkeys of Chinese origin are suitable for studies of mucosal lentivirus transmission by comparing the relative ability of these animals and rhesus macaques of Indian origin to become infected by vaginal (IVAG) inoculation with SIVmac251. In addition, we sought to test the hypothesis that differences in viral load during the first few weeks after inoculation were due to the relative strength of the anti-SIV immune responses in the two populations of rhesus macaques. Significant difference was not observed between the number of Indian and Chinese origin monkeys that were infected after IVAG SIV inoculation in this study. For 8–9 weeks after infection there was considerable overlap in the range of viral loads among the Indian and Chinese animals and the variation among the Indian origin animals was greater than the variation among the Chinese origin monkeys. By 6 weeks postinfection, viral loads in SIV-infected Chinese origin monkeys tended to be at the lower end of the range of viral loads observed in SIV-infected Indian origin monkeys. The strength of the anti-SIV antibody response was also more variable in the Indian origin rhesus macaques, but at 6–8 weeks postinfection, Chinese and Indian origin rhesus macaques had similar titers of anti-SIV antibodies. Microsatellite allele frequencies differed between Chinese and Indian rhesus macaques; however, the majority of alleles present in Indian-origin animals were also found in Chinese macaques. Together these results show that host factors, other than geographic origin, determine the ability of a rhesus macaque to be infected after IVAG SIV exposure and that geographic origin does not predict the viral load of SIV-infected animals during the first 8–9 weeks after IVAG inoculation.
Introduction
The Use of Rhesus Monkeys in AIDS research has led to a growing demand for these animals. This demand has outstripped the supply of domestically bred animals, forcing researchers to consider using monkeys from other sources. Most of the domestically bred rhesus macaques are derived from monkeys originally imported from India. Because it is no longer possible to import animals from India, importing monkeys from China seems to be the most reliable option for obtaining additional animals. However, there is some evidence that the clinical course of SIV infection is slower and more variable in Chinese origin monkeys compared with Indian origin monkeys.1 The goal of this study was primarily to determine whether Chinese origin rhesus monkeys are suitable for studies of mucosal lentivirus transmission or whether they were more inherently resistant to infection after IVAG SIV inoculation compared with Indian origin rhesus macaques.
We observed no significant difference in the number of SIV-infected Indian and Chinese monkeys after vaginal SIV inoculation in our study. However, infected Chinese origin monkeys tended to have lower viral loads by 6 weeks postinfection compared with infected Indian origin monkeys. During the 8- to 9-week observation period there was considerable overlap in the range of viral loads among the Indian and Chinese animals. In addition, the variation in plasma SIV RNA levels and anti-SIV antibodies was greater among Indian origin animals than among Chinese origin monkeys at 6–8 weeks postinfection. Only 4 of 13 highly polymorphic microsatellite loci had allele frequencies that were sufficiently different between Chinese and Indian origin macaques to be useful in identifying the geographic origin of individual animals. We found no microsatellite alleles that were diagnostic for Chinese or Indian origin. Together these results support the notion that geographic origin neither determines the ability of a rhesus macaque to be infected after intravaginal (IVAG) SIV exposure nor predicts the viral load or anti-SIV antibody responses of SIV-infected animals during the first 8–9 weeks after IVAG inoculation. However, to estimate the complete range of differences in response to SIV infection between Chinese and Indian origin rhesus macaques will require long-term studies that assess viral loads and immune responses over the course of infection from the time of inoculation to the onset of immunodeficiency.
Materials and Methods
Animal inoculations and virus stock
All the animals used in this study were adult, multiparous female rhesus macaques. The Indian origin rhesus macaques were colony bred at the California Regional Primate Research Center (CRPRC, Davis, CA). The Chinese origin rhesus macaques were imported from a primate center in Kunming Province, People's Republic of China. Before beginning the study, the animals were observed for 3 months at the CRPRC and regular menstrual cycles were documented. All animals were seronegative for HIV-2, SIV, type D retrovirus, and simian T cell lymphotropic virus type 1 at the beginning of the study. At the time of SIV inoculation the age range of animals was 3.9 to 16.1 years (Table 1) and their weight range was 4.0 to 9.2 kg. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. We adhered to the Guide for Care and Use of Laboratory Animals.2 When necessary animals were anesthetized with ketamine hydrochloride (10 mg/kg; Parke-Davis, Morris Plains, NJ) injected intramuscularly.
Table 1.
Experimental Design, Virus Isolation from PBMCs, and Plasma Antibody Responses of Indian and Chinese Origin Rhesus Macaques IVAG Inoculated with SIVmac251
| Ancestral country | Animal no. | Ageb (years) | No. of inoculationsc | Virus isolation results (weeks pi) | Anti-SIV Ab titer (reciprocal)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 6 weeks pi | 8 weeks pi | ||||
| India | 27762d | 5.7 | 1 | − | − | − | ND | ND | Neg | Neg |
| 28209d | 4.9 | 1 | − | − | − | ND | ND | Neg | Neg | |
| 28336 | 4.8 | 1 | + | + | + | + | ND | 200 | 200 | |
| 28349 | 4.8 | 1 | − | + | + | + | ND | 32,000 | 400,000 | |
| 28418 | 4.8 | 1 | − | + | + | + | ND | 32,000 | 3,200 | |
| 28549 | 4.5 | 1 | + | + | + | + | ND | 16,000 | 800,000 | |
| 27307 | 6.2 | 1 | + | + | + | + | + | 32,000 | 800,000 | |
| 28209d | 4.9 | 1 | + | + | + | + | + | 32,000 | 400,000 | |
| 29058d | 3.9 | 1 | − | − | − | − | − | Neg | Neg | |
| 31167 | 13.1 | 1 | + | + | + | + | + | 16,000 | 320,000 | |
| 31168 | 16.0 | 1 | − | + | + | + | + | Nege | Nege | |
| 22162 | 16.1 | 2 | + | + | + | ND | + | 3,200 | NA | |
| 22237 | 13.2 | 2 | + | + | + | ND | + | 16,000 | NA | |
| 22990 | 12.4 | 2 | + | + | + | ND | + | 32,000 | NA | |
| 23657 | 12.3 | 2 | + | + | + | ND | + | 64,000 | NA | |
| 23737 | 12.0 | 2 | + | + | + | ND | + | 200,000 | NA | |
| 24185 | 11.3 | 2 | + | + | + | ND | + | 80,000 | NA | |
| 27762d | 5.7 | 2 | + | + | + | ND | + | 200 | NA | |
| 29058d | 3.9 | 2 | + | + | + | ND | + | 200 | NA | |
| China | 31403 | 4.6 | 1 | + | + | + | + | ND | 100,000 | 400,000 |
| 31404 | 4.8 | 1 | − | − | − | − | ND | Neg | ||
| 31424 | 4.8 | 1 | + | + | + | + | ND | 100,000 | 400,000 | |
| 31426 | 5.5 | 1 | − | − | − | − | ND | Neg | Neg | |
| 31427 | 4.8 | 1 | − | − | − | − | ND | Neg | Neg | |
| 31428 | 4.8 | 1 | + | + | + | + | ND | 16,000 | 160,000 | |
| 31405 | 4.4 | 2 | + | + | + | − | + | 100,000 | 400,000 | |
| 31406 | 5.4 | 2 | + | + | + | − | + | 200,000 | 400,000 | |
| 31409 | 5.5 | 2 | + | + | + | + | + | 64,000 | 800,000 | |
| 31410 | 4.6 | 2 | + | + | + | + | + | 16,000 | 32,000 | |
| 31415 | 5.3 | 2 | + | + | +e | ND | ND | Nege | NAe | |
| 31433 | 4.8 | 2 | − | − | − | − | − | Neg | Neg | |
| 31436 | 4.9 | 2 | + | + | + | + | + | 64,000 | 160,000 | |
| 31437 | 5.3 | 2 | + | + | + | + | 100,000 | 800,000 | ||
Abbreviations: NA, Sample was not available; Neg, no SIV-specific antibodies were detected; ND, not determined; pi, postinfection.
Antibody end-point dilution titers; the titer shown is the reciprocal of the highest serum dilution that was positive.
Age of animals in years at the time of the first IVAG inoculation.
Number of IVAG inoculations with SIVmac251-5/98.
After these animals failed to become infected following the first SIV inoculation, they were reinoculated.
Although SIV infected, these animals failed to make an anti-SIV antibody response and they rapidly progressed to terminal AIDS by 6 (31415) or 12 weeks pi (31168).
Rhesus macaques were inoculated intravaginally with 1 ml of undiluted SIVmac251-5/98 virus stock containing 1 × 105 TCID50 (50% tissue culture infective doses)/ml and 4 × 109 copies of RNA per milliliter. This SIVmac251 stock was produced by short-term expansion of an older virus stock on rhesus peripheral blood mononuclear cells (PBMCs). The technique used for intravaginal inoculation has been published.3,4 The animals were inoculated in experimental groups without regard for the stage of the menstrual cycle. In this study, and in retrospective analysis of a large number of rhesus macaques, we have not observed that stage of the menstrual cycle is associated with differences in the ability of animals to be come infected after IVAG inoculation with SIV (C.J. Miller, unpublished results). Blood samples were collected into heparinized tubes at weeks 0, 2, 4, 6, 8, and 10 postinoculation.
In vitro titration of SIVmac251-5/98 stock on primary PBMCs from Indian and Chinese origin rhesus monkeys
PBMCs were isolated from the blood of SIV-naive rhesus monkeys as described above. The cells were cultured until the cell numbers had doubled (7–10 days) in complete RPMI 1640 containing recombinant human interleukin 2 (IL-2, 50 U/ml; Cetus, Emeryville, CA) and Staphylococcus enterotoxin A (0.5 μg/ml; Toxin Technologies, Sarasota, FL). We have demonstrated that this method reliably stimulates rhesus PBMCs as needed to propagate a variety of SIVmac isolates.5–7 To titrate the virus stock, the activated PBMCs were resuspended in RPMI at a concentration of 2 × 106 cells/ml and 100 μl of the cell suspension was added to wells of a 96-well plate. Serial 10-fold dilutions of the virus stock (100 μl) were added directly to wells in quadruplicate. The virus stock dilution series ran from 10−1 to 10−5. Thus, on the basis of the TCID50 of this stock in CEMx174 cells, the multiplicity of infection (MOI) in the dilution series used to infect primary PBMCs ranged from 0.5 × 10−2 to 0.5 × 10−6. The cultures were maintained for 1 week without changing the medium and then aliquots of medium were assayed for the presence of SIV major core protein (p27) by antigen capture enzyme-linked immunosorbent assay (ELISA).7,8 Each well was scored as positive or negative for p27 and the TCID50 of the stock in an individual animal's PBMCs was calculated by the method of Reed and Muench.9 PBMCs from five Indian origin and five Chinese origin rhesus macaques were tested in this assay. Note that the PBMC TCID50 assay described above differs from the standard titration assay to generate the TCID50 in CEMx174 cells. Although the MOIs tested were the same, for titration in CEMx174 cells, the culture volume is expanded to 5 ml in flasks and media are changed every 3–4 days for 4 weeks. Thus, the TCID50 values generated in the PBMC assay and the CEMx174 assay are not directly comparable.
Virus isolation
PBMCs were isolated from whole blood of SIV-inoculated animals by Ficoll gradient separation (lymphocyte separation medium; Organon Teknika, Westchester, PA) and cocultured with CEMx174 cells as previously described.8 Aliquots of the culture medium were assayed regularly for the presence of SIV major core protein (p27) by antigen capture ELISA.7,8
SIV provirus PCR analysis
Nested polymerase chain reaction (PCR) was carried out on genomic PBMC DNA in a DNA thermal cycler (Perkin-Elmer Cetus) as previously described.10 Briefly, cryopreserved PBMCs isolated from whole blood of each monkey in the experiment were washed three times in Tris buffer at 4°C and resuspended at 107 cells/ml. Ten microliters of the cell suspension were added to 10 μl of PCR lysis buffer (50 mM Tris-HCl [pH 8.3], 0.45% Nonidet P-40 [NP-40], 0.45% Tween 20) with proteinase K (200 μg/ml). The cells were incubated for 3 hr at 55°C, followed by 10 min at 96°C. Two rounds of 30 cycles of amplification were performed on aliquots of plasmid DNA containing the complete genome of SIVmac1A11 (positive control) or aliquots of cell lysates under conditions described elsewhere.10 The primers used specifically amplify SIV gag. DNA from uninfected CEMx174 cells was amplified as a negative control in all assays to monitor potential reagent contamination. β-Actin DNA sequences were amplified by two rounds of PCR (30 cycles per round) from all PBMC lysates to direct potential inhibitors of Taq polymerase. After the second round of amplification, a 10-μl aliquot of the reaction product was removed and run on a 1.5% agarose gel. Amplified products in the gel were visualized by ethidium bromide staining. Blood samples for PCR analysis were collected at the times indicated in Table 1.
Assessment of plasma SIV RNA levels
SIV RNA in plasma and in the SIVmac251-5/98 stock were quantified by an SIV-specific branched DNA (bDNA) signal amplification assay.11 This assay is similar to the Quantiplex HIV RNA assay except that target probes were designed to hybridize with the pol region of the SIVmac group of strains including SIVmac251 and SIVmac239. SIV pol RNA in plasma samples was quantified by comparison with a standard curve produced with serial dilutions of cell-free SIV-infected tissue culture supernatant. The SIV RNA in tissue culture fluid was quantified by comparison with a standard curve of purified DNA-free, in vitro-transcribed SIVmac239 pol RNA. SIV RNA associated with viral particles was measured after concentration from 1 ml of heparinized plasma by centrifugation (23,500 × g for 1 hr at 4°C). The lower limit of detection of the assay was 1500 copies of SIV RNA per milliliter of plasma.
Detection of plasma SIV-specific antibodies
Plasma samples were collected at 0, 6, and 8 weeks postinfection and tested for the presence of anti-SIV IgG antibodies; SIV IgG antibody titers were then determined for positive samples as described.12
Genetic assessments
We screened rhesus macaques for the presence of the major histocompatibility complex (MHC) class I alleles MamuA*01 and MamuB*01, using a PCR-based technique.13,14 Genetic variation in the Chinese and Indian origin macaques was assessed by DNA testing of 13 microsatellite loci distributed throughout 10 of the 21 chromosomes of the rhesus macaque genome. Standard, multiplexed PCR-based DNA genotyping methods were used.15 Macaque microsatellites were assayed from genomic DNA extracted from whole blood collected in anticoagulant EDTA. Heterologous human primers for loci D3S1768, D6S276, D6S291, D6S1691, D7S513, D7S794, D8S1106, D10S1412, D11S925, D13S765, D16S403, D17S804, and D18S572 were used for PCR amplification. Primers were obtained from Research Genetics (Huntsville, AL). For purposes of multiplexing markers in PCR tests, primers for D6S276 (Z16711), D11S925 (Z17002), D17S804 (Z17033), and D18S72 (Z17153) were redesigned from human clone sequences deposited in GenBank (available from the Internet: URL http://www.ncbi.nlm.nih.gov/) (M.C.T. Penedo, unpublished). Numbers in parentheses are GenBank accession numbers. Genotypes for each locus were used to calculate number of alleles detected, frequency of most common allele and heterozygosity. This panel of microsatellite markers has been validated by using rhesus macaques of known pedigree.16–18
Statistical analysis
To assess differences in susceptibility among Chinese and Indian rhesus macaques to SIV infection after one or two intravaginal inoculations, logistic regression analysis was performed for the number of SIV-infected animals in each of the two categories (geographic origin and number of intravaginal inoculations). All tests of the levels of SIV RNA in plasma were made on log-transformed values of SIV RNA copy number for each animal. Comparisons of viral load in plasma over the entire observation period between SIV-infected Chinese and Indian rhesus macaques were performed with a mixed model, repeated measures analysis of variance (ANOVA). Comparisons of viral load in Indian and Chinese origin animals at specific times after SIV infection were done by using t tests with Welch's correction.19,20
For the statistical analyses of the outcome of SIV inoculation, results were considered significant if the probability (p) of obtaining a result at least as extreme as the observed result was less than 0.05 (i.e., p < 0.05). The probability values reported are for two-tailed tests. The SAS (version 8.0; SAS, Cary, NC) and Instat (version 2.03 for the Mac; GraphPad Software, San Diego, CA) statistical software packages were used for these analyses. One-tailed paired-sample t tests21 were used to test the significance of differences between Chinese and Indian monkeys for average number of alleles per locus and heterozygosity.
Results
PBMCs from Chinese and Indian origin rhesus macaques are equally susceptible to SIV infection in vitro
In an in vitro study, the SIVmac251-5/98 stock replicated in PBMCs from all six Indian origin and all six Chinese origin rhesus monkeys (Fig. 1). None of these 21 animals was subsequently inoculated IVAG with SIV. There was considerable variability in the viral p27 antigen levels produced and the p27 concentration was dependent on the specific PBMC donor used. However, the variability and range of p27 concentration produced by PBMCs from the Indian and Chinese origin monkeys were similar. Thus, there was no evidence of any difference in the levels of SIV replication in PBMCs from Chinese or Indian rhesus monkeys in vitro.
Fig. 1.
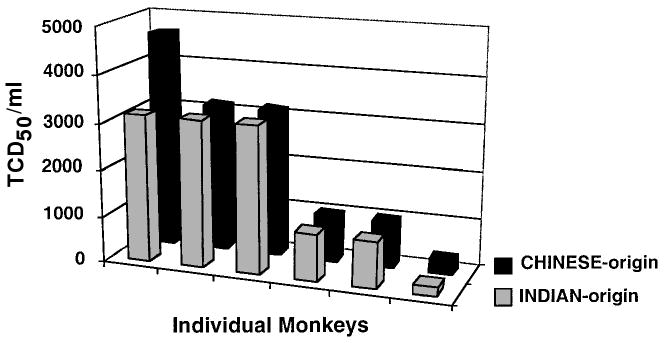
Comparison of in vitro titration of SIVmac251-5/98 stock in primary PBMCs from Indian and Chinese origin rhesus monkeys. Note that the range of p27 antigen levels in PBMCs from all of the animals is similar.
IVAG inoculation is equally efficient in Chinese and Indian rhesus macaques
After a single IVAG inoculation with 1 ml of the undiluted SIVmac251-5/98 stock, 3 of 6 Chinese origin rhesus monkeys (50%) and 8 of 12 Indian origin rhesus monkeys (67%) became virus isolation positive (Table 1). After two IVAG inoculations (spaced 4 hr apart) with 1 ml of the undiluted SIVmac251-5/98 stock, seven of eight Chinese origin rhesus monkeys (88%) and eight of eight Indian origin rhesus monkeys (100%) became virus isolation positive (Table 1). The results of PCR for proviral SIV gag, using genomic DNA extracted from PBMCs, uniformly confirmed the virus isolation results (data not shown). Although the results of IVAG inoculation could be interpreted to suggest a trend toward a slightly decreased ability to be SIV infected in Chinese origin monkeys compared with Indian origin animals, the difference is not statistically significant (p = 0.290). SIV transmission was significantly more efficient in all rhesus monkeys, Chinese and Indian, IVAG inoculated twice compared with monkeys inoculated only once (p = 0.036).
Plasma viral RNA levels differ in SIV-infected Chinese and Indian rhesus macaques
Regardless of geographic origin, peak plasma SIV RNA levels in SIV-infected animals occurred at week 2 postinfection in 25 of 26 monkeys with samples available at all study time points (Fig. 2). In one Chinese origin animal (31410) SIV RNA did not peak in plasma until week 4 postinfection (Fig. 2D). The range in peak plasma levels was greater for Chinese origin animals (1.1 × 105 to 1.2 × 108 copies of SIV RNA per milliliter) than for Indian origin animals (1.1 × 107 to 3.4 × 108 copies of SIV RNA per milliliter). Although the mean peak level of SIV RNA in plasma was 2-fold higher for Indian origin animals (5.0 × 107 copies of SIV RNA per milliliter) than for Chinese origin animals (2.5 × 107 copies of SIV RNA per milliliter) (Fig. 3A), these means were not significantly different (Table 2, p = 0.094).
Fig. 2.

SIV RNA levels in plasma of rhesus macaques of Chinese and Indian origin.
Fig. 3.

(A) Levels of SIV RNA in plasma 2 and 6 weeks after IVAG inoculation for macaques of Chinese origin (filled symbols) and Indian origin (open symbols). The means (indicated by arrows) for Chinese and Indian origin animals are significantly different at 6 weeks postinfection, but not at 2 weeks postinfection (see Table 3). (B) Decrease in SIV RNA plasma levels from 2 to 6 weeks after IVAG inoculation with SIV for macaques of Chinese origin (filled symbols) and Indian origin (open symbols). The means (indicated by arrows) for Chinese and Indian origin animals are significantly different (see Table 3).
Table 2.
Repeated Measures Analysis of Variance to Test for Significant Effects of Geographic Origin, Number of IVAG SIV Inoculations, and Time after Inoculation on SIV RNA Levels in Plasma of SIV-Infected Rhesus Macaques
| Factora | F value | Degrees of freedomb | Probability of a larger F value |
|---|---|---|---|
| Ancestral country | 10.15 | 1 | 0.004c |
| Number of IVAG SIV inoculations | 0.01 | 1 | 0.937 |
| Weeks pid | 31.45 | 2 | <0.0001c |
| Ancestral country × weeks pie | 1.40 | 2 | 0.258 |
| IVAG inoculations × weeks pif | 0.12 | 2 | 0.890 |
Analyses were performed on the log10 of the number of copies of SIV RNA per milliliter of plasma.
Degrees of freedom for F value.
p ≤ 0.050 was considered statistically significant.
Comparison of the temporal trend in SIV plasma RNA levels among SIV-infected animals of Indian and Chinese origin. Data for weeks 2, 4, and 6 postinfection were analyzed; missing data for some animals precluded analyses of all times postinfection.
Comparison of the temporal trend in SIV plasma RNA levels between animals of different geographic origin.
Comparison of the temporal trend in SIV plasma RNA levels between animals that received different numbers of IVAG SIV inoculations.
The range in plasma SIV RNA levels at 6 weeks postinfection was greater for Indian origin animals (1.3 × 105 to 5.9 × 107 copies of SIV RNA per milliliter) than for Chinese origin animals (5.4 × 103 to 8.2 × 105 copies of SIV RNA per milliliter). In addition, the mean level of SIV RNA in plasma at 6 weeks postinfection was 25-fold higher for Indian origin macaques (1.5 × 106 copies of SIV RNA per milliliter) than for Chinese origin animals (6.3 × 105 copies of SIV RNA per milliliter) (Fig. 3A); these means were significantly different (Table 3, p < 0.001). Viral RNA levels in plasma of one Chinese origin monkey (31410) never exceeded 5.1 × 105 copies/ml (Fig. 2D); this pattern of low plasma viral load was not observed for any of the SIV-infected Indian origin macaques.
Table 3.
Unpaired t Test Comparisons of SIV RNA Levels in Plasma in Infected Rhesus Macaques of Chinese and Indian Origin at Specific Times after IVAG Inoculation
| Time after IVAG inoculation | Chinese origin meana ± SEMb(nc) | Indian origin mean ± SEM (n) | Mean differenced | Probability of a larger t value |
|---|---|---|---|---|
| 2 weeks pi | 7.2 ± 0.3 (10) | 7.7 ± 0.1 (16) | 0.5 | 0.094 |
| 6 weeks pi | 5.0 ± 0.2 (9) | 6.3 ± 0.2 (16) | 1.3e | <0.001e |
| 2–6 weeks pif | 2.1 ± 0.2 (9) | 1.4 ± 0.2 (16) | −0.7e | 0.020e |
Analyses were performed on the log10 of the number of copies of SIV RNA per milliliter of plasma; mean values shown are log (copies of SIV RNA per milliliter of plasma).
Standard error of the mean.
n = number of animals from which data were analyzed.
(Mean value for Indian origin animals) minus (mean value for Chinese origin animals).
p ≤ 0.050 was considered statistically significant.
The decrease between SIV plasma RNA levels at week 2 and week 6 postinfection for individual animals was analyzed; missing data for 6 weeks postinfection precluded one Chinese origin animal (31415) from analysis.
As previously observed for SIV-infected rhesus macaques, SIV RNA levels in plasma decreased from 2 weeks to 6 weeks postinfection in most animals (Figs. 2 and 3B). A greater range of decline in SIV plasma RNA levels from 2 weeks to 6 weeks postinfection was found among Indian origin macaques (Fig. 3B). However, the mean reduction in SIV plasma RNA levels from 2 weeks to 6 weeks postinfection (Fig. 3B) was significantly greater (Table 3, p = 0.020) for animals of Chinese origin (∼100-fold decrease) compared with animals of Indian origin (∼25-fold decrease). Thus, Chinese origin macaques tended to have lower levels of SIV RNA in plasma at 6 weeks postinfection compared with those of Indian origin animals. Nevertheless, the lower mean levels of SIV RNA observed in plasma of SIV-infected Chinese origin macaques at 6 weeks postinfection cannot be used to infer that viral loads several months later in infection would also have been lower compared with SIV-infected Indian origin animals.
Antibody responses to SIV infection
Of the 10 Chinese origin rhesus macaques that became SIV infected, 9 developed detectable serum anti-SIV antibody responses by 8 weeks postinfection (Table 1). Similarly, 15 of 16 of the SIV-infected Indian origin rhesus monkeys had detectable serum anti-SIV antibody responses by 8 weeks postinfection (Table 1). The range of antibody titers in SIV-infected Indian origin rhesus monkeys was highly variable (200–800,000), spanning a 4000-fold range. Rising titers of SIV-specific antibodies levels were not observed for two SIV-infected Indian origin animals during the course of this study. From 6 weeks to 8 weeks postinfection SIV antibody levels in plasma remained low (200) for monkey 28336 and declined 10-fold (32,000 to 3200) for monkey 28418.
Compared with Indian origin animals, the anti-SIV antibody titers among the SIV-infected, Chinese origin rhesus macaques during the first 8 weeks postinfection were more consistent (16,000–800,000), differing by 10- to 50-fold. The two SIV-infected monkeys that failed to mount anti-SIV antibody responses rapidly developed severe clinical AIDS. Chinese origin monkey 31415 was killed at 6 weeks postinfection with severe nonresponsive enterocolitis and wasting, and Indian origin monkey 31168 was killed at 12 weeks postinfection with Pneumocystis pneumonia. Thus, rapid progression to AIDS can occur in both Chinese and Indian origin monkeys that fail to develop anti-SIV immunity.
Genetic diversity among rhesus macaques from different ancestral countries and impact on SIV infection
Screening for the presence of two well-characterized alleles of rhesus macaque MHC class I region, MamuA*01 and MamuB*01, was done to verify reports that these alleles are rare or absent in rhesus macaques of Chinese origin. In populations of Indian origin rhesus macaques the frequency of MamuA*01 is 20 to 25%13 and the frequency of MamuB*01 is 25 to 50% (D. Watkins, personal communication; and M.L. Marthas and M.C.T. Penedo, unpublished results). In this study, MamuA*01 was not detected in 10 Chinese origin macaques and was present in only 3 of 16 Indian macaques; MamuB*01 was found in 1 Chinese origin animal and 7 Indian origin animals (Table 4). These observed frequencies of MamuA*01 (19%) and MamuB*01 (44%) were similar to expected frequencies for Indian origin macaques. The MamuA*01 and MamuB*01 alleles were rare in Chinese origin macaques, consistent with previous findings (D. Watkins, personal communication). In addition, there was no association of resistance to infection after IVAG SIV inoculation or low plasma viral load after SIV infection in animals carrying MamuA*01 or MamuB*01 alleles. The one Chinese origin macaque positive for MamuB*01 (31406) had a peak viral load (3.8 × 107 copies of SIV RNA per milliliter) that fell within the range of peak viral load for the seven MamuB*01-positive Indian origin macaques (Table 1 and Fig. 1D). The three Indian origin MamuA*01-positive animals (28209, 28349, and 28418) became SIV infected and maintained high viral loads in plasma (Fig. 1A).
Table 4.
MHC Alleles and Microsatellite Genotypes of Indian and Chinese Origin Rhesus Macaques IVAG Inoculated with SIVmac251
| Ancestral country | Animal no. | Rhesus MHC alleles | Microsatellite locus genotypea | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mamu A*01 | Mamu B*01 | D7S794b | D17S804 | D7S513 | D3S1768 | D13S765 | D11S925 | D6S291 | D6S276 | D6S1691 | D10S1412 | D18S72 | D16S403 | D8S1106 | ||
| India | 27762 | − | − | 124/128b | 118 | 189/207 | 209/221 | 216/224 | 308 | 204 | 225/235 | 197 | 157/163 | 308/330 | 168 | 164/172 |
| 28209 | + | − | 120/128 | 118 | 197/211 | 193/229 | 208/236 | 308/330 | 200/214 | 233/235 | 197/203 | 163 | 306/330 | 168 | 144/152 | |
| 28336 | − | + | 108/124 | 114/118 | 195/207 | 205/225 | 224/228 | 312/330 | 204 | 221/235 | 197/213 | 160 | 306/328 | 156/158 | 152/156 | |
| 28349 | + | + | 108 | 118 | 187/205 | 217/221 | 232/236 | 310/330 | 206 | 225 | 213/221 | 157/163 | 306/308 | 164/168 | 160/164 | |
| 28418 | + | − | 120/124 | 118 | 189/203 | 193/217 | 224/228 | 308 | 204 | 227/235 | 197 | 157/163 | 306 | 156/174 | 140/144 | |
| 28549 | − | − | 124 | 118 | 187/219 | 205/229 | 212/224 | 308/312 | 206/214 | 233 | 199 | 157 | 308 | 164 | 144/152 | |
| 27307 | − | + | 120/124 | 118 | 195/207 | 213 | 224/232 | 312/330 | 204/206 | 215/227 | 177/197 | 157/163 | 310/324 | 152/168 | 160 | |
| 29058 | − | + | 128 | 118 | 197/203 | 225 | 208/224 | 312/338 | 206/214 | 225/231 | 197/209 | 157 | 306 | 164/174 | 144/152 | |
| 31167 | − | − | 128 | 118 | 187/189 | 201/213 | 224/236 | 312/342 | 206/214 | 225/235 | 197 | 157/163 | 306/308 | 164/168 | 144/160 | |
| 31168 | − | + | 120/128 | 118 | 191/203 | 213/221 | 216/232 | 308/338 | 206/208 | 221/235 | 197/215 | 157/166 | 306/308 | 156/158 | 148/168 | |
| 22162 | − | − | 108/124 | 118 | 203/207 | 205/221 | 208/232 | 308/338 | 206/214 | 205/221 | 197/203 | 157/160 | 306/314 | 164/170 | 144/172 | |
| 22237 | − | − | 108/128 | 118 | 195/207 | 201/205 | 212/220 | 312/330 | 204 | 227/235 | 195/197 | 157/160 | 302/328 | 144/164 | 132/144 | |
| 22990 | − | − | 128 | 118 | 187/207 | 201/225 | 224/228 | 330 | 206/212 | 225/233 | 191/197 | 157/163 | 308/330 | 152/164 | 144/168 | |
| 23657 | − | + | 128/136 | 118 | 187/207 | 225/229 | 224 | 338 | 206/214 | 233 | 199/211 | 157 | 306/308 | 164/168 | 148 | |
| 23737 | − | + | 124/128 | 118 | 219/233 | 201/225 | 232/260 | 308/338 | 204/206 | 235/237 | 195/201 | 157/160 | 306/308 | 156/168 | 152/156 | |
| 24185 | − | − | 108/128 | 118 | 205/211 | 205/217 | 228/260 | 308/312 | 204 | 221/225 | 205/213 | 157/163 | 306/328 | 164 | 144/160 | |
| 3/16 | 7/16 | |||||||||||||||
| No. of allelesc | 5 | 2 | 11 | 9 | 9 | 6 | 6 | 9 | 14 | 4 | 8 | 8 | 10 | |||
| Most common alleled (freq.) | 128 (13/32) 0.41 | 118 (31/32) 0.97 | 207 (7/32) 0.22 | 225 (6/32) 0.13 | 224 (10/32) 0.31 | 308 (10/32) 0.31 | 204 (12/32) 0.38 | 235 (8/32) 0.25 | 197 (14/32) 0.44 | 157 (17/32) 0.53 | 306 (13/32) 0.41 | 164 (11/32) 0.34 | 144 (9/32) 0.28 | |||
| Freq. het.se | 11/16 0.69 | 1/16 0.06 | 16/16 1.00 | 14/16 0.88 | 15/16 0.94 | 12/16 0.75 | 10/16 0.63 | 13/16 0.81 | 12/16 0.75 | 11/16 0.69 | 13/16 0.81 | 12/16 0.75 | 14/16 0.88 | |||
| China | 31403 | − | − | 124/128b | 106/114 | 189/207 | 213/221 | 220/232 | 330/332 | 204/206 | 211/215 | 197/217 | 154/157 | 316/320 | 166 | 144/152 |
| 31404 | − | − | 112/124 | 108/118 | 207/219 | 221/225 | 220/236 | 344/350 | 204/206 | 211/227 | 197/211 | 160 | 312/314 | 158/186 | 160 | |
| 31424 | ND | ND | 124 | 118 | 177/207 | 185/189 | 224/228 | 308/312 | 202/218 | 223/237 | 211/217 | 157/160 | 312/326 | 140/152 | 144/148 | |
| 31426 | − | − | 128 | 114 | 198/297 | 205/217 | 220/228 | 328/332 | 204/206 | 225 | 209/211 | 157/163 | 316/332 | 142/156 | 156/160 | |
| 31427 | ND | ND | 128 | 120/126 | 199/217 | 197 | 228/264 | 328/330 | 204 | 231 | 177/203 | 157 | 312/316 | 156/160 | 148/160 | |
| 31428 | ND | ND | 108/128 | 118/122 | 205/207 | 209 | 224/272 | 328/330 | 208/222 | 223/227 | 177/213 | 157 | 308 | 152/166 | 148/160 | |
| 31405 | − | − | 124 | 114/122 | 195/209 | 213/221 | 220/232 | 330/332 | 204/206 | 211/215 | 197/217 | 154/157 | 316/320 | 166 | 144/152 | |
| 31406 | − | + | 124 | 114/122 | 193/199 | 185/213 | 220/224 | 328/332 | 208/216 | 211/213 | 195/213 | 160 | 312/320 | 150/172 | 160 | |
| 31409 | − | − | 124/128 | 114 | 191/207 | 201/217 | 220/224 | 308/310 | 204/206 | 211 | 201/205 | 154/163 | 306 | 158/160 | 144/156 | |
| 31410 | − | − | 108/128 | 114/120 | 199/211 | 185/229 | 232/236 | 320/324 | 204 | 223/229 | 203/205 | 154/160 | 314 | 142/158 | 152/156 | |
| 31415 | − | − | 108/128 | 114/118 | 193/197 | 193/213 | 200/224 | 322/330 | 202/214 | 211 | 207 | 157/160 | 318/324 | 142/166 | 144/160 | |
| 31433 | − | − | 124/132 | 112/122 | 199/209 | 205/221 | 264/268 | 312/348 | 202/204 | 211 | 207/209 | 157/172 | 312/336 | 158/162 | 140/160 | |
| 31436 | − | − | 128 | 108/118 | 205 | 185/201 | 224/228 | 330/332 | 200/202 | 211 | 193/199 | 157 | 310/322 | 144/166 | 144 | |
| 31437 | − | − | 124 | 116 | 189/193 | 209/217 | 228 | 312/348 | 200/204 | 223 | 203/211 | 154/157 | 312/318 | 146/162 | 148/152 | |
| 0/10 | 1/10 | |||||||||||||||
| No. of allelesc | 6 | 9 | 13 | 12 | 9 | 12 | 9 | 9 | 13 | 5 | 12 | 13 | 6 | |||
| Mostd common allele (freq.) | 124 (12/28) 0.43 | 114 (9/28) 0.32 | 207 (6/28) 0.21 | 185, 213, 221 (4/28) 0.14 | 220, 224, 228 (6/28) 0.21 | 330 (6/28) 0.21 | 204 (11/28) 0.39 | 211 (12/28) 0.43 | 211 (4/28) 0.14 | 157 (13/28) 0.46 | 312 (6/28) 0.21 | 166 (7/28) 0.25 | 160 (9/28) 0.32 | |||
| Freq. het.se | 7/14 0.50 | 10/14 0.71 | 13/14 0.93 | 11/14 0.79 | 13/14 0.93 | 14/14 1.00 | 12/14 0.86 | 7/14 0.50 | 13/14 0.93 | 9/14 0.64 | 11/14 0.79 | 12/14 0.86 | 11/14 0.79 | |||
Abbreviation: ND, Not done.
As detected with heterologous human primers.
Each locus is designated as for human microsatellite marker locus; alleles at each locus are designated by a number indicating mobility of PCR product on a polyacrylamide gel; two numbers separated by a slash (/) indicates a heterozygote; a single number indicates only one allele, which is most likely a homozygote; alternatively, a null allele could also be present.
Number of alleles observed in all animals from one ancestral country.
Allele (or alleles) that are most frequently detected in animals from one ancestral country. The number of alleles over the total number of alleles (2 × number of individuals for diploids) is given in parentheses. Animals for which a single allele was detected were inferred to be homozygous and, thus, were assumed to carry two copies of the same allele.
Frequency of heterozygous animals (i.e., two different alleles detected) at each locus defined as: the number of animals heterozygous at a locus over the total number of animals.
Microsatellite marker loci were used to evaluate the degree of genetic similarity between macaques of Chinese and Indian origin chosen for this study (Table 4). Differences in allelic composition were found between the two groups. The average number of alleles per locus was significantly higher in Chinese (9.85 ± 2.88) than in Indian (7.77 ± 3.17) macaques (t12 = 2.46, p < 0.025). Similarly, the proportion of heterozygotes per locus was significantly higher in Chinese (0.79 ± 0.16) than in Indian (0.74 ± 0.23) macaques (t12 = 3.60, p < 0.0025). These results are in agreement with findings from another comparison of genetic diversity among Chinese and Indian rhesus macaques based on a set of 15 microsatellite loci, only 1 of which (D17S804) was common to both studies.22
The genotype of an animal at the four loci with alleles that were unique to either Chinese or Indian origin (D6S276, D18S72, D16S403, and D17S804; Table 4) could correctly identify the ancestral country of the 30 individuals in this study. However, the genotype of an animal at these four microsatellite loci may not be a reliable predictor of the ancestral country of origin for other rhesus macaques. Also, this panel of 13 microsatellite loci could not be used to identify individual rhesus macaques of mixed Chinese/Indian descent, that is, animals with ancestors of both Chinese and Indian origin.
Discussion
The major goal of this study was to determine whether rhesus macaques of Chinese origin were resistant to SIV infection by IVAG inoculation. We found that there was no significant difference among Indian and Chinese origin rhesus macaques with regard to the number of animals that were infected after IVAG inoculation with either one or two doses of SIV. Thus, monkeys from both countries are useful for SIV studies. We also found no difference in the in vitro ability of PBMCs to become SIV infected, based on the geographic origin of the donor monkey. However, we did find that two IVAG doses of SIV resulted in significantly more SIV-infected macaques than one IVAG inoculation; this finding is consistent with previous reports for inoculation with IVAG SIVmac251.23,24
In addition, we sought to determine whether the level of viremia in SIV infection is markedly different in Chinese and Indian origin rhesus monkeys during the first 8–9 weeks of infection. As previously reported, viral load among SIV-infected macaques was variable and the variation was greater among the Indian origin rhesus monkeys than among the Chinese origin rhesus monkeys. The high SIV RNA levels at 2 weeks postinfection found in plasma of Chinese and Indian origin animals were not significantly different. However, by 6 weeks postinfection the plasma SIV RNA levels tended to be lower in Chinese compared with Indian origin rhesus macaques; this difference was statistically significant despite substantial overlap in the range of viral loads found in monkeys of Indian and Chinese origin (Fig. 1). This result is consistent with earlier observations from smaller numbers of Chinese and Indian rhesus macaques inoculated parenterally with SIV.1 The observation that SIV-infected animals with similar levels of plasma viremia at 2 weeks postinfection can have substantially different plasma viral levels at 6–8 weeks postinfection has been reported in a previous study of Indian origin rhesus macaques inoculated IVAG with uncloned SIVmac251.25 The SIVmac251 isolate used for IVAG inoculation in this study is composed of a genetically heterogeneous population of viral variants25 (M.L. Marthas, unpublished results). We have shown that the genetic complexity of SIVmac251 variants transmitted by IVAG inoculation varies substantially among rhesus macaques.25 This study was not designed to evaluate IVAG transmission of SIV variants. However, on the basis of our previous findings, it is possible that plasma SIV RNA levels during the first 6 weeks postinfection among the SIV-infected animals of Chinese and Indian origin were modulated by the population of viral variants transmitted IVAG25 as well as by the immune response of individual macaques to SIV infection. Finally, it is important to note that the differences observed in plasma SIV RNA levels at 6 weeks postinfection between Chinese and Indian origin monkeys in this study cannot be used to infer that viral loads several months later in infection would also differ.
The range of SIV-specific antibody titers in plasma at 6–8 weeks postinfection was similar among Chinese and Indian origin macaques; however, anti-SIV antibody levels were more consistent among SIV-infected Chinese origin animals compared with Indian origin animals. Because this study was not designed to evaluate cell-mediated immune responses to SIV infection, it is possible that cell-mediated immunity to SIV might differ between Chinese and Indian origin macaques. In addition, it is possible that the levels or breadth of SIV-specific cell-mediated immunity might be associated with the lower plasma viral loads observed in Chinese origin compared with Indian origin animals.
Indian rhesus monkeys that fail to make an antibody response to SIV or SHIV infection have a rapid disease course.26–29 We now show that rapid disease progression also occurs in SIV-infected Chinese rhesus monkeys and is associated with the failure to make an anti-SIV antibody response. The frequency of rapid progressors in the two monkey populations was similar: 1 of 16 Indian origin monkeys and 1 of 10 Chinese origin monkeys developed terminal AIDS within 3 months of SIV infection. Although our study was not designed to compare the long-term clinical course of SIV disease in Chinese and Indian origin rhesus monkeys, the similar frequency of rapid progressors in the two populations provides some circumstantial evidence that the clinical course of SIV infection is similar.
A panel of 13 microsatellite markers was used to assess the degree of genetic similarity between monkeys of Chinese and Indian origin. Consistent with other studies of rhesus macaque populations, microsatellite marker allele frequencies differed between Chinese and Indian origin rhesus macaques; however, the majority of alleles present in Indian origin animals were also found in Chinese origin macaques.22 Our findings that the Chinese macaques were genetically more diverse than the Indian macaques as measured by average number of alleles per locus and average heterozygosity were also in agreement with previous studies.22 Even though significant differences were observed between the Chinese and Indian origin macaques used in this study, additional studies are needed to examine the reliability of this panel of microsatellites to predict the ancestral country of origin for all rhesus macaques and to identify animals of mixed descent.
In summary, we observed no significant difference between Chinese and Indian rhesus macaques in their ability to become SIV infected after IVAG inoculation. However, compared with Indian origin animals, monkeys of Chinese origin that become SIV infected after IVAG inoculation, on average, exhibited lower plasma virus levels by 6 weeks postinfection. Together, these results suggest that the difference in viremia detected between Indian and Chinese origin rhesus macaques early in infection may reflect genetic differences in host response to SIV infection between these populations. Most importantly, because there is more variation in response to SIV infection among individuals within a group of outbred Indian origin or outbred Chinese origin macaques than between the two groups, it is not possible to predict the virus load for any individual macaque on the basis of the animal's ancestral country of origin. To evaluate the full range of differences in the pathogenesis and natural history of SIV infection in Chinese and Indian origin rhesus macaques will require long-term studies of larger numbers of animals that assess viral loads and cellular and humoral immune responses over the course of infection from the time of inoculation through the development of fatal immunodeficiency.
Acknowledgments
We are grateful for the expert technical support of Steven Joye, Yichuan Wang, Judith Torten, Kristen Bost, Linda Hirst, David Bennet, and Wilhelm Von Morgenland, whose assistance was crucial to the successful completion of these studies. Dr. Neil Willits (Statistical Laboratory, UC Davis) provided expert assistance for statistical analyses. This work was supported by Public Health Science grant RR00169 from the National Center for Research Resources; grants AI39109 (MLM), AI39435 (CJM), and AI35545 (CJM) NO1 AI65312 from the National Institute of Allergy and Infectious Diseases; and Elizabeth Glaser Scientist award #8-97 (MLM) from the Elizabeth Glaser Pediatric AIDS Foundation.
References
- 1.Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: Effects of splenectomy on virus burden. Virology. 1994;200:436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources. Guide for Care and Use of Laboratory Animals. National Resource Council; Washington, D.C.: 1998. [Google Scholar]
- 3.Miller C, Marthas M, Torten J, et al. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CJ, Marthas M, Greenier J, et al. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banapour B, Marthas M, Ramos R, et al. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus (SIVmac) J Virol. 1991;65:5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger R, Marthas M, Padrid P, Pratt-Lowe E, Padrid PA, Luciw PA. The nef gene of simian immunodeficiency virus SIVmac1A11. J Virol. 1992;66:5432–5442. doi: 10.1128/jvi.66.9.5432-5442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marthas M, Ramos R, Lohman B, et al. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohman B, Higgins J, Marthas M, Marx P, Pedersen N. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 10.Miller CJ, McChesney ML, Lü X, et al. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dailey PJ, Zamroud M, Kelso R, Kolberg J, Urdea M. 13th Annual Symposium on Nonhuman Primate Models of AIDS. Monterey, California: 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. [Google Scholar]
- 12.Lu X, Kiyono H, Lu D, et al. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans DT, Knapp LA, Jing PC, et al. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol Lett. 1999;66:53–59. doi: 10.1016/s0165-2478(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 15.Bowling AT, Eggleston-Stott ML, Byrns G, et al. Validation of microsatellite markers for routine horse parentage testing. Anim Genet. 1997;28:247–252. doi: 10.1111/j.1365-2052.1997.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Chan AWS, Dominko T, Lutjens CM, et al. Clonal propagation of primate offspring by embryo splitting. Science. 2000;287:317–319. doi: 10.1126/science.287.5451.317. [DOI] [PubMed] [Google Scholar]
- 17.Penedo MC, Cordova KI, Ward T, et al. Animal Genetics Symposium. Minneapolis, Minnesota: 2000. A parentage test for rhesus macaques using human primers for microsatellite markers. [Google Scholar]
- 18.Penedo MC, Bertolini L, Marthas ML, Bowling AT. Microsatellites for the MHC region of rhesus macaques. Human and Animal Immunogenetics: Comparative Evolution of the Mammalian MHC Conference; University of Manchester, Manchester, England. September 8–10 2000. [Google Scholar]
- 19.Aspin AA, Welch BL. Tables for use in comparisons whose accuracy involves two variances, separately estimated. Biometrika. 1949;36:290–296. [PubMed] [Google Scholar]
- 20.Trickett WH, Welch BL, James GS. Further critical values for the two-means problem. Biometrika. 1956;43:203–205. [Google Scholar]
- 21.Zar JH. Biostatistical Analysis. 2nd. Prentice-Hall; Englewood Cliffs, New Jersey: 1984. [Google Scholar]
- 22.Morin PA, Kanthaswamy S, Smith DG. Simple sequence repeat (SSR) polymorphisms for colony management and population genetics in rhesus macaques (Macaca mulatta) Am J Primatol. 1997;42:199–213. doi: 10.1002/(SICI)1098-2345(1997)42:3<199::AID-AJP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Miller C, Alexander N, Sutjipto S, et al. Effect of virus dose and Nonoxynol-9 on the genital transmission of SIV in rhesus macaques. J Med Primatol. 1990;19:401–409. [PubMed] [Google Scholar]
- 24.Miller C, Alexander N, Gettie A, Hendrickx A, Marx P. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil Steril. 1992;57:1126–1128. [PubMed] [Google Scholar]
- 25.Greenier JL, Miller CJ, Lu D, et al. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J Virol. 2001;75:3753–3765. doi: 10.1128/JVI.75.8.3753-3765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel MD, Letvin NL, Sehgal PK, et al. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- 27.Lewis M, Bellah S, McKinnon K, et al. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–292. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Pauza CD, Lü X, Montefiori DC, Miller CJ. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J AIDS Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kimata JT, Kuller L, Anderson DB, Dailey P, Overbaugh J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat Med. 1999;5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]


