Abstract
Photochromic channel blockers provide a conceptually simple and convenient way to modulate neuronal activity with light. We have recently described a family of azobenzenes that function as tonic blockers of Kv channels but require UV-A light to unblock and need to be actively switched by toggling between two different wavelengths. We now introduce red-shifted compounds that fully operate in the visible region of the spectrum and quickly turn themselves off in the dark. Furthermore, we have developed a version that does not block effectively in the dark-adapted state, can be switched to a blocking state with blue light, and reverts to the inactive state automatically. Photochromic blockers of this type could be useful for the photopharmacological control of neuronal activity under mild conditions.
Keywords: photopharmacology, ion channel blockers, voltage-gated potassium channels, photochromic molecules, azobenzenes
The merger of artificial photoswitches with natural receptor proteins has proven to be an effective way to control neural activity with light.1−4 This approach combines the virtually limitless repertoire of synthetic chemistry with a detailed understanding of the transmembrane proteins that underlie the generation and modulation of action potentials. As such, it provides a useful alternative to naturally occurring light-gated ion channels and light-powered pumps, which are currently driving the field of optogenetics.5 Most of these are based on a single photoswitch, retinal, which is often endogenously produced and does not need to be added externally. While this provides advantages in terms of practicality, it confines the tuning of these systems to mutations in the protein surrounding the chromophore.6 By contrast, a combination of synthetic photoswitches and natural receptors should allow for more flexibility, since both components can be manipulated through chemistry and genetic engineering, respectively.
Artificial photoswitches can be combined with endogenous neural receptors through covalent or noncovalent bonding.7 So-called photochromic ligands (PCLs) bind noncovalently and contain a photoswitchable moiety whose configuration can be changed upon irradiation.7−10 As such, they change their efficacy in a light-dependent way and essentially function as photochromic neurotransmitters or neuromodulators. Since they are distributed like small-molecule drugs, they are able to photosensitize naïve tissues within minutes, as opposed to days in the case of optogenetic probes. PCLs can be rapidly switched by using two different wavelengths of light that greatly favor one isomer or the other. Alternatively, one could use photoswitches that are actively switched to one isomer with light but thermally revert to the more stable form in the dark. This obviates the need to work with two different wavelengths but requires switches that turn themselves off at appropriate rates.
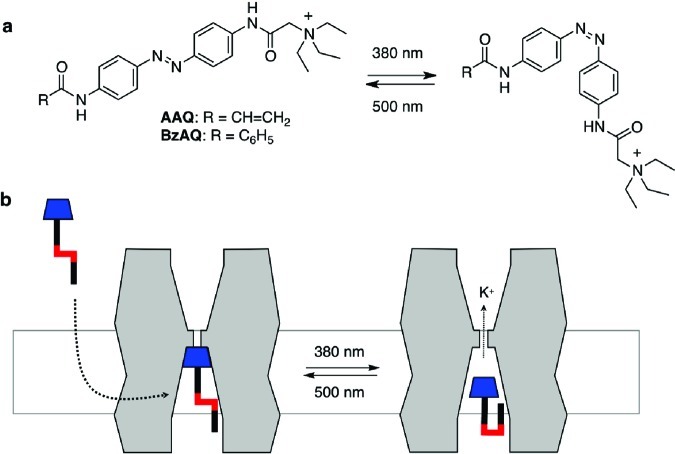
Recently, we introduced a family of simple azobenzene derivatives that function as PCLs for tetrameric voltage-gated ion channels, in particular potassium channels.7,9 These molecules, represented by AAQ and BzAQ (Figure 1), operate as photochromic open channel blockers, that bind in a light-dependent manner to the tetraethylammonium (TEA) binding site located in the inner cavity of potassium channels.7 In their extended trans form, AAQ and BzAQ fit into this cavity, but in their bent cis form their apparent affinity drops sharply. To reach their binding site, these amphiphilic molecules can either partition into the membrane or they can be imported through a patch pipet, whose content can rapidly exchange with the cytosol. Although many details of this reversible molecular encapsulation of azobenzenes by channel proteins remain to be clarified, they have already proven themselves as effective modulators of neural activity. For instance, Purkinje neurons and pacemaker neurons in the heart of Hirudo medicinalis could be controlled with photochromic neuromodulators of this type.9
Figure 1.
(a) Molecular structures of AAQ and BzAQ, two PCLs for Kv channels; (b) AAQ, BzAQ, and related PCLs are membrane permeable and function as photochromic open-channel blockers.
One of the greatest advantages of azobenzene photoswitches is the well-understood effect of substitutions and molecular extensions on their photophysical properties and thermal stability.11 So-called “regular azobenzenes”, represented by the parent molecule, as well as the bis-acylated azodianilines AAQ and BzAQ, are thermodynamically more stable in their trans-configuration, which completely predominates in the dark-adapted state. Their photostationary cis/trans ratios (PSRs) assume their maximum values in the UV-A region of the electromagnetic spectrum (315–380 nm). At these wavelengths, cis/trans ratios exceeding 9:1 can be observed.12 Once the light is turned off, the cis-isomers of regular azobenzenes are thermally relatively stable. The half-life of AAQ in physiological solution at room temperature, for instance, is 7–8 min.
Azobenzenes with strongly electron-donating substituents on both rings are known to absorb at increased wavelengths and have an increased rate of thermal back-isomerization from cis to trans.11,13 In addition to these, one could use azobenzenes that feature an electron-donating substituent on one end and an electron-withdrawing one on the other. These “push–pull” azobenzenes, which are also referred to as “pseudo-stilbenes”, are marked by greatly red-shifted absorption spectra. They are also thermally instable in their cis form and revert at room temperature to the thermodynamically more stable trans form on a millisecond to second time scale. The rate of this reversal is greatly dependent on the solvent, with polar protic solvents promoting very fast isomerization.14 As such, push–pull azobenzenes are ideally suited as photochromic ion channel blockers that can be activated with visible light and turn themselves off once the light intensity drops below a certain level. Studies using red-shifted blockers would generally benefit from the deeper tissue penetration of light with longer wavelength. Since longer wavelengths are also associated with less phototoxicity, photochromic compounds of this type would be particularly useful in chemical approaches toward restoring vision.
Results and Discussion
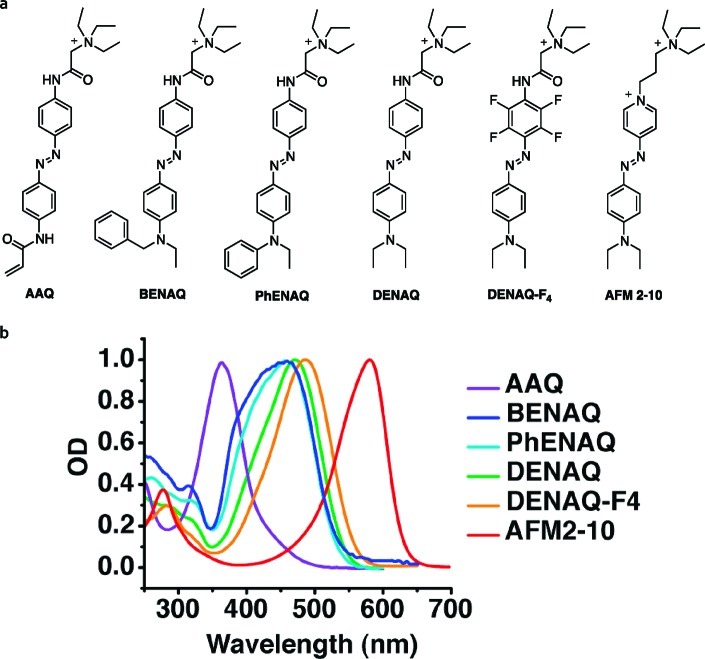
We now present a family of push–pull azobenzenes that have red-shifted action spectra and decreased thermal stability and function as photochromic blockers of voltage-gated ion channels (Figure 2). These molecules feature a strongly electron-donating dialkylamino or aryl alkylamino group on one side and a mildly electron-withdrawing acylamino moiety that terminates in a quaternary ammonium ion on the other side of the azobenzene. This positively charged “head group” interacts with the TEA binding site in the inner lumen of the channel, which blocks the flow of ions. The “tail” of the molecules, that is, the electron-donating substituent, determines the spectral properties of the photoswitch as well as the thermal stability of the cis-isomer.
Figure 2.
(a) Potential photochromic Kv blockers investigated in this study. (b) UV/Vis spectra of the azobenzenes investigated. Spectra were recorded at room temperature in phosphate buffered saline solution at pH 7.4.
Spectroscopic Characterization of Red-Shifted PCLs
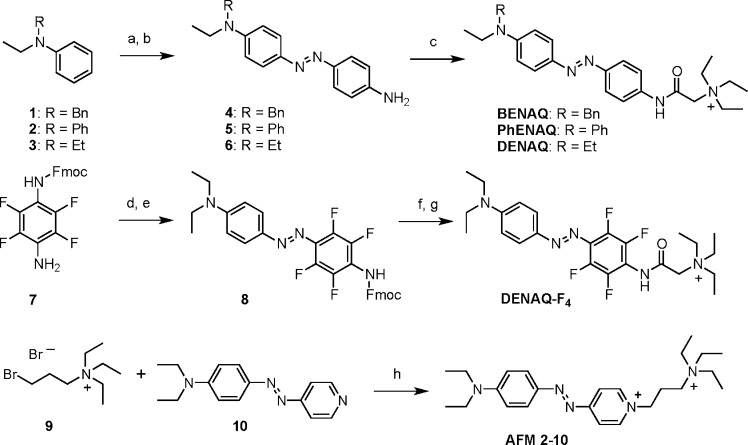
AAQ, a representative of our original type, which is marked by a bis-acylated azodianiline core, serves as a point of reference (Figure 2). Spectroscopically, it can be classified as a “regular azobenzene” with a strong π–π* band at 362 nm in water (trans isomer). Replacement of the lower acylamino group with an alkylamino group increases the electron density of one side and shifts the absorption spectrum of the trans-isomer toward the red. For instance, the absorption spectrum of trans-PhENAQ, which bears a phenylethylamino group, is shifted to 456 nm in phosphate buffered saline solution. BENAQ, which bears a benzylethylamino substituent, has a similar absorption spectrum peaking at 459 nm. The diethylamino derivative DENAQ is shifted to an larger extent, as its absorption spectrum peaks at 470 nm. To increase the push–pull effect without significantly altering the sterics of the azobenzene moiety, we also explored fluorinated derivatives, such as DENAQ-F4. This compound shows a relatively small bathochromic shift with respect to DENAQ (484 nm). Our most red-shifted compound, AFM2-10, which peaks at 580 nm, is in essence an azobenzene version of the well-known fluorescent styrene dye FM2-10. The synthesis of these compounds follows standard protocols and is summarized in Scheme 1 (for further details see the Supporting Information).
Scheme 1. Synthesis of BENAQ, PhENAQ, DENAQ, DENAQ-F4, and AFM 2-10.
Reagents and conditions: (a) 4-nitroaniline, isoamylnitrite, HCl, MeOH (87% for 1, 79% for 2, 62% for 3); (b) Na2S, H2O, 1,4-dioxane, 90 °C (88% for 4, 42% for 5, 88% for 6); (c) 2-triethylammonium acetic acid chloride, DIPEA, DMF, 0 °C to RT (67% for BENAQ, 66% for PhENAQ, 92% for DENAQ); (d) BF3 Et2O, isoamylnitrite, THF −40 to −5 °C; (e) diethylaniline, NaOAc, 0 °C to RT (36% over two steps); (f) piperidine, Et2O, 0 °C to RT (85%); (g) 2-triethylammonium acetic acid chloride, DIPEA, DMF, 0 °C to RT (38%); (h) DMF, 80 °C (92%).
In general, the absorption spectra of the trans-isomers of azobenzenes are correlated with their PSRs at different wavelengths. Hence, red-shifting of the absorption maximum of their trans isomer through appropriate substitutions should also red-shift the PSR maximum as a function of the wavelength. This is difficult to measure with our push–pull azobenzenes because their thermal relaxation is too fast to enrich the cis isomer in aqueous solution for detection by standard spectrophotometric methods.15 Similar observations were made by Uyeda et al.16 who investigated structurally related azobenzenes that bore a dimethylamino substituent on one side and acylamino substituents on the other. In this case, thermal cis- to trans-isomerization was very fast in aqueous solution and only detectable by flash laser photolysis. By contrast, the thermal isomerization was found to be relatively slow in dimethyl sulfoxide (DMSO), and PSRs up to 78% cis could be observed in this solvent. Nevertheless, these photoswitches performed well in aqueous solution when incorporated in DNA.17 Preliminary experiments with our compounds show similar PSRs in DMSO (see Supporting Information Figure 1), but it is difficult to extrapolate this to aqueous buffer solutions.
Electrophysiological Characterization of Red-Shifted PCLs
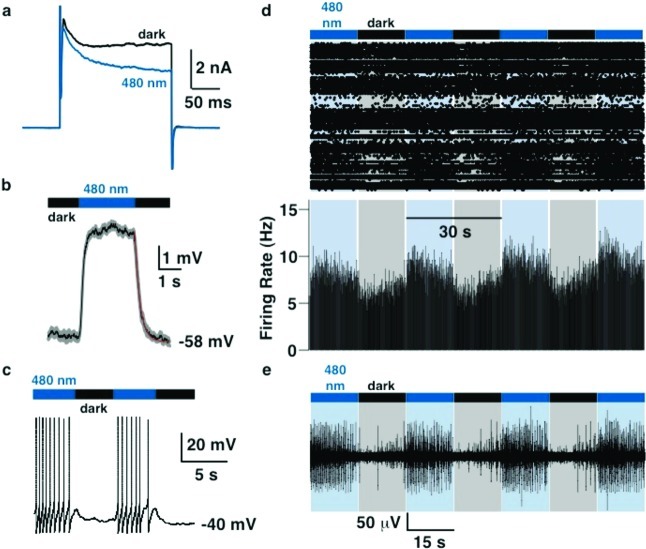
The electrophysiological action spectrum of photochromic channel blockers should mirror their absorption spectra and PSRs in solution, provided the interaction with the channel protein does not greatly influence these. Push–pull azobenzenes, such as DENAQ or PhENAQ, with their red-shifted absorption spectra, are therefore expected to show a red-shifted action spectrum and fully operate in the visible region of the electromagnetic spectrum. This is indeed the case. Figure 3 shows the effects of 100 μM DENAQ on the conductance of a voltage-gated potassium channel at different wavelengths. Recordings were performed in HEK 293 cells transiently expressing Kv3.1 in whole-cell voltage clamp mode after transient treatment with the PCL. Our data show that DENAQ is a much better blocker in the dark adapted-state than at 480 nm (Figure 3a), which is true at all membrane potentials tested (Figure 3b). Unblock could be achieved at all wavelengths between 380 and 540 nm, with maximum unblock observed around 480 nm (Figure 3c and d). At this wavelength, percent photoswitching, as determined by the difference between the maximum current under irradiation and in the dark, divided by the maximum current under irradiation, is 63.2 ± 7.2% (n = 5 cells). Thus, the action spectrum of DENAQ is shifted by ca. 100 nm with respect to AAQ and BzAQ. Remarkably, the blocking effect of trans-DENAQ is restored within seconds once the light is turned off (t1/2 = 305 ± 57 ms, averaged over n = 4 cells, Figure 3e). DENAQ is therefore a trans-blocker with a strongly red-shifted action spectrum and decreased thermal stability with respect to AAQ and BzAQ.
Figure 3.
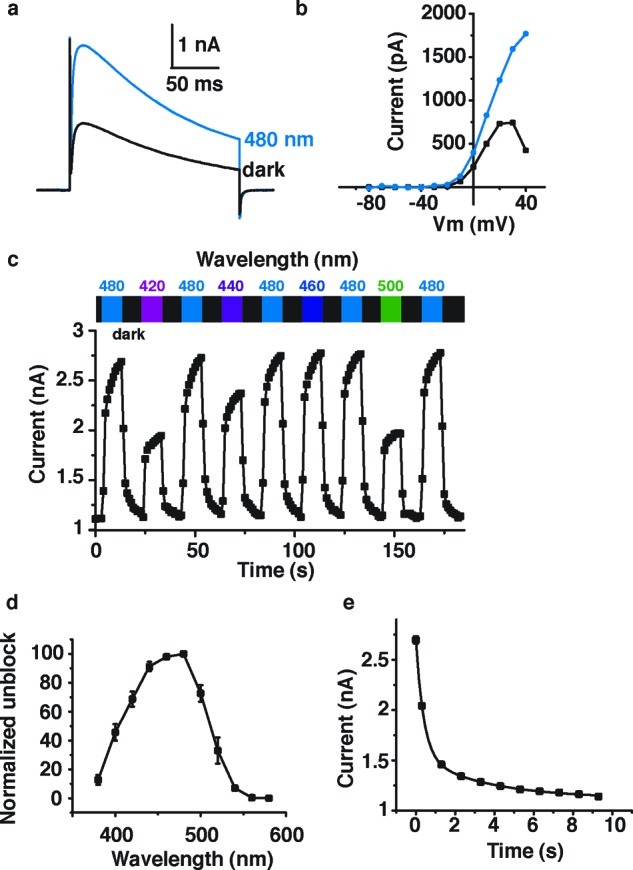
Effect of DENAQ on Kv3.1 expressed in HEK293 cells. (a) Cells were treated with 100 μM DENAQ. Kv current was measured in whole cell mode using a 200 ms depolarization from −60 to +40 mV, in the dark and under 480 nm light. Capacitive currents have been cut off for clarity. (b) Membrane-voltage dependence of block and unblock. Steady-state current (at the end of the 200 ms depolarization) is plotted as a function of membrane potential. (c) Reversibility of photoswitching and action spectrum of DENAQ on Kv3.1. Potassium current was measured using the protocol described in (a) looped at 1 Hz. Peak current is plotted as a function of time. Cycles of dark and illumination (420–500 nm) are indicated. (d) Unblock as a function of wavelength. Unblock was normalized to 100% for 480 nm light (n = 3–4 cells). (e) Apparent thermal relaxation rate of DENAQ measured by electrophysiology. Kv3.1 peak current is plotted as function of time after light is switched off. Four light-dark cycles are averaged for this single cell. Data points were fitted with a biexponential decay equation: y = y0 + A1 exp(−x/τ1) + A2 exp(−x/τ2) with y0 = 1122 ± 23 pA, A1 = 1148 ± 59 pA, τ1 = 392 ± 37 ms, A2 = 422 ± 39 pA, and τ2 = 3.5 ± 0.8 s.
The compound PhENAQ has similar spectral properties but shows a key difference in terms of its blocking state. It is a red-shifted azobenzene that preferentially blocks in its thermodynamically less stable cis-form, that is, at 480 nm (Figure 4a). In this case, electrophysiological recordings were performed in HEK 293 cells transiently expressing Shaker-IR in whole-cell voltage clamp mode. Percent photoswitching, as determined by the difference between the steady-state current in the dark and in 480 nm light, divided by the current in the dark, was found to be 29.4 ± 4.8% (n = 3 cells). cis-Block occurred at all membrane potentials tested (Figure 4b) and was fully reversible over many cycles (Figure 4c). Once the light is turned off, PhENAQ quickly reverts to the less blocking trans state (t1/2 = 2.6 ± 0.1 s, averaged for n = 3 cells) (Figure 4d). Thus, PhENAQ has little effect when added in the dark (or at longer wavelengths) but becomes an efficient blocker when irradiated with blue light.
Figure 4.
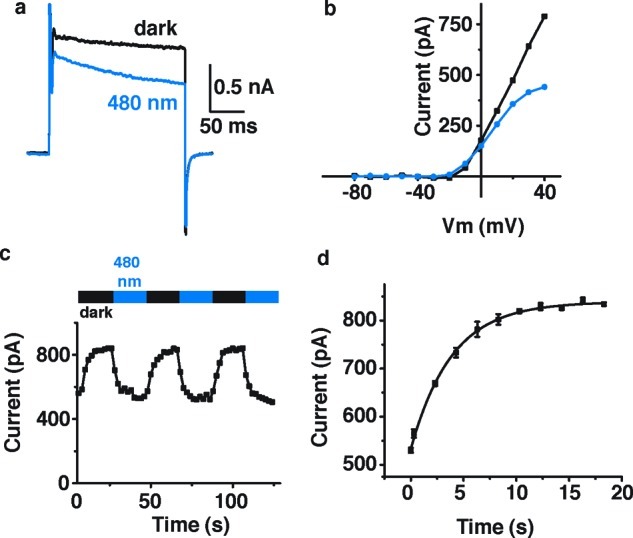
Effects of PhENAQ on Shaker-IR expressed in HEK 293 cells. Cells were treated with 50 μM PhENAQ. (a) Kv current was measured using a 200 ms depolarization from −60 to +40 mV, in the dark and under 480 nm light. (b) Current versus voltage dependence of block and unblock, under 480 nm light and in the dark, respectively. Steady state current was plotted as a function of membrane potential. (c) Reversibility of PhENAQ using cycles of 480 nm light and dark. Peak current was measured using the protocol described in (a) looped at 0.5 Hz. (d) Apparent thermal relaxation rate of PhENAQ measured by electrophysiology. Shaker-IR peak current is plotted as a function of time after the light is switched off. Three light/dark cycles are averaged for this single cell. Data points were fitted with a monoexponential decay equation: y = y0 + A exp(−x/τ) with y0 = 838 ± 3 pA, A = −306 ± 5 pA, and τ = 3.9 ± 0.2 s.
In comparison, DENAQ and PhENAQ show some key differences, beyond their trans versus cis activity. Structurally, they are only distinguished by the presence of an ethyl and phenyl substituent, respectively. However, DENAQ does not appear to affect Shaker-IR channels, which we routinely use in our investigations on photochromic Kv blockers, yet it cleanly blocks Kv3.1 channels. By contrast, PhENAQ blocks Shaker-IR and a range of other Kv channels (not shown). Why it does so preferentially in its cis-form remains an open question. It could be due to an attractive interaction of the phenyl ring with an amino acid residue lining the inner lumen of the channel, which can only take effect when the azobenzene is cis-configured. Also noticeable is the difference in the rate of thermal relaxation (t1/2 = 305 ± 57 ms vs 2.6 ± 0.1 s). This could reflect either differences in the dissociation from the channel protein as the rate-determining step or different rates of the thermal isomerization in solution. Detailed structural and kinetic investigations will be needed to clarify these points.
The remaining azobenzenes shown in Figure 2 were tested on HEK cells expressing various channels but failed to show functional features that significantly go beyond DENAQ or PhENAQ. Despite its seemingly small chemical modification, DENAQ-F4 proved too to toxic to be viable as a photochromic blocker. AFM2-10 had virtually no effect on the conductance of potassium channels, even at very high concentrations (500 μM). It appears that subtle changes in the composition the molecules influence not only the photophysical and thermal but also the pharmacological properties of our photochromic blockers.
Optical Regulation of Neuronal Excitability
cis-Blockers, such as PhENAQ, have the inherent advantage that they have little effect on ion channels in their thermodynamically more stable, dark-adapted state, which should make them less toxic to excitable cells. We therefore decided to focus on PhENAQ and investigate its effect on neuronal firing patterns. In accordance with its effect on Shaker-IR in HEK 293 cells, PhENAQ behaves as a cis-blocker of voltage-gated potassium channels endogenously expressed in hippocampal neurons (percent photoswitching = 24.5 ± 4.5%, n = 3 cells, Figure 5a). To look at optical modulation of membrane potential, we performed current clamp experiments. When the membrane potential of the cell was close to the resting potential, PhENAQ rapidly and reliably elicited membrane depolarization upon 480 nm light irradiation (Δ = 6.0 ± 0.4 mV, n = 4 cells) without inducing cell firing (Figure 5b). When switched back to the dark, repolarization occurred within a second (t1/2 = 163 ± 2 ms, n = 4 cells). When current was injected to bring the cell closer to threshold for firing, light-induced depolarization triggered action potential firing, which ceased rapidly when the light was switched off (Figure 5c).
Figure 5.
Effect of PhENAQ on neuronal firing. (a) Voltage-clamp recording from a hippocampal neuron treated with 50 μM PhENAQ. Voltage-gated potassium current was elicited using a 200 ms depolarization from −60 to +40 mV, under 480 nm light irradiation or in the dark. (b) Reproducibility of light-evoked membrane depolarization in neurons. Current clamp recordings were made from a hippocampal neuron treated with 50 μM PhENAQ. Average trace (black) and standard deviation (gray) of four light cycles are shown. Apparent thermal relaxation rate was fitted with a monoexponential decay equation (red): y = y0 + A exp(−x/τ) with y0 = −46.78 ± 0.01 mV, A = 3.86 ± 0.02 mV, and τ = 237 ± 3 ms. (c) Optical regulation of action potential firing. (d) Multielectrode array recording from an acute rat cerebellar slice. Top, raster plot of spiking; bottom, average firing rate calculated in 100 ms time bins. (e) Extracellular recording from a single cell using the multielectrode array. Color bars represent illumination with 480 nm (blue) or periods of darkness (black).
The depolarization required to induce spiking depends not only on the various ion channels expressed in a given type of cell but also on the strength and variability of its synaptic input. To test whether PhENAQ can modulate action potential firing of cells that are not artificially depolarized, we recorded extracellularly from cerebellar slices using a multielectrode array (MEA) with three-dimensional electrodes. Cerebellar slices are marked by high levels of spontaneous neuronal activity, probably originating from Purkinje neurons.18 As can be seen in Figure 5d, neuronal firing was increased upon irradiation with 480 nm but decreased markedly once the light was turned off. Although the effects are not large, they are reversible and reproducible. They are probably limited by the low solubility of PhENAQ in buffer solution, which makes it difficult to deliver uniformly in brain slices. Figure 5e shows an example of a single cerebellar neuron whose activity was strongly photomodulated. As a control, we checked that light itself has no effect on the activity of naïve cerebellar slices (Supporting Information Figure 2).
Conclusion
Following a general paradigm for tuning azobenzene photoswitches, we have developed compounds that function as red-shifted photochromic blockers of potassium channels, which turn themselves off automatically in the dark. The lessons learned during this study could be applied to other types of photoswitches, including photochromic versions of the neurotransmitter glutamate19,20 and covalently tethered blockers, agonists, and antagonists of neural receptor proteins (so-called photochromic tethered ligands, or PTLs).12,21−24
One of our most advanced compounds, DENAQ, is a blocker of Kv3.1 channels that is active in its dark-adapted trans state. By contrast, PhENAQ is a cis-blocker that becomes active upon irradiation with blue light, which makes it an attractive molecule for the optical regulation of neuronal excitability. A photochromic cis-blocker of Shaker channels has been previously observed, but it was neither red-shifted nor thermally instable and it proved to be too toxic to be of any practical use.7 Although tonic blockers have performed remarkably well in complex neural systems, cis-blockers such as PhENAQ could have advantages where a minimum of perturbation upon addition of the compound is desirable. Both DENAQ and PhENAQ revert thermally, that is, in the dark, to their default trans states within hundreds of milliseconds to seconds.
The use of these compounds in the photopharmacological control of electrical activity with visible light in neural tissue such as the retina is currently under investigation. Their effects on other voltage-gated ion channels, such as Nav and Cav channels, are also under study in our laboratories. Finally, the incorporation of red-shifted, thermally destabilized azobenzenes into other soluble and tethered photochromic ligands is under active investigation and will be reported in due course.
Methods
Synthesis
See the Supporting Information.
Spectroscopic Analysis
UV/Vis spectra were measured at room temperature using a SmartSpec Plus photometer (Biorad). UV/Vis spectra of thermally unstable compounds were measured on a NanoDrop 2000c (Thermo Scientific) instrument at concentrations of approximately 1 mM in DMSO solvent. Photoisomerization was achieved by directly illuminating a 1 μL sample on the pedestal with 473 nm light from a 200 μm, 0.22 NA fiber optic cable placed perpendicular to the optical path such that the sample was at the focal point of the fiber tip. 150 mW was delivered to the sample from a 200 mW DPSS blue 473 nm laser (MBL-III-473, Opto Engine).
Cell Culture
HEK 293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Cells were plated on 12 mm diameter poly-l-lysine coated glass coverslips at a density of 20 000 cells/cm2. Transfection was performed using the calcium phosphate method, as already described.9 We transfected cells either with Shaker-IR (Inactivation Removed)25 or rat Kv3.1 cDNA, using a bicistronic GFP-expression vector (pIRES). Cells were recorded 12–48 h after transfection. Primary cultures of neonatal rat hippocampal neurons were performed using standard procedures, as previously described.9 Recordings were performed 2–3 weeks after plating.
Cerebellar Slice Preparation
Sagittal cerebellar slices were prepared from 13–17 day-old Sprague–Dawley rats. Briefly, the animal was decapitated under isoflurane anesthesia and the brain was quickly removed. Sagittal slices (340 μm thick) were cut with a microtome (Leica VT1000S; Leica Microsystems, Wetzlar, Germany) in an ice-cold artificial cerebrospinal fluid (ACSF) and then placed in an incubating chamber for 30 min at 34 °C. Thereafter, slices were kept at room temperature. Bicarbonate-buffered ACSF was used as the slicing, storage, and recording solution; composition in mM: NaCl 126, KCl 2.5, NaHCO3 26, NaH2PO4 1.25, CaCl2 2.5, MgSO4 1.3, and glucose 10, saturated with O2/CO2 (95/5%).
Animal care and experimental protocols were approved by the University of California Berkeley Animal Care and Use Committee.
Electrophysiology
Cells were incubated with 10 μM to 1 mM (as indicated) photoswitch for 15 min in the cell incubator (37 °C, 7% CO2, dark). The photoswitch was diluted in extracellular solution (contains in mM: NaCl 138, KCl 1.5, MgCl2 1.2, CaCl2 2.5, HEPES free acid 5, and glucose 10, pH 7.4). Final DMSO content was always ≤1% vol/vol. Cells were then washed twice with 500 μL of extracellular solution and directly used for electrophysiological recordings. Whole-cell recordings were performed using an PC-505B amplifier (Warner Instruments, CT) linked to a personal computer equipped with pClamp8 (Molecular Devices). Patch pipettes had resistances between 3 and 4 MΩ and were filled with intracellular solution (contains in mM: NaCl 10, K+ gluconate 135, HEPES free acid 10, MgCl2 2, MgATP 2, EGTA 1, pH 7.4). For neuronal recordings only, synaptic transmission was blocked using 25 μM DNQX and 20 μM biccuculine, and voltage-gated sodium channels were blocked using 1 μM tetrodotoxin in the extracellular solution (in voltage-clamp mode only). To measure voltage-gated K+ current from HEK cells or neurons, the holding membrane potential was set to −60 mV and stepped to +40 mV (unless otherwise indicated) for 200 ms at 0.5 or 1 Hz. In current clamp mode, injection of current was used to depolarize the cell and induce action potential firing. For illumination, we used a monochromator (Polychrome V, Till photonics) controlled using Clampex and connected to the back of the microscope using a UV/Vis quartz fiber. Light output measured using a hand-held power meter (Newport 840-C) and through a 20× objective was 4–7 mW/mm2. Data were filtered at 2 kHz and acquired at a sampling frequency of 10 kHz.
Multielectrode Array (MEA) Recordings
Experiments were performed at room temperature in ACSF saturated with O2/CO2. Slices were incubated with 50 μM PhENAQ (DMSO concentration 0.5% vol/vol) for 15 min at room temperature and then washed 5 min prior to recording. Slices were then placed on a tridimensional microarray made of 60 pyramid-shape microelectrodes (MEA60 200 3D GND, Ayanda Biosystems SA, Lausanne, Switzerland). Recordings were acquired with an MEA-1060 amplifier board (gain 1200, sampling frequency 20 kHz, Butterworth second order highpass filter 300 Hz, Multi Channel Systems, Reutlingen, Germany) positioned on the stage of an inverted microscope (Olympus IX71). Principal component analysis of spike waveforms was used for sorting spikes generated by individual cells (Offline Sorter; Plexon, Denton, TX). Light was delivered using the 100 W halogen lamp of the microscope and a 480/40 bandpass filter, and was focused on the slice using a 4× objective (Olympus UPLanFL N, NA 0.13). Light was computer-controlled using a filter-wheel controller (Lambda 10-3, Sutter Instruments) and an ultrafast shutter (Uniblitz VCM-D1, Vincent Associates). Light intensity measured at the back of the 4× objective and through the MEA was 17–28 mW/mm2. Slices were continuously superfused during recording with fresh ACSF.
Statistics and Data Analysis
Data were analyzed using Clampfit 10 (Molecular Devices, Sunnyvale, CA), MC Rack (Multi Channel Systems, Reutlingen, Germany), Offline Sorter (Plexon, Denton, TX), and Origin (OriginLab, Northampton, MA) software. Statistical analysis was performed using Origin. All values reported are mean ± SEM except when stated.
Acknowledgments
We thank Caleb M. Smith for his help with the MEA data analysis.
Support for the work was provided by the Nanomedicine Development Center for the Optical Control of Biological Function, PN2EY018241 (D.T. and R.H.K.), the National Institutes of Health (RO1 EY018957 and RO1MH088484, R.H.K.), and the Deutsche Forschungsgemeinschaft (SFB 749, D.T.).
Author Contributions
M.R.B., A.M., and D.T. designed the research. A.M. and D.T. wrote the paper. M.A.K., M.S., and F.M.E.H. synthesized the compounds. A.M. and T.F. carried out the biological investigations.
Supporting Information Available
Additional figures and experimental procedures, and general experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ These authors contributed equally to this work.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Banghart M. R.; Volgraf M.; Trauner D. (2006) Engineering light-gated ion channels. Biochemistry 45(51), 15129–15141. [DOI] [PubMed] [Google Scholar]
- Gorostiza P.; Isacoff E. Y. (2008) Optical switches for remote and noninvasive control of cell signaling. Science 322(5900), 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H.; Fortin D. L.; Trauner D. (2009) New photochemical tools for controlling neuronal activity. Curr. Opin. Neurobiol. 19(5), 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G.; Heckel A. (2006) Biologically active molecules with a “light switch. Angew. Chem., Int. Ed. 45(30), 4900–4921. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. (2011) Optogenetics. Nat. Methods 8(1), 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P.; Moglich A. (2011) Channelrhodopsin engineering and exploration of new optogenetic tools. Nat. Methods 8(1), 39–42. [DOI] [PubMed] [Google Scholar]
- Banghart M. R.; Mourot A.; Fortin D. L.; Yao J. Z.; Kramer R. H.; Trauner D. (2009) Photochromic blockers of voltage-gated potassium channels. Angew. Chem., Int. Ed. 48(48), 9097–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E.; Wassermann N. H.; Erlanger B. F. (1971) Photochromic activators of the acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 68(8), 1820–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin D. L.; Banghart M. R.; Dunn T. W.; Borges K.; Wagenaar D. A.; Gaudry Q.; Karakossian M. H.; Otis T. S.; Kristan W. B.; Trauner D.; Kramer R. H. (2008) Photochemical control of endogenous ion channels and cellular excitability. Nat. Methods 5(4), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A.; Chang H. W. (1977) Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature 266(5600), 373–374. [DOI] [PubMed] [Google Scholar]
- Knoll, H. Photoisomerization of Azobenzenes. In CRC Handbook of Organic Photochemistry and Photobiology; Horspool W. M., Lenci, F., Eds.; CRC Press: Boca Raton, FL, 1996, 2nd ed., pp 89.1−89.16. [Google Scholar]
- Gorostiza P.; Volgraf M.; Numano R.; Szobota S.; Trauner D.; Isacoff E. Y. (2007) Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc. Natl. Acad. Sci. U.S.A. 104(26), 10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovski O.; Beharry A. A.; Zhang F.; Woolley G. A. (2009) Spectral tuning of azobenzene photoswitches for biological applications. Angew. Chem., Int. Ed. 48(8), 1484–1486. [DOI] [PubMed] [Google Scholar]
- Schanze K. S.; Mattox T. F.; Whitten D. G. (1983) Solvent Effects upon the Thermal Cis-Trans Isomerization and Charge-Transfer Absorption of 4-(Diethylamino)-4′-nitrobenzene. J. Org. Chem. 48(17), 2808–2813. [Google Scholar]
- Whitten D. G.; Wildes P. D.; Pacifici J. G.; Irick G. Jr. (1971) Solvent and substituent on the thermal isomerization of substituted azobenzenes. Flash spectroscopic study. J. Am. Chem. Soc. 93(8), 2004–2008. [Google Scholar]
- Kamei T.; Kudo M.; Akiyama H.; Wada M.; Nagasawa J.; Funahashi M.; Tamaoki N.; Uyeda T. Q. P. (2007) Visible-Light Photoresponsivity of a 4-(Dimethylamino)azobenzene Unit Incorporated into Single-Stranded DNA: Demonstration of a Large Spectral Change Accompanying Isomerization in DMSO and Detection of Rapid (Z)-to-(E) Isomerization in Aqueous Solution. Eur. J. Org. Chem. 2007(11), 1846–1853. [Google Scholar]
- Kamei T.; Akiyama H.; Morii H.; Tamaoki N.; Uyeda T. Q. (2009) Visible-light photocontrol of (E)/(Z) isomerization of the 4-(dimethylamino)azobenzene pseudo-nucleotide unit incorporated into an oligonucleotide and DNA hybridization in aqueous media. Nucleosides, Nucleotides Nucleic Acids 28(1), 12–28. [DOI] [PubMed] [Google Scholar]
- Kessler M.; Kiliman B.; Humes C.; Arai A. C. (2008) Spontaneous activity in Purkinje cells: multi-electrode recording from organotypic cerebellar slice cultures. Brain Res. 1218, 54–69. [DOI] [PubMed] [Google Scholar]
- Volgraf M.; Gorostiza P.; Szobota S.; Helix M. R.; Isacoff E. Y.; Trauner D. (2007) Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J. Am. Chem. Soc. 129(2), 260–261. [DOI] [PubMed] [Google Scholar]
- Abrams Z. R.; Warrier A.; Trauner D.; Zhang X. (2010) A Signal Processing Analysis of Purkinje Cells in vitro. Front. Neural Circuits 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart M.; Borges K.; Isacoff E.; Trauner D.; Kramer R. H. (2004) Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7(12), 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. J.; Banghart M. R.; Trauner D.; Kramer R. H. (2006) Light-induced depolarization of neurons using a modified Shaker K(+) channel and a molecular photoswitch. J. Neurophysiol. 96(5), 2792–2796. [DOI] [PubMed] [Google Scholar]
- Szobota S.; Gorostiza P.; Del Bene F.; Wyart C.; Fortin D. L.; Kolstad K. D.; Tulyathan O.; Volgraf M.; Numano R.; Aaron H. L.; Scott E. K.; Kramer R. H.; Flannery J.; Baier H.; Trauner D.; Isacoff E. Y. (2007) Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54(4), 535–545. [DOI] [PubMed] [Google Scholar]
- Volgraf M.; Gorostiza P.; Numano R.; Kramer R. H.; Isacoff E. Y.; Trauner D. (2006) Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2(1), 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T.; Zagotta W. N.; Aldrich R. W. (1990) Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250(4980), 533–538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.