Abstract
HIV-specific CD8+ T cells that secrete multiple cytokines in response to Ag stimulation are associated with the control of virus replication during chronic HIV infection. To determine whether the presence of polyfunctional CD8+ T cell responses distinguishes protected and unprotected monkeys in a live attenuated lentivirus model, SIV Gag peptide-specific CD8+ T cell responses of simian HIV (SHIV) 89.6-vaccinated, SIVmac239-challenged rhesus macaques were compared in two monkeys that controlled challenge virus replication and two that did not. The ratio of Bcl-2+ Gag-specific CD8+ T cells to caspase-3+ Gag-specific CD8+ T cells was higher in the vaccinated-protected animals compared with unprotected monkeys. In addition, polyfunctional SIV-specific CD8+ T cells were consistently detected through 12 wk postchallenge in the protected animals but not in the unprotected animals. In the unprotected monkeys, there was an increased frequency of CD8+ T cells expressing markers associated with effector memory T cells. Further, there was increased annexin V expression in central memory T cells of the unprotected animals before challenge. Thus, monkeys that control viral replication after live attenuated SHIV infection have polyfunctional SIV-specific CD8+ T cells with an increased survival potential. Importantly, the differences in the nature of the SIV-specific CD8+ T cell response in the protected and unprotected animals are present during acute stages postchallenge, before different antigenic levels are established. Thus, the polyfunctional capacity and increased survival potential of CD8+ SIV-specific T cells may account for live attenuated, SHIV89.6-mediated protection from uncontrolled SIV replication.
In the macaque model of AIDS, live attenuated viruses provide the most consistent protection against challenge with pathogenic SIV. Although this vaccine approach is not suitable for humans, understanding the protective responses that are elicited by attenuated lentivirus infection would be a major advance in HIV vaccine development. It is assumed that virus-specific CD8+ T lymphocytes play a central role in controlling HIV and SIV replication, because HIV/SIV-specific CD8+ T cell responses temporally correlate with the postpeak decline of viremia in acute infection (1–3), and depletion of CD8+ T cells in chronic SIV infection results in a rapid rise in plasma viral RNA (vRNA)3 levels (4, 5).
CD8+ T lymphocytes interact with virus-infected cells by recognizing viral peptides presented on the cell surface by MHC class I molecules. Once this cognate interaction occurs, there is induction of numerous genes involved in cell cycle, proliferation, apoptosis, and cytokine secretion. Recent studies have emphasized the qualitative aspects of the T cell response, and the preservation of “polyfunctional” HIV-specific CD8+ T cells that secrete multiple cytokines is associated with the control of viremia in infected people (6) and with protection from uncontrolled viral replication in vaccinated rhesus monkeys after simian HIV (SHIV) infection (7). Studies of HIV-infected, long-term nonprogressors found that Ag-specific production of cytokines such as IL-2 and TNF-α, in addition to IFN-γ, may be the most specific indicators of an effective anti-HIV CD8+ T cell response (6, 8, 9).
A major goal of AIDS vaccine research is to generate a durable and effective HIV-specific memory T cell response. Memory T lymphocytes have been divided into two distinct populations: central memory (CM) and effector memory (EM) T cells (10). CM T cells have limited effector function, but they have the capacity to home to lymphoid organs and, upon secondary exposure to their cognate Ag, rapidly proliferate to become effector T cells. In contrast, EM T lymphocytes home to peripheral tissues and, following antigenic stimulation, have limited proliferative capacity but possess the ability to degranulate and rapidly produce effector cytokines such as IFN-γ.
The B cell lymphoma/leukemia-2 (Bcl-2) protein is an important survival signal that protects cells from apoptosis. Moreover, although protective members of the Bcl-2 family are up-regulated in CM T cells, their levels are down-regulated in EM T cells, implying a role for these molecules in modulating the susceptibility of T cells to apoptosis (11). Caspase-3 is the cytoplasmic enzyme that initiates the final common metabolic pathway resulting in apoptosis. After TCR-mediated activation of T cells the caspase-3 levels increase, enhancing susceptibility to apoptosis in the EM T cell subset but not in the CM T cell subset (12). Recent studies have suggested that clinical disease progression in HIV-infected individuals may be associated with functional and phenotypic alterations of HIV/SIV-specific CD8+ T cells (8, 9, 13–17). This functional impairment includes not only abnormalities in the ability of effector cells to generate a cytotoxic response (8, 13), but defects in phenotypic maturation, cell cycle progression, and apoptosis are also involved (13–17). Increased apoptotic susceptibility in the EM subset of HIV-specific CD8 T cells, which lack the terminally differentiated phenotype observed in other persistent viral infections, has been described (17).
We have previously shown that the majority of rhesus macaques immunized with nonpathogenic SHIV89.6 and subsequently challenged with pathogenic SIVmac239 can control challenge virus replication (18). However, the failure of vaccine-induced immunity to control SIV replication in 40% of SHIV89.6 vaccinated animals offers the opportunity to study the effector mechanisms involved in vaccine efficacy. No comparison of the qualitative aspects of the SIV-specific CD8+ T cell response in immunized animals that can or cannot control the challenge virus has been previously reported. In the present study, we used polychromatic flow cytometry to determine the functional capabilities and survival capacity of CD8+ T cells upon Gag-specific stimulation in a small group of SHIV-immunized macaques that were challenged with SIVmac239. We found that vaccinated-protected animals maintained polyfunctional, SIV Gag-specific CD8+ T cells that had increased survival capacity throughout the first 12 wk postchallenge (PC). Also, the presence of an expanded CM T cell population was associated with a reciprocal decrease in the frequency of EM T cells in vaccine-protected animals. Thus, induction of an effector memory T cell pool more susceptible to apoptosis and less capable of polyfunctional cytokine production may explain why some SHIV-immunized animals are unable to control challenge virus replication.
Materials and Methods
Animals, vaccination, and challenge
Male rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (Davis, CA) in accordance with the American Association for Accreditation of Laboratory Animal Care standards. The experiments were approved by the Institutional Animal Use and Care Committee of the University of California (Davis, CA). All animals were negative for Abs to HIV-2, SIV, type D retrovirus, and simian T cell lymphotropic virus type 1 at the time the study was initiated. In SIV-infected animals at the California National Primate Research Center, AIDS is defined as weight loss of > 15% in 2 wk, opportunistic infections that do not respond to therapy, or persistent leucopenia (total leukocytes of <3000 per microliter of blood).
Four animals were selected from a larger live attenuated SHIV vaccine study (19). Approximately 1 year after i.v inoculation with virulence attenuated SHIV89.6 (18), the animals were challenged by i.v. inoculation with highly pathogenic SIVmac239. The pathogenic SIVmac239 stock used in the present study was produced in rhesus PBMC as previously described (20) and contained ~105 tissue culture infective dose per ml. The virus challenge consisted of a single i.v. inoculation with 1 ml of the SIVmac239 stock diluted 1/100 in sterile saline to produce an inoculum containing 103 tissue culture infective dose.
PBMC and lymph node mononuclear cells (LNMC) isolation
PBMC or LNMC were isolated using lymphocyte separation medium (ICN Biomedicals). PBMC and LNMC samples were frozen in 10% DMSO (Sigma-Aldrich) and 90% FBS (Gemini BioProducts) and stored in liquid nitrogen until future analysis in immunological and virological assays (20).
Plasma vRNA measurement
Plasma samples were analyzed for vRNA by a quantitative branched DNA assay (21). Virus load in plasma samples was reported as vRNA copy numbers per milliliter of plasma. The detection limit of this assay is 125 vRNA copies per milliliter of plasma.
MHC genotyping by PCR
MHC genotyping was performed by the Rhesus Macaque MHC Typing Core, University of Wisconsin Hospital and Clinics (Madison, WI).
Peptides
A 125-peptide set of 15-mer peptides overlapping by 11 amino acids corresponding to the sequence of SIVmac239 Gag was provided by the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (Bethesda, MD). The same peptides were used to prepare a pool containing the highly immunogenic region from the Gag protein p27. For fine mapping of CTL epitopes, 9- and 10-mer peptides were synthesized (Genemed Synthesis) corresponding to the SIVmac239 Gag regions of interest. All of the peptides were dissolved in DMSO (Sigma-Aldrich).
SIV-specific IFN-γ ELISPOT assay
As described previously (18, 22), the number of IFN-γ spot-forming cells (SFC) in PBMC responding to SIVmac239 Gag peptides was determined by using an IFN-γ ELISPOT kit (U-CyTech; Utrecht University, Utrecht, Netherlands). Both peptide pools and single peptides (either 15-, 9-, or 10-mers), were tested at a final concentration of 10 µg/ml. Additionally, a p27 Gag peptide pool was included in all of the samples tested. Negative controls consisted of cells that were cultured in the same concentration of DMSO used for peptide dilution (0.5–0.6%). A sample was considered positive if the number of IFN-γ SFC was greater than the mean of the matched medium-only wells ± 2 SD and if the frequency of IFN-γ secreting cells was ≥50 SFC/1 × 106 PBMC. In addition to stimulating each PBMC sample with PMA/ionomycin, PBMC from monkeys infected with SIVmac239 Δnef and known to have a strong anti-SIV p27-specific IFN-γ response were included as positive controls in every assay (range: 150–600 SFC/1 × 106 PBMC; mean: 420 SFC/1 × 106 PBMC). Further, PBMC samples from SIV-naive monkeys were also included as negative controls in every assay (mean: 0 SFC/1 × 106).
Epitope mapping
A matrix approach was used for peptide deconvolution as described elsewhere (23). Briefly, the SIV gag 15-mer peptides were combined in 23 pools designed in a way that each peptide was present in two different pools, and screened by IFN-γ ELISPOT assay. Peptides common to two pools eliciting responses were considered for further analysis. The optimal 9- to 10-mers were determined by analysis of truncated peptides. A minimum of five ELISPOT assays were performed to define at least one minimal peptide per animal. T cell responses against the specific peptides were confirmed by intracellular cytokine staining. The amino acid sequence of selected peptides was evaluated by two different CTL epitope-prediction programs, The Immune Epitope Database and Analysis Resource (available at http://www.immuneepitope.org/home.do) and Prediction of Peptide Binding to Macaque MHC Class-I Molecules (available at http://www.mamu.liai.org/index.html).
Intracellular staining
PBMC or LNMC were thawed and rested overnight at 37°C in 5% CO2 atmosphere in AIM V medium (Invitrogen Life Technologies) containing 20% FCS. The next day, cells were adjusted to 1 × 106 per milliliter in RPMI 1640 with 10% FCS containing anti-CD28 and anti-CD49d Abs (1 µg/ml final concentration; BD Biosciences) as costimulatory molecules. In all experiments, the following samples were prepared: Gag specific 9-to 10-mer immunodominant peptides at 0.1 and 1 µg/ml; background controls containing costimulatory molecules and DMSO; a negative control with medium only; and a positive control stimulated with staphylococcal enterotoxin B (0.2 µg/ml; Sigma-Aldrich). Cells were divided further into one of two panels (see below) and in the second one a mixture of anti-CD107a and anti-CD107b-FITC (clones H4A3 and H4B4 respectively; BD Pharmingen) was added to the cells at a pretitrated volume. Cells were incubated for 5 h at 37°C in the presence of brefeldin A (Sigma-Aldrich) and monensin (GolgiStop; BD Biosciences). Following incubation, cells were washed and surface stained for CD3, CD4, and CD8 with different combinations of Ab depending on the panel: 1) anti-CD3-Pacific Blue, anti- CD4-allophycocyanin, and anti-CD8-allophycocyanin-Cy7; or 2) anti-CD3-Pacific Blue, anti-CD4-PerCP-Cy5.5, and anti-CD8-allophycocyanin-Cy7. 7-Aminoactinomycin D (7-AAD; 1/10 dilution; Molecular Probes) was added before permeabilization as a dead cell marker. Samples were fixed (1% para-formaldehyde), permeabilized (0.5% saponin), and then stained intracellularly with the following: Bcl-2-FITC clone Bcl-2/100 and caspase-3-PE clone C92-605 (both from BD-Pharmingen) for 1 h at 4°C (panel 1); or IFN-γ-allophycocyanin clone B27, TNF-α-PE-Cy7 clone Mab11, and IL-2-PE clone MQ1–17H12 (all from BD Biosciences) for 20 min at room temperature (panel 2). After washing with permeabilizing buffer, cells were fixed in PBS containing 1% paraformaldehyde. Data were acquired using a FACSAria flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star) in a Macintosh G5 computer (Apple). Approximately 250,000 events in the lymphocyte gate were acquired. The background level of cytokine staining varied from sample to sample but was typically <0.05% of the CD8+ T lymphocytes. The only samples considered positive were those in which, after subtracting the DMSO background control, the frequency was >0.05% for a single marker and 0.02% for two or more simultaneous markers. All data are reported after subtraction of the DMSO medium control cultures. The SPICEv4.1.5 and PESTLEv1.5.2 software programs (a gift from M. Roederer, Vaccine Research Center, NIAID/National Institutes of Health) were used to create the pie charts that represent each individual response.
Immunophenotypic staining to identify T cell subsets
Two different strategies were used for lymphocyte phenotype staining, depending on the sample type and the aim of the analysis. All mAbs were from BD Biosciences unless specified. Data was acquired using a FACSAria flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star) in a Macintosh G5 computer (Apple). For samples collected before SIV challenge, cryopreserved PBMC were thawed and rested overnight in AIM V medium (Invitrogen Life Technologies) with 20% FCS. Then, 1 × 106 of PBMC were suspended in a 200-µl volume of complete RPMI 1640 and stained using a mixture containing pretitrated amounts of the following mAbs: anti-CD3-Cy7 allophycocyanin, anti-CD4-PerCP-Cy5.5, anti-CD8-Alexa Fluor 700 (clone RPA-T8), anti-CD28-PE (clone L293), anti-CD45RA-Cy7PE (clone L48), and anti-CD95-FITC (clone DX2). Cells were washed with FACS buffer and stained with 2 µl of 7-AAD and 5 µl of Pacific Blue annexin V in 250 µl of annexin binding buffer by using the Vybrant apoptosis assay kit (Molecular Probes). Samples were incubated for 30 min at room temperature followed by a wash with annexin binding buffer and fixation in 1% paraformaldehyde. A minimum of 200,000 small lymphocyte events was collected per sample. Samples were gated on the live (7-AAD) CD3+ population for analysis. CD8+ T cell phenotypes are defined as naive (CD45RA+CD28+), central memory (CD45RA−CD28+), effector memory (CD45RA−CCD28−), or terminally differentiated effector memory (C45RA+CD28−). For CD4+ T cells, phenotypes are defined as naive (CD95−CD28+), central memory (CD95+CD28+), and effector memory (CD95+CD28−).
For samples collected after SIV challenge, freshly isolated PBMC and LNMC were stained with anti-CD3-Cy55 PerCp, anti-CD8-Alexa Fluor 700, anti-CD28-PE, and anti-CCR7-Cy7 PE (clone 3D12) and fixed with 1% paraformaldehyde. At least 100,000 small lymphocyte events were collected from each tube analyzed. Samples were gated on the CD3+ CD8+ T cell population and phenotypes in this case are defined as naive and central memory (CCR7+CD28+), intermediate memory (CCR7−CCD28+), or effector memory (CCR7−CD28+).
Results
Outcome of SIVmac239 challenge
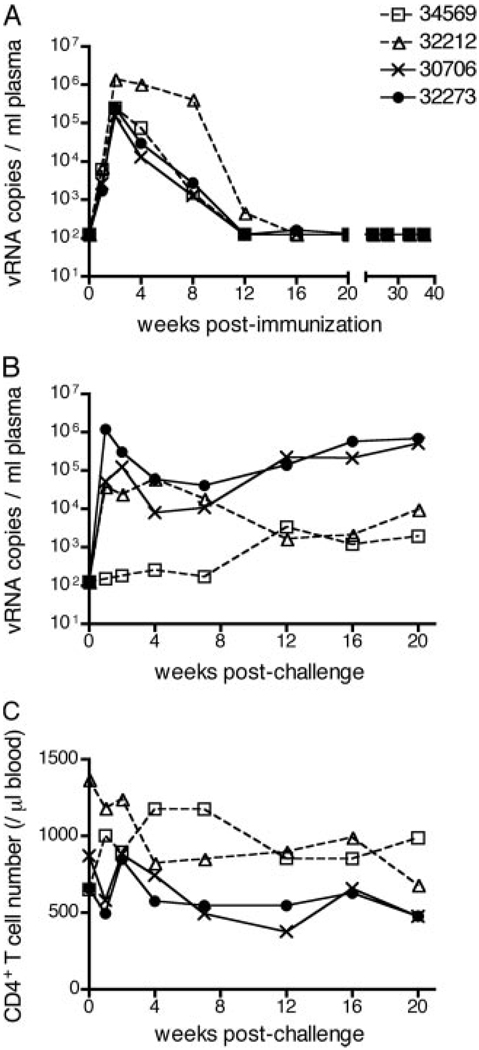
All four animals in the study were infected (immunized) with attenuated SHIV89.6 for ~1 year before i.v. challenge with SIVmac239. Overall, the SHIV89.6 infection followed a similar pattern in all four animals, reaching a peak of plasma vRNA levels of ~105 to 106 vRNA copies/ml 2 wk after inoculation that decreased to undetectable levels between 12 and 21 wk (Fig. 1A). Eventually, all the animals had undetectable levels of vRNA (wk 21–40).
FIGURE 1.
Plasma SIV RNA levels and peripheral CD4+ T cell levels in SHIV-immunized male rhesus macaques before and after i.v. SIV challenge. A, vRNA levels in plasma during the immunization period. B, vRNA levels in plasma after SIVmac239 challenge. C, Absolute number of CD4+ T cells in peripheral blood (cells per microliter) after SIVmac239 challenge. Note that the solid symbols denote “unprotected” animals that did not control SIV replication and the open symbols denote “protected” animals that did control SIV replication. The limit of detection of vRNA was 102 copies/ml (see Materials and Methods).
After SIVmac239 challenge, there were two clearly distinguishable patterns of plasma viremia and CD4 decline over the 20-wk PC observation period (Fig. 1B). The vaccinated-protected monkeys (34569 and 32212) partially controlled SIV replication (vRNA: <104 copies/ml plasma from 12 to 20 wk PC) and maintained relatively stable CD4+ T cell counts (Fig. 1C). In contrast, the vaccinated-unprotected monkeys (32273 and 30706) poorly controlled SIV replication, had relatively high levels of viremia after 7 wk PC (vRNA: > 104 copies/ml plasma), and a steady decline of CD4+ T cell counts (Fig. 1, B and C).
High levels of differentiated effector phenotypes in CD8+ T cells are associated with poor control of SIV replication
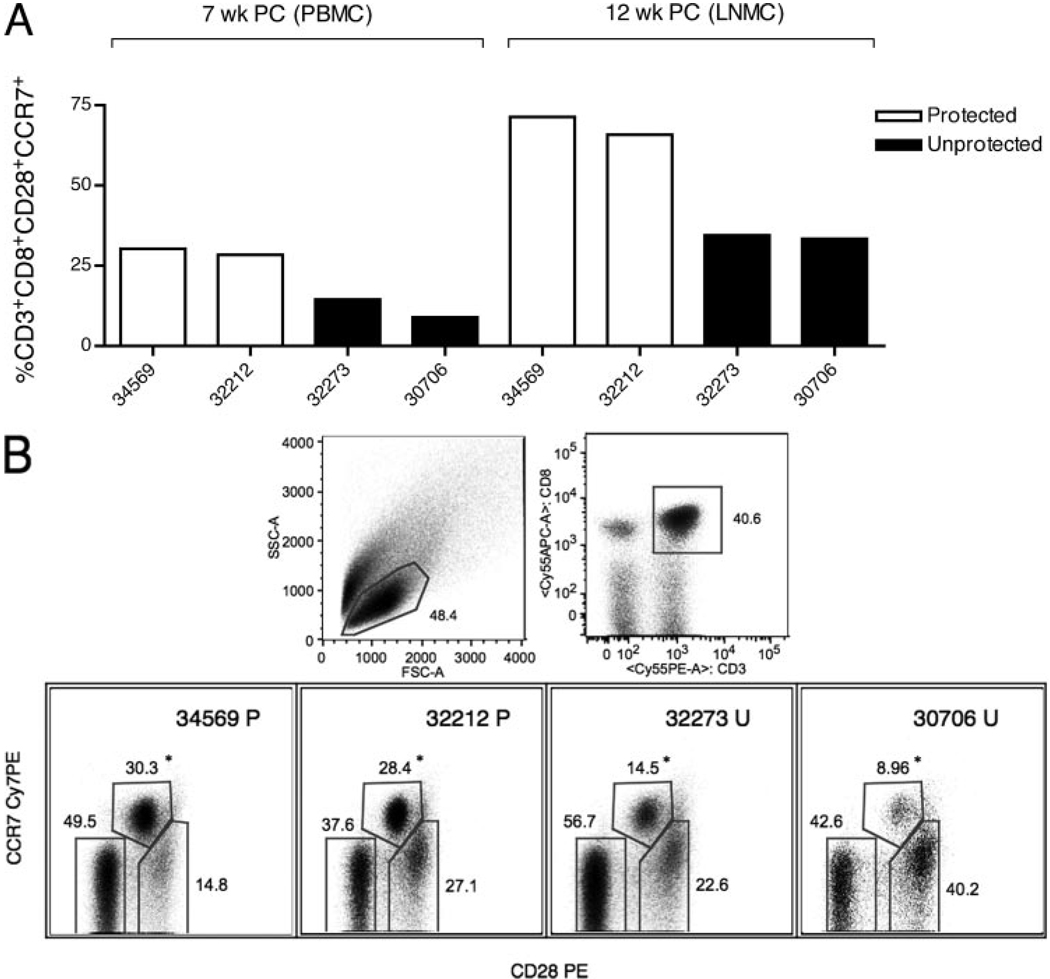
In Table I, a summary of the frequency of the CD3+CD8+ T phenotypic populations in PBMC for all animals at all time points is shown. There were striking differences in CD28 and CCR7 expression on the CD8+ T cells of protected and unprotected monkeys. The CD8+CD28+CCR7+ T cell population, which contains naive and central memory CD8+ T cells, was twice as frequent in PBMC of the protected animals at 7 wk PC in PBMC (29.3 ± 0.9 vs 11.7 ± 2.7) and in axillary LNMC at 12 wk PC (68.6 ± 2.8 vs 33.9 ± 0.55) compared with unprotected animals (Fig. 2A). Gating strategy for the distinct phenotypes within the CD3+CD8+ T cell population in PBMC at 7 wk PC is shown in Fig. 2B.
Table I.
Frequency of naive and memory CD3+CD8+ T cells in PBMC of SHIV-immunized rhesus macaques after i.v. pathogenic SIVmac239 challenge
| Animal | Weeks PC | N Plus CM (%)a | IM (%)b | EM (%)c |
|---|---|---|---|---|
| 34569 Pd | 4 | 19.8 | 23.1 | 54.0 |
| 7 | 30.3 | 14.8 | 49.5 | |
| 12 | 37.9 | 14.2 | 47.1 | |
| 32212 Pd | 4 | 23.9 | 23.9 | 49.3 |
| 7 | 28.4 | 27.1 | 37.6 | |
| 12 | 36.3 | 14.5 | 44.2 | |
| 32273 Ue | 4 | 23.6 | 32.6 | 43.0 |
| 7 | 14.5 | 22.6 | 56.7 | |
| 12 | 33.5 | 26.2 | 39.5 | |
| 30706 Ue | 4 | 22.7 | 38.6 | 37.8 |
| 7 | 8.96 | 40.2 | 42.6 | |
| 12 | 19.5 | 35.9 | 43.1 |
Naive (N) and CM CD3+CD8+ T cells (percentage of CD28+CCR7+).
Intermediate memory (IM) CD3+CD8+ T cells (percentage of CD28+CCR7−).
EM CD3+CD8+ T cells (percentage of CD28+CCR7−).
Protected.
Unprotected.
FIGURE 2.
Gating strategy and frequency of the naive and central memory phenotypes (defined as CD28+ CCR7+) in the CD3+CD8+ T cell population. A, CD28+CCR7+ frequencies at 7 wk PC in PBMC and at 12 wk PC in axillary LNMC. B, Gating strategy for characterizing expression of CD28 and CCR7 molecules at 7 wk PC in CD3+CD8+ T cells; the values shown in A for 7 wk PC correspond to the CCR7+CD28+ cell population that is highlighted by an asterisk in the bottom row of B. P, Protected; U, unprotected; FSC, forward scatter; SSC, side scatter.
Increased susceptibility to apoptosis in central memory T cells before SIV challenge is associated with uncontrolled viral replication
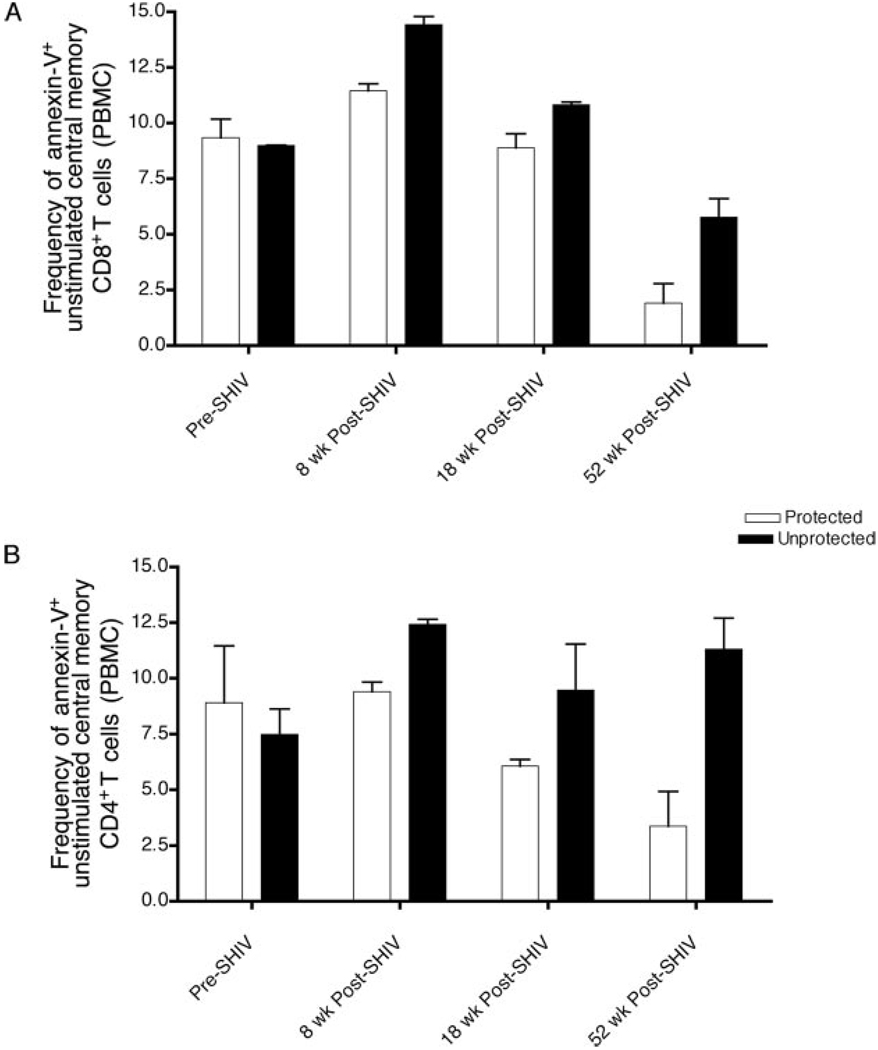
In PBMC collected before challenge (at wk 8, 18, and 52 postimmunization), the frequency of apoptotic T cells (annexin V+/7-AAD−) was higher in the unprotected animals. The increased levels of apoptosis extended to both CD4+ and CD8+ T cells and, overall, the same trend was observed in all of the different phenotypes. As shown in Fig. 3, central memory T cells most consistently expressed increased levels of apoptosis in both the CD4+ and CD8+ T cell subsets at all time points, although the baseline levels before immunization were the same for the two groups. Therefore, although no differences were observed regarding the phenotypic distribution of the T cell populations during the immunization period, the apoptotic potential of these phenotypes, particularly that of the central memory subset, was already different before challenge in the protected and unprotected animals.
FIGURE 3.
Apoptotic susceptibility of central memory T cells in unstimulated PBMC before (Pre-SHIV) and after SHIV89.6 infection (Post-SHIV) (before pathogenic SIV challenge). A, The frequency of apoptotic T cells (annexin V+/7-AAD−) in the CM CD8+ T cell subset, defined as CD45RA− and CD28+. B, The frequency of apoptotic T cells in the CM CD4+ T cell subset, defined as CD95+ and CD28+.
Control of SIV replication was associated with the induction and maintenance of Gag-specific, polyfunctional CD8 + T cells
The following alleles were present in the indicated study animal: 34569 (no known Mamu MHC class I alleles); 32212 (Mamu-A*01); 32273 (Mamu-A*02); and 30706 (Mamu-A*08 and Mamu-B*17/29). To define at least one optimal 9- to 10 mer peptide for each animal, PBMC from at least five time points, beginning at 7 wk PC, were tested in IFN-γ ELISPOT assays using the matrix of SIV Gag p55 peptide pools (23). Flow cytometry was used to confirm that the IFN-γ response to the optimal peptide was mediated by CD8+ T cells. The specific sequence for the immunodominant peptide in each animal was as follows: 34569, WKCGKMDHVM (Gag 415–424); 32212, CTPYDINQM (Gag 181–189); 32273, CVRMYNPTNI (Gag 274–283); 30706 LLIQNANPD (Gag 321–329).
A flow cytometric assay was used to measure multiple CD8+ T functions, i.e., CD107ab mobilization (degranulation) and IFN-γ, TNF-α, and IL-2 production in response to specific peptide stimulation. Fig. 4 shows the maximum response to SIV Gag peptide stimulation (0.1 or 1 µg/ml peptide) at wk 4, 7, and 12 PC. Note that at 4 wk PC (Fig. 4A) only three of the animals were analyzed due to sample availability; nevertheless, differences between protected and unprotected animals were already apparent. The one unprotected animal studied had a low frequency of monofunctional (IFN-γ) SIV Gag-specific CD8+ T cells in response to SIV peptide stimulation; however, both of the protected animals had relatively high frequencies of monofunctional SIV Gag-specific CD8+ T cells capable of degranulating or secreting IL-2 or IFN-γ alone. Moreover, in PBMC from the protected animals there was a small fraction of polyfunctional CD8+ T cells, positive for CD107ab and IL-2 simultaneously.
FIGURE 4.
Frequency and distribution of polyfunctional SIV Gag-specific CD8+ T cells after SIV challenge. The maximum response to SIV gag peptide stimulation (0.1 or 1 µg/ml peptide) is shown as a frequency of CD8+ T cells calculated by subtracting the frequency of positive cells in the DMSO control wells from the frequency of positive cells in the peptide-stimulated wells. A–C, Four (A), 7 (B), and 12 wk (C) PC. The scatter graphs on the left show the frequency of CD8+ T cells that were positive for any combination of the four functions. The pie charts summarize the same data in the context of the overall SIV-specific CD8+ T cell response and the extent to which the CD8+ T cell response was polyfunctional. Each portion of a pie chart indicates the percentage of SIV-specific T cells that responded with one, two, three, or four functions, and the arcs around the pie show the function or combination of functions to which the specific response corresponds (see color legend). ID, Identifier.
By 7 wk PC (Fig. 4B), the differences between the SIV-specific T cell responses of protected and unprotected animals were much more pronounced despite the fact that the plasma vRNA levels at 7 wk PC were similar in all animals. In the unprotected animals monofunctional SIV Gag-specific CD8+ T cells predominated, whereas in the PBMC of protected animals there were high frequencies of polyfunctional CD8+ T cells (64 and 38% in the protected monkeys vs 3 and 22% in the unprotected monkeys). Finally, at 12 wk PC (Fig. 4C) there was no apparent difference between the groups, and all animals had CD8+ T cells capable of displaying multiple functions in response to specific peptide stimulation. The strength and nature of the SIV-specific CD8+ T cell response was highly variable from animal to animal depending on the time point analyzed. However, the protected animals consistently had polyfunctional CD8+ T cells throughput the SIV challenge period while the unprotected animals failed to mount a poly-functional T cell response on at least one time point tested. A detailed summary of the SIV Gag-specific CD8+ T cells responses of each animal is shown in Table II.
Table II.
Summary of SIV Gag peptide specific CD8+ T cell responses (frequency of the responding cells) after i.v. SIVmac239 challenge
| Functional Profilea | Apoptotic Signalsa | ||||||
|---|---|---|---|---|---|---|---|
| Animal | Weeks PC | IL-2 (%) | TNF-α (%) | IFN-γ (%) | CD107ab (%) | Bcl-2 (%) | Caspase-3 (%) |
| 34569 Pb | 4 | 0.14 | —c | 0.23 | 0.13 | 1.1 | −0.9d |
| 7 | 0.27 | 0.79 | 0.48 | 0.05 | 3.7 | 0.25 | |
| 12 | —b | 0.23 | 0.3 | —c | 2.5 | 3.1 | |
| 32212 P | 4 | 0.17 | —b | 0.08 | 0.4 | 4.1 | −0.5d |
| 7 | 0.12 | 0.40 | 0.43 | 1.11 | 5.1 | −1.54d | |
| 12 | 0.4 | 0.37 | 0.3 | 1.87 | 3.8 | 1.5 | |
| 32273 Ue | 4 | —c | —c | 0.08 | —c | 0.4 | 6.9 |
| 7 | —c | —c | —c | 1.02 | 2.0 | 2.0 | |
| 12 | 0.4 | 1.73 | 1.67 | 0.81 | −1.4d | 16.7 | |
| 30706 Ue | 4 | ND | ND | ND | ND | ND | ND |
| 7 | 0.29 | 0.17 | 0.20 | 1.25 | 2.1 | 0.1 | |
| 12 | —c | —c | 0.06 | —c | 1.5 | 4.1 | |
Data show the maximum value at 0.1 or 1 µg/ml peptide.
Protected.
Values less than the cutoff (<0.05%).
Negative net change (value in the DMSO control was higher than in the peptide stimulated sample).
Unprotected.
Control of SIV replication is associated with induction of Bcl-2 in Gag-specific CD8+ T cells
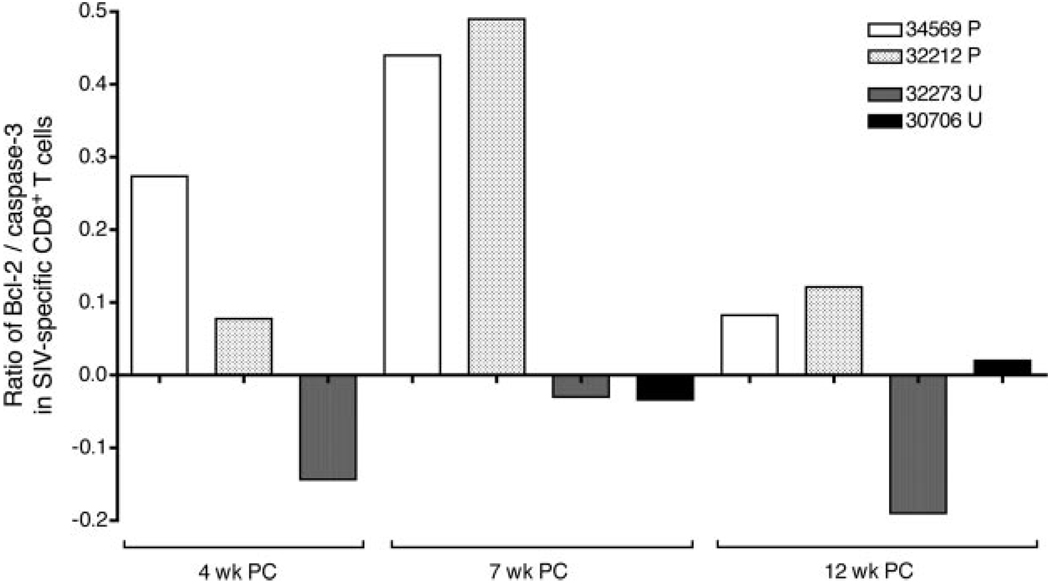
The ratio of Bcl-2+ Gag peptide-specific CD8+ T cells to caspase-3+ Gag peptide-specific CD8+ T cells was determined at several time points (Fig. 5). At all of the time points tested, upon SIV Gag peptide stimulation there was strong induction of Bcl-2 expression in the CD8+ T cells of protected, but not unprotected, animals. In contrast, Gag peptide stimulation induced cleaved caspase-3 expression in the CD8+ T cells of unprotected animals (Table II). In fact, in protected animals the Bcl-2+ to caspase-3+ ratio among SIV Gag-specific CD8+ T cells was always positive (Fig. 5), and this result was obtained at either of the peptide concentrations (0.1 and 1 µg/ml) tested. Thus, optimal SIV Gag peptides stimulate survival signals in the SIV-specific CD8+ T cells of protected monkeys but apoptosis in the SIV-specific CD8+ T cells of unprotected animals. In fact, there was induction of Bcl-2 in the SIV-specific CD8+ T cells of animals after optimal peptide stimulation as early as 1 wk PC (data not shown).
FIGURE 5.
Ratio of Bcl-2+ SIV Gag-specific CD8+ T cell frequency to caspase-3+ SIV Gag-specific CD8+ T cell frequency at 4, 7, and 12 wk postchallenge. Values shown are after stimulation with 0.1 µg/ml peptide, and the frequencies were calculated after subtraction of the corresponding DMSO control. P, Protected; U, unprotected.
An early SIV-specific CD8 + T cell response in lymph nodes is associated with later immune exhaustion
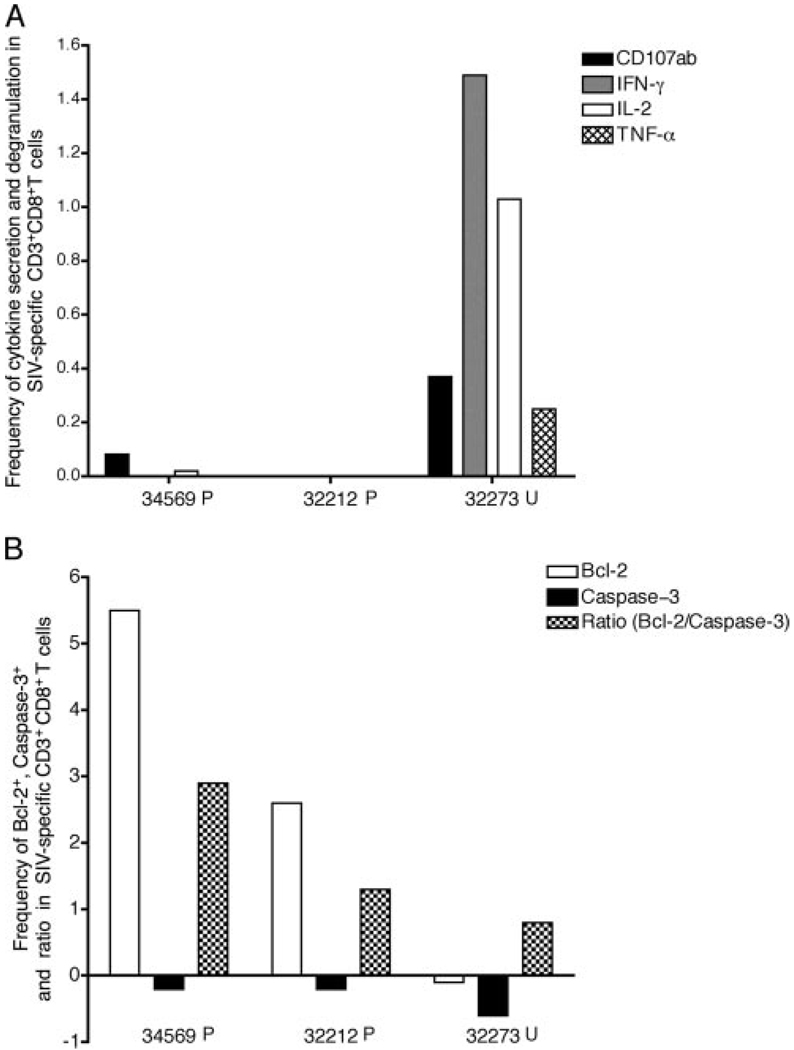
Because viral replication occurs mainly in lymphoid tissues, we assessed the Gag-specific CD8+ T cells isolated from the axillary lymph nodes at wk 2 PC (Fig. 6). There were low frequency poly-functional SIV-specific CD8 + T cells in lymph nodes samples of three of the four monkeys, and the unprotected animal (32273) had the highest frequency of SIV-specific CD8+ T cells secreting IFN-γ or IL-2 alone (0.77 and 0.35%, respectively) or in combination (0.25%). In addition, the lowest ratio of Bcl-2+ to caspase-3+ T cells was found in the SIV-specific CD8+ T cells from the axillary LNMC of this unprotected animal (Fig. 6B). Thus, specific stimulation with the immunodominant peptide was unable to induce Bcl-2 expression in CD8+ T cells from the lymph nodes of the unprotected animal 32273, whereas there was a marked increase in this survival signal upon specific peptide stimulation in the protected monkeys’ specific CD8+ T cells.
FIGURE 6.
Functional profile and apoptotic potential of SIV Gag-specific CD8+ T cells in the axillary lymph node 2 wk after SIV challenge. CD8+ T cells after stimulation with 0.1 µg/ml peptide are shown. The frequency was calculated by subtracting the DMSO control from the peptide-stimulated samples. A, Frequency of SIV-specific cytokine-positive and degranulation-positive (CD107ab marker) CD8+ T cells. B, Frequency and ratio of Bcl-2+ SIV Gag-specific CD8+ T cells and caspase-3+ SIV-specific CD8+ T cells.
The Gag-specific CD8+ T cell response before challenge does not predict viral load PC
In an attempt to determine, whether the differences in SIV-specific CD8+ T cell responses observed PC were established before challenge, we analyzed the SIV Gag-specific CD8+ T cell response at 8 and 18 wk postimmunization in the four study animals. At 8 wk postimmunization only two of the four animals, one protected (32212) and the other unprotected (32273), had detectable Gag-specific CD8+ T cells and the responses were all monofunctional (data not shown). At 18 wk postimmunization however, only IFN-γ monofunctional cells were detected in three of the four animals. The frequencies were 0.31 and 0.83% of the CD3 CD8+ T cells for the protected animals 34569 and 32212, respectively, and substantially lower (0.032%) for one of the two unprotected animals (30706; data not shown). At 8 wk postimmunization there was no induction of Bcl-2 in the SIV Gag-specific CD8+ T cells of any of the animals, and only one animal (30706) studied had moderate levels of caspase-3+ SIV Gag specific CD8+ T cells (data not shown).
Discussion
CD8+ T lymphocytes contribute to the limited viral containment that occurs in HIV/SIV infection (24, 25), but the role of CD8+ T cell responses in protection elicited by attenuated lentivirus infection is unclear (26, 27). We now report that, following i.v. SIV challenge 1 year after attenuated SHIV89.6 immunization, two distinct patterns of SIV Gag-specific CD8+ T cell responses were detected in protected and unprotected individuals. Although the number of animals in this study was small, the two SHIV-immunized monkeys that did not control challenge virus replication were consistently unable to mount a polyfunctional SIV Gag-specific CD8+ T cell response after SIV challenge. Further, the ratio of Bcl-2+ to caspase-3+ effector SIV Gag-specific CD8+ T cells was low (<0.1) in the unprotected animals. In contrast, the SHIV-immunized animals that controlled SIV challenge had persistent polyfunctional CD8+ T cell responses and the frequency of Bcl-2+ CD8+ T cells was high, which kept the Bcl-2+ to caspase-3+ CD8+ T cell ratio at >0.1 in the protected animals. Importantly, Ag load did not explain these differences in CD8+ T cell responses, as the differences in survival and functionality were apparent before the differences in viral replication were detectable. Thus, fragile SIV-specific CD8+ T cell responses, unsupported by a large CM pool and long-lived effector cells, may at least partly explain why some animals fail to contain replication of SIV despite their previous infection with attenuated SHIV.
The ability of virus-specific T cells to secrete IFN-γ and maintain IL-2 and TNF-α secretion is associated with control of viral replication during chronic HIV infection (6). IL-2 is the principal cytokine involved in promoting T cell proliferation and clonal expansion of Ag-specific cells and is required for the differentiation and survival of effector T cells (28). Moreover, HIV-1-specific CD8+ T cells may support their own proliferation, independently of CD4+ T cells, only if they are able to secrete IL-2 (29). Cells producing both IL-2 and TNF-α were more frequent and persistent in the protected animals compared with the unprotected animals. In fact, only protected animals had SIV-specific polyfunctional CD8+ T cells with IL-2 secretion at all of the time points tested. In the unprotected animals, the predominant response of SIV-specific CD8+ T cells was degranulation and IFN-γ secretion. In HIV-infected individuals CD8+ T cells capable of degranulating without concurrent cytokine production are observed (30), and IFN-γ secretion is the last antiviral response that is lost in an exhausted HIV-specific CD8+ T cell response (9). In the current study, the unprotected, SHIV-immunized, animals had easily detectable SIV-specific CD8+ T cell responses, but they had limited functions. These findings emphasize the potential for assays measuring only IFN-γ production to mischaracterize the potential effectiveness of a candidate HIV vaccine in preclinical and clinical testing.
The increased susceptibility of both specific and bystander CD4+ and CD8+ T lymphocytes to apoptotic cell death is a major contributor to the pathogenesis of AIDS (31, 32). In addition, it has been reported that HIV-specific CD8+ T cells have reduced levels of Bcl-2 protective proteins, which increases their sensitivity to apoptosis after antigenic stimulation (33). We found that stimulation of T cells with the optimal Gag peptides induced marked differences in expression of Bcl-2 and caspase-3 proteins in circulating lymphocytes of vaccinated-protected and vaccinated-unprotected animals. Although the protected animals had marked SIV-specific induction of Bcl-2 in CD8+ T cells in the acute through chronic phases of the challenge virus infection, the CD8+ T cells from unprotected animals did not increase expression of this protective molecule but expressed higher levels of cleaved caspase-3, a mediator of apoptosis. Proapoptotic proteins encoded by the viral genome and the hyperactivation induced by high levels of viremia (31, 32) are key factors driving apoptosis in lentiviral infections. In this regard, the levels of T cell activation, based on surface CD38 and HLA-DR expression were similar in all four monkeys (data not shown). It is also important to note that the differences in the apoptotic potential of the CD8+ T cells in the protected and unprotected monkeys were observed during the acute phase of the infection, before different antigenic levels were established. Hence, although both activation and virus-induced apoptosis may exacerbate T cell death, other host factors may be more important in the setting of an attenuated lentiviral infection.
The high frequency of annexin V+ cells in the CD8+ CM T cell population in the unprotected animals before challenge indicated that increased susceptibility to apoptotic cell death develops during the immunization phase. In the lymphocytic choriomeningitis virus murine model, many virus-specific CD8+ T cells in lymphoid organs are annexin V+, excluding them from the development of functional memory (34). The mechanism leading to the differential susceptibility of T cells to apoptosis is unknown. A plausible explanation is that changes in the cytokine milieu or differential TCR engagement avidity (33–35) occurring at the time that long-lived memory T cell pools are created alters susceptibility to apoptosis. It is also possible that concurrent immune activation and inflammatory responses during the induction of antiviral immune responses play a role in determining the relative susceptibility of Ag-specific T cells to apoptotic stimuli. The relative levels of proapoptotic factors and how they might affect the entire CD8+ T cell subset or a specific CTL population remains to be elucidated. Nevertheless, the fact that differences in apoptotic potential exist before challenge but not before the SHIV89.6 infection suggests: 1) that these differences are not an intrinsic idiosyncrasy of the individual’s lymphocyte survival capacity; and 2) that they are not related to levels of Ag exposure PC. Other host factors in the setting of a live attenuated lentiviral infection may also affect CM T cell survival. In any case, we speculate that the increased apoptosis observed in CM T cells before challenge in unprotected animals is directly related to the inability to maintain a long-lived, polyfunctional CD8+ T cell pool.
In summary, the present study shows for the first time that control of SIV challenge virus replication in animals immunized with a lentiviral vaccine is associated with polyfunctional, SIV-specific CD8+ T cells with a high ratio of Bcl-2+ T cells to caspase-3+ T cells. Further, before SIV challenge a higher frequency of pro-apoptotic T cells was found in animals that would not control challenge virus replication. In the protected monkeys the increased survival capacity of SIV-specific CD8+ effector T cells likely protects CD4+ Th cells from infection; these CD4+ T cells in turn help maintain polyfunctional antiviral CD8+ T cells. In contrast, the large proportion of effector memory T cells susceptible to apoptosis and less capable of sustained polyfunctional cytokine production may account for live attenuated vaccine failure in the unprotected monkeys. These results suggest that the quality of antiviral CD8+ T cell responses are critical in providing protective immunity in attenuated lentiviral vaccine systems. Although further studies with more animals are needed to confirm these results, eliciting similar T cell responses through other vaccine modalities should be a goal in HIV vaccine candidates.
Acknowledgments
We thank the Immunology Core Laboratory and Primate Services Unit at the California National Primate Research Center and Ding Lu, Lili Guo, and Roxana Colon for excellent technical assistance. We also thank Mario Roederer, National Institutes of Health Vaccine Research Center, for providing the SPICE and PESTLE software.
Footnotes
This work was supported by Public Health Services Grants RR00169 from the National Center for Research Resources and P01 AI066314 and R01 AI44480 from the National Institute of Allergy and Infectious Diseases.
Abbreviations used in this paper: vRNA, viral RNA; 7-AAD, 7-aminoactinomycin D; Bcl-2, B-cell lymphoma/leukemia-2; CM, central memory; EM, effector memory; LNMC, lymph node mononuclear cell; PC, postchallenge; SFC, spot-forming cell; SHIV, simian human immunodeficiency virus.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lifton MA, Lord CI, Forman MA, Letvin NL. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 4.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 5.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acierno PM, Schmitz JE, Gorgone DA, Sun Y, Santra S, Seaman MS, Newberg MH, Mascola JR, Nabel GJ, Panicali D, Letvin NL. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 2006;176:5338–5345. doi: 10.4049/jimmunol.176.9.5338. [DOI] [PubMed] [Google Scholar]
- 8.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 9.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 11.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in Ag-specific CD8+ T cells during viral infection. J. Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 12.Sabbagh L, Kaech SM, Bourbonniere M, Woo M, Cohen LY, Haddad EK, Labrecque N, Ahmed R, Sekaly RP. The selective increase in caspase-3 expression in effector but not memory T cells allows susceptibility to apoptosis. J. Immunol. 2004;173:5425–5433. doi: 10.4049/jimmunol.173.9.5425. [DOI] [PubMed] [Google Scholar]
- 13.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and Ag-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 16.Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar P, Mounzer KC, Altman JD, Katsikis PD. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8+ T cells. Blood. 2007;109:2505–2513. doi: 10.1182/blood-2006-05-021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- 18.Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and α interferon responses. J. Virol. 2003;77:3099–3118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genescà M, Li J, Fritts L, Chohan P, Bost K, Rourke T, Blozis SA, McChesney MB, Miller CJ. Depo-provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239. J. Med. Primatol. 2007;36:266–275. doi: 10.1111/j.1600-0684.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CJ, McChesney MB, Lu X, Dailey PJ, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dailey PJ, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 1995;24:209. [Google Scholar]
- 22.Pahar BJ, Li J, Rourke T, Miller CJ, McChesney MB. Detection of Ag-specific T cell interferon γ expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J. Immunol. Methods. 2003;282:103–115. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmeister B, Kiecker F, Tesfa L, Volk HD, Picker LJ, Kern F. Mapping T cell epitopes by flow cytometry. Methods. 2003;29:270–281. doi: 10.1016/s1046-2023(02)00349-3. [DOI] [PubMed] [Google Scholar]
- 24.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 25.Barouch DH, Powers J, Truitt DM, Kishko MG, Arthur JC, Peyerl FW, Kuroda MJ, Gorgone DA, Lifton MA, Lord CI, et al. Dynamic immune responses maintain cytotoxic T lymphocyte epitope mutations in transmitted simian immunodeficiency virus variants. Nat. Immunol. 2005;6:247–252. doi: 10.1038/ni1167. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239δ3-vaccinated rhesus macaques. J. Virol. 2005;79:8131–8141. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stebbings R, Stott J, Almond N, Hull R, Lines J, Silvera P, Sangster R, Corcoran T, Rose J, Cobbold S, et al. Mechanisms of protection induced by attenuated simian immunodeficiency virus, II: lymphocyte depletion does not abrogate protection. AIDS Res. Hum. Retroviruses. 1999;14:1187–1198. doi: 10.1089/aid.1998.14.1187. [DOI] [PubMed] [Google Scholar]
- 28.Nacsa J, Edghill-Smith Y, Tsai WP, Venzon D, Tryniszewska E, Hryniewicz A, Moniuszko M, Kinter A, Smith KA, Franchini G. Contrasting effects of low-dose IL-2 on vaccine-boosted simian immunodeficiency virus SIV-specific CD4+ and CD8+ T cells in macaques chronically infected with SIVmac251. J. Immunol. 2005;174:1913–1921. doi: 10.4049/jimmunol.174.4.1913. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-γIL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA. 2005;102:7239–7244. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 31.Gougeon ML. To kill or be killed: how HIV exhausts the immune system. Cell Death Differ. 2005;12 (Suppl. 1):845–854. doi: 10.1038/sj.cdd.4401616. [DOI] [PubMed] [Google Scholar]
- 32.Hurtrel B, Petit F, Arnoult D, Muller-Trutwin M, Silvestri G, Estaquier J. Apoptosis in SIV infection. Cell Death Differ. 2005;12 (Suppl. 1):979–990. doi: 10.1038/sj.cdd.4401600. [DOI] [PubMed] [Google Scholar]
- 33.Petrovas C, Mueller YM, Dimitriou ID, Bojczuk PM, Mounzer KC, Witek J, Altman JD, Katsikis PD. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J. Immunol. 2004;172:4444–4453. doi: 10.4049/jimmunol.172.7.4444. [DOI] [PubMed] [Google Scholar]
- 34.Wang XZ, Brehm MA, Welsh RM. Preapoptotic phenotype of viral epitope-specific CD8 T cells precludes memory development and is an intrinsic property of the epitope. J. Immunol. 2004;173:5138–5147. doi: 10.4049/jimmunol.173.8.5138. [DOI] [PubMed] [Google Scholar]
- 35.Franco A, Tilly DA, Gramaglia I, Croft M, Cipolla L, Meldal M, Grey HM. Epitope affinity for MHC class I determines helper requirement for CTL priming. Nat. Immunol. 2000;1:145–150. doi: 10.1038/77827. [DOI] [PubMed] [Google Scholar]