Abstract
Purpose
Stereotactic body radiation therapy (SBRT) provides excellent local control with acceptable toxicity for patients with early-stage non–small cell lung cancer. However, the efficacy and safety of SBRT for patients previously given thoracic radiation therapy is not known. In this study, we retrospectively reviewed outcomes after SBRT for recurrent disease among patients previously given radiation therapy to the chest.
Materials and Methods
A search of medical records for patients treated with SBRT to the thorax after prior fractionated radiation therapy to the chest at The University of Texas M. D. Anderson Cancer Center revealed 36 such cases. The median follow-up time after SBRT was 15 months. The endpoints analyzed were overall survival, local control, and the incidence and severity of treatment-related toxicity.
Results
SBRT provided in-field local control for 92% of patients; at 2 years, the actuarial overall survival rate was 59%, and the actuarial progression-free survival rate was 26%, with the primary site of failure being intrathoracic relapse. Fifty percent of patients experienced worsening of dyspnea after SBRT, with 19% requiring oxygen supplementation; 30% of patients experienced chest wall pain and 8% Grade 3 esophagitis. No Grade 4 or 5 toxic effects were noted.
Conclusions
SBRT can provide excellent in-field tumor control in patients who have received prior radiation therapy. Toxicity was significant but manageable. The high rate of intrathoracic failure indicates the need for further study to identify patients who would derive the most benefit from SBRT for this purpose.
Keywords: Stereotactic body radiation therapy, retreatment, recurrent lung cancer
INTRODUCTION
Approximately 70% of patients presenting with lung cancer will receive external beam radiation therapy (EBRT) to the thorax as either definitive or adjuvant treatment. Despite advances in tumor localization, radiation dose escalation, and the use of concurrent chemotherapy, the rates of locoregional relapse among such patients remains high (1). Moreover, even among patients for whom radiation is successful, the development of second lung cancers is a common problem (2, 3). As such, the treatment of recurrent or second lung cancers in patients who have undergone previous irradiation to the thorax is a common dilemma facing oncologists today.
Because patients with locoregional relapse or second cancers after thoracic irradiation typically are not candidates for surgery (4), the most common form of salvage therapy offered is chemotherapy. However, the response rates to second-line chemotherapy are generally low, and durable control is uncommon (5). For this reason, some groups have explored the use of reirradiation for such patients. These studies have examined a variety of techniques, doses, and fraction sizes, but nevertheless overall survival has been limited, and the side effects are generally significant (6–11).
Image-guided hypofractionated stereotactic body radiation therapy (SBRT) can deliver high biologically effective doses to tumors while minimizing the dose to the surrounding tissues (12). This unique therapeutic advantage has been exploited with good results for early-stage, inoperable non-small cell lung cancer (NSCLC) (13–20). However, the role of SBRT for patients who have previously received EBRT to the thorax has not been examined. In this study, we report our early institutional experience with using SBRT for patients who have had intrathoracic relapse after conventional thoracic radiation.
METHODS AND MATERIALS
Patients
We reviewed the records of 246 consecutive patients treated with SBRT for lung cancer between October 2004 and November 2008 at the University of Texas M. D. Anderson Cancer Center. From this group, we identified 37 patients who had previously received standard fractionated EBRT to the thorax for lung cancer and subsequently underwent SBRT radiation therapy targeted to recurrent or second lung cancers within the thorax. One patient did not return to M. D. Anderson Cancer Center for follow-up after SBRT and was excluded from the analysis.
Treatment
Our techniques for patient immobilization and treatment planning have been described in detail in previous reports (15, 16). Briefly, all patients were treated while supine and immobilized in either a customized Vac-LOK cradle (CIVCO Medical Solutions, Kalona, IA) during EBRT or a BodyFix cradle (Elekta, Stockholm, Sweden) during SBRT. Four-dimensional (4D) computed tomography (CT) images were obtained with a Discovery ST Positron Emission Tomography (PET)/CT system (GE Medical Systems, Milwaukee, WI). The envelope of motion of the gross tumor volume was delineated by using a maximal intensity projection of the 4D CT and then modifying these contours by visual verification of the coverage on each phase of the 4D CT scans. This structure has not been defined by the International Commission on Radiation Units and Measurements and is referred to here as the internal gross tumor volume (iGTV). The clinical target volume was then created by expanding the iGTV isotropically by 8-mm margin followed by editing as necessary on the basis of the treating physician's judgment of tumor spread. A further expansion of 3 mm was added to create a planning target volume (PTV) to account for residual setup error and patient motion after the image-guided setup. Patients were treated with six to nine beams of 6-MV X-rays. Coplanar beams were used when possible to reduce the amount of normal lung being irradiated, but noncoplanar beams were used occasionally to minimize overlap of radiation fields on the skin. Patient were positioned each day by using volumetric imaging on the treatment couch with either CT-on-rail or cone-beam CT systems. The setup was established with the goal of covering the tumor or sparing nearby critical structures based on the judgment of the treating radiation oncologist. On each treatment day, a pair of orthogonal port films was taken to confirm that all shifts were accomplished correctly and that the patient had not moved; if any discrepancies were discovered, the volumetric imaging was repeated.
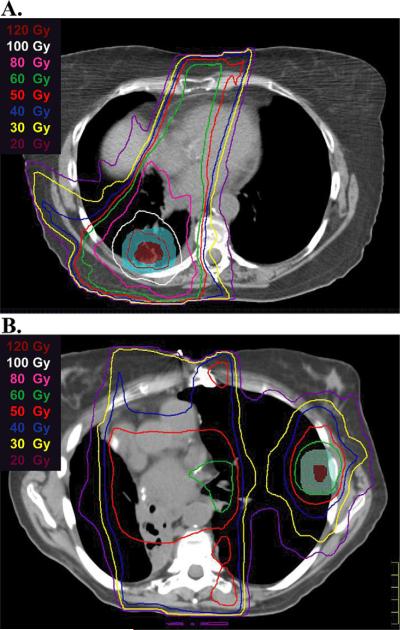
For the SBRT plans, the dose–volume constraints used for critical structures were consistent with our previously published guidelines (Table 1) (15). In addition, for the majority (91%) of patients, composite plans were generated, and adjustments were made to limit the radiation dose to critical structures on an individual basis to account for any prior EBRT, again at the discretion of the treating physician. These composite plans were used to determine the cumulative maximum point dose and maximum cord point doses (reported later in the article). These plans were also used to determine whether the lesion targeted by SBRT was an “in-field relapse,” defined as a recurrent lesion in an area that had previously received more than 30 Gy during the initial EBRT course (Fig. 1A), or an “out-of-field relapse,” defined as a recurrent lesion or second primary tumor in an area that had received less than 30 Gy during the initial EBRT course (Fig. 1B).
Table 1.
Critical organ dose–volume limits for non–small cell lung cancer lesions treated using stereotactic body radiation therapy delivering 50 Gy in four fractions
| Organ | Volume (cc) | Total dose* (Gy) |
|---|---|---|
| Esophagus | ≤1 | 35 (8.8) |
| ≤10 | 30 (7.5) | |
| Brachial plexus | Any point | <40 |
| ≤1 | 35 (8.8) | |
| ≤10 | 30 (7.5) | |
| Trachea | ≤1 | 35 (8.8) |
| ≤10 | 30 (7.5) | |
| Main bronchus and bronchial tree | ≤1 | 40 (10) |
| ≤10 | 35 (8.8) | |
| Heart | ≤1 | 40 (10) |
| ≤10 | 35 (8.8) | |
| Whole lung (excluding GTV) | V20 | <20% |
| V10 | <30% | |
| V5 | <40% | |
| Major vessels | ≤1 | 40 (10) |
| ≤10 | 35 (8.8) | |
| Skin (to 5 mm) | ≤1 | 40 (10) |
| ≤10 | 35 (8.8) | |
| Spinal cord | ≤1 | 20 (5) |
| ≤10 | 15 (3.8) |
Abbreviation: GTV = gross tumor volume
Dose per fraction.
Fig. 1.
Representative examples of stereotactic body radiation therapy (SBRT) retreatment plans for an in-field relapse (A) and an out-of-field relapse (B). Representative isodose lines are shown. The SBRT gross tumor volume is shown in red color wash; the SBRT planning target volume is shown in blue or green color wash.
Follow-up
Patients underwent chest CT scanning every 3 months for 2 years after the SBRT and then every 6 months for another 3 years. PET scans were recommended at 3–5 months after SBRT. Toxic effects were scored according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Effects version 3 (CTCAE v3). The presence of chest wall pain was also documented and scored based on whether the patient required narcotics for pain control. Clinical responses were evaluated according to the Response Evaluation Criteria in Solid Tumor criteria based on findings from both PET and CT images. Rates and times of overall survival, progression-free survival, intrathoracic relapse, in-field recurrence, and distant metastasis were recorded. Intrathoracic tumor recurrence was defined as progressive abnormalities on CT images corresponding to one or more avid lesions on PET scans or positive biopsy findings anywhere in the thorax after the SBRT. The beginning of the follow-up period was defined as the date of last SBRT treatment. The timing of recurrence was scored as the time at which the first image (PET or CT) showed abnormalities.
Statistical analysis
Actuarial overall survival and intrathoracic relapse were calculated using the Kaplan-Meier method, and tests for significance were based on the log-rank statistic. The completion of SBRT was used as time zero. For analyses of radiation-associated toxicity, the significance of differences between proportions was tested with Fisher's exact test.
RESULTS
Patients
Patient characteristics are detailed in Table 2. The 36 patients comprised 21 women and 16 men. The primary lung tumors varied in histology and stage, with most patients having Stage III/IV disease. For the purpose of this study, a single focus of disease in the contralateral lung from a larger primary tumor but no evidence of distant metastatic disease were considered to have cosynchronous primary tumors and not intrathoracic metastasis; five patients fit this description. Twenty-four patients (66%) had received definitive EBRT, seven had received postoperative adjuvant EBRT, and five patients had received palliative EBRT. The median dose delivered at the time of initial treatment was 61.5 Gy (range, 30–79.2 Gy). Most patients (28, or 78%) had received chemotherapy at some point during the treatment of the primary lung tumor, with 14 (38%) patients receiving concurrent chemoradiation. Nine patients had undergone wedge resection or lobectomy.
Table 2.
Characteristics of Patients
| Sex, n | |
| Male | 16 |
| Female | 20 |
| Median age at time of SBRT, years (range) | 67.5 (52–92) |
| Median KPS at time of SBRT (range) | 80 (60–100) |
| Median follow-up, months (range) | 15 (4–45) |
| Median interval between treatments, months (range) | 22 (0–92) |
| Initial Stage, n (%) | |
| I–II | 16 (44%) |
| III | 17 (47%) |
| IV | 3 (8%) |
| Type of initial radiation, n (%) | |
| Definitive | 24 (67%) |
| Postoperative | 7 (19%) |
| Palliative | 5 (14%) |
| Method of initial radiation, n (%) | |
| Three-dimensional conformal | 25 (69%) |
| IMRT | 11 (31%) |
| Median dose of initial radiation, Gy (range) | 61.5 (30–79.2) |
| Surgical history, n (%) | |
| Wedge resection | 2 (6%) |
| Lobectomy | 7 (19%) |
| Chemotherapy with initial treatment, n (%) | |
| Neo-adjuvant | 11 (31%) |
| Concurrent | 14 (38%) |
| Adjuvant | 7 (19%) |
| Size of recurrence, long axis, cm (range) | 1.7 (0.6–3.8) |
| Histology of recurrence, n (%) | |
| Adenocarcinoma | 14 (39%) |
| Squamous carcinoma | 12 (33%) |
| NSCLC, NOS | 8 (22% |
| Other | 2 (6%) |
| Type of recurrence, n (%) | |
| Isolated in-field recurrence | 11 (31%) |
| Isolated out-of-field recurrence | 13 (36%) |
| Recurrence in setting of disseminated disease | 12 (33%) |
| SBRT dose and fractionation | |
| 50 Gy in 4 fractions | 26 (72%) |
| 40 Gy in 4 fractions | 6 (17%) |
| Other | 4 (11%) |
| Maximum point dose combined radiation treatments, Gy (range) | 81.5 (59.4–134.6) |
Abbreviations: IMRT = intensity-modulated radiation therapy; KPS = Karnofsky performance status; NSCLC = non–small cell lung cancer; SBRT = stereotactic body radiation therapy.
SBRT
Median patient age at the time of reirradiation was 67.5 years (range, 52–92 years). The median time between EBRT and SBRT was 22.0 months (range, 0–92 months). A single lesion was targeted in each patient. Eleven patients (30%) were treated for what were thought to be isolated infield relapses, because the SBRT target was within the high-dose (>30 Gy) region of the previously treated field. Thirteen patients (36%) were treated for isolated out-of-field relapses or cosynchronous primary cancers in which the other primary tumor was previously addressed with definitive fractionation EBRT and had no evidence of metastatic disease. The other 12 patients (33%) were treated for out-of-field relapses and had documented distant metastatic disease or multiple sites of intrathoracic disease that had not been previously irradiated. At the time of SBRT, these individuals were thought to have stable disease except for the focus targeted by SBRT. The median size of tumors at the time of SBRT was 1.7 cm (range, 0.6–3.8 cm) along the longest axis. The most common radiation dose-fractionation schedule was 50 Gy in four fractions prescribed to the PTV (26 patients; 72%). Two patients received alternative but biologically equivalent doses, and the other eight patients received dose and fractionation schedules that are now believed to be suboptimal (13, 15). In addition, for those patients in whom dosing was considered optimal, three had compromised coverage in that less than 50% of the PTV was covered by the prescription dose. Thus, the total number of patients who received optimal biologically effective doses without compromised PTV coverage was 25 (69%). Suboptimal or compromised coverage was statistically more common in the groups receiving SBRT for in-field recurrences (p = 0.03). The median of individual patient maximum point dose from the composite plans was 81.5 Gy (range, 59.4–134.6 Gy). The median of individual maximum spinal cord dose was 35.6 Gy (range, 11.0–52.4 Gy). All patients completed SBRT therapy as scheduled. No significant side effects were noted during treatment.
Survival and local control endpoints
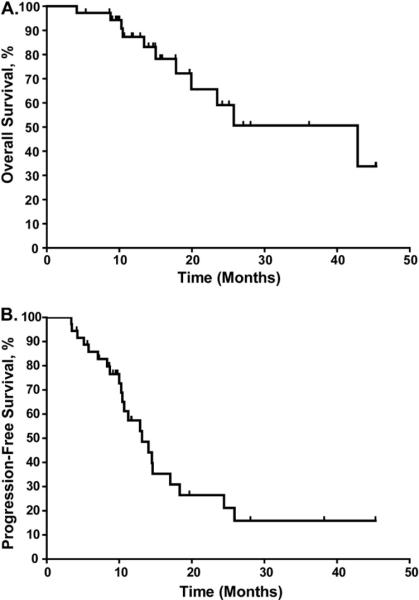
Radiographic response to radiation was seen in all patients, and the 2-year actuarial overall survival rate for the entire group was 59% (Fig. 2A). However, the 2-year actuarial progression-free survival rate was only 26% (Fig.2B). The predominant site of failure was intrathoracic relapse, with 18 patients experiencing failure in the thorax alone and four patients in the thorax and at distant sites. However, only three of the 22 intrathoracic failures occurred within the SBRT field (i.e., within the PTV of the SBRT field). Of these three patients, one had compromised tumor coverage (only 22% of the PTV covered at 50 Gy), and the other had received suboptimal dose and fractionation plus compromised tumor coverage (40 Gy in four fractions and only 24% of the clinical target volume covered at 40 Gy). Hence, the local control rate for all patients was 92%, and that for patients who received optimal biologically effective doses without compromised PTV was 96%. Of the 22 patients with relapse, 15 received salvage chemotherapy, one received a combination of salvage chemotherapy and radiation, and one received radiation alone.
Fig. 2.
Kaplan-Meier analyses of overall survival (A) and progression-free survival (B) after stereotactic body radiation therapy.
The substantial rate of progression led us to perform subgroup analyses to determine whether we could identify a population who benefited particularly from SBRT. We found that the patients treated for isolated out-of-field relapses who had no evidence of metastatic disease had significantly longer progression-free survival time than did patients treated for in-field relapses or patients treated for out-of-field relapses with known metastatic disease or multiple foci of intrathoracic disease (p = 0.04; Fig. 3). Progression-free survival did not seem to be associated with tumor histology, time between treatments, tumor size, or SBRT dose. However, given the small numbers of patients in this trial, the power to detect such a difference was low.
Fig. 3.
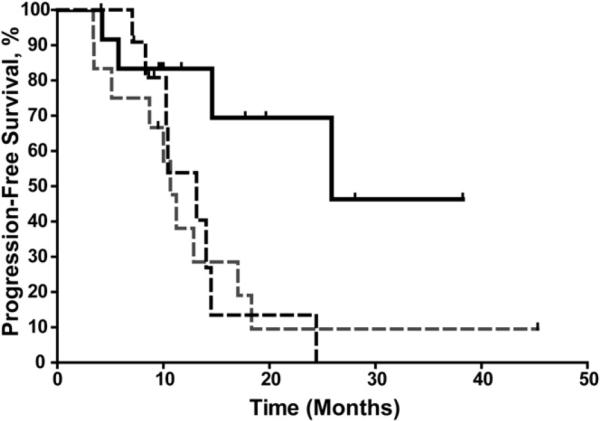
Kaplan-Meier analysis of progression-free survival for patients with in-field relapse (black dashed line), isolated out-of-field relapse (solid black line), and disseminated disease (gray dashed line). Progression-free survival was significantly better among patients with isolated recurrence outside the previous treatment field (p = 0.04 by log-rank test).
Toxicity
Twelve patients (33%) experienced at least one form of Grade 3 toxicity (Table 3). Of those patients, seven developed Grade 3 pneumonitis (worsening dyspnea requiring oxygen supplementation), three developed Grade 3 esophagitis (dehydration requiring intravenous hydration and hospitalization), two developed Grade 3 chest wall ulcers, and two had Grade 3 cough. No patients experienced Grade 4 or 5 toxicity, although one patient did die of progressive polymicrobial pneumonia at 3 months after treatment.
Table 3.
Incidence of Grade 2 and 3 toxicity by group
| In-field relapse (n = 11) | Out-of-field relapse (n = 25) | Total (n = 36) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Cough | |||
| Grade 2 | 0 | 3 (12%) | 3 (8%) |
| Grade 3 | 0 | 1 (4%) | 1 (3%) |
| Pneumonitis | |||
| Grade 2 | 5 (45%) | 6 (24%) | 11 (36%) |
| Grade 3 | 0 | 7 (28%) | 7 (28%) |
| Esophagitis | |||
| Grade 2 | 1 (9%) | 1 (4%) | 2 (6%) |
| Grade 3 | 1 (9%) | 2* (8%) | 3 (8%) |
| Skin | |||
| Grade 2 | 1 (9%) | 0 | 1 (3%) |
| Grade 3 | 2 (18%) | 0 | 2 (6%) |
| Chest Wall Pain | |||
| Not requiring narcotic | 4 (36%) | 1 (4%) | 5 (14%) |
| Requiring narcotic | 3 (27%) | 3 (12%) | 6 (17%) |
No Grade 4 or 5 toxicity was seen.
In addition, one patient developed esophageal stricture requiring dilation.
In addition to the seven patients experiencing Grade 3 pneumonitis, 11 patients experienced Grade 2 pneumonitis (worsening dyspnea but not requiring oxygen supplementation; Table 3). As such, 50% of the study population experienced some form of symptomatic pneumonitis, making it the most common side effect in this study. Interestingly, no Grade 3 pneumonitis was seen among the patients who underwent SBRT for an in-field relapse (and hence would have had the largest degree of overlap between the treatment fields; Fig. 4). Further analysis revealed a statistically significant (p = 0.03) association between treatment of out-of-field relapse and Grade 3 pneumonitis, but Grade 3 pneumonitis was not associated with other factors such as tumor size, central location (i.e., being <2 cm from the mediastinum, bronchial tree, or major vessels), SBRT dose, history of pneumonitis with prior treatment, or short time between treatments (<1 year), although the small numbers of patients in this study limit the power of such analyses.
Fig. 4.
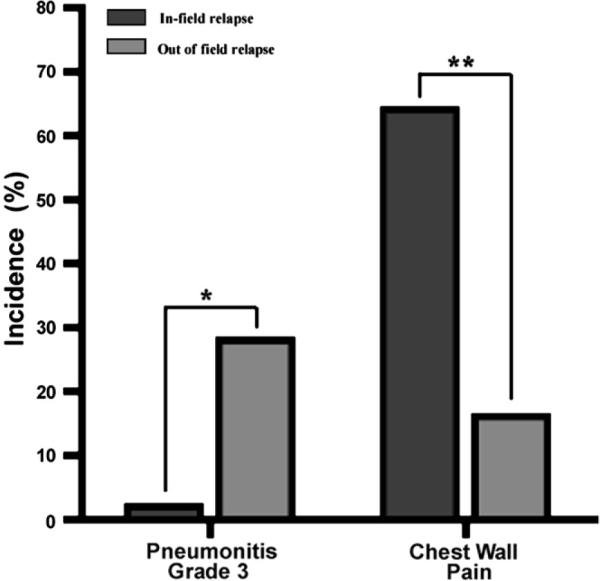
Grade 3 pneumonitis and chest wall pain stratified by in-field relapse or out-of-field relapse. *p = 0.03, **p = 0.02 by Fisher's exact test.
Chest wall pain is common among individuals receiving SBRT for lung tumors; because chest wall pain is not covered in the NCI CTCAE v3, we documented its occurrence separately. Overall, 11 patients (31%) experienced chest wall pain, of whom six required narcotics to control this symptom. As was true of pneumonitis, chest wall pain was not associated with tumor size, SBRT dose, or short time between treatments (<1 year). However, unlike pneumonitis, chest wall pain was more common in the group undergoing SBRT for in-field relapse (Fig. 4).
DISCUSSION
The treatment of locoregional relapses or second lung cancers in patients who have received prior radiation therapy to the thorax is a difficult clinical problem. These patients are rarely surgical candidates (4), and traditional chemotherapy and radiation therapy approaches are generally unsuccessful in providing durable disease control (5–11). As such, new therapies, and probably combinations of therapies, are required for more effective treatment in such cases. Previous studies have demonstrated the effectiveness of SBRT for managing early-stage NSCLC (13–20). In this study, we present our early experience with SBRT for locoregional relapse or second cancers appearing after thoracic irradiation.
Consistent with previous reports of SBRT for early-stage NSCLC (15, 16), SBRT also provided a high rate in field local control in the group reported here. Interestingly, all three of the in-field failures occurred in the subset of patients undergoing reirradiation for in-field relapses. It is possible this reflects some form of radiation resistance, as has been described regarding reirradiation at other sites (21–23). It is also possible that disruption of the tissue architecture in the previously treated field made SBRT targeting difficult. However, because only one of these three patients received what previous studies have demonstrated to be a sufficient biologically effective dose to the PTV, it is also possible that our findings reflect SBRT underdosing in these two cases. Larger studies would be required to determine the relative contributions of these possible factors.
Despite the high rate of in-field control, the rate of intrathoracic relapse seen in this study was considerable, with an estimated 2-year relapse rate of 74%. This high rate of intrathoracic relapse is consistent with previous reports (6–8) and likely speaks to the underlying tumor biology in the patients being treated. The timing of these relapses suggests that these patients had harbored microscopic if not macroscopic disease at the time of SBRT. Consistent with this hypothesis was our finding of significantly better progression-free survival in the subset of patients who underwent SBRT for isolated recurrence (or second cancer) outside the previous treatment field. Taken together, these results suggest that it may be possible to identify patients for whom SBRT would be a good salvage therapy, and perhaps also patients who may benefit from SBRT in combination with systemic therapy.
The overall rates of SBRT-associated toxicity in this study were considerable and significantly higher than previously seen for patients with early SNCLC cancer treated with SBRT (15, 16). However, some of the worsening dyspnea, the most common side effect, may be part of the natural process of chronic obstructive pulmonary disease and radiation-induced pneumonitis from prior radiation therapy, as may also be the case for the chest wall pain. We found an interesting dichotomy in the toxicity profile: patients who were retreated for in-field relapses experienced higher rates of chest wall pain and lower rates of pneumonitis than did those given SBRT to targets outside the previous treatment field. Conversely, patients who were treated for lesions that lay outside the previous treatment field experienced higher rates of pneumonitis but lower rates of chest wall pain. The reason for this difference is not clear but may speak to differences in the biologic basis of these conditions. Radiation pneumonitis is believed to be an inflammatory process, and some have hypothesized that previously irradiated areas have already undergone fibrosis and are less susceptible to radiation-induced inflammation (7). The underlying biology of SBRT-associated chest wall pain is not well understood (24, 25) but probably represents a mix of conditions including nerve damage, fractures, and myositis. Prior radiation and high cumulative doses would likely predispose individuals to any of these conditions. However, this hypothesis is based on the results gathered from a small number of patients; it is possible that these biologic considerations may prove less important when the relationship between these side effects and the anatomic location, dose, and volume of the treatment is understood. Another limitation of our study is the relatively short follow-up period, which precludes assessment of very late toxicity. Additional information on this endpoint will require continued contact with this initial cohort of SBRT patients.
Comparisons between this study of SBRT and other retrospective studies of conventional reirradiation for lung tumors are difficult for several reasons. First, these studies involved heterogeneous groups of patients treated with both definitive and palliative intent who presented with both symptomatic and asymptomatic disease (6–11). Because all of the patients on our study were treated for radiographically identified disease and all were treated with definitive intent, our group may well represent a population with more favorable prognosis. Second, many of these studies seem to have only included lesions inside the prior radiation field (6–11), whereas our study included both in-field and out-of-field relapses. Nevertheless, the excellent in-field control rate in our cohort as a whole (92%) and the 75% control rate in the subset of patients undergoing reirradiation for in-field relapses compare favorably to the response rates for patients given definitive treatment in the other reports (6–8). As such, SBRT likely represents a substantial improvement over fractionated EBRT for those patients who would benefit from improved in field control.
The comparative cost of this high control rate in terms of toxicity is difficult to assess. Certainly, chest wall pain is a side affect of SBRT that is not associated with fractionated radiation therapy. With an incidence of 31%, this is a significant toxicity that must be considered when comparing these modalities for reirradiation. In contrast, both modalities are associated with a low rate of clinically significant esophagitis (<10%), and it is likely that the actual incidence of this side effect depends more on tumor location than the treatment modality used (6–8). How the choice of treatment modality affects the rate of symptomatic pneumonitis is less clear. The 50% incidence of symptomatic pneumonitis seen in this study with SBRT-based reirradiation is significant. However, the rates of symptomatic pneumonitis after definitive fractionated reirradiation have been reported from 8% to 55% (6–8). Because all these studies contain small numbers of patients with heterogeneous populations in terms of performance status and pretreatment lung function, a definitive comparison between these modalities is not possible.
CONCLUSIONS
We describe here the early outcomes and toxicity after SBRT for patients who had previously received EBRT for lung cancer. Although the overall failure rates and morbidity associated with the SBRT were significant, SBRT did provide durable in-field control and seemed to benefit a certain subset of patients. As such, for appropriately selected individuals, SBRT, used cautiously and with close follow-up, may be a valid treatment option for this challenging clinical presentation. On the basis of these results, we currently offer SBRT after previous EBRT to patients with a reasonable performance status (Karnofsky Performance Status >60) and who after multidisciplinary evaluation, it is believed would benefit from control of the targeted lesion. However, we believe that future prospective trials with greater numbers of patients and longer follow-up are required.
Acknowledgments
We thank the thoracic radiation oncology team for their help and support and the Department of Scientific Publications at M. D. Anderson for assistance in the preparation of this article.
Dr. Chang is a recipient of the Research Scholar Award from the Radiological Society of North America and the Development Award from The University of Texas M. D. Anderson Cancer Center National Institutes of Health Lung Cancer Specialized Programs of Research Excellence (P50 CA70907).
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol. 2007;25:4146–4152. doi: 10.1200/JCO.2007.12.6581. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 3.Jeremic B, Shibamoto Y, Acimovic L, et al. Second cancers occurring in patients with early stage non-small-cell lung cancer treated with chest radiation therapy alone. J Clin Oncol. 2001;19:1056–1063. doi: 10.1200/JCO.2001.19.4.1056. [DOI] [PubMed] [Google Scholar]
- 4.Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non–small cell lung cancer: Surgical and oncologic outcomes. Ann Thorac Surg. 2008;86:1632–1638. doi: 10.1016/j.athoracsur.2008.07.042. discussion 1638–1639. [DOI] [PubMed] [Google Scholar]
- 5.Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: A systematic review and practice guideline. J Thorac Oncol. 2006;1:1042–1058. [PubMed] [Google Scholar]
- 6.Wu KL, Jiang GL, Qian H, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: A prospective Phase I–II clinical trial. Int J Radiat Oncol Biol Phys. 2003;57:1345–1350. doi: 10.1016/s0360-3016(03)00768-5. [DOI] [PubMed] [Google Scholar]
- 7.Tada T, Fukuda H, Matsui K, et al. Non-small-cell lung cancer: Reirradiation for loco-regional relapse previously treated with radiation therapy. Int J Clin Oncol. 2005;10:247–250. doi: 10.1007/s10147-005-0501-1. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto Y, Murakami M, Yoden E, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2002;52:390–396. doi: 10.1016/s0360-3016(01)02644-x. [DOI] [PubMed] [Google Scholar]
- 9.Green N, Melbye RW. Lung cancer: Retreatment of local recurrence after definitive irradiation. Cancer. 1982;49:865–868. doi: 10.1002/1097-0142(19820301)49:5<865::aid-cncr2820490507>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Montebello JF, Aron BS, Manatunga AK, et al. The reirradiation of recurrent bronchogenic carcinoma with external beam irradiation. Am J Clin Oncol. 1993;16:482–488. doi: 10.1097/00000421-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kramer GW, Gans S, Ullmann E, et al. Hypofractionated external beam radiotherapy as retreatment for symptomatic non-small-cell lung carcinoma: An effective treatment? Int J Radiat Oncol Biol Phys. 2004;58:1388–1393. doi: 10.1016/j.ijrobp.2003.09.087. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 13.Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for Stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a Phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 15.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located Stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JY, Roth JA. Stereotactic body radiation therapy for Stage I non-small cell lung cancer. Thorac Surg Clin. 2007;17:251–259. doi: 10.1016/j.thorsurg.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 17.McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010–1015. doi: 10.1016/j.ijrobp.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 18.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a Phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–125. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 21.Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 22.Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys. 2009;73:399–409. doi: 10.1016/j.ijrobp.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144–1150. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 24.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 25.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.02.027. In press. [DOI] [PubMed] [Google Scholar]