Abstract
In nonhuman primate models of acquired immunodeficiency syndrome, live attenuated lentiviruses provide the most reliable protection from systemic and mucosal challenge with pathogenic simian immunodeficiency virus (SIV). Although live attenuated lentiviruses may never be used in humans because of safety concerns, understanding the nature of the protective immune mechanisms induced by live attenuated vaccines in primate models will be useful for developing other vaccine approaches. Approximately 60% of rhesus macaques immunized with nonpathogenic simian-human immunodeficiency virus (SHIV) strain 89.6 are protected from infection or clinical disease after intravaginal (IVAG) challenge with pathogenic SIVmac239. The goal of the present study was to determine whether administration of Depo-Provera before IVAG challenge with SIV decreases the protective efficacy of infection with SHIV89.6. The rate of protection after IVAG challenge with SIVmac239 was significantly lower (P < .05), and the acute postchallenge plasma viral RNA levels were significantly higher (P < .006), in Depo-Provera–treated, SHIV89.6-immunized macaques than in Depo-Provera–naive, SHIV89.6-immunized macaques. In the primate model of sexual transmission of human immunodeficiency virus, treatment with progesterone before IVAG challenge with a pathogenic virus can decrease the efficacy of a model “vaccine.”
The sexual transmission of HIV has produced a pandemic that continues to expand unabated. The best hope of controlling HIV lies in the development of vaccines or microbicides that prevent systemic infection from becoming established after sexual exposure. HIV is a mucosally transmitted pathogen; thus, mucosal immune responses would be beneficial in an HIV vaccine. Two studies of the simian immunodeficiency virus (SIV) model found that systemically administered antiserum or attenuated SIV vaccines that protect macaques against intravenous (iv) challenge with SIV do not protect macaques against mucosal challenge [1, 2]. However, oral or subcutaneous immunization with an attenuated simian-human immunodeficiency virus (SHIV) protects macaques against intravaginal (IVAG) challenge with pathogenic SHIV [3], and there is no significant difference in the rates of protection against IVAG challenge with SIV among groups of macaques immunized with an attenuated SHIV by either the IVAG, intranasal, or iv routes [4]. In the studies mentioned above, “protection” was defined as either the inability to detect challenge virus in blood or a significant reduction in plasma viral RNA (vRNA) and a concomitant lack of disease progression [4]. Plasma vRNA levels in macaques and humans are highly predictive of clinical outcome [5–7] and of the likelihood of mucosal HIV transmission to an uninfected partner [8, 9]. Thus, if the observed reduction in plasma vRNA levels after vaccination in macaques can be duplicated with an HIV vaccine in humans, then the rates of HIV transmission would decrease, and the pandemic would slow.
Because strong systemic anti-HIV immune responses may be sufficient to decrease HIV transmission rates by vaccination, specific strategies to elicit mucosal responses may not be necessary. However, if the goal of vaccination is to prevent the sexual transmission of HIV, then anti-HIV–specific immunity in the female genital tract mucosa will likely be critical to the success of the vaccine. Thus, the success of HIV vaccine candidates may best correlate with their ability to induce such responses mucosally, as has been described in recent primate studies demonstrating that only animals with local HIV- or SIV-specific IgG, IgA, or cytotoxic T lymphocytes were protected against challenge with a more-virulent strain [10–15].
To elicit HIV-specific immune responses in the female genital tract by vaccination, several factors need to be considered, including route of immunization, nature of the antigen/adjuvant or vector, and hormonal status of the vaccine recipient. Sex steroid hormones are important in regulating both the systemic and secretory immune system (reviewed in [16]). Thus, the effects of sex steroids on immune responses must be considered when assessing immune responses to HIV and HIV vaccines. Furthermore, because women who use long-acting progestins, such as Depo-Provera (Pharmacia), for contraception are a critical target population for HIV vaccination, the effects of exogenous hormones on vaccine-induced immune responses and vaccine efficacy need to be considered in preclinical and clinical HIV vaccine development.
In nonhuman primate models of AIDS, live attenuated lentiviruses provide the most reliable protection against systemic and mucosal challenge with pathogenic SIV [2, 4, 10, 17–21], and understanding the nature of the protective immune mechanisms induced by live attenuated vaccines in primate models will be useful for developing other vaccine approaches. We have previously shown that ~60% of rhesus macaques immunized with nonpathogenic SHIV89.6 are protected against infection or clinical disease after IVAG challenge with pathogenic SIVmac239 [4, 22]. The goal of the present study was to determine whether administration of Depo-Provera before IVAG challenge with SIV decreases the protective efficacy of SHIV89.6 infection. We found that the rate of protection after IVAG challenge with SIVmac239 was significantly lower (P < .05) and that the acute postchallenge plasma vRNA levels were significantly higher (P < .006) in Depo-Provera–treated, SHIV89.6-immunized macaques than in Depo-Provera–naive, SHIV89.6-immunized macaques.
MATERIALS AND METHODS
Animals
The female, multiparous, regularly cycling rhesus macaques (Macaca mulatta) used in the present study were housed at the California National Primate Research Center (Davis, CA), in accordance with the regulations of the American Association for Accreditation of Laboratory Animal Care standards. All macaques were negative for antibodies to HIV-2, SIV, type-D retrovirus, and simian T cell lymphotropic virus type 1 at the time the study was initiated.
Immunization and treatment with Depo-Provera
Ten macaques were immunized by iv inoculation with live attenuated SHIV89.6. At weeks −1, 0, 1, 2, 4, 6, and 8 after immunization and monthly thereafter, blood was collected and analyzed for vRNA levels and antiviral immune responses. Approximately 1 year after infection with SHIV89.6 and 4 weeks before challenge with SIV, a single dose of Depo-Provera (30 mg/kg) was administered by intramuscular injection to 6 macaques randomly selected from the group of 10 macaques. Hereafter, these macaques are referred to as “Depo-Provera–SHIV macaques,” and the 4 Depo-Provera–naive, SHIV-immunized macaques are referred to as “SHIV macaques.” In preclinical trails of candidate HIV vaccines and microbicides, this dose and timing regimen of Depo-Provera has been widely used to increase the susceptibility of female rhesus macaques to IVAG challenge with SIV [23–28].
IVAG challenge with SIV
The pathogenic SIVmac239 stock used in the present study was produced in rhesus peripheral blood mononuclear cells (PBMCs), as described elsewhere [19], and contained ~105 TCID50/mL. The virus challenge of the SHIV89.6-immunized macaques consisted of 2 IVAG inoculations with 1 mL of the undiluted SIVmac239 stock. Two unimmunized control macaques were challenged IVAG with SIVmac239 contemporaneously with the 6 Depo-Provera–SHIV macaques and 4 SHIV macaques. Blood samples were collected at weeks 1, 2, and 4 after infection, monthly thereafter, and at necropsy. Six months after challenge, the 6 Depo-Provera–SHIV macaques were killed by phenobarbitol overdose, and blood and tissue samples were collected. Two unimmunized control macaques and 4 SHIV macaques were necropsied 12 weeks after challenge with SIV, after the viral set point had been reached and the progressive nature of the SIV infection had been established.
To increase the power of the study, the plasma vRNA data from the 6 Depo-Provera–SHIV macaques were compared with the plasma vRNA data from the 4 contemporaneous SHIV macaques and with our previously published plasma vRNA data from 43 SHIV macaques challenged IVAG with SIVmac239 and from 18 unimmunized control macaques [4]. The vRNA assays, immunization protocols, and virus stocks that were used in the previous study were the same as those used in the present study. Note that the postchallenge plasma vRNA levels of the 2 contemporaneous control macaques and 4 SHIV macaques fell within the range of those of the historical control and SHIV macaques. Thus, the plasma vRNA data from contemporaneous and historical SHIV macaques were combined for comparison to the plasma vRNA data in the Depo-Provera–SHIV macaques.
Isolation of PBMCs
PBMCs were isolated from heparinized blood by use of Lymphocyte Separation Medium (ICN Biomedicals). PBMC samples were frozen in 10% DMSO (Sigma)/90% fetal bovine serum (Gemini BioProducts) and stored in liquid nitrogen until future analysis in immunological and virological assays [19].
Virus load measurement
Plasma samples were analyzed for vRNA by use of a quantitative branched DNA assay [29]. Virus load in plasma samples is reported as vRNA copy numbers per milliliter of plasma. The detection limit of this assay is 125 vRNA copies/mL of plasma.
Measurement of anti-SIV antibody titers
Anti-SIV binding antibody titers in serum and cervicovaginal secretions were measured by use of ELISA plates coated with detergent-disrupted SIVmac239, as described elsewhere [30]. The results of the anti-SIV antibody ELISA are reported as the dilution of a sample that produced optical density values above the cutoff value.
Interferon (IFN)–γ ELISPOT assay
As described elsewhere [4], the number of IFN-γ–secreting cells in frozen PBMCs responding to an SIVmac239 Gag p27 peptide pool was determined by use of an IFN-γ ELISPOT kit (U-CyTech; Utrecht University). Negative controls consisted of cells that were cultured in medium only and peptide-stimulated cells from uninfected macaques. A sample was considered to be positive only if the number of IFN-γ–secreting cells per well was >50 cells/1 × 106 PBMCs and if the number of positive IFN-γ spot-forming cells (sfcs) was greater than the mean (±2 SD) of the medium-only wells. Data are reported as the number of IFN-γ sfcs/1× 106 PBMCs, and the background number of sfcs in medium-only wells were subtracted from the number of sfcs in SIV peptide-stimulated wells. By use of these criteria, PBMC samples collected from study macaques before the initial immunization were consistently negative for SIV p27–specific IFN-γ secretion (data not shown). In addition to stimulating each PBMC sample with PMA/ionomycin, we included fresh PBMC samples from at least 2 macaques infected with SIVmac239Δnef and known to have strong anti-SIV p27–specific IFN-γ responses as positive controls in every assay. Furthermore, fresh PBMC samples from at least 2 SIV-naive macaques were included as negative controls in every assay. All the positive and negative controls gave appropriate results in all experiments.
T cell proliferation assay
SIV-specific T cell proliferative responses were measured as described elsewhere [31]. The SIV antigen used for this assay, whole aldrithol-2–inactivated SIV-mac239, was provided by Dr. J. Lifson (Laboratory of Retroviral Pathogenesis, SAIC Frederick, Bethesda, MD). The following antigen concentrations were used: 1 and 10 ng of p27/well. Each PBMC sample was stimulated with concanavalin A, and fresh PBMC samples from at least 2 macaques infected with SIVmac239Δnef and known to have strong anti-SIV T cell proliferative responses were included as positive controls in every assay. Furthermore, fresh PBMC samples from at least 2 SIV-naive macaques were included as negative controls in every assay. All the positive and negative controls gave appropriate results in all experiments.
Statistical analysis
The proportion of protected Depo-Provera–SHIV macaques was compared with the proportion of protected SHIV macaques by use of Fisher’s exact test. Plasma vRNA data generated after challenge with SIV were log10 transformed, and the mean vRNA levels of the Depo-Provera–SHIV and SHIV macaque groups at single postchallenge time points were compared by use of unpaired Student’s t test. To compare viral load in the Depo-Provera–SHIV and SHIV macaque groups over the entire 20 weeks after challenge, the vRNA level of each macaque was transformed into an area under the curve (AUC). The mean vRNA AUC of each group was calculated, and the AUC values of the groups were compared by use of Student’s t test. The value assigned to samples below the assay cutoff of 125 copies/mL was 50 copies/mL. InStat software (version 4.0; Graph Pad Software) and Macintosh G4 computers (Apple) were used for all analyses.
RESULTS
Plasma vRNA Levels Before and After Challenge with SIV
All 10 of the SHIV89.6-inoculated rhesus macaques in the present study developed peak plasma SHIV vRNA levels of 106–107 vRNA copies/mL by 2 weeks after immunization, which quickly decreased to undetectable levels by 16–24 weeks after immunization (data not shown). Four weeks after administration of Depo-Provera to 6 randomly selected macaques, all 10 macaques underwent IVAG challenge with SIVmac239. Before and after administration of Depo-Provera, low-level plasma viremia was detected intermittently in all the macaques, regardless of treatment. Two vaccine-naive macaques were challenged IVAG with SIVmac239 at the same time.
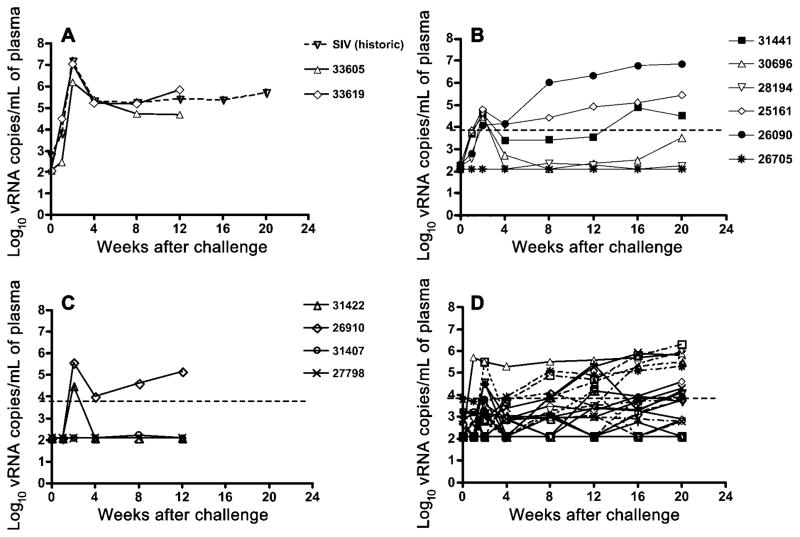
At week 1 after challenge with SIVmac239, 5 of 6 Depo-Provera–SHIV macaques had detectable plasma vRNA, and the vRNA levels in the 5 viremic macaques increased to >104 vRNA copies/mL by 2 weeks after challenge (figure 1). Thereafter, plasma vRNA levels decreased by varying degrees, but, from 8 weeks after challenge onward, plasma vRNA levels steadily increased in 2 macaques (21561 and 26090). Increasing plasma vRNA levels were detected in 1 macaque (31441), by 16 weeks after challenge, and in another macaque (30696), by 20 weeks after challenge (figure 1). One Depo-Provera–SHIV macaque (26075) was negative for vRNA in all plasma samples tested.
Figure 1.
Plasma viral RNA (vRNA) levels after intravaginal inoculation with simian immunodeficiency virus (SIV) strain mac239. Shown are mean plasma vRNA levels of concurrent unvaccinated control macaques (n = 2) and historical unvaccinated control macaques (n = 18) from a previous study [4] (A), Depo-Provera–simian-human immunodeficiency virus (SHIV) macaques (n = 6) (B), concurrent SHIV macaques (n = 4) (C), and historical SHIV macaques (n = 43) (D). Note that the difference between Depo-Provera–SHIV (B) and SHIV macaques (C and D) at 1 and 2 weeks was significant (P < .006). The limit of detection for the assay is 125 copies/mL of plasma.
At week 2 after challenge with SIVmac239, 2 of 4 SHIV macaques had plasma vRNA levels >104 vRNA copies/mL (figure 1). In macaque 26910, plasma vRNA levels decreased at 4 weeks after challenge but steadily increased from 8 weeks after challenge. In the other macaque (31442), plasma vRNA was undetectable from 4 weeks to the end of the study (figure 1). One SHIV macaque (31407) had a low plasma vRNA level (< 500 vRNA copies/mL plasma) at 2 time points, and the other SHIV macaque (27798) was negative for vRNA in all plasma samples tested. Two unimmunized control macaques also underwent IVAG inoculation with SIVmac239 (figure 1). At 2 weeks after inoculation, they had peak plasma vRNA levels >106 vRNA copies/mL, and these vRNA levels remained high (>50,000 vRNA copies/mL) throughout a 3-month postchallenge observation period.
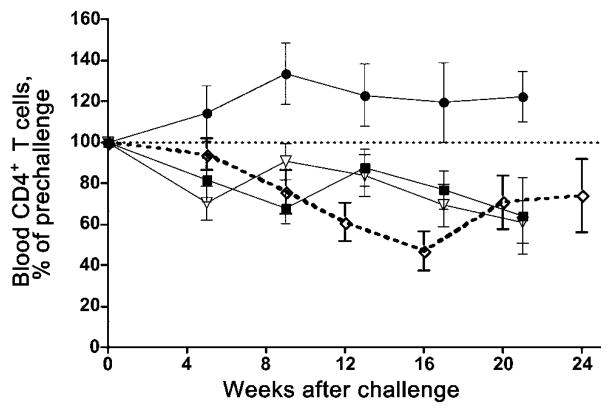
In previous studies in this vaccine/challenge system, we correlated plasma vRNA levels, lymphocyte counts, and clinical outcome [4, 22]. Thus, we used plasma vRNA levels as the criteria for determining whether a macaque is considered to be “protected.” If a macaque had a viral load of < 104 vRNA copies/mL during the 6-month postchallenge observation period, it was considered to be protected against the IVAG challenge with SIVmac239. These macaques showed no evidence of decreasing CD4+ T cell counts in blood or tissues and had no signs of clinical disease. On the basis of these criteria, 2 (50%) of 4 contemporaneous and 27 (63%) of 43 historical SHIV macaques [4] were protected against the IVAG SIVmac239 inoculum. Thus, when contemporaneous and historical studies are combined, 29 (62%) of 47 of SHIV macaques were protected against IVAG challenge with SIVmac239. In contrast, only 1 (16%) of 6 Depo-Provera–SHIV macaques were protected against IVAG challenge with SIVmac239. The difference in the proportion of vaccine-protected macaques between the SHIV macaques and the Depo-Provera–SHIV macaques was statistically significant (P < .05). During the postchallenge period, Depo-Provera–SHIV macaques had steady decreases in peripheral CD4+ T cell counts similar to those in unprotected SHIV macaques, whereas protected SHIV macaques had steady CD4+ T cell counts (figure 2).
Figure 2.
Mean CD4+ T cell levels relative to levels on the day of challenge in peripheral blood of simian-human immunodeficiency virus (SHIV) 89.6 vaccinated–protected macaques (●; n = 11); SHIV89.6 vaccinated–unprotected macaques (▽; n = 6); SHIV89.6-vaccinated, Depo-Provera–treated macaques (◇; n = 6); and SHIV89.6-naive control macaques (■; n = 6–9). Note that the data from the SHIV89.6-vaccinated–protected macaques, the SHIV89.6 vaccinated/unprotected macaques, and naive control macaques have been published previously [4] and that the SHIV89.6-vaccinated, Depo-Provera–treated macaques are the 6 macaques in the present study.
To increase the power of the study, the plasma vRNA data from the 6 Depo-Provera–SHIV macaques were compared with the plasma vRNA data from the 4 contemporaneous SHIV macaques and our previously published plasma vRNA data from 43 SHIV macaques and 18 unimmunized control macaques challenged IVAG with SIVmac239 [4], by use of Student’s t test. At week 1 after challenge with SIV, the mean plasma vRNA level of the Depo-Provera–SHIV macaques (3.14 log10 vRNA copies/mL; n = 6) was significantly higher (P < .003) than the mean plasma vRNA level of the combined SHIV macaques (2.33 log10 vRNA copies/mL; n = 47). At week 2 after challenge with SIV, the mean plasma vRNA level of the Depo-Provera–SHIV macaques (4.12 n = 6) was significantly higher (P < .006) log10 vRNA copies/mL; than the mean plasma vRNA level of the combined SHIV macaques (2.81 log10 vRNA copies/mL; n = 47). When vRNA level was converted to AUC, the mean AUC of the Depo-Provera–SHIV macaques (69.4; n = 6) was significantly higher (P≤.029) than the mean AUC of the combined SHIV macaques (48.4; n = 47) during the first 20 weeks after challenge. Thus, treatment with Depo-Provera resulted in decreased vaccine-mediated control of challenge virus replication that was most striking during the acute postchallenge period.
Antiviral Immune Responses
Plasma binding antibody response to whole SIV after challenge with SIVmac239
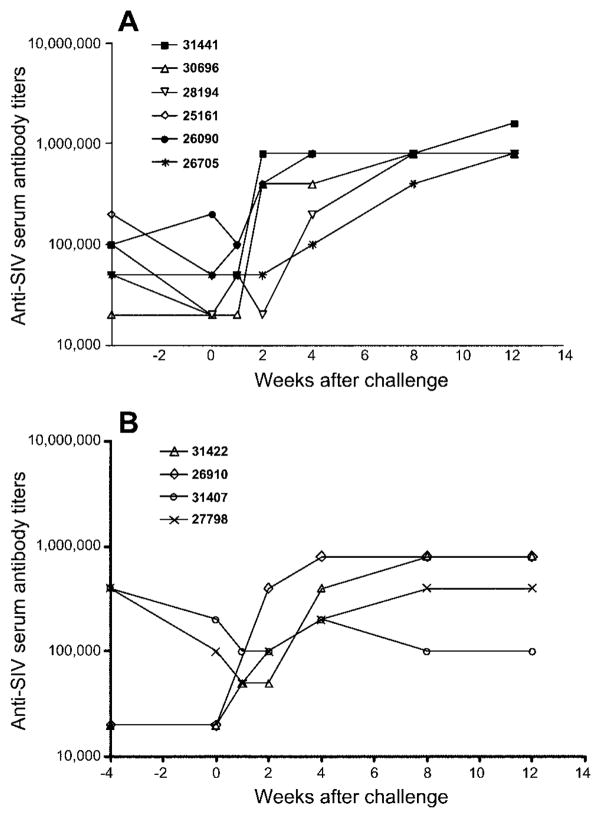
Consistent with systemic SHIV infection, all SHIV89.6-immunized macaques developed anti-SIV binding antibodies during the first 4 weeks of infection, and live attenuated virus and anti-SIV binding antibody titers further increased during the first 3 months after SHIV89.6 infection (data not shown). Anti-SIV binding antibodies persisted at high levels in the serum of all SHIV89.6-immunized macaques throughout the chronic phase of SHIV89.6 infection, even when vRNA was undetectable in the plasma. At the time of treatment with Depo-Provera (1 year after immunization with SHIV89.6), serum anti-SIV binding antibody titers ranged from 1:20,000 to 1:400,000. Consistent with high plasma vRNA levels in the Depo-Provera–SHIV macaques after IVAG challenge with SIVmac239, serum anti-SIV binding antibody titers increased in all 6 macaques after challenge with SIV (figure 3). In 4 of the 6 macaques, increased antibody titers were detectable by 2 weeks after challenge; in 2 of the 6 vaccinated macaques (28194 and 26705), increased antibody titers were detectable by 4 weeks after challenge. Importantly, increased serum anti-SIV binding antibody titers were also observed in macaque 26705, which had no detectable vRNA in the plasma, suggesting that the virus was actively replicating at very low levels in tissues. Among the 4 concurrent SHIV macaques, after challenge, serum anti-SIV binding antibody titers increased in only 2 of the macaques (41442 and 26910), with postchallenge plasma vRNA levels >104 copies/mL plasma.
Figure 3.
Plasma anti–simian immunodeficiency virus (SIV) antibody titers after intravaginal inoculation with SIVmac239, in Depo-Provera–simian-human immunodeficiency virus (SHIV) macaques (n = 6) (A) and in concurrent SHIV macaques (n = 4) (B).
Both of the 2 unvaccinated control macaques developed anti-SIV binding antibodies by week 4 after challenge (1: 200,000). At week 12 after challenge, serum anti-SIV binding antibody titers in the Depo-Provera–SHIV macaques (1: 800,000–1:1,600,000) and the unvaccinated control macaques (1:800,000) were similar (data not shown).
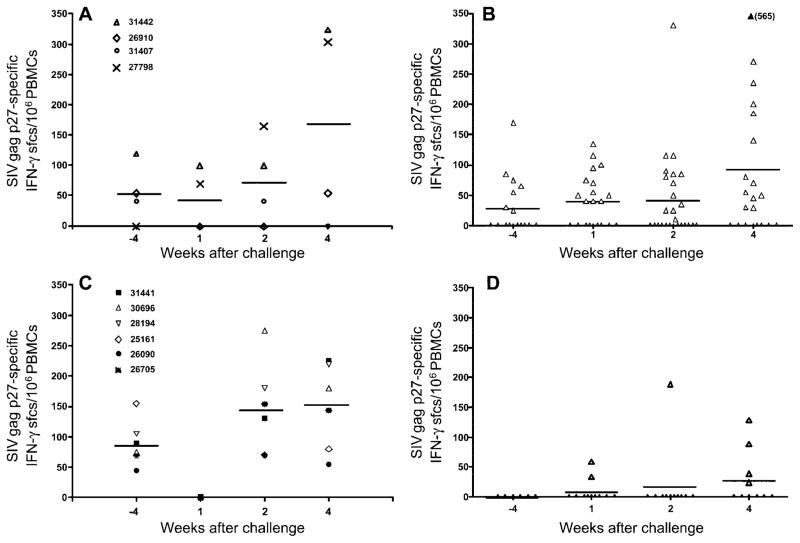
SIV Gag p27–specific IFN-γ T cell responses after challenge with SIVmac239
At the time Depo-Provera treatment was started (1 year after SHIV89.6 infection), all 6 Depo-Provera–SHIV macaques had detectable SIV Gag p27–specific IFN-γ T cell responses in PBMCs (figure 4). Unexpectedly, at 1 week after challenge, PBMCs from the 6 Depo-Provera–SHIV macaques lost the ability to secrete IFN-γ in response to stimulation with SIV Gag p27 peptide (figure 4). At weeks 2 and 4 after challenge, SIV Gag–specific IFN-γ–secreting T cells were again detectable in PBMCs of all 6 Depo-Provera–SHIV macaques. It should be noted that, despite undetectable post-challenge plasma vRNA levels, the PBMC SIV Gag–specific IFN-γ T cell responses in macaque 26705 were similar to those in the other 5 Depo-Provera–SHIV macaques.
Figure 4.
Anti–simian immunodeficiency virus (SIV) Gag p27 interferon (IFN)–γ–secreting T cell frequencies in peripheral blood mononuclear cells (PBMCs) around the time of intravaginal challenge with SIVmac239, in concurrent simian-human immunodeficiency virus (SHIV) macaques (n = 4) (A), historical SHIV macaques (n = 43) (B), Depo-Provera–SHIV macaques (n = 6) (C), and concurrent and historical unvaccinated control macaques (n = 10) (D). sfcs, spot-forming cells.
Of the 4 concurrent SHIV macaques, 3 had SIV Gag–specific IFN-γ T cell responses at the time of challenge. Although macaque 27798 had no detectable SIV Gag–specific IFN-γ T cell responses before IVAG challenge with SIVmac239, this macaque did develop good anamnestic IFN-γ T cell responses and was able to control postchallenge virus replication. Similar to what was observed in the Depo-Provera–SHIV macaques, in 2 of the 3 macaques with detectable IFN-γ T cell responses at the time of challenge, SIV Gag–specific IFN-γ T cell responses were no longer detectable at week 1 after challenge but became detectable again at week 2 after challenge.
In contrast to the apparent anamnestic IFN-γ T cell responses observed in the PBMCs of the SHIV89.6-immunized macaques, 1 of the 2 unvaccinated control macaques (33605) did not respond with IFN-γ secretion to in vitro SIV Gag peptide stimulation at any of the time points tested; in the other macaque (33619), SIV Gag–specific IFN-γ–secreting T cells were detected only at week 2 after challenge. At this time point, the frequency of SIV Gag–specific IFN-γ–secreting T cells in macaque 33619 (190 cells/106 PBMCs) was comparable to the frequencies of SIV Gag–specific IFN-γ–secreting T cells in the vaccinated macaques (70–275 cells/106 PBMCs). The delayed response of SIV Gag–specific IFN-γ–secreting T cells in unvaccinated macaques, compared with that in vaccinated macaques, was consistent with results obtained in our previous study [4].
SIV-specific T cell proliferative responses after challenge with SIVmac239
At 1 week after challenge, 4 of 6 Depo-Provera–SHIV macaques had positive anti-SIV lymphoproliferative (LP) responses. At 2 and 4 weeks after inoculation, respectively, only 1 of 6 and 2 of 6 of the Depo-Provera–SHIV macaques maintained these responses. In contrast, 2 of 4 SHIV macaques had positive anti-SIV LP responses at 1 and 2 weeks after inoculation; this increased to 3 of 4 macaques with positive reactions at 4 weeks after inoculation. Thus, Depo-Provera–SHIV macaques tended to lose the ability to proliferate after challenge with SIV, whereas SHIV macaques maintained their anti-SIV LP capacity throughout the postchallenge period.
DISCUSSION
The results of the present study have demonstrated that prior administration of Depo-Provera to female rhesus macaques significantly lowers the efficacy of a live attenuated lentiviral infection that protects the majority of Depo-Provera–naive female rhesus macaques against IVAG challenge with virulent SIVmac239. This loss of protection was manifested as a decrease in the proportion of macaques that had undetectable or low levels of SIV in plasma and no evidence of CD4+ T cell loss during a 6-month follow-up period. Furthermore, Depo-Provera–SHIV macaques had significantly higher viral loads in the postchallenge phase than did Depo-Provera–naive macaques. The higher plasma vRNA levels were associated with the loss of SIV Gag–specific IFN-γ T cell responses in PBMCs at 1 week after challenge in the Depo-Provera–SHIV macaques. Of note, vaccine failure was also observed in 1 of the 2 concurrent SHIV macaques that had undetectable SIV Gag–specific IFN-γ T cell responses at 1 week after challenge. Overall, the frequencies of SIV Gag–specific IFN-γ T cells in PBMCs of the SHIV macaques in the present study were comparable to the frequencies of SIV Gag–specific IFN-γ T cells observed in our previous study [4]. This result is especially troubling because, even though live attenuated lentiviral vaccines are inherently unsafe because of their potential for retention of virulence and integration-induced carcinogenesis [32, 33], they provide the most consistent protection against systemic and mucosal challenge with virulent viruses in the macaque models of AIDS. The effect of Depo-Provera may be even greater with vaccines that are less effective than live attenuated viruses.
At least 2 mechanisms can explain the deleterious effect of Depo-Provera on the effectiveness of live attenuated vaccines. Either Depo-Provera dramatically increases the effective dose of the challenge inoculum, or it dramatically blunts the development of anamnestic antiviral immune responses, or both. There is abundant evidence that exogenous progestins increase susceptibility to genital tract infections in unvaccinated women and macaques. The use of exogenous hormones for contraception, especially injectable progestins, increases a woman’s susceptibility to HIV infection and other sexually transmitted diseases (STDs) [34, 35]. In rhesus macaques, exogenous pro-gesterone increases the susceptibility of macaques to IVAG inoculation with SIV or SHIV [36], which has been attributed to progesterone-dependent thinning of the cervicovaginal epithelium. However, progesterone does not have a dramatic effect on the thickness of human genital mucosa [37]; thus, epithelial thinning is unlikely to be the complete explanation for why women who use injectable progestins are at increased risk for acquiring HIV infection and STDs. Although Depo-Provera may increase the effective dose of the inoculum by increasing the probability of replication-competent virion/target cell interaction, Depo-Provera also seems to suppress the initial immune response to vaginally transmitted HIV or SIV.
In both women and female rhesus macaques, the ovarian hormones estrogen and progesterone are secreted in a tightly regulated fashion, producing the menstrual cycle, and these cyclic hormones also dramatically affect immunity. In vitro, estrogens enhance nonspecific differentiation of antibody-secreting cells [38, 39] by suppressor T cells [39]. In both female rhesus macaques and women, immunoglobulin and antibody levels in genital secretions are relatively low around the time of ovulation [40–43] and are relatively high during menstruation and the luteal phase. This fluctuation in antibody levels is not associated with shifts in mucosal immune cell populations, because the number of B cells in the cervicovaginal mucosa remains constant during the menstrual cycle [44, 45]. The cyclic fluctuation in the ability of B cells to secrete immunoglobulin in female macaques [46] and in women [47] involves a mechanism that requires the presence of CD8+ T cells [46].
Exogenous progesterone has profound immunosuppressive effects, and a single dose enhances renal allograft survival in dogs and skin allograft survival in rabbits [48] and produces uncontrolled growth of Moloney sarcoma virus–induced tumors in mice [49]. Progesterone enhances susceptibility and decreases immune responses to vaginal herpes infection in mice [50–52]. Furthermore, exposure to progesterone for >15 days prevents the induction of protective mucosal immune responses in mice immunized IVAG with attenuated herpes simplex virus–2 [53]. In the present study, we noted that Depo-Provera–SHIV macaques, in contrast to protected SHIV macaques, were incapable of making anti–SIV Gag ELISPOT responses at 1 week after challenge. In a previous study, expression of IFN-α mRNA was higher in PBMCs of protected SHIV macaques, compared with those of unprotected SHIV macaques [4], and studies are planned to assess differences in innate antiviral immunity between Depo-Provera–SHIV macaques and SHIV macaques.
Clearly, treatment with progesterone before IVAG challenge with a pathogenic virus can decrease the efficacy of a model vaccine in the primate model of HIV sexual transmission. Thus, care should be taken in designing and interpreting the results of preclinical primate vaccine studies that use treatment with progesterone before IVAG challenge with a pathogenic virus. It would also seem prudent in designing a phase 3 trial to consider whether the use of exogenous progestins for contraception can decrease the efficacy of an HIV vaccine in women. Women using injectable progestins shed more HIV in cervical secretions, are at increased risk for acquiring HIV infection, and have higher plasma vRNA levels in the early stages of HIV infection and more-rapid disease progression [54–56]. Although we did not conclusively document the nature of the Depo-Provera–induced immune suppression in the present study, the findings from all these studies suggest that exogenous progesterone affects multiple steps in viral transmission, including the initial susceptibility to infection, the initial immune response to the virus invasion in the naive host, and the ability of the immune host to mount effective antiviral recall responses.
Acknowledgments
Public Health Service (grant RR00169); National Center for Research Resources (grant RR14555); National Institute of Allergy and Infectious Diseases (grant AI44480).
We thank the Immunology Core Laboratory at the California National Primate Research Center (Davis, CA) and Lara Compton, Ding Lu, Blia Vang, and Rino Dizon for excellent technical assistance.
References
- 1.Joag SV, Li Z, Wang C, et al. Passively administered neutralizing serum that protected macaques against infection with parenterally inoculated pathogenic simian-human immunodeficiency virus failed to protect against mucosally inoculated virus. AIDS Res Hum Retroviruses. 1999;15:391–4. doi: 10.1089/088922299311367. [DOI] [PubMed] [Google Scholar]
- 2.Marthas M, Sutjipto S, Miller C, et al. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against intravenous and intravaginal challenge. In: Brown F, Chanock RM, Ginsberg HS, Lerner RA, editors. Vaccines 1992. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 117–22. [Google Scholar]
- 3.Joag SV, Liu ZQ, Stephens EB, et al. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J Virol. 1998;72:9069–78. doi: 10.1128/jvi.72.11.9069-9078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel K, Compton L, Rourke T, et al. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellors JW, Kingsley LA, Rinaldo CR, Jr, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch VM, Fuerst TR, Sutter G, et al. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)–infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–52. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelbrot L, Burgard M, Teglas JP, et al. Frequent detection of HIV-1 in the gastric aspirates of neonates born to HIV-infected mothers. AIDS. 1999;13:2143–9. doi: 10.1097/00002030-199910220-00018. [DOI] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 10.Murphey-Corb M, Wilson LA, Trichel AM, et al. Selective induction of protective MHC class I–restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162:540–9. [PubMed] [Google Scholar]
- 11.Haigwood NL. Progress and challenges in therapies for AIDS in non-human primate models. J Med Primatol. 1999;28:154–63. doi: 10.1111/j.1600-0684.1999.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 12.Heeney J, Akerblom L, Barnett S, et al. HIV-1 vaccine–induced immune responses which correlate with protection from SHIV infection: compiled preclinical efficacy data from trials with ten different HIV-1 vaccine candidates. Immunol Lett. 1999;66:189–95. doi: 10.1016/s0165-2478(98)00157-6. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson C, Makitalo B, Thorstensson R, et al. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS. 1998;12:2261–70. doi: 10.1097/00002030-199817000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bagarazzi ML, Boyer JD, Javadian MA, et al. Systemic and mucosal immunity is elicited after both intramuscular and intravaginal delivery of human immunodeficiency virus type 1 DNA plasmid vaccines to pregnant chimpanzees. J Infect Dis. 1999;180:1351–5. doi: 10.1086/314978. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino N, Ami Y, Someya K, et al. Protective immune responses induced by a non-pathogenic simian/human immunodeficiency virus (SHIV) against a challenge of a pathogenic SHIV in monkeys. Microbiol Immunol. 2000;44:363–72. doi: 10.1111/j.1348-0421.2000.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 16.Grossman CJ. Regulation of the immune system by sex steroids. Endocr Rev. 1984;5:435–55. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- 17.Almond N, Rose J, Sangster R, et al. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919–22. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Mukherjee S, Shen J, et al. Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologousSHIVs and simian immunodeficiency virus. Virology. 2002;301:189–205. doi: 10.1006/viro.2002.1544. [DOI] [PubMed] [Google Scholar]
- 19.Miller CJ, McChesney MB, Lü XS, et al. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–21. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RP, Lifson JD, Czajak SC, et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–61. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel MD, Kirchhoff F, Czajak SC, Sehagal PB, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–41. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 22.Abel K, La Franco-Scheuch L, Rourke T, et al. Gamma interferon–mediated inflammation is associated with lack of protection from intra-vaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6–immunized rhesus macaques. J Virol. 2004;78:841–54. doi: 10.1128/JVI.78.2.841-854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrose Z, Thompson J, Larsen K, et al. Evidence for immune-mediated reduction of viral replication in Macaca nemestrina mucosally immunized with inactivated SHIV89. 6. Virology. 2003;308:178–90. doi: 10.1016/s0042-6822(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 24.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 25.Mascola JR, Lewis MG, VanCott TC, et al. Cellular immunity elicited by human immunodeficiency virus type 1/simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies. J Virol. 2003;77:10348–56. doi: 10.1128/JVI.77.19.10348-10356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veazey RS, Klasse PJ, Ketas TJ, et al. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198:1551–62. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veazey RS, Shattock RJ, Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 28.Wilson LA, Murphey-Corb M, Martin LN, Harrison RM, Ratterree MS, Bohm RP. Identification of SIV env-specific CTL in the jejunal mucosa in vaginally exposed, seronegative rhesus macaques (Macaca mulatta) J Med Primatol. 2000;29:173–81. doi: 10.1034/j.1600-0684.2000.290311.x. [DOI] [PubMed] [Google Scholar]
- 29.Dailey PJ, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay [abstract 99]. Program and abstracts of the 13th annual Symposium on Nonhuman Primate Models of AIDS; Monterey, CA. Davis, CA: California National Primate Research Center; 1995. p. 180. [Google Scholar]
- 30.Lü XS, Kiyono H, Lu D, et al. Targeted lymph node immunization with whole-inactivated SIV or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McChesney MB, Collins JR, Lu D, et al. Occult systemic infection and persistent SIV-specific CD4+ T cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol. 1998;72:10029–35. doi: 10.1128/jvi.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruprecht RM. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol Rev. 1999;170:135–49. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann-Lehmann R, Vlasak J, Williams AL, et al. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS. 2003;17:157–66. doi: 10.1097/00002030-200301240-00004. [DOI] [PubMed] [Google Scholar]
- 34.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–9. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 35.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 36.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 37.Mauck CK, Callahan MM, Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 38.Sthoeger ZM, Chiorazzi N, Lahita RG. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen–induced human B cell differentiation. J Immunol. 1988;141:91–8. [PubMed] [Google Scholar]
- 39.Paavonen T, Andersson LC, Adlercreutz H. Sex hormone regulation of in vitro immune response: estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen–stimulated cultures. J Exp Med. 1981;154:1935–45. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schumacher GFB, Yang SL. Cyclic changes of immunoglobulins and specific antibodies in human and rhesus monkey cervical mucus. In: Insler V, Bettendorf G, editors. The uterine cervix in reproduction. Stuttgart: Georg Thieme Verlag; 1977. pp. 187–203. [Google Scholar]
- 41.Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–42. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jalanti R, Isliker H. Immunoglobulins in human cervico-vaginal secretions. Int Arch Allergy Appl Immunol. 1977;53:402–8. doi: 10.1159/000231778. [DOI] [PubMed] [Google Scholar]
- 43.Lü FX, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller CJ. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–8. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–73. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 45.Ma Z, Lu FX, Torten M, Miller CJ. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol. 2001;100:240–9. doi: 10.1006/clim.2001.5058. [DOI] [PubMed] [Google Scholar]
- 46.Lu FX, Abel K, Ma Z, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu FX, Ma Z, Moser S, Evans TG, Miller CJ. Effects of ovarian steroids on immunoglobulin-secreting cell function in healthy women. Clin Diagn Lab Immunol. 2003;10:944–9. doi: 10.1128/CDLI.10.5.944-949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turcotte JG, Haines RF, Brody GL, Meyer TJ, Schwartz SA. Immunosuppression with medroxyprogesterone acetate. Transplantation. 1968;6:248–60. doi: 10.1097/00007890-196803000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Markham RB, White A, Goldstein AL. Selective immunosuppressive activity of steroids in mice inoculated with the Moloney sarcoma virus (38503) Proc Soc Exp Biol Med. 1975;148:190–3. doi: 10.3181/00379727-148-38503. [DOI] [PubMed] [Google Scholar]
- 50.McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:747–53. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677–85. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–65. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang CC, McClelland RS, Overbaugh J, et al. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–9. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 55.Lavreys L, Baeten JM, Martin HL, Jr, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS. 2004;18:695–7. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 56.Lavreys L, Baeten JM, Kreiss JK, et al. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis. 2004;189:303–11. doi: 10.1086/380974. [DOI] [PubMed] [Google Scholar]